Abstract
PURPOSE:
To determine if energy intake and appetite regulation differ in response to an acute bout of resistance exercise (REx) vs aerobic exercise (AEx).
METHODS:
Physically inactive adults (n=24, 35±2% body fat, 50% female) completed 3 conditions: AEx (walking at 65–70% heart rate max for 45 minutes); REx (1-set to failure of 12 exercises); and sedentary control (SED). Each condition was initiated in the post-prandial state (35 minutes post breakfast). Appetite (visual analogue scale [VAS] for hunger, satiety, and prospective food consumption and hormones (ghrelin, PYY, and GLP-1) were measured before and 30, 90, 120, 150, and 180-minutes following a standardized breakfast. Area under the curve (AUC) was calculated using the trapezoid method. Ad libitum energy intake was evaluated at a lunch meal following the 180-minute measurements.
RESULTS:
No differences in ad libitum energy intake (REx: 991±68; AEx: 937±65; SED: 944±76 kcals, p=0.50), nor appetite ratings (all p>0.05) were detected. AUC for ghrelin, PYY, and GLP-1 were all lower following REx vs. AEx (Ghrelin: REx: 130,737±4,928; AEx: 143,708±7,500, p=0.006; PYY: REx: 20,540±1,177; AEx: 23,812±1,592, p=0.001; and GLP-1: REx: 1,314±93; AEx: 1,615±110, p=0.013). Neither exercise condition significantly differed from SED.
CONCLUSIONS:
Acute REx lowers both orexigenic (ghrelin) and anorectic (PYY and GLP-1) gut peptides compared to acute AEx. Ad libitum energy intake did not increase compared to SED in either exercise condition, indicating both exercise modalities have appetite and energy intake suppressing effects. Future work is needed to determine if exercise of differing modalities influences chronic appetite regulation.
Keywords: eating behaviors, resistance exercise, ghrelin, PYY, GLP-1, appetite ratings
INTRODUCTION:
Obesity is a significant public health concern due to the increased risk of adverse co-morbid health outcomes and associated economic strain on the healthcare system. Weight loss is indicated for adults with overweight/obesity (OW/OB), but unfortunately weight loss and maintenance of weight loss are challenging and often unsuccessful(1). Multiple mechanisms interact to determine energy intake regulation and ultimately, weight status(2). These mechanisms include physiological or homeostatic mechanisms which consist of short-term signals such as gut peptides, gastric distension, and metabolites that are influenced by acute food intake, as well as long-term signals such as leptin and insulin levels that are influenced by current adiposity levels(3). However, homeostatic mechanisms are often overridden by non-homeostatic mechanisms such as reward pathways, food cravings, dietary restraint and disinhibition, and food-related environmental and social cues(4, 5). Given our modern environment which promotes an increase in energy intake and decrease in energy expenditure, it is not surprising the prevalence of obesity has increased(5). Strategies which effectively target the multiple, integrated mechanisms of appetite regulation and allow for appropriate levels of energy intake to achieve and maintain healthy body weight are greatly needed.
Diet-induced weight loss results in changes to homeostatic and non-homeostatic appetite regulation signals that promote increased energy intake(1, 6, 7). Exercise may be a strategy to dampen these effects and promote successful weight management(8). Acute aerobic exercise (AEx) has been shown to attenuate the anticipated increase in appetite and energy intake created by a caloric deficit from increased energy expenditure(9–17). This is regulated, in part, by changes in gut peptides such as increased peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) and decreased ghrelin(17–20). However, non-homeostatic mechanisms such as subjective appetite and food cravings may also be influenced by exercise in a manner that would promote appetite and energy intake regulation. For instance, bouts of AEx have been shown to result in reduced neuronal responses in brain regions associated with food reward pathways and are often accompanied by reduced hunger and increased satiety(21, 22). Chronic AEx interventions have produced conflicting results, showing no effect or modest effects on appetite-related indices (e.g., gut peptides, self-reported hunger) and energy intake(10, 11, 16, 23–29).
Even less is known about how resistance exercise (REx) acutely or chronically influences the mechanisms underlying energy intake(12, 13, 30, 31). Findings from a few studies suggest that acute REx suppresses ghrelin(13, 32) and decreases “liking” and “wanting” of high-fat foods compared to an acute AEx session(33). However, trials that have evaluated energy intake following acute REx have primarily been conducted in normal-weight males, and likely are not representative of the influence REx has adults with OW/OB. We previously observed a decrease in self-reported energy intake following a 12-week REx intervention in adults with OW/OB(34, 35) and others have shown decreased fat intake following a REx training program in adults with OW/OB(36). These findings suggest REx may alter food reward pathways, but clearly indicate further investigation is warranted. Therefore, the purpose of this study was to compare the effects of an acute bout of REx, AEx, and a sedentary (SED) control condition on hormonal and behavioral indices of appetite regulation and energy intake in adults with OW/OB. We hypothesized that hunger, ghrelin, food cravings, and energy intake would be lower following a bout of REx compared to AEx.
METHODS:
Participants:
Adults (18–55 years old) with OW/OB (BMI 25–35 kg/m2) whom were weight-stable (<±5% in the past 6 months, as evaluated by on-line or phone screening questionnaire, as well as objectively measured weight at the consent visit), physically inactive (not meeting current ACSM physical activity guidelines of 150 min/wk of moderate-intensity activity and 2x/wk whole body resistance exercise, as evaluated by a modified-version of the International Physical Activity Questionnaire-Short Form(37)), and otherwise healthy were eligible for enrollment in the trial. Women that were currently pregnant, lactating, less than 6 months post-partum, or peri- or post-menopausal were excluded. The Colorado Multiple Institutional Review Board approved the study protocol, and the trial was pre-registered on clinicaltrials.gov (NCT03143868). Participants provided written informed consent prior to participation.
