Abstract
KCNQ2 and KCNQ3 pathogenic channel variants have been associated with a spectrum of developmentally regulated diseases that vary in age of onset, severity, and whether it is transient (i.e Benign Familial Neonatal Seizures) or long lasting (i.e Developmental and Epileptic Encephalopathy). KCNQ2 and KCNQ3 channels have also emerged as a target for novel antiepileptic drugs as their activaton could reduce epileptic acivity. Consequently, a great effort has taken place over the last two decades to understand the mechanisms that control the assembly, gating, and modulation of KCNQ2 and KCNQ3 channels. The current view that KCNQ2 and KCNQ3 channels assemble as heteromeric channels (KCNQ2/3) forms the basis of our understanding of KCNQ2 and KCNQ3 channelopathies and drug design. Here, we review the evidence that supports the formation of KCNQ2/3 heteromers in neurons. We also highlight functional and transcriptomic studies that suggest channel composition might not be necessarily fixed in the nervous system, but rather is dynamic and flexible, allowing some neurons to express KCNQ2 and KCNQ3 homomers. We propose that to fully understand KCNQ2 and KCNQ3 channelopathies, we need to adopt a more flexible view of KCNQ2 and KCNQ3 channel stoichiometry, which might differ across development, brain regions, cell types, and disease states.
Keywords: KCNQ2, KCNQ3, autism, epilepsy, potassium channels
Introduction
Over the last five years, trio-based whole-exome or targeted sequencing studies have identified numerous genes that contribute to the risk of neurodevelopmental disorders including KCNQ2 and KCNQ3 potassium channels [1–3]. Although the role of KCNQ2 and KCNQ3 in benigh forms of pediatric epilepsy was known since the late 90s[4], it was the targeted sequencing in 2012 of patients with unresolved epilepsy that first led to the discovery that KCNQ2 channel dysfunction is also associated with severe forms of epilepsy[5]. Since then, multiple studies have implicated KCNQ2 and KCNQ3 channel dysfunction in multiple monogenic neurodevelopmental disorders[6, 7].
Thus, work from many groups have now shown that KCNQ2 or KCNQ3 loss of function (i.e reduced potassium channel activity) could lead to early-onset epilepsy in children, that could be either benign or severe depending on the nature of the pathogenic variant[6]. Loss-of-function variants usually alter KCNQ2 and KCNQ3 gating, trafficiking or even subcellular localization. However, researchers have recently identified multiple KCNQ2 and KCNQ3 gain-of-function variants in patients with epileptic encephalopathy and autism spectrum disorders (https://gene.sfari.org/database)[8, 9, 3], respectively. KCNQ2 and KCNQ3 gain-of-function, reviewed in detail elsewhere[10], leads to greater KCNQ2 and KCNQ3 activity in heterologous cells[8]. This boosted KCNQ2 and KCNQ3 activity presumably results to reduce neuronal excitability. However, how decreasing neuronal excitability also leads to neuronal hypersychrony is not yet known.
In general, no cellular mechanisms have been proposed that fully explain the symptoms of patients carrying KCNQ2 and KCNQ3 loss or gain of function pathogenic variants, nor have any therapies been established. A leading hypothesis explaining the general genotype–phenotype relationship between KCNQ2 and KCNQ3 variants and neurodevelopmental disorders is based on a model in which KCNQ2 and KCNQ3 form heteromers (KCNQ2/3), and dysfunction of these heteromers leads to disease[7]. In this review, we discuss the data that have led to this model and explore the consequences of KCNQ2 and KCNQ3 channel composition regarding the genotype and phenotype relationships of KCNQ2- and KCNQ3-associated neurodevelopmental disorders.
KCNQ2 and KCNQ3: a brief history
In 1980, Brown and Adams recorded a slow-activating voltage-gated potassium current that was blocked by muscarine in sympathetic neurons, which they termed the M-current[11]. Since then, studies have demonstrated the M-current in almost all regions of the central and peripheral nervous system[12–14]. Along with apamin-sensitive SK channels, the M-current also forms the medium afterhyperpolarization that follows brief bouts of activity or an action potential afterdepolarization[15, 16]. In 1998, researchers identified the channel subunits that underlie the classical M-current; KCNQ2 and KCNQ3 heteromeric channels[17], which were previously identified through positional cloning in patients experiencing benign familial neonatal seizures[4]. The KCNQ channel family includes three other members: KCNQ1, KCNQ4, and KCNQ5[4]. KCNQ1 is present at minimal levels in the brain, while KCNQ4 is predominantly located in the inner ear, raphe nuclei, and some brainstem nuclei. The role of KCNQ5, which is ubiquitously expressed in the forebrain, has not been a major focus with the exception of a few studies. Previous work has identified KCNQ5 variants in patients with epilepsy[18] and more recent studies have suggested KCNQ5 variants as a possible locus for neuropsychiatric disorders[19]. KCNQ5 and KCNQ3 can also form heteromers that resemble the M-current[20, 21]; but the curent consensus is that KCNQ2/3 and not KCNQ3/5 heteromers mediate the classical the M-current[14, 7].
The current dogma is that KCNQ2 and KCNQ3 channels form heteromers (KCNQ2/3), which act as the predominant KCNQ channels in the central nervous system[17, 22]. As we discuss below, this viewpoint is supported by various findings, starting with the original study demonstrating that KCNQ2 and KCNQ3 form heteromers with properties that best resemble those of the native current in superior cervical ganglion neurons[17, 22, 23]. Most notably, KCNQ2/3 heteromers exhibit the same pharmacology as the native M-current, i.e., both KCNQ2 and KCNQ3 show similar sensitivities to extracellular application of Tetraethylammonium (TEA), XE991, and linopirdine, which are known M-current blockers[17, 22, 23].
