Abstract
BACKGROUND/OBJECTIVES:
In older persons with dementia (PWD), extensive medication use is often unnecessary, discordant with goals of care, and possibly harmful. The objective of this study was to determine the prevalence and medication constituents of polypharmacy among older PWD attending outpatient visits in the US.
DESIGN:
Cross-sectional analysis.
SETTING & PARTICIPANTS:
PWD and persons without dementia (PWOD) age ≥65 years attending outpatient visits recorded in the nationally representative National Ambulatory Medical Care Survey (NAMCS), 2014-2016.
MEASUREMENTS:
PWD were identified as those with a diagnosis of dementia on the NAMCS encounter form and/or those receiving an anti-dementia medication. Visits with PWD and PWOD were compared in terms of sociodemographic, practice/physician factors, comorbidities, and prescribing outcomes. Regression analyses examined the effect of dementia diagnosis on contributions by clinically relevant medication categories to polypharmacy (defined as being prescribed ≥5 prescription and/or non-prescription medications).
RESULTS:
The unweighted sample involved 918 visits for PWD and 26,543 visits for PWOD, representing 29.0 and 780 million outpatient visits. PWD had a median age of 81 and on average had 2.8 comorbidities other than dementia; 63% were female. The median number of medications in PWD was 8 compared to 3 in PWOD (p<0.001). After adjustment, PWD had significantly higher odds of being prescribed ≥5 medications (AOR 3.0; 95% CI: 2.1-4.3) or ≥10 medications (AOR 2.8; 95% CI: 2.0-4.2) compared to PWOD. The largest sources of medications among PWD were cardiovascular and central nervous system medications; usage from other categories was generally elevated in PWD compared to PWOD. PWD had higher odds of receiving at least one highly sedating or anticholinergic medication (AOR 2.5; 95% CI: 1.6-3.9).
CONCLUSION:
In a representative sample of outpatient visits, polypharmacy was extremely common among PWD, driven by a wide array of medication categories. Addressing polypharmacy in PWD will require cross-cutting and multidisciplinary approaches.
Keywords: polypharmacy, dementia, prescribing, outpatient care
INTRODUCTION
Polypharmacy is associated with a host of adverse outcomes among older adults, including drug reactions and use of potentially inappropriate medications (PIMs), significant morbidity such as falls and cognitive decline, and mortality.1,2,3,4 While many medications may be prescribed in line with chronic disease-specific guidelines, some medications have limited value, are discordant with goals of care, and are associated with more harm than benefit in older adults.5 This challenging clinical and public health situation is encapsulated by the care of people with dementia (PWD), a growing population and widely recognized health system priority in the US and internationally.6,7 People living with Alzheimer’s disease and related dementias often have multimorbidity8 and are particularly vulnerable to the occurrence and risks of polypharmacy given the potential for communication barriers between providers, patients, and caregivers, cognitive and functional changes associated with the disease, and evolving goals of care in the context of reduced life expectancy.9 Moreover, specific medications including anticholinergic and sedative medications have been linked with increased risk of hospitalization and mortality among PWD.10 As a result, recent literature has highlighted the importance of safe prescribing11 and the promise of deprescribing interventions12 for this vulnerable population.
Surprisingly, nationally representative data regarding overall prescribing practices among the estimated 3.4 million PWD living in the community13 in the US are sparse.1,9 Studies have documented high prevalence of polypharmacy and exposure to PIMs among PWD, but these primarily represent nursing home settings and more advanced stages of dementia or do not involve a random national sample of PWD, limiting generalizability.9,14,15,16,17,18 Additionally, many studies have focused specifically on the role of central nervous system-active polypharmacy19 or PIMs such as anticholinergic medications in prescribing practices related to PWD.20,21,22 Given that the majority of adverse drug reactions affecting older adults result from medications that are not necessarily considered inappropriate in this age group23,24, a broad perspective on the full breadth of medication categories contributing to polypharmacy among PWD is necessary.