Study Design:
Each eligible participant completed baseline evaluations including: height, weight, body composition via dual energy x-ray absorptiometry (DEXA; Hologic Discovery W, Bedford, MA), and the Three Factor Eating Questionnaire(38) to evaluate dietary restraint, disinhibition, and hedonic hunger. Participants next completed four exercise familiarization sessions (two for REx and two for AEx) over the course of two weeks in order to learn proper use of exercise equipment and to allow for individualized exercise prescriptions to be determined for the REx and AEx-specific study days. Following baseline testing and the exercise familiarization sessions, participants completed, in randomized order, stratified by sex, a REx study day, an AEx study day, and a SED study day. A washout period of at least 7 days separated the testing days for male participants, and at least 1 month for female participants in order to ensure all testing sessions occurred during the follicular phase of the menstrual cycle (determined via menstrual cycle logs). Each study day visit was proceeded by a 1-day run-in diet, to ensure energy and macronutrient balance. The caloric value of the diet was individualized for each participant and determined using the Mifflin-St. Joer equation multiplied by an activity factor of 1.3, and the macronutrient composition was 20% protein, 30% fat, and 50% carbohydrate. All food was prepared by the Colorado Clinical and Translational Research Center (CTRC)’s metabolic kitchen. Participants were instructed to consume only the food provided and were queried on adherence the following day.
Study Days:
An overview of the Study Day visits is presented in Figure 1.
Figure 1. Study Day Overview.
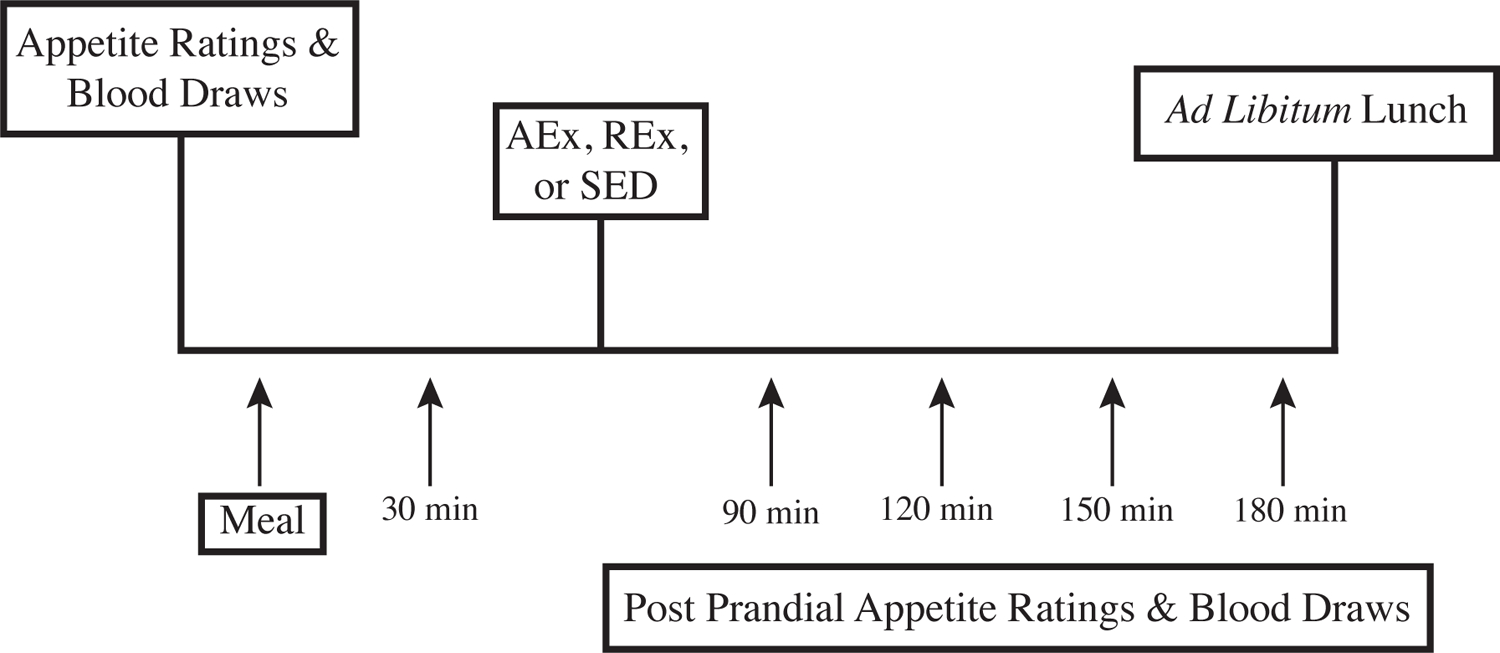
REx: resistance exercise; AEx: aerobic exercise; SED: sedentary control condition; FCI: Food Craving Inventory
Following the 1-day run-in diet, participants presented to the outpatient CTRC in the morning after an overnight fast of at least 10 hours and having refrained from exercise for the prior 48-hours and alcohol consumption for the prior 24-hours. Upon arrival, an intravenous (IV) catheter was placed for serial blood sampling followed by fasting blood sampling for analysis of ghrelin, PYY, and GLP-1. Participants then completed fasting appetite evaluations measured by 100 mm visual analogue scale (VAS). These included ratings of hunger, prospective food consumption, and satiety, as previously described(39). Participants next consumed a standard breakfast meal over 15 minutes prepared by the CTRC Metabolic Kitchen. The caloric content was equal to 25% of each participant’s total daily requirement and had a macronutrient composition identical to the run-in diet. The breakfast meal typically consisted of toast with butter, scrambled eggs, yogurt, fruit, though differed slightly for each participant in order to ensure the same relative caloric value was provided and not the same absolute caloric value. Repeat blood draws and appetite ratings were performed at 30, 90, 120, 150, and 180 minutes after the meal, and the area under the curve (AUC) was calculated using the trapezoid method(40). Radioimmunoassays were used to measure total ghrelin (Millipore; %CV: 4.5%) and PYY (Millipore, %CV: 5.3%), and ELISA was used to measure GLP-1 (Mercodia; %CV: 6.7%) by the CTRC Core Laboratory. Following the 30 minute blood draw and appetite rating participants began their assigned 45-minute exercise (or sedentary control) session [see below for details]. Following the exercise bout, at the 90-minute blood draw and appetite ratings assessment point, participants also completed the Food Craving Inventory Inventory(41) questionnaire. After the final blood draw and appetite rating (180 min post breakfast) participants were offered an 1800-calorie lunch to evaluate ad libitum energy intake. The lunch meal consisted of lasagna, salad, two types of salad dressing (a vinaigrette and a ranch option), dinner rolls, butter, cheese, pound cake, strawberries, diet soda, and a regular soda).