Further evidence indicating that KCNQ2/3 heteromers mediate the M-current has arisen from a series of observations in heterologous cells, which showed that the expression of KCNQ2 and KCNQ3 homomers was very limited. In particular, KCNQ3 expression in oocytes led to no or very low (barely above background) voltage-activated potassium currents, whereas KCNQ2 expression led to small currents with a much lower density than KCNQ2/3 heteromers[24]. Initially, reports indicated that the co-expression of KCNQ2 and KCNQ3 channels could lead to an almost ten-fold higher current density than the expression of KCNQ2 channels alone[17, 25, 24]. However, subsequent studies have suggested that this increase could be smaller, closer to three- or four-fold rather than ten-fold. Independent of the precise amplification difference, co-expression of KCNQ2 with KCNQ3 leads to a larger current density than expression of KCNQ2 or KCNQ3 channels alone. This effect is not restricted to expression systems, but extends to neurons as well. For instance, Rasmussen et al (2007) [26] showed that co-transfecting KCNQ2 with KCNQ3 in cultured pyramidal neurons led to a 15-fold increase in the M-current density, much larger than that obtained by expressing KCNQ2 or KCNQ3 alone.
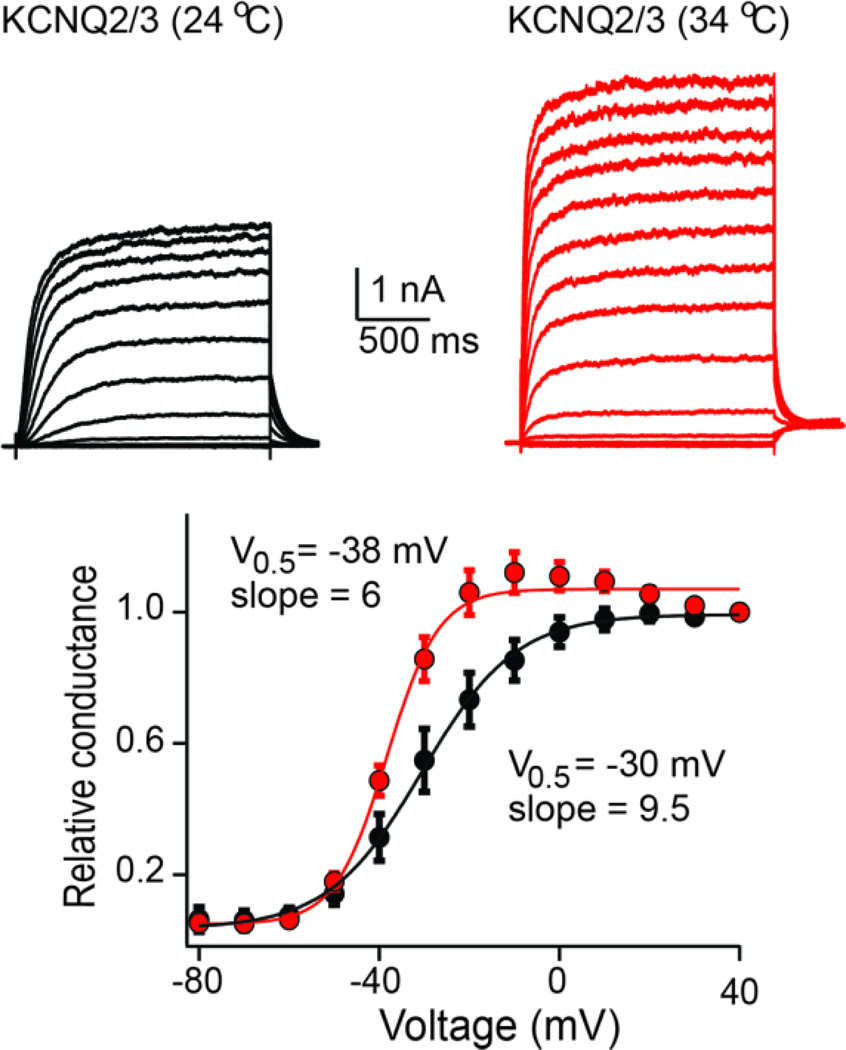
In subsequent years, researchers sought to understand the mechanisms that control KCNQ2/3 current levels in neurons. This work has shown that the magnitude of the KCNQ2/3 current in neurons depends on three primary factors. (i) Membrane potential. KCNQ2 and KCNQ3 channels are slow activating non-inactivating voltage-gated potassium channels whose probability of opening depends on the membrane potential[17, 25]. KCNQ2/3 channels start activating below the threshold for action potentials reaching peak activation above −20 mV, thus acting as a break[27] (Figure 1). (ii) Membrane PIP2 levels. KCNQ2 and KCNQ3 channels can be considered as voltage-gated as well as PIP2-activated potassium channels[13, 28]. KCNQ2 and KCNQ3 channels do not function in the absence of the phospholipid PIP2[28]. However, the affinity of KCNQ2 and KCNQ3 for PIP2 differs substantially, with KCNQ2 channels having a ~10-fold lower affinity than KCNQ3 channels[29]. As PIP2 can independently gate KCNQ2 and KCNQ3 channels, the PIP2 affinity of KCNQ2/3 is not simply an intermediate between that of KCNQ2 and KCNQ3; rather, it shows a two-component concentration relationship[30]. (iii) Co-expression of KCNQ2 with KCNQ3. Co-expression of these two channels leads to increased trafficking to the membrane[24, 31]. KCNQ3 channels are primarily localized in the endoplasmic reticulum (ER). Tetramerization of KCNQ2 with KCNQ3 channels masks an ER retention motif in KCNQ3 channels, allowing efficient expression in the membrane[32, 33]. Notably, not all studies have shown a lack of expression of KCNQ3 channels in the plasma membrane. Multiple groups have recorded KCNQ3 homomeric channels in cells other than oocytes, such as HEK293T or CHO cells[34, 35, 8]. However, the magnitude of the KCNQ3 current is typically smaller than that of KCNQ2 homomeric and KCNQ2/3 heteromeric channels. Additionally, Shapiro and colleagues found that KCNQ3 channels express well on the surface, but enter a quiescent silent conformation due to instability of the selectivity pore[36, 37]. Co-expression of KCNQ2 channels with KCNQ3 stabilizes KCNQ3 pore residues, allowing for robust KCNQ2/3-mediated currents. Work by many groups have also shown calmodulin has a key role in KCNQ2 channel trafficking, heteromeridization with KCNQ3 as well as regulation of KCNQ2/3 PIP2 affinity. A discussion on the role of calmodulin and KCNQ2/3 channels expression and properties can be found in other expert reviews[38, 14, 39].
Fig. 1.