Using nationally representative data, we aimed to profile polypharmacy among PWD by comparing polypharmacy prevalence and medication categories contributing to polypharmacy between older adults with dementia and people without dementia (PWOD) attending outpatient visits in the US. Additionally, we aimed to compare exposure to highly anticholinergic and sedating medications in these groups. We explored associations between the diagnosis of dementia and both polypharmacy and prescribing of clinically relevant medication categories, accounting for factors including age, sex, and comorbidity burden. Better understanding the overall medication use of community-dwelling PWD is critical to designing clinical and health system interventions to reduce potentially unnecessary, harmful, or goal-discordant medication exposure in this population.
METHODS
Overall Design
This was a cross-sectional analysis of the National Ambulatory Medical Care Survey (NAMCS). NAMCS is an annual national probability sample survey of nonfederal office-based healthcare visits conducted by the National Center for Health Statistics (NCHS).25 The survey utilizes a stratified two-stage approach with outpatient physicians selected in the first stage and patient visits in the second stage. The outpatient visit is the unit of observation, and data are weighted to provide unbiased national estimates and to account for nonresponse by sampled physicians. For sampled visits, trained U.S. Census Bureau field representatives, with input from outpatient physicians and/or physician office staff, complete a computerized patient record form based on documentation from the sampled office visit and the electronic medical record, including patient characteristics and diagnoses, practice information, and medications. Medication coding is performed centrally by SRA International, Inc. (Durham, NC) and is subject to quality control procedures. Medications are categorized according to the Multum Lexicon Drug Database scheme.26
Study Population
The study involved all sampled visits in NAMCS involving patients age 65 years or older. The period of 2014-2016 was selected given that the maximum recordable number of medications expanded from 10 to 30 in 2014 and that these represent the most recent years of survey data. In line with prior studies20,27, PWD were identified as those with a diagnosis of dementia on the encounter form and/or those receiving an anti-dementia medication. For the diagnosis of dementia on the NAMCS encounter form, PWD were identified either by the indication of “Alzheimer’s disease/Dementia” in the medical history section, or if a diagnosis of dementia was coded as one of up to five diagnoses assigned to the sampled visit in the diagnosis section. Dementia was defined using the relevant ICD-9-CM (2014-2015) and ICD-10-CM (2016) codes (Supplementary Methods). Anti-dementia medications included cholinesterase inhibitors and memantine.
Measurements
We collected information regarding the number and types of prescription and nonprescription medications reported by providers as newly prescribed or continued during the sample visits, including medications intended for regular or as-needed use, over-the-counter medications, and vitamins/dietary supplements. Physicians were asked to categorize reported medications as either newly prescribed during the sampled visit or as continued. Continued medications represented those that had been prescribed or noted at a prior visit and which the patient was expected to continue taking. Polypharmacy was defined as a binary variable as having five or more continued or newly prescribed regular or as-needed medications (including all prescription and over-the counter medications and vitamins) recorded at a visit and, in an additional analysis, as ten or more similarly defined medications. Additional prescribing outcomes included the presence of at least one highly anticholinergic medication as defined by Rhee et al.28 who drew on the American Geriatrics Society Beers Criteria29 and the Anticholinergic Risk Scale30 or at least one highly sedating medication as defined by the sedative load model (Supplementary Methods).31–33 For highly anticholinergic and sedating medications for which there were adequate counts to generate reliable national estimates (>30 unweighted prescriptions, per NCHS guidance), we estimated an average number of prescriptions per visit as well as number of prescriptions over the study period for PWD. Building on the Multum Lexicon Drug Database scheme, we classified all prescribed medications into one of 11 mutually exclusive categories of medications that are clinically relevant in the management of chronic disease in older adults.
Socioeconomic demographics included age, sex, race, ethnicity, and source of insurance. We used age as a categorical variable given that it is top coded at 92 years to maintain confidentiality in the publicly available NAMCS dataset. There were missing data for approximately one quarter of survey responses regarding race and ethnicity; in these cases, NCHS imputes missing data using a model-based, single, sequential regression imputation method. Practice and provider factors included the specialty of the visit clinician, whether the patient had been seen previously in the practice, and the region of the country. NAMCS records the absence or presence of 23 specific medical comorbidities during each sampled visit; we created a comorbidity count based on the total number of comorbidities other than dementia present at each visit.