REx Study Day:
Participants completed a whole-body REx workout consisting of 1-set to failure (e.g. RPE of 9–10 on 10-point scale on final reps) on 12 exercises in the Exercise Research Laboratory, with a 3-minute rest between exercises. Resistance for the REx trial was determined based on the Exercise Familiarization sessions. Specifically, we selected a resistance level needed for each participant to complete ~12–15 repetitions per exercise, using proper form, with an RPE of 9–10 on a 10-point scale on final reps. This protocol eliminated the need for max testing to set an exercise protocol off of a specific intensity based on % of 1RM. We believed this protocol was the best choice to study appetite response to an acute REx bout as it 1) adheres to ACSM guidelines(42), particularly for untrained individuals; 2) decreases participant burden by negating the need for a maximal exercise testing session prior to study days; and 3) in our experience can be completed in ~45 minutes(43). When not engaging in REx, participants rested quietly in a seated position, to mirror the sedentary condition. They were permitted to use personal mobile devices or computers, or read.
AEx Study Day:
Participants were fitted with a chest-worn heart rate (HR) monitor and then walked on a treadmill for 45 minutes (5-minute warm-up, 35-minute workout, and 5-minute cool-down) at 65–70% age-predicted HR max. Speed and grade were self-selected by participants, with oversight and input from study staff in order to ensure the target HR range was maintained. This protocol was selected because it: 1) adheres to ACSM guidelines(42); 2) was of equivalent duration of the REx protocol; and 3) decreases participant burden by negating the need for a maximal exercise testing session prior to study days. When not engaging in AEx, participants rested quietly in a seated position, to mirror the sedentary condition. They were permitted to use personal mobile devices or computers, or read.
SED Study Day:
Participants rested quietly in a seated position throughout the study day for this condition. They were permitted to use personal mobile devices or computers, or read.
Statistical Analyses:
This pilot trial was designed to obtain data for sample size estimations for a larger-scale trial. Thus, sample size was initially determined in regards to differences in PYY AUC using preliminary data from a separate trial in our lab in a similar participant population. We estimated that we would have 80% power to detect differences in PYY between REx and AEx with a sample size of n=13. Future trials will build upon findings here to power trials to detect differences in other measures of appetite regulation.
Descriptive univariate analyses were conducted on all study variables. One-way repeated measures ANOVAs were used to compare differences in appetite and energy intake outcomes between study conditions, with a Bonferroni correction for posthoc comparisons. This procedure was also used to test for a potential visit order effect, and none was detected (p>0.05 for all outcome variables). The primary pairwise comparison of interest was between REx and AEx. All statistical tests were two-tailed with significance set at P < 0.05. Data are reported as means and standard errors unless otherwise noted. Pearson correlation analysis was conducted among ad libitum intake, VAS scores (AUC for hunger, satiety, and prospective food consumption), hormone levels (AUC for ghrelin, PYY, and GLP-1), food craving inventory, percent body fat, and baseline self-reported eating behavior scores (TFEQ). Data were analyzed using SPSS version 26 (IBM Corp).
Body composition and GLP-1 data are available for n=21 and n=19 participants, respectively, as these measures were added following study onset. Data for other bloodwork is available for n=23 participants for AEx and SED visits, and n=22 participants for REx visits as we were unable to obtain catheter access for one and two participants, respectively. Lastly, 180 minute bloodwork and AUC at the REx visit is not available for an additional participant due to the IV catheter failing prior to that time point. These were removed from analysis.
RESULTS:
Participant Characteristics:
A total of 369 individuals expressed interest in the trial. Of the initial interest list 38 responded to the research team, passed the phone screening, and attended the informed consent and in-person screening visit. Two did not qualify at the in-person screening (EATS-26 too high; and initiated an exercise program after phone screening); 7 participants dropped before completing exercise familiarization sessions due to time constraints; 4 canceled exercise familiarization visits and did not respond to rescheduling attempts; and 1 participant dropped after completing exercise familiarization sessions and prior to study day visits (family emergency). Thus, a total of 24 participants (50% female) completed study day visits and were included in the trial. Baseline characteristics are presented in Table 1.
Table 1.
Baseline participant characteristics
| n, (%F) | 24 (50) |
| Race, % white | 75 |
| Age, yr. | 35 ± 2 |
| Body mass index, kg/m2 | 28.5 ± 0.9 |
| Fat mass, % | 35 ± 2 |
| TFEQ | |
| TFEQ - Hunger | 4.9 ± 0.7 |
| TFEQ - Restraint | 7.9 ± 0.9 |
| TFEQ - Disinhibition | 5.9 ± 0.7 |
%F: percent of study sample that was female. Remaining data presented as mean±SE. TFEQ: Three-Factor Eating Questionnaire
Ad libitum Energy Intake
There was no difference in either absolute or relative (% of total energy needs) ad libitum energy intake following REx, AEx, or SED (REx: 991±68 kcals, 47.5±3.4% total energy needs; AEx: 937±65 kcals, 44.6±3.0% total energy needs; SED: 944±76 kcals, 45.1±3.8% total energy needs, p=0.50). Macronutrient composition of the lunch meal also did not differ (data not shown).
Subjective Appetite Ratings and Cravings
VAS measurements for each study condition are shown in Figure 2. There was no difference in fasting nor 30 minute appetite ratings between study day visits as neither the exercise nor SED conditions had been initiated at this point (p>0.05 for all). There was also no difference in any of the appetite ratings at the 90, 120, 150, or 180 minute time points nor AUC following REx, AEx, or SED (all p>0.05). Food cravings, measured post-exercise or SED, also did not differ between study conditions (REx: 35.4±2.0; AEx: 36.7±1.7; SED: 33.8±1.7, p=0.39).
Figure 2. Subjective Appetite Responses.