(A) Example of KCNQ2/3 mediated currents expressed in HEK293T cells at room and at near physiological temperature. Notice the slow activation kinetics independent of temperature. Bottom panel shows summary of conductance-to-voltage relationship of KCNQ2/3 current at room and near physiological temperatures. Independent of temperature KCNQ2/3 currents start activating at ~ −60 to −50 mV. The data were fit with a Boltzmann equation (unpublished data, Tzingounis).
KCNQ2 and KCNQ3 heteromers in neurons: animal models
Further support for the KCNQ2/3 model stems from studies using animal models. The expression of KCNQ2-dominant-negative subunits decreases the M-current of CA1 pyramidal neurons by almost 75–80%, suggesting that the native M-current is mediated by KCNQ2-containing channels in the hippocampus[40]. Additionally, KCNQ2 deletion leads to perinatal lethality within one hour[41]. Death is due to failure to breathe. Following birth, Kcnq2 homozygous knockout mice initiate breathing but die within an hour due to pulmonary atelectasis (i.e lungs are deflated)[41]. The cellular mechanisms and cell types responsible for the failure to breathe in the absence of KCNQ2 channels is unknown. In contrast to Kcnq2 knockout mice, Kcnq3 knockout mice survive to adulthood[42]. This result is likely due to the differential effect of KCNQ2 or KCNQ3 ablation on the M-current in neurons. For instance, conditional deletion of KCNQ2 channels from CA1 excitatory neurons leads to an ~85% loss of M-current, whereas deletion of Kcnq3 reduces the M-current by ~50%[43]. Additionally, the total KCNQ3 protein level in the hippocampus is significantly lower in Kcnq2 conditional knockout mice[43] as well as in Kcnq2 deleted fast-spiking parvalbumin interneurons[44], unlike KCNQ2 protein levels in Kcnq3 null hippocampi. However, ablation of KCNQ2 might not lead to KCNQ3 protein loss in all cell-types as shown for dentate gyrus granule cells and nodes of Ranvier of peripheral sensory neurons[45, 46]. In another study, Brown and colleagues demonstrated that deletion of KCNQ2 channels abolishes the M-current in sympathetic neurons[47]. In addition to mouse studies, recent work in humans has identified epilepsy patients that lack both copies of functional KCNQ3 channels[48]. Currently, no patients with homozygous KCNQ2 variants have been identified. Therefore, clinical data and mouse studies support a model in which KCNQ2 channels are required for M-current expression in contrast to KCNQ3 channels, which might act to boost the probability of opening and PIP2 affinity of KCNQ2 channels.
Although previous data support the current KCNQ2/3 model, this model might not apply for all cell types and nervous system regions. For instance, in a subpopulation of dorsal root ganglion neurons[45], large sciatic nerve axons[49], and cortical vasoactive intestinal polypeptide (VIP) interneurons[50], KCNQ2, but not KCNQ3, channels are expressed, suggesting that the KCNQ stoichiometry might not be constrained to KCNQ2/3 heteromers. Additionally, conditional deletion of Kcnq2 from granule cells of the dentate gyrus diminished the M-current by only 50%[46]. This result was due to compensation through increased expression of KCNQ3 channels, which led to the formation of KCNQ3 homomers or KCNQ2/3 heteromers with a 1:3 KCNQ2:KCNQ3 channel stoichiometry[46]. Additionally, Sun et al. (2019) found that vagal bronchopulmonary neurons express Kcnq3 mRNA, with no detectable expression of Kcnq2 or Kcnq5 mRNA[51]. Consistent with this finding, the M-current properties in these neurons match those of KCNQ3 homomers. Thus, these recent data suggest that despite their low level of expression, KCNQ3 homomers may mediate M-like currents in neurons. How could this be possible? As mentioned earlier, KCNQ3 channels can form functional channels that may traffic to the surface, just not as readily as their KCNQ2 counterparts. Importantly, the cell types for which KCNQ3 homomeric expression has been indicated also have a very high input resistance, potentially allowing a small number of KCNQ3 channels to exert an influence on the membrane potential[52].
KCNQ2 and KCNQ3 channel composition across development and cell types
Recent data showing that KCNQ2, KCNQ2/3, and KCNQ3 can mediate M-like currents in some cell types raise some pertinent questions: How should we navigate the genotype–phenotype relationship of pathogenic KCNQ2 and KCNQ3 variants? Should we explain everything through the lens of KCNQ2/3 heteromers or should we adapt a more dynamic and flexible KCNQ stoichiometry depending on the expression pattern of KCNQ2 and KCNQ3 for different time points, cell types, and regions?
To answer these questions, we must first determine the developmental expression profile and pattern of KCNQ2 and KCNQ3 channels in the nervous system. In particular, we must identify the extent and timing of the co-expression of KCNQ2 and KCNQ3 channels. The current model regarding the developmental profile of KCNQ2 channels suggests that KCNQ2 channels are expressed first, followed by a delayed ramp-up of expression of KCNQ3 channels[53]. Consistent with this view, Brown and colleagues found that Kcnq3 mRNA levels increase substantially from E18/19 to P45 (with Kcnq2 mRNA levels remaining constant) in sympathetic neurons[23] (see also Tinel et al (1998)[54] for Kcnq2 and Kcnq3 transcriptic changes across development in mouse brain). In parallel, the sensitivity of the M-current to extracellular application of TEA also decreases, consistent with the incorporation of KCNQ3 channel subunits in KCNQ2-mediated complexes. We note that KCNQ3 has a much lower TEA affinity than KCNQ2 channels[55] (M-current TEA affinity is conferred by KCNQ2 channels[55]). However, the strongest evidence for an earlier expression of KCNQ2 channels comes from studies using human brain tissue, which have shown that KCNQ2 protein expression starts at 22–24 gestational weeks[56], whereas KCNQ3 protein levels are not detectable until 29 gestational weeks[56]. Moreover, researchers have observed simultaneous expression of KCNQ2 and KCNQ3 from late fetal life to infancy, independent of the region[56]. Consistent with the dual expression of KCNQ2 and KCNQ3 channels in late embryonic life, Yus-Najera et al (2003)[57] found that KCNQ2 co-immunoprecipitates from rat brains with KCNQ3 as early as postnatal day 2 (P2), suggesting that KCNQ2 and KCNQ3 heteromers may form as early as the first week of life in rodents, a time point corresponding to the last trimester in utero in humans.