Statistical analysis
Data were analyzed using NAMCS sampling weights and other survey design features to account for its complex, multistage survey design that incorporates several stages of clustering, stratification, and probabilistic sampling. Descriptive analyses evaluated sampled visits, comparing PWD and PWOD. We reported survey-weighted medication counts with medians and interquartile ranges given the non-normal distribution of these data. We used survey-weighted multivariate logistic regression models to estimate unadjusted and adjusted odds ratios and 95% confidence intervals (CIs), assessing the relationship between the diagnosis of dementia and polypharmacy, with adjustment for categorical factors selected based on prior studies and content knowledge8,34 including age, sex, and comorbidity count. We performed marginal analyses to determine the predicted mean number of medications used per visit and probability of being prescribed at least one drug per visit across medication categories. For the former, we fit a linear regression model with dementia status, age, sex, and comorbidity count as predictors and number of medications by category as the outcome. We used bootstrap techniques with 1,000 repetitions to confirm that the resulting confidence intervals were virtually identical to those obtained via the linear regression models, as a result of the large sample size. For the latter marginal analysis, we fit a logistic regression model with these same factors as predictors and the presence of a medication from each category as the outcome. Marginal analyses standardized age, sex, and comorbidity count to the average values among PWD in the overall analytic sample to elucidate the effect of a documented diagnosis of dementia on prescribing practices beyond these other factors that have important and established effects on prescribing. We used non-parametric Wilcoxon signed rank tests to assess the null hypothesis that greater predicted mean number of medications and probability of being prescribed at least one drug per visit would be split evenly between PWD and PWOD across the 11 medication categories.
We conducted several sensitivity analyses. First, we limited the analytic sample to visits involving primary care providers given the possibility that there was differential coding of medications based on provider type. Second, we varied the definition of dementia to exclude those who were identified solely through use of anti-dementia medications, given the possibility that this would bias the outcome of polypharmacy. All analyses were performed using Stata SE, version 16.1 and SAS, version 9.4.
The study was exempted from review by the institutional review boards of University of California, San Francisco and San Francisco VA Medical Center.
RESULTS
Characteristics of the study population
Over the 3-year study period, there were 918 sampled outpatient visits by PWD, corresponding to 29.0 million visits, and 26,543 visits by PWOD, corresponding to 780 million visits. The median age among PWD attending sampled visits was 81 years, 63% were female, and they had on average 2.8 comorbidities other than dementia. PWOD were on average younger, less likely to be female, and had fewer comorbid conditions (Table 1). PWD were more likely to be seen in an outpatient visit by a primary care physician than PWOD (59% vs. 38%, p<0.001).
Table 1:
Characteristics of people >=65 years old with and without dementia attending outpatient visits in US, 2014-2016
| Characteristic | With Dementia 29.0 million visits (weighted) | Without Dementia 780 million visits (weighted) | p value for comparison |
|---|---|---|---|
| Age --65-74 --75-84 -->=85 |
22% (0.17-0.29) 40% (0.32-0.49) 38% (0.28-0.49) |
55% (0.54-0.56) 33% (0.32-0.34) 12% (0.11-0.13) |
p<0.001 |
| Female sex | 63% (0.58-0.68) | 56% (0.55-0.57) | p=0.01 |
| Race --White --Black --Other |
89% (0.82-0.93) 6% (0.04-0.10) 5% (0.02-0.13) |
85% (0.82-0.87) 9% (0.08-0.10) 6% (0.04-0.10) |
p=0.43 |