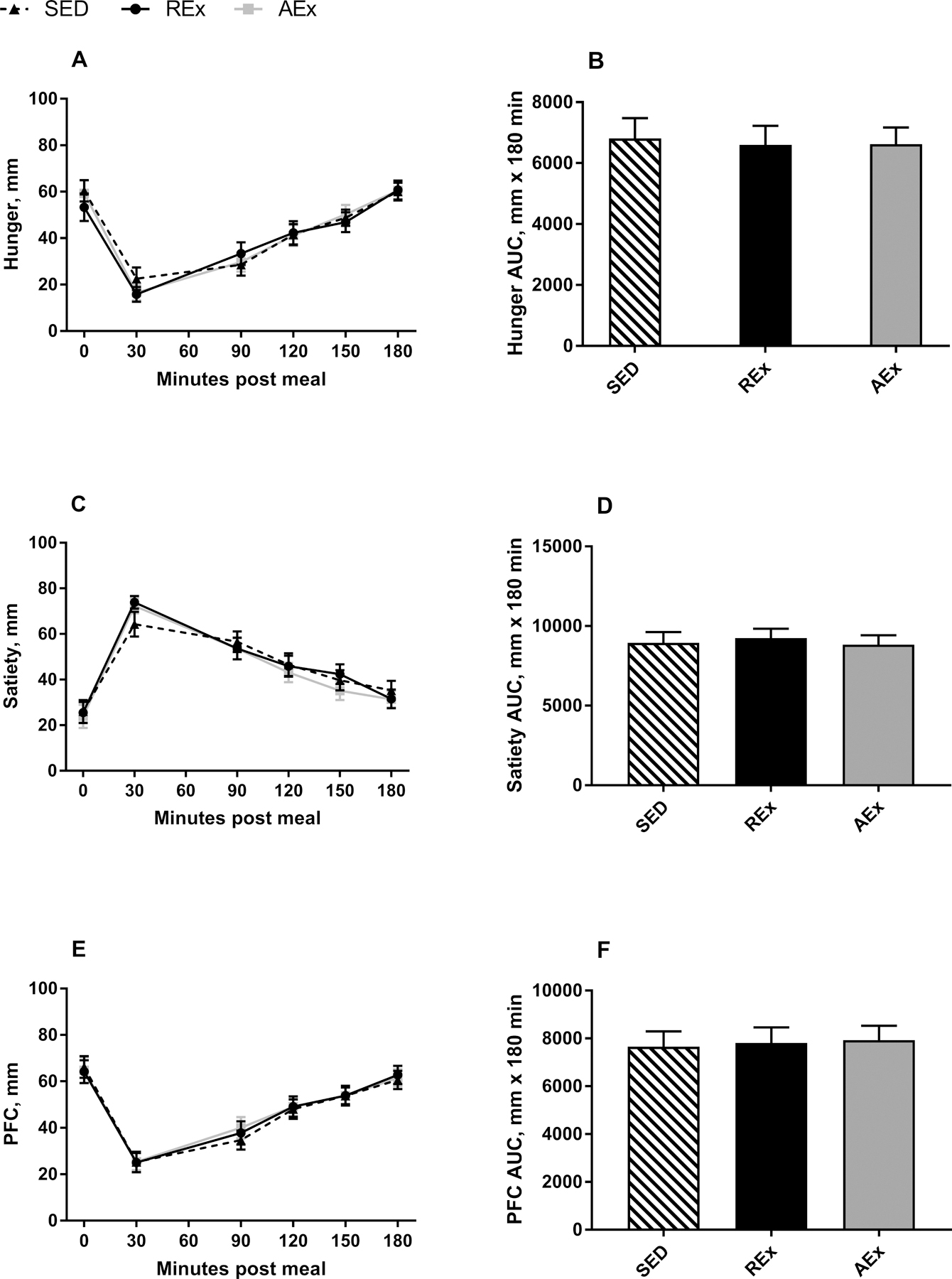
Curves for hunger (A), satiety (C), and PFC (E) and total AUC for hunger (B), satiety (D), and PFC (F). Data are presented as mean±SE
Appetite ratings were evaluated with 100 mm visual analogue scales. Exercise occurred between 35–80 minutes post meal.
SED: sedentary control; REx: resistance exercise; AEx: aerobic exercise; AUC: area under the curve; PFC: prospective food consumption
Ghrelin, PYY, and GLP-1
Figure 3 depicts ghrelin, PYY, and GLP-1 concentrations for each study condition. As expected, there was no difference in fasting nor 30 minute appetite-related hormone values between study day visits (p>0.05 for all). However, AUC for ghrelin, PYY, and GLP-1 did differ between conditions (Table 2). Post hoc analysis revealed that ghrelin, PYY, and GLP-1 AUC were all lower following REx as compared to AEx (p=0.006; p=0.001; and p=0.013, respectively). These AUC differences were due to lower hormone concentrations at multiple post-exercise blood collection time points in the REx compared to the AEx condition (Figure 3; Table 2). There were no significant differences in AUC between either exercise condition and SED, though there was a trend for ghrelin and GLP-1 AUC to be lower in the REx vs. SED condition (p=0.063 and 0.05, respectively), and for PYY AUC to be lower in the SED vs. AEx condition (p=0.075
Figure 3. Hormonal appetite responses.