Whereas data on KCNQ2 and KCNQ3 protein levels and M-currents in early development are scarce, there is a wealth of data from large transcriptomic studies. Sestan and colleagues have compiled mRNA data from human brains across development, starting from post-conception day 50 through 10000 days[58]. They report that Kcnq2 and Kcnq3 mRNA is expressed early on (day 50; (http://development.psychencode.org/#), and these levels remain constant across development in multiple brain regions independent of gender. Similarly, in situ hybridization of Kcnq3 from the Allen Brain Atlas has shown that Kcnq3 mRNA levels start to increase from embryonic day 15.5 (E15.5) and E18.5 peaking at P4 following a small decline to adult levels (https://developingmouse.brain-map.org/). Overall, the current data indicate that the expression of KCNQ2 and KCNQ3 channels begins in early development, with KCNQ2 channels starting at a slightly earlier time point.
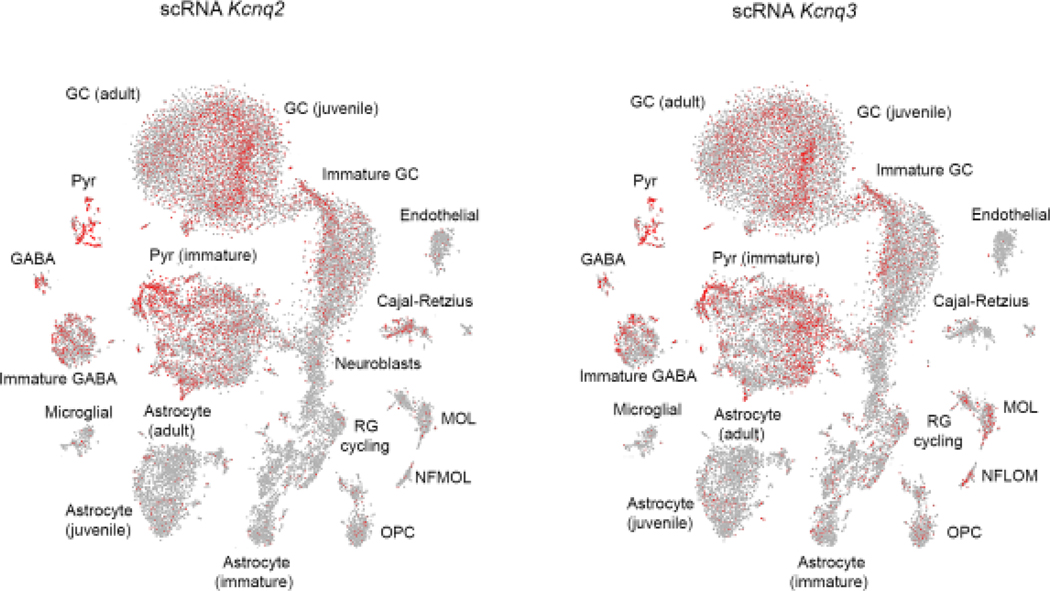
With a few exceptions, the data thus far do not provide any information regarding the coexpression of these channels in a single cell type across development. Therefore, we assessed a recent study that determined the RNA transcriptome in the mouse dentate gyrus of the hippocampus[59]. This region undergoes neurogenesis postnatally, allowing exploration of the transcriptome across different cell types and different developmental time points. Hochgerner et al. (2018)[59] determined the transcriptome of over 20,000 cells across multiple development points in mice (E16.5 to P132), providing a timeline of cell-type development in the dentate gyrus of the hippocampus. Figure 2 shows a visualization of the single-cell RNA data from this dataset for Kcnq2 and Kcnq3. One can see that the expression pattern of Kcnq2 and Kcnq3 is highly similar to that of both Kcnq2 and Kcnq3 co-expressed in mature pyramidal neurons and granule cells. However, some notable differences exist. First, a clear differential expression of KCNQ2 and KCNQ3 channels occurs in Cajal-Retzius cells (CRs). In particular, Kcnq2, but not Kcnq3, is primarily expressed in CRs. If confirmed by FISH and immunohistochemistry, this finding could be very important, as CRs play a fundamental role in the development of the cortex, acting as the primary source of reelin, a glycoprotein involved in instructing the radial migration of projection neurons[60, 61]. Additionally, CRs, and unlike the neocortex, persist postnatally in dentate gyrus[60]. Thus, hippocampal CRs might initiate a cascade of events that can reshape network excitability across multiple developmental time points. Second, as granule cells progress from neuroblasts to immature cells, they express both Kcnq3 and Kcnq2 mRNA early on; which might explain the propensity of KCNQ3 to compensate for the loss of KCNQ2 in granule cells[46]. Third, in contrast to granule cell development, a greater proportion of immature juvenile CA3 pyramidal neurons express Kcnq3 mRNA before Kcnq2 mRNA. Interestingly, the application of retigabine, a KCNQ activator[62], does not increase the amplitude of the classical M-current in CA3 pyramidal neurons from P1 rats[63], unlike its effect on the M-current in juvenile rats that readily express KCNQ2/3 heteromers[63]. Carver et al. (2019)[46] also observed this lack of M-current potentiation caused by retigabine when they deleted KCNQ2 channels from granule cells. As noted above, deletion of Kcnq2 from granule cells leads to the emergence of an M-current produced by either KCNQ3 homomers or KCNQ2/3 heteromers, following a 1:3 KCNQ2:KCNQ3 ratio. Together, this raises the possibility that immature CA3 pyramidal neurons might express a more KCNQ3 enriched M-current. This could be directly tested in the future using application of TEA. Fourth, newly formed and mature oligodendrocytes primarily express Kcnq3 mRNA, rather than Kcnq2 mRNA. Currently, the role of KCNQ3 channels has not been explored in relation to oligodendrocyte excitability and myelination.
Fig. 2.
Developmetal expression of Kcnq2 and Kcnq3 mRNA in dentate gyrus of the hippocampus. Panels show t-distributed stochastic neighbor embedding (t-SNE) plots of dentate gyrus cell types across development. Each dot point represents a cell expressing a designated mRNA. Panels show the scRNA data for Kcnq2 and Kcnq3 mRNA. The illustration depicts the developmental trajectory of the different cell types identified in Dentate gyrus. In particular, the t-SNE represents visualisation of 24,185 cells from mice of various ages, perinatal (E16.5–P5), juvenile (P18–P23), and adult (P120–P132) mice. Data for this illustration obtained from http://linnarssonlab.org/dentate/. See Hochgerner et al (2018) [59] for details. GC:granule cells, Pyr:Pyramidal neurons, CRs: Cajal-Retzius cells, OPC:oligodendrocyte progenitor cells, NFOL: newly formed oligodenrdocytes, MOL: Mature oligodendrocytes, RG: radial glial.