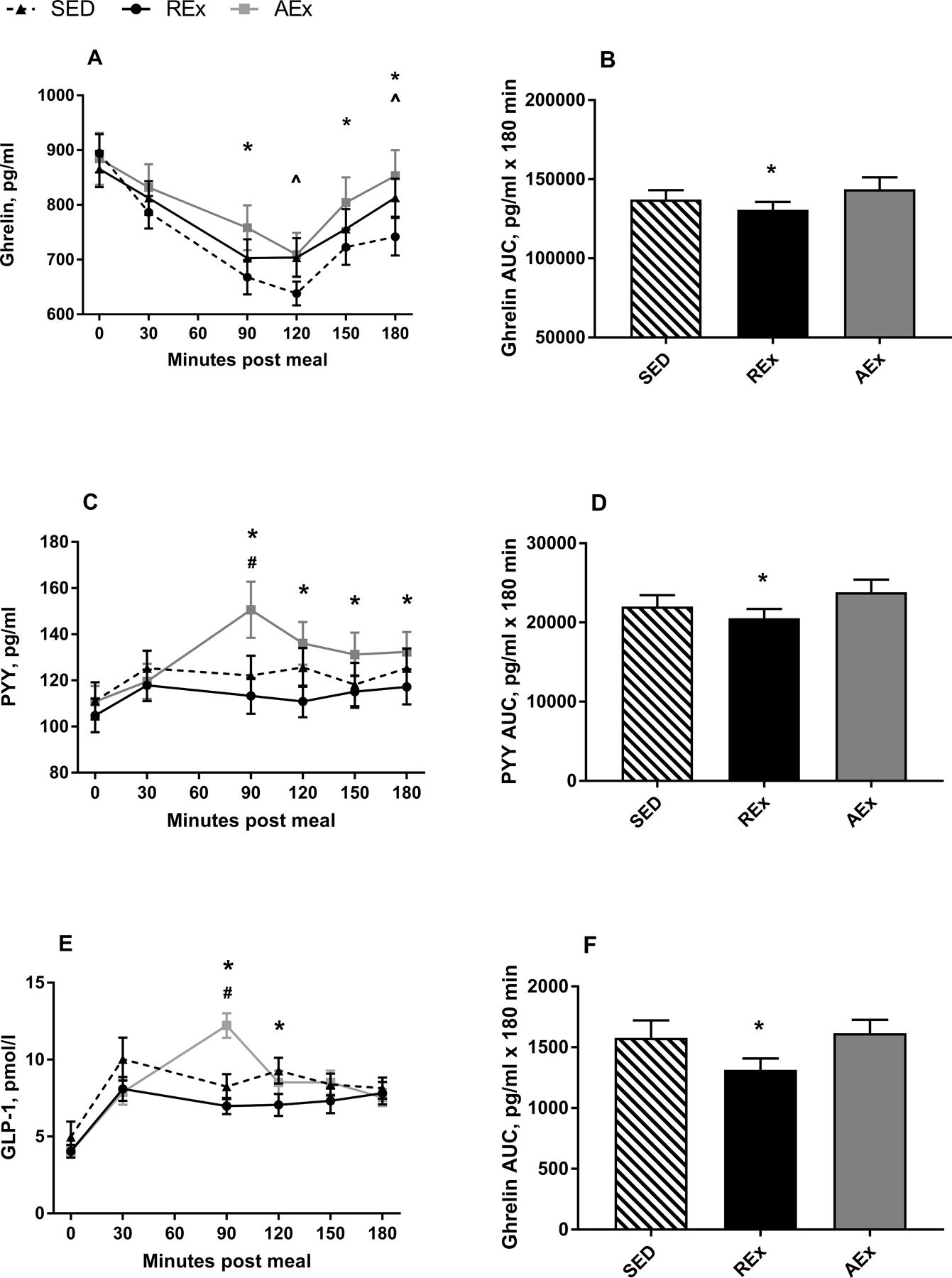
Curves for ghrelin (A), PYY (C), and GLP-1 (E) and total AUC for ghrelin (B), PYY (D), and GLP-1 (F). Data are presented as mean±SE . *REx significantly different from AEx (p<0.05); ^REx significantly different from SED; #AEx significantly different from SED (p<0.05)
Exercise occurred between 35–80 minutes post meal.
REx: resistance exercise; SED: sedentary control; AEx: aerobic exercise; AUC: area under the curve; PYY: peptide tyrosine tyrosine; GLP-1: glucagon like peptide-1
Table 2.
Appetite hormonal response to acute exercise
| REx | AEx | SED | p-value (ANOVA) |
p-value (REx vs. AEx) |
p-value (REx vs. SED) |
p-value (AEx vs. SED) |
|
|---|---|---|---|---|---|---|---|
| Ghrelin, pg/mL | |||||||
| AUC | 130,737±4,928 | 143,708±7,500 | 137,182±5,923 | 0.001 | 0.006 | 0.063 | 0.140 |
| 90 min | 668±31 | 758±41 | 703±35 | 0.002 | 0.008 | 0.286 | 0.134 |
| 120 min | 638±22 | 709±40 | 704±35 | 0.009 | 0.091 | 0.037 | 1.0 |
| 150 min | 723±32 | 804±46 | 756±36 | 0.009 | 0.034 | 0.322 | 0.226 |
| 180 min | 742±34 | 853±47 | 813±35 | 0.002 | 0.007 | 0.048 | 0.646 |
| PYY, pg/mL | |||||||
| AUC | 20,540±1,177 | 23,812±1,592 | 22,022±1,416 | <0.001 | 0.001 | 0.192 | 0.075 |
| 90 min | 113± 8 | 151± 12 | 122± 9 | <0.001 | 0.002 | 0.467 | 0.006 |
| 120 min | 111± 7 | 136± 9 | 126± 8 | 0.001 | 0.002 | 0.072 | 0.222 |
| 150 min | 115± 7 | 131± 9 | 118± 9 | 0.020 | 0.023 | 0.115 | 1.0 |
| 180 min | 117±8 | 132±9 | 125±8 | 0.036 | 0.067 | 0.245 | 0.798 |
| GLP-1, pmol/L | |||||||
| AUC | 1,314±93 | 1,615±110 | 1,578±143 | 0.017 | 0.013 | 0.05 | 1.0 |
| 90 min | 7.0±0.5 | 12.2±0.8 | 8.2±0.8 | <0.001 | <0.001 | 0.256 | 0.001 |
| 120 min | 7.1±0.7 | 8.5±0.8 | 9.3±0.8 | 0.007 | 0.201 | 0.010 | 0.620 |
| 150 min | 7.3±0.8 | 8.5±0.8 | 8.4±0.7 | 0.204 | - | - | - |
| 180 min | 7.8±0.7 | 7.5±0.6 | 8.1±0.7 | 0.501 | - | - | - |
Data are presented as mean±SE. REx: resistance exercise; AEx: aerobic exercise; SED: sedentary control; AUC: area under the curve
Correlational Analyses
Ad libitum energy intake was positively correlated with post-exercise hunger, prospective food consumption, and food cravings, following both AEx and REx, but these associations were not present following the SED condition (Table 3). Additionally, ad libitum energy intake was negatively correlated with percent body fat percent in both the REx and SED conditions, but not the AEx condition. Across conditions, none of the appetite hormones (ghrelin, PYY, nor GLP-1) AUCs were significantly correlated with the other indices of appetite regulation (p>0.05 for all, data not shown).
Table 3.
Bivariate Correlations with Ad Libitum Energy Intake
| Ad Libitum Energy Intake (kcals) | |||
|---|---|---|---|
| AEx | SED | REx | |
| Hunger AUC, mm * 180 min | .668 (p<0.001) | .207 (p=0.33) | .660 (p<0.001) |
| Satiety AUC, mm * 180 min | −.233 (p=0.27) | −.333 (p=0.11) | −.582(p=0.003) |
| PFC AUC, mm * 180 min | .619 (p=0.001) | .310 (p=.14) | .600 (p=0.002) |
| Food Craving Inventory | .414 (p=0.04) | −.040 (p=0.85) | .524 (p=0.009) |
| Fat Mass, % | −.351 (p=0.12) | −.587 (p=0.005) | −.660 (p=0.001) |
Significant correlations are indicated in bold.
AUC: Area under the curve; PFC: Prospective food consumption
DISCUSSION:
This study examined gut peptide concentrations, appetite, food cravings, and energy intake at a single ad libitum meal following an acute bout of AEx, REx, and SED completed in the postprandial state in adults with OW/OB whom reported not meeting current physical activity guidelines(44). Findings indicate that circulating concentrations of the anorexic gut peptides, PYY and GLP-1 are increased following AEx as compared to REx, and that circulating concentrations of the orexigenic gut peptide, ghrelin, are also greater following AEx and SED vs. REx. Interestingly, despite differences in hormonal indices of appetite, subjective appetite ratings did not differ between condition, nor did post-exercise food cravings or ad libitum energy intake. In addition, despite no differences in these variables between conditions, subjective huger, prospective food consumption, and food cravings were positively associated with energy intake following both REx and AEx conditions, and satiety was negatively associated with energy intake following REx.
While few trials have directly compared appetite hormonal responses between aerobic and resistance exercise, our results are in agreement with prior work in this area. Specifically, Broom et al (45) found blunted ghrelin responses to 90 minutes of REx as compared to 60 minutes of AEx and a SED condition and increased PYY levels following AEx. Similarly, Balaguera-Cortes et al also found blunted ghrelin responses to 45 minutes of REx compared to both AEx and SED(46). These consistent results occurred despite heterogeneity in study design. The trials by Broom and Balaguera-Cortes were conducted in young (~21 year old), physically active (average VO2 max of ~62 and ~58 ml/kg/min, respectively) men, with exercise conducted in the fasted state. Conversely, the current trial was conducted in physically inactive men and women, across a broader age range, and exercise was conducted in the post-prandial state. It is plausible that the reason for the different appetite hormonal response to acute exercise could be due to differences in total energy expenditure between AEx and REx, not specifically exercise modality. For instance, prior work examining changes in appetite hormones to acute bouts of aerobic exercise have shown that ghrelin, PYY, and GLP-1 values are increased with increasing energy cost of exercise(19, 47–52). Thus, the suppressed ghrelin, PYY, and GLP-1 response to acute REx as compared to acute AEx found in the present trial and prior work may be due to the higher caloric cost of AEx compared to REX, though this is not consistently shown(13, 53).
Despite differences in hormonal regulators of appetite and energy intake between AEx and REx, neither subjective measures of appetite nor food cravings differed between acute bouts of AEx, REx, or SED. This is in agreement with some, but not all, prior studies showing that acute exercise does not increase hunger or prospective food consumption compared to non-exercise control conditions(10, 54). Trials that have shown transient suppression of appetite with acute exercise compared to a control condition have primarily been in studies that utilized vigorous-intensity exercise, endurance-trained male participants, and were conducted in the fasted state(10, 17), which differ from the current trial in design and participant make-up. While McNeil et al. previously reported a decrease in preference for high-fat foods following a single bout of REx compared to a no-exercise control session, to our knowledge, the current study is the first to evaluate subjective appetite following REx compared to AEx and SED.
In this and prior trials, the lack of correlation between hormonal and subjective measures of appetite could be due to a disconnect between biological and subjective hunger, because differences in ghrelin, PYY, and GLP-1 were detected between exercise and sedentary conditions with no differences in subjective appetite ratings or ad libitum energy intake. Interestingly, appetite ratings and food cravings were correlated with ad libitum energy intake following the exercise conditions, but not the sedentary condition, which suggests that perhaps exercise does influence appetite, but perhaps VAS measures are not sensitive effect to detect changes in adults in response to a single exercise bout conducted in the postprandial state.
Our findings showing no difference in absolute energy intake at an ad libitum meal following acute REx, AEx, or SED conditions is, to our knowledge, the first time this has been studied in adults with OW/OB. One prior trial measuring differences in ad libitum energy intake following REx, AEx, and SED conducted in physically active men also showed no difference in post-exercise energy intake(46). The larger body of literature examining energy intake in response to exercise has evaluated AEx vs SED or different intensities of AEx, and has also found that a single bout of AEx does not acutely increase absolute energy intake compared to sedentary conditions or AEx of lower intensities (12, 47, 53). Furthermore, in some but not all, trials, when accounting for the energy cost of the exercise bout, the relative energy intake in the exercise condition results in an energy deficit (energy intake – exercise energy expenditure = negative value)(12, 47). Together these consistent findings have led to the common conclusion that exercise suppresses appetite since there is not an increase in compensatory energy intake compared to the sedentary condition. Our trial is also in agreement with these findings, and extends prior work by including a REx condition and conducting the trial in adults with OW/OB. However, it is worth noting that in our trial, the absolute energy intake was ~950 kcals (45% energy needs) across conditions, and this was following provision of a test breakfast equivalent to 25% of total energy needs. Thus, while participants in our trial, like others, did not consume greater amounts of calories following exercise vs SED, the exercise expenditure (while not measured via indirect calorimetry) was not large enough with our prescription (45 minutes AEx or REx) to be greater than their energy intake (12). This is likely due to provision of ad libitum or buffet style meals, in which excess calories are provided. These situations are known to result in greater energy intake than is typical, and is likely not indicative of how individuals would eat in a more natural setting(55).