Taken together, the current data indicate that different cell types may express very different KCNQ2 and KCNQ3 channel compositions as development progresses, the model of KCNQ2 first followed by KCNQ2/3 heteromers might not apply to all cell-types. Thus, genotype–phenotype relationships viewed simply through the lens of KCNQ2/3 heteromers are more likely to reflect adult brains rather than developing cells.
Beyond the forebrain
In our interpretation of the genotype–phenotype relationship through the prism of KCNQ2/3 heteromers, one caveat is that our current knowledge on KCNQ2 and KCNQ3 mRNA and protein colocalization stems from work primarily performed in sympathetic neurons and in neurons in the neocortex and hippocampus, with only limited information on subcortical regions (see for example [64–66]), spinal cord[67, 68], and the peripheral nervous system [45]. In the hippocampus and cortex, KCNQ2 and KCNQ3 colocalization in excitatory and inhibitory cells is highly prevalent, with very few exceptions. A notable exception is the VIP interneuron GABAergic cell, a cell type that controls the activity of interneuron networks[69, 50]. VIP interneurons express KCNQ2 but very limited number of KCNQ3 channels, as demonstrated at the single cell mRNA (see Allen brain atlas)[70] and protein level[50]. Although the majority of cells in the forebrain and thalamus express both KCNQ2 and KCNQ3 channels[71], this trend may not occur in subcortical regions. For instance, a previous immunohistochemistry study using a KCNQ3 antibody found low expression of KCNQ3 channels in the brainstem[72]; moreover, the Allen Brain Atlas shows limited expression of Kcnq3 mRNA in the brainstem (https://mouse.brain-map.org/), consistent with earlier in situ hybridization reports showng weak Kcnq3 expression [54, 71].
In addition to the possibility that regions of the brainstem may not express KCNQ2 and KCNQ3 channels equally, a subset of neurons in the peripheral nervous system may also exhibit varying expression levels. A recent transcriptome analysis of adult vagal sensory ganglia showed that KCNQ2 and KCNQ3 channels are not always found in the same cell populations[73]. Most notably, KCNQ3 channels were highly enriched in polymodal nodose ganglion cells whereas KCNQ2 was enriched in Piezo2-expressing nodose neurons, which are important for sensing pulmonary volume and stretching (https://ernforsgroup.shinyapps.io/vagalsensoryneurons/). Additionally, a recent report showed that KCNQ2 channels are enriched in neurons important for locomotion in the spinal cord[68], a finding similar to previous work that found robust expression of KCNQ2 but of KCNQ3 in spinal cord neurons[67, 68]. The differential enrichment of KCNQ2 channels over KCNQ3 channels in the brainstem and some vagus neurons may also contribute to the very different symptoms exhibited by patients carrying Kcnq2 and Kcnq3 gain-of-function mutations[7]. Recently, studies have identified a gain-of-function variant (KCNQ3R230C or KCNQ3R230H) in multiple patients with autism spectrum disorders[9, 3] (https://gene.sfari.org/database/human-gene/KCNQ3) and sleep-activated multifocal epileptiform discharges[9]. Kcnq3 gain-of-function mutations lead to constitutively open KCNQ3 channels across a range of hyperpolarized membrane potentials[8, 9]. Similarly, Kcnq2 gain-of-function mutations lead to constitutively open channels[8]. However, patients with KCNQ2 gain of function primarily suffer from severe respiratory problems and heightened startle reflexes, symptoms that start soon after birth[74], supporting the notion that KCNQ2 channels may be expressed at much higher levels in the brainstem and spinal cord than KCNQ3 channels.
KCNQ5, the enigmatic neuronal KCNQ channel
KCNQ5 was the last member of the KCNQ family identified[20, 21]. KCNQ5 like KCNQ2 and KCNQ3, is expressed in the forebrain and in both glutamatergic and gabaergic cells[72, 75]. Northern blot analysis in the human brain revealed that KCNQ5 is present in several regions including the occipital, frontal and temporal lobe of the cerebral cortex, as well as the putamen and hippocampus[20]. However, KCNQ5 channels were not found in thalamus and were absent in the dentate gyrus region of the hippocampus[21].
KCNQ5 channels can form heteromers with KCNQ3 channels, but not KCNQ2 channels when expressed in oocytes[21]. Thus, the current model is that KCNQ5 channels might be primarily found as KCNQ3/5 heteromers in the brain. This is based on the finding that co-expressing KCNQ5 with KCNQ3 channels increases the KCNQ5 current almost four-fold[20, 21]. Although known M-current blockers such as linopiridine poorly inhibit KCNQ5 channels[21], they are readily blocked when co-expressed with KCNQ3 channels[76] raising the possibility that KCNQ3/5 might form M like currents in some neurons.
Previous work using knockin mice expressing a dominant negative KCNQ5 channel variant has shown that KCNQ5 channels contribute to the medium and calcium-activated slow afterhyperpolarization currents in CA3 pyramidal neurons of the hippocampus[72]. Additionally, using the same mouse line as in the earlier study, Jentsch and colleagues showed that KCNQ5 channels control excitabilty of interneurons and loss of KCNQ5 containing channel activity alter hippocampal oscillations[75]. Similarly, an earlier report using pharmacology suggested that KCNQ5 channels might control the excitability of some glutamatergic synaptic terminals[77].
Consistent with the suggested role of KCNQ5 channels in controlling neuronal excitability, Lehman et al (2017)[18] reported that loss or gain of function KCNQ5 channels variants could lead to epileptic encephalopathy.