Despite the strong randomized cross-over design, dietary control, and direct measurement of energy intake, we acknowledge a number of limitations to this study. First, we did not measure the energy cost of REx, AEx, or SED, nor did we attempt to match energy expenditure between REx and AEx conditions. We instead elected to match the exercise conditions on duration, in order to reflect greater practical utility. Therefore, the energy expenditure in the AEx condition was greater than REx in the present study, as is commonly the case in studies comparing strength vs aerobic activities. Second, we measured total ghrelin values, and not the acylated form specifically which is known to impact energy intake. However, acylated ghrelin typically accounts for ~5–10% of total ghrelin, and a prior investigation which did measure only acylated ghrelin also found lower active ghrelin in REx vs SED and AEx(46). Third, our sample was predominately Caucasian, and therefore out findings cannot be extrapolated to other races, and work evaluating race-based differences in appetite regulation are required. Finally, as this trial evaluated appetite responses to an acute bout of exercise and SED, we cannot generalize these results to chronic alterations in appetite and energy intake that may occur with prolonged training.
In conclusion, both REx and AEx cause suppression of appetite regulatory hormonal responses. However, exercise modality influences this response, with REx suppressing ghrelin to a greater extent, and AEx increasing PYY and GLP-1 to a greater extent. Energy intake did not differ between exercise nor SED conditions. This suggests that the energy expended with either REx or AEx vs SED is not compensated for by an increase in hunger, food cravings, or energy intake. Future work should extend assessment of energy intake following acute exercise in order to determine if energy intake differs by exercise modality for the remainder of the day or the following day(s), as well as examine changes in appetite and energy intake regulation following chronic exercise training of differing modalities.
ACKNOWLEDGEMENTS:
This work was supported by the National Institutes of Health Grant Numbers: NIH/NCATS under award numbers UL1TR002535, 1UL01TR002538, KL2TR002539, NIDDK P30DK048520, NIH/NIDDK Grant T32DK07658 and T32DK007446. This work was also supported by a pilot grant award from the University of Colorado’s Center for Women’s Health Research. This trial was pre-registered at clinicaltrials.gov (NCT03143868). The manuscript content is the authors’ sole responsibility and do not necessarily represent official NIH views. Drs. Melanson and Cornier are supported by resources from the Geriatric Research, Education, and the Clinical Center at the Denver VA Medical Center. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. We acknowledge and thank the University of Colorado’s Clinical and Translational Research Center for their support in conducting this trial.
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflicts of interest. The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES:
- 1.Maclean PS, Bergouignan A, Cornier MA, Jackman MR. Biology’s response to dieting: the impetus for weight regain. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;301(3):R581–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young-Hyman D Introduction to special issue: Self-regulation of appetite—it’s complicated. Obesity. 2017;25:S5–S7. [DOI] [PubMed] [Google Scholar]
- 3.MacLean PS, Blundell JE, Mennella JA, Batterham RL. Biological control of appetite: A daunting complexity. Obesity. 2017;25:S8–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoeckel LE, Birch LL, Heatherton T et al. Psychological and neural contributions to appetite self-regulation. Obesity. 2017;25:S17–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz MB, Just DR, Chriqui JF, Ammerman AS. Appetite self-regulation: Environmental and policy influences on eating behaviors. Obesity. 2017;25:S26–S38. [DOI] [PubMed] [Google Scholar]
- 6.Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite. 2004;43(3):253–9. [DOI] [PubMed] [Google Scholar]
- 7.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The Effects of Overfeeding on the Neuronal Response to Visual Food Cues in Thin and Reduced-Obese Individuals. PloS one. 2009;4(7):e6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLean PS, Wing RR, Davidson T et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring, Md.). 2015;23(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King NA, Caudwell P, Hopkins M et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity (Silver Spring, Md.). 2007;15(6):1373–83. [DOI] [PubMed] [Google Scholar]
- 10.Martins C, Morgan L, Truby H. A review of the effects of exercise on appetite regulation: an obesity perspective. Int J Obes. 2008;32(9):1337–47. [DOI] [PubMed] [Google Scholar]
- 11.King JA, Wasse LK, Ewens J et al. Differential Acylated Ghrelin, Peptide YY3–36, Appetite, and Food Intake Responses to Equivalent Energy Deficits Created by Exercise and Food Restriction. The Journal of clinical endocrinology and metabolism. 2011;96(4):1114–21. [DOI] [PubMed] [Google Scholar]
- 12.Schubert MM, Desbrow B, Sabapathy S, Leveritt M. Acute exercise and subsequent energy intake. A meta-analysis. Appetite. 2013;63:92–104. [DOI] [PubMed] [Google Scholar]
- 13.Schubert MM, Sabapathy S, Leveritt M, Desbrow B. Acute Exercise and Hormones Related to Appetite Regulation: A Meta-Analysis. Sports Medicine. 2014;44(3):387–403. [DOI] [PubMed] [Google Scholar]
- 14.King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. Eur J Clin Nutr. 1994;48(10):715–24. [PubMed] [Google Scholar]
- 15.King NA, Horner K, Hills AP et al. Exercise, appetite and weight management: understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. British journal of sports medicine. 2012;46(5):315–22. [DOI] [PubMed] [Google Scholar]
- 16.Unick JL, Otto AD, Goodpaster BH, Helsel DL, Pellegrini CA, Jakicic JM. Acute effect of walking on energy intake in overweight/obese women. Appetite. 2010;55(3):413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. Journal of applied physiology (Bethesda, Md. : 1985). 2007;102(6):2165–71. [DOI] [PubMed] [Google Scholar]
- 18.Hagobian TA, Sharoff CG, Braun B. Effects of short-term exercise and energy surplus on hormones related to regulation of energy balance. Metabolism. 2008;57(3):393–8. [DOI] [PubMed] [Google Scholar]
- 19.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. The Journal of endocrinology. 2007;193(2):251–8. [DOI] [PubMed] [Google Scholar]
- 20.Martins C, Kulseng B, King NA, Holst JJ, Blundell JE. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. The Journal of clinical endocrinology and metabolism. 2010;95(4):1609–16. [DOI] [PubMed] [Google Scholar]
- 21.Crabtree DR, Chambers ES, Hardwick RM, Blannin AK. The effects of high-intensity exercise on neural responses to images of food. The American journal of clinical nutrition. 2014;99(2):258–67. [DOI] [PubMed] [Google Scholar]