Although KCNQ2 and KCNQ5 might not form heteromers when co-expressed in oocytes, co-expression of KCNQ5 along with KCNQ3 and KCNQ2 channels in CHO cells might lead to the formation of tri-heteromeric KCNQ2/KCNQ3/KCNQ5 channels[78]. However, the functional properties of these channels are not known. What is also not known is the subcompartment location of KCNQ5 channels. Some studies have suggested that KCNQ5 channels are expressed in the pre-synaptic glutamatergic terminals[79, 77]; however, this has come in question in follow up work done by another group[75]. KCNQ5 is expressed in the soma[75] and recent work suggested expression in dendrites and spines[80], but no reports of KCNQ5 channel expression in axons or the axon initial segment. We note that some earlier work has suggested the presence of M-like currents and KCNQ channels in dendrites[81, 82], but whether these dendritic channels include KCNQ5, KCNQ3/5, or KCNQ2/3 channels is not known. Imporantly, deletion of KCNQ2 from forebrain excitatory neurons also leads to the reduction in KCNQ5 protein levels[43]; raising the possibility KCNQ2 and KCNQ5 or KCNQ2/KCNQ3/KCNQ5 might be together in a complex in some neurons.
Considering the high expression of KCNQ2 and KCNQ3 channels in axons[83, 84] and the lack of knowledge on whether KCNQ5 channels express in axons, it is currently, unknown whether neurons express KCNQ5 as homomers, KCNQ3/5 heteromers or KCNQ2/KCNQ3/KCNQ5 tri-heteromers. This is an important question to resolve as KCNQ5 incorporation to KCNQ2/3 channels might fundamentally change the impact of KCNQ2 and KCNQ3 pathogenic variants and their pharmacological properties of KCNQ2/3 channels as KCNQ5 channels have a different pharmacological profie than KCNQ2/3 channels.
Conclusion
The accelerated frequency of KCNQ2 and KCNQ3 pathogenic variant identification over the last five years has led to an urgent need to decipher the mechanisms by which KCNQ2 and KCNQ3 dysfunction lead to neurodevelopmental disorders. The dominant paradigm that KCNQ2 and KCNQ3 channels are found as KCNQ2/3 heteromers in neurons has guided genotype-phenotype relationships over the last twenty years. Although this has led to an improved understanding of KCNQ2 and KCNQ3 channelopathies and development of new KCNQ2/3 activators, this framework is based on a limited number of studies focusing on very few cell types, brain regions, and developmental time points, primarily adult. As we discussed in this review KCNQ2 and KCNQ3 channel composition likely differs during development, nervous system region, and from cell type to cell type. Therefore, depending on their spatial-temporal expression KCNQ2 and KCNQ3 pathogenic variants would initiate unique and differing transcriptional network changes at different brain regions and cell-types. This might result in KCNQ2 and KCNQ3 channel composition remodeling that could partially compensate for the loss or gain of KCNQ2/3 channel activity and possibly make pharmacological targeting of KCNQ2/3 heteromeric channels inadequate. Future studies focusing on understanding the impact of KCNQ2 and KCNQ3 channels across development and across the nervous system, central and periphery, are neccessary not only for uncovering new biological signatures of KCNQ2 and KCNQ3 pathology but to also develop new therapeutic strategies tailored to KCNQ2 and KCNQ3 channelopathy patients. To date, the majority of studies have focused on understanding the effect of variants to KCNQ2 and KCNQ3 channels in heterologous cells and in developing activators for KCNQ2/3 heteromers. To complement this work, development of new compounds (activators and inhibitors) that are selective for KCNQ2 and KCNQ3 channels tested on patient derived neurons, organoids, and knockin mouse models may present additional opportunities for new therapeutic strategies for KCNQ2 and KCNQ3 channelopathies.
Acknowledgments
Funding Sources
This work was funded by NIH grants HL137094, NS101596, NS118262, and NS118262 to AVT.
Footnotes
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Heyne HO, Artomov M, Battke F, Bianchini C, Smith DR, Liebmann N, et al. Targeted gene sequencing in 6994 individuals with neurodevelopmental disorder with epilepsy. Genet Med. 2019November;21(11):2496–503. [DOI] [PubMed] [Google Scholar]
- 2.Moyses-Oliveira M, Yadav R, Erdin S, Talkowski ME. New gene discoveries highlight functional convergence in autism and related neurodevelopmental disorders. Curr Opin Genet Dev. 2020. Dec;65:195–206. [DOI] [PubMed] [Google Scholar]
- 3.Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell. 2020. Feb 6;180(3):568–84 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000. Oct;1(1):21–30. [DOI] [PubMed] [Google Scholar]
- 5.Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012January;71(1):15–25. [DOI] [PubMed] [Google Scholar]
- 6.El Kosseifi C, Cornet MC, Cilio MR. Neonatal Developmental and Epileptic Encephalopathies. Semin Pediatr Neurol. 2019December;32:100770. [DOI] [PubMed] [Google Scholar]
- 7.Nappi P, Miceli F, Soldovieri MV, Ambrosino P, Barrese V, Taglialatela M. Epileptic channelopathies caused by neuronal Kv7 (KCNQ) channel dysfunction. Pflugers Arch. 2020. Jul;472(7):881–98. [DOI] [PubMed] [Google Scholar]
- 8.Miceli F, Soldovieri MV, Ambrosino P, De Maria M, Migliore M, Migliore R, et al. Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits. J Neurosci. 2015March4;35(9):3782–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sands TT, Miceli F, Lesca G, Beck AE, Sadleir LG, Arrington DK, et al. Autism and developmental disability caused by KCNQ3 gain-of-function variants. Ann Neurol. 2019August;86(2):181–92. [DOI] [PubMed] [Google Scholar]
- 10.Niday Z, Tzingounis AV. Potassium Channel Gain of Function in Epilepsy: An Unresolved Paradox. Neuroscientist. 2018. Aug;24(4):368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980February14;283(5748):673–6. [DOI] [PubMed] [Google Scholar]
- 12.Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. [DOI] [PubMed] [Google Scholar]
- 13.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005. Nov;6(11):850–62. [DOI] [PubMed] [Google Scholar]
- 14.Greene DL, Hoshi N. Modulation of Kv7 channels and excitability in the brain. Cell Mol Life Sci. 2017February;74(3):495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–87. [DOI] [PubMed] [Google Scholar]
- 16.Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007. Jun;8(6):451–65. [DOI] [PubMed] [Google Scholar]
- 17.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998December4;282(5395):1890–3. [DOI] [PubMed] [Google Scholar]