- 22.Evero N, Hackett LC, Clark RD, Phelan S, Hagobian TA. Aerobic exercise reduces neuronal responses in food reward brain regions. Journal of applied physiology (Bethesda, Md. : 1985). 2012;112(9):1612–9. [DOI] [PubMed] [Google Scholar]
- 23.Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? The Proceedings of the Nutrition Society. 2003;62(3):651–61. [DOI] [PubMed] [Google Scholar]
- 24.Caudwell P, Gibbons C, Hopkins M, King N, Finlayson G, Blundell J. No sex difference in body fat in response to supervised and measured exercise. Med Sci Sports Exerc. 2013;45(2):351–8. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly JE, Hill JO, DJ; Jet al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: The midwest exercise trial. Archives of Internal Medicine. 2003;163(11):1343–50. [DOI] [PubMed] [Google Scholar]
- 26.Stubbs RJ, Sepp A, Hughes DA et al. The effect of graded levels of exercise on energy intake and balance in free-living men, consuming their normal diet. Eur J Clin Nutr. 2002;56(2):129–40. [DOI] [PubMed] [Google Scholar]
- 27.Stubbs RJ, Sepp A, Hughes DA et al. The effect of graded levels of exercise on energy intake and balance in free-living women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(6):866–9. [DOI] [PubMed] [Google Scholar]
- 28.Cornier MA, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. The Effects of Exercise on the Neuronal Response to Food Cues. Physiology and Behavior. 2012;105(4):1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakicic JM, Otto AD, Lang W et al. The effect of physical activity on 18-month weight change in overweight adults. Obesity (Silver Spring, Md.). 2011;19(1):100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: compensatory behavioral adaptations. Medicine and science in sports and exercise. 2013;45(8):1600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly JE, Herrmann SD, Lambourne K, Szabo AN, Honas JJ, Washburn RA. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PloS one. 2014;9(1):e83498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghanbari-Niaki A. Ghrelin and glucoregulatory hormone responses to a single circuit resistance exercise in male college students. Clinical biochemistry. 2006;39(10):966–70. [DOI] [PubMed] [Google Scholar]
- 33.McNeil J, Cadieux S, Finlayson G, Blundell JE, Doucet E. The effects of a single bout of aerobic or resistance exercise on food reward. Appetite. 2015;84:264–70. [DOI] [PubMed] [Google Scholar]
- 34.Halliday TM, Davy BM, Clark AG et al. Dietary intake modification in response to a participation in a resistance training program for sedentary older adults with prediabetes: findings from the Resist Diabetes study. Eating behaviors. 2014;15(3):379–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halliday TM, Savla J, Marinik EL, Hedrick VE, Winett RA, Davy BM. Resistance training is associated with spontaneous changes in aerobic physical activity but not overall diet quality in adults with prediabetes. Physiology & behavior. 2017;177:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bales CW, Hawk VH, Granville EO et al. Aerobic and resistance training effects on energy intake: the STRRIDE-AT/RT study. Med Sci Sports Exerc. 2012;44(10):2033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth ML. Assessment of Physical Activity: An International Perspective. Research Quarterly for Exercise and Sport. 2000;71(2):s114–20. [PubMed] [Google Scholar]
- 38.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of psychosomatic research. 1985;29(1):71–83. [DOI] [PubMed] [Google Scholar]
- 39.Thomas EA, Bechtell JL, Vestal BE et al. Eating-related behaviors and appetite during energy imbalance in obese-prone and obese-resistant individuals. Appetite. 2013;65:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245–50. [DOI] [PubMed] [Google Scholar]
- 41.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obesity research. 2002;10(2):107–14. [DOI] [PubMed] [Google Scholar]
- 42.ACSM’s guidelines for exercise testing and prescription. 7th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005. [Google Scholar]
- 43.Davy BM, Winett RA, Savla J et al. Resist diabetes: A randomized clinical trial for resistance training maintenance in adults with prediabetes. PloS one. 2017;12(2):e0172610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Physical Activity Guidelines for Americans, 2nd edition. In: UDoHaH Services editor. Washington DC: 2018. [Google Scholar]
- 45.Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. American journal of physiology. Regulatory, integrative and comparative physiology. 2009;296(1):R29–35. [DOI] [PubMed] [Google Scholar]
- 46.Balaguera-Cortes L, Wallman KE, Fairchild TJ, Guelfi KJ. Energy intake and appetite-related hormones following acute aerobic and resistance exercise. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2011;36(6):958–66. [DOI] [PubMed] [Google Scholar]
- 47.Larson-Meyer DE, Palm S, Bansal A, Austin KJ, Hart AM, Alexander BM. Influence of running and walking on hormonal regulators of appetite in women. Journal of obesity. 2012;2012:730409-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christ ER, Zehnder M, Boesch C et al. The effect of increased lipid intake on hormonal responses during aerobic exercise in endurance-trained men. European journal of endocrinology. 2006;154(3):397–403. [DOI] [PubMed] [Google Scholar]
- 49.Russel RR, Willis KS, Ravussin E, Larson-Meyer ED. Effects of endurance running and dietary fat on circulating ghrelin and peptide YY. Journal of sports science & medicine. 2009;8(4):574–83. [PMC free article] [PubMed] [Google Scholar]
- 50.Deighton K, Barry R, Connon CE, Stensel DJ. Appetite, gut hormone and energy intake responses to low volume sprint interval and traditional endurance exercise. European journal of applied physiology. 2013;113(5):1147–56. [DOI] [PubMed] [Google Scholar]
- 51.Cooper JA, Watras AC, Paton CM, Wegner FH, Adams AK, Schoeller DA. Impact of exercise and dietary fatty acid composition from a high-fat diet on markers of hunger and satiety. Appetite. 2011;56(1):171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueda SY, Yoshikawa T, Katsura Y, Usui T, Nakao H, Fujimoto S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. The Journal of endocrinology. 2009;201(1):151–9. [DOI] [PubMed] [Google Scholar]
- 53.Howe SM, Hand TM, Larson-Meyer DE, Austin KJ, Alexander BM, Manore MM. No Effect of Exercise Intensity on Appetite in Highly-Trained Endurance Women. Nutrients. 2016;8(4):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alajmi N, Deighton K, King JA et al. Appetite and Energy Intake Responses to Acute Energy Deficits in Females versus Males. Medicine and science in sports and exercise. 2016;48(3):412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larson DE, Rising R, Ferraro RT, Ravussin E. Spontaneous overfeeding with a ‘cafeteria diet’ in men: effects on 24-hour energy expenditure and substrate oxidation. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1995;19(5):331–7. [PubMed] [Google Scholar]