- 18.Lehman A, Thouta S, Mancini GMS, Naidu S, van Slegtenhorst M, McWalter K, et al. Loss-of-Function and Gain-of-Function Mutations in KCNQ5 Cause Intellectual Disability or Epileptic Encephalopathy. Am J Hum Genet. 2017July6;101(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee PH, Anttila V, Won H, Feng Y-CA, Rosenthal J, Zhu Z, et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell. 2019;179(7):1469–82. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, et al. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem. 2000July21;275(29):22395–400. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000August4;275(31):24089–95. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro MS, Roche JP, Kaftan EJ, Cruzblanca H, Mackie K, Hille B. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K(+) channels that underlie the neuronal M current. J Neurosci. 2000March1;20(5):1710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadley JK, Passmore GM, Tatulian L, Al-Qatari M, Ye F, Wickenden AD, et al. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J Neurosci. 2003June15;23(12):5012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwake M, Pusch M, Kharkovets T, Jentsch TJ. Surface expression and single channel properties of KCNQ2/KCNQ3, M-type K+ channels involved in epilepsy. J Biol Chem. 2000May5;275(18):13343–8. [DOI] [PubMed] [Google Scholar]
- 25.Yang WP, Levesque PC, Little WA, Conder ML, Ramakrishnan P, Neubauer MG, et al. Functional expression of two KvLQT1-related potassium channels responsible for an inherited idiopathic epilepsy. J Biol Chem. 1998July31;273(31):19419–23. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, et al. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007March15;120(Pt 6):953–63. [DOI] [PubMed] [Google Scholar]
- 27.Maljevic S, Wuttke TV, Lerche H. Nervous system KV7 disorders: breakdown of a subthreshold brake. J Physiol. 2008April1;586(7):1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006December1;314(5804):1454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Gamper N, Hilgemann DW, Shapiro MS. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005October26;25(43):9825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telezhkin V, Brown DA, Gibb AJ. Distinct subunit contributions to the activation of M-type potassium channels by PI(4,5)P2. J Gen Physiol. 2012July;140(1):41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci U S A. 2006June6;103(23):8870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwake M, Jentsch TJ, Friedrich T. A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep. 2003January;4(1):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwake M, Athanasiadu D, Beimgraben C, Blanz J, Beck C, Jentsch TJ, et al. Structural determinants of M-type KCNQ (Kv7) K+ channel assembly. J Neurosci. 2006April5;26(14):3757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soh H, Tzingounis AV. The specific slow afterhyperpolarization inhibitor UCL2077 is a subtype-selective blocker of the epilepsy associated KCNQ channels. Mol Pharmacol. 2010December;78(6):1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W, Devaux JJ. Calmodulin orchestrates the heteromeric assembly and the trafficking of KCNQ2/3 (Kv7.2/3) channels in neurons. Mol Cell Neurosci. 2014January;58:40–52. [DOI] [PubMed] [Google Scholar]
- 36.Zaika O, Hernandez CC, Bal M, Tolstykh GP, Shapiro MS. Determinants within the turret and pore-loop domains of KCNQ3 K+ channels governing functional activity. Biophys J. 2008December;95(11):5121–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choveau FS, Shapiro MS. Regions of KCNQ K(+) channels controlling functional expression. Front Physiol. 2012;3:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung HJ. Role of calmodulin in neuronal Kv7/KCNQ potassium channels and epilepsy. Frontiers in biology. 2014;9(3):205–15. [Google Scholar]
- 39.Alaimo A, Villarroel A. Calmodulin: A Multitasking Protein in Kv7.2 Potassium Channel Functions. Biomolecules. 2018July18;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005January;8(1):51–60. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe H, Nagata E, Kosakai A, Nakamura M, Yokoyama M, Tanaka K, et al. Disruption of the epilepsy KCNQ2 gene results in neural hyperexcitability. J Neurochem. 2000July;75(1):28–33. [DOI] [PubMed] [Google Scholar]
- 42.Tzingounis AV, Nicoll RA. Contribution of KCNQ2 and KCNQ3 to the medium and slow afterhyperpolarization currents. Proc Natl Acad Sci U S A. 2008December16;105(50):19974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soh H, Pant R, LoTurco JJ, Tzingounis AV. Conditional deletions of epilepsy-associated KCNQ2 and KCNQ3 channels from cerebral cortex cause differential effects on neuronal excitability. J Neurosci. 2014April9;34(15):5311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunbar C, Jing J, Sonesra A, Park S, Soh H, Lee M, et al. Removal of KCNQ2 from Parvalbumin-expressing Interneurons Improves Anti-Seizure Efficacy of Retigabine. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King CH, Lancaster E, Salomon D, Peles E, Scherer SS. Kv7.2 regulates the function of peripheral sensory neurons. J Comp Neurol. 2014October1;522(14):3262–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carver CM, Shapiro MS. Gq-Coupled Muscarinic Receptor Enhancement of KCNQ2/3 Channels and Activation of TRPC Channels in Multimodal Control of Excitability in Dentate Gyrus Granule Cells. J Neurosci. 2019February27;39(9):1566–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robbins J, Passmore GM, Abogadie FC, Reilly JM, Brown DA. Effects of KCNQ2 gene truncation on M-type Kv7 potassium currents. PLoS One. 2013;8(8):e71809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lauritano A, Moutton S, Longobardi E, Tran Mau-Them F, Laudati G, Nappi P, et al. A novel homozygous KCNQ3 loss-of-function variant causes non-syndromic intellectual disability and neonatal-onset pharmacodependent epilepsy. Epilepsia Open. 2019September;4(3):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, et al. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006May15;573(Pt 1):17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goff KM, Goldberg EM. Vasoactive intestinal peptide-expressing interneurons are impaired in a mouse model of Dravet syndrome. Elife. 2019July8;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun H, Lin AH, Ru F, Patil MJ, Meeker S, Lee LY, et al. KCNQ/M-channels regulate mouse vagal bronchopulmonary C-fiber excitability and cough sensitivity. JCI Insight. 2019March7;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kreitzer AC, Carter AG, Regehr WG. Inhibition of interneuron firing extends the spread of endocannabinoid signaling in the cerebellum. Neuron. 2002May30;34(5):787–96. [DOI] [PubMed] [Google Scholar]
- 53.Dirkx N, Miceli F, Taglialatela M, Weckhuysen S. The Role of Kv7.2 in Neurodevelopment: Insights and Gaps in Our Understanding. Front Physiol. 2020;11:570588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tinel N, Lauritzen I, Chouabe C, Lazdunski M, Borsotto M. The KCNQ2 potassium channel: splice variants, functional and developmental expression. Brain localization and comparison with KCNQ3. FEBS Lett. 1998November6;438(3):171–6. [DOI] [PubMed] [Google Scholar]
- 55.Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1–4 potassium channels. Br J Pharmacol. 2000February;129(3):413–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanaumi T, Takashima S, Iwasaki H, Itoh M, Mitsudome A, Hirose S. Developmental changes in KCNQ2 and KCNQ3 expression in human brain: possible contribution to the age-dependent etiology of benign familial neonatal convulsions. Brain Dev. 2008May;30(5):362–9. [DOI] [PubMed] [Google Scholar]
- 57.Yus-Najera E, Munoz A, Salvador N, Jensen BS, Rasmussen HB, Defelipe J, et al. Localization of KCNQ5 in the normal and epileptic human temporal neocortex and hippocampal formation. Neuroscience. 2003;120(2):353–64. [DOI] [PubMed] [Google Scholar]
- 58.Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, Pochareddy S, et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science. 2018December14;362(6420). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hochgerner H, Zeisel A, Lonnerberg P, Linnarsson S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat Neurosci. 2018February;21(2):290–99. [DOI] [PubMed] [Google Scholar]
- 60.Anstotz M, Huang H, Marchionni I, Haumann I, Maccaferri G, Lubke JH. Developmental Profile, Morphology, and Synaptic Connectivity of Cajal-Retzius Cells in the Postnatal Mouse Hippocampus. Cereb Cortex. 2016February;26(2):855–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anstotz M, Quattrocolo G, Maccaferri G. Cajal-Retzius cells and GABAergic interneurons of the developing hippocampus: Close electrophysiological encounters of the third kind. Brain Res. 2018October15;1697:124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001August1;21(15):5535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Safiulina VF, Zacchi P, Taglialatela M, Yaari Y, Cherubini E. Low expression of Kv7/M channels facilitates intrinsic and network bursting in the developing rat hippocampus. J Physiol. 2008November15;586(22):5437–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martire M, D’Amico M, Panza E, Miceli F, Viggiano D, Lavergata F, et al. Involvement of KCNQ2 subunits in [3H]dopamine release triggered by depolarization and pre-synaptic muscarinic receptor activation from rat striatal synaptosomes. J Neurochem. 2007July;102(1):179–93. [DOI] [PubMed] [Google Scholar]
- 65.Cerina M, Szkudlarek HJ, Coulon P, Meuth P, Kanyshkova T, Nguyen XV, et al. Thalamic Kv 7 channels: pharmacological properties and activity control during noxious signal processing. Br J Pharmacol. 2015June;172(12):3126–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGuier NS, Griffin WC 3rd, Gass JT, Padula AE, Chesler EJ, Mulholland PJ. Kv7 channels in the nucleus accumbens are altered by chronic drinking and are targets for reducing alcohol consumption. Addict Biol. 2016November;21(6):1097–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004February4;24(5):1236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verneuil J, Brocard C, Trouplin V, Villard L, Peyronnet-Roux J, Brocard F. The M-current works in tandem with the persistent sodium current to set the speed of locomotion. PLoS Biol. 2020. Nov;18(11):e3000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011. Jan 1;71(1):45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nature neuroscience. 2016;19(2):335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001July1;21(13):4609–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tzingounis AV, Heidenreich M, Kharkovets T, Spitzmaul G, Jensen HS, Nicoll RA, et al. The KCNQ5 potassium channel mediates a component of the afterhyperpolarization current in mouse hippocampus. Proc Natl Acad Sci U S A. 2010June1;107(22):10232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kupari J, Haring M, Agirre E, Castelo-Branco G, Ernfors P. An Atlas of Vagal Sensory Neurons and Their Molecular Specialization. Cell Rep. 2019May21;27(8):2508–23 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mulkey SB, Ben-Zeev B, Nicolai J, Carroll JL, Gronborg S, Jiang YH, et al. Neonatal nonepileptic myoclonus is a prominent clinical feature of KCNQ2 gain-of-function variants R201C and R201 H. Epilepsia. 2017March;58(3):436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fidzinski P, Korotkova T, Heidenreich M, Maier N, Schuetze S, Kobler O, et al. KCNQ5 K(+) channels control hippocampal synaptic inhibition and fast network oscillations. Nat Commun. 2015. Feb 4;6:6254. [DOI] [PubMed] [Google Scholar]
- 76.Wickenden AD, Zou A, Wagoner PK, Jegla T. Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br J Pharmacol. 2001January;132(2):381–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang H, Trussell LO. KCNQ5 channels control resting properties and release probability of a synapse. Nat Neurosci. 2011June12;14(7):840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Carver CM, Choveau FS, Shapiro MS. Clustering and Functional Coupling of Diverse Ion Channels and Signaling Proteins Revealed by Super-resolution STORM Microscopy in Neurons. Neuron. 2016October19;92(2):461–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garcia-Pino E, Caminos E, Juiz JM. KCNQ5 reaches synaptic endings in the auditory brainstem at hearing onset and targeting maintenance is activity-dependent. J Comp Neurol. 2010April15;518(8):1301–14. [DOI] [PubMed] [Google Scholar]
- 80.Galvin VC, Yang ST, Paspalas CD, Yang Y, Jin LE, Datta D, et al. Muscarinic M1 Receptors Modulate Working Memory Performance and Activity via KCNQ Potassium Channels in the Primate Prefrontal Cortex. Neuron. 2020. May 20;106(4):649–61 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen X, Johnston D. Properties of single voltage-dependent K+ channels in dendrites of CA1 pyramidal neurones of rat hippocampus. J Physiol. 2004August15;559(Pt 1):187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol. 2006June;95(6):3480–95. [DOI] [PubMed] [Google Scholar]
- 83.Battefeld A, Tran BT, Gavrilis J, Cooper EC, Kole MH. Heteromeric Kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J Neurosci. 2014March5;34(10):3719–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kole MH, Cooper EC. Axonal Kv7.2/7.3 channels: caught in the act. Channels (Austin). 2014;8(4):288–9. [DOI] [PMC free article] [PubMed] [Google Scholar]