| Hispanic or Latino ethnicity --Not Hispanic or Latino --Hispanic or Latino |
85% (0.77-0.90) 15% (0.10-0.23) |
90% (0.88-0.92) 10% (0.08-0.12) |
p=0.05 |
| Specialty of clinician --Primary Care --Medical Specialty --Surgical |
59% (0.50-0.67) 28% (0.22-0.35) 13% (0.10-0.17) |
38% (0.35-0.41) 34% (0.31-0.38) 28% (0.26-0.31) |
p<0.001 |
| Seen before in practice | 88% (0.81-0.92) | 88% (0.87-0.89) | p=0.81 |
| # visits with sampled physician’s practice in past 12 months (among visits to primary care clinics) --0 --1-2 --3-5 --6+ |
10% (0.04-0.22) 20% (0.13-0.29) 34% (0.26-0.43) 36% (0.25-0.48) |
10% (0.09-0.13) 27% (0.24-0.31) 34% (0.31-0.37) 29% (0.25-0.32) |
p=0.32 |
| # visits with sampled physician’s practice in past 12 months (among visits to non-primary care clinics) --0 --1-2 --3-5 --6+ |
23% (0.18-0.29) 35% (0.28-0.42) 24% (0.19-0.31) 18% (0.13-0.25) |
22% (0.21-0.24) 33% (0.31-0.35) 26% (0.24-0.28) 19% (0.17-0.20) |
p=0.96 |
| Region of country --NE --Midwest --South --West |
12% (0.08-0.17) 18% (0.13-0.24) 43% (0.34-0.53) 27% (0.17-0.40) |
20% (0.18-0.22) 20% (0.18-0.22) 35% (0.32-0.38) 25% (0.21-0.29) |
p=0.07 |
| Source of payment --Private --Public --Self-pay/other |
10% (0.06-0.17) 84% (0.76-0.89) 6% (0.03-0.11) |
15% (0.14-0.17) 79% (0.77-0.81) 6% (0.05-0.07) |
p=0.21 |
| # of comorbidities --0 --1 --2 --3 -->=4 |
13% (0.09-0.19) 15% (0.11-0.21) 22% (0.18-0.28) 19% (0.15-0.25) 30% (0.22-0.38) |
17% (0.15-0.18) 21% (0.20-0.23) 21% (0.20-0.22) 18% (0.17-0.19) 23% (0.21-0.25) |
p=0.08 |
| Number of medications (median, IQR) |
8 (4-13) | 3 (1-8) | p<0.001 |
| Polypharmacy -->=5 medications -->=10 medications |
72% (0.64-0.79) 43% (0.34-0.54) |
44% (0.42-0.47) 20% (0.18-0.22) |
p<0.001 p<0.001 |
All results are adjusted for weights and survey design to produce nationally representative estimates; the unweighted sample included 918 visits for PWD and 26,543 visits for PWOD. For all percentages, the denominator is total number of visits. The only exception is for the number of repeat visits in the past 12 months: since this information is only available for the sampled physician’s practice, this variable is presented separately for primary care and non-primary care clinics, and the denominators in those cases refer to the total number of visits involving a primary care physician or visits involving a non-primary care physician. Pearson Chi-squared tests were used for categorical variables and t-tests for continuous variables.
Frequency and constituents of polypharmacy
The median number of total medications reported at visits was 8 in PWD, compared to 3 in PWOD (p<0.001). Five or more medications were prescribed more often in PWD than PWOD (72% vs. 44%, p<0.001) as were ten or more medications (43% vs. 20%, p<0.001; Table 1). In analyses adjusting for sociodemographic factors, provider and practice characteristics, and comorbidity count, compared to PWOD, PWD attending outpatient visits had 3.0-fold (95% CI, 2.1-4.3) greater odds of receiving five or more medications and 2.8-fold (95% CI, 2.0-4.2) greater odds of receiving ten or more medications. These findings were very similar to those from unadjusted analyses (Supplementary Table S1).
To better understand what medications contributed to polypharmacy in PWD and how this compared to PWOD with otherwise similar characteristics, we next analyzed the predicted number of medications in each group that would be used by a patient of standardized age, sex, and comorbidity count (wherein those characteristics matched the average values among PWD). These findings are summarized in Table 2. Among PWD, there was a higher predicted mean number of medications per visit in 10 of 11 medication categories (Wilcoxon signed-rank, p=0.007). Similarly, among PWD there was a higher predicted probability of being prescribed at least one medication across all 11 categories (Wilcoxon signed-rank, p<0.001). The largest relative differences were among the central nervous system (CNS), genitourinary (GU), analgesic, and gastrointestinal (GI) medication categories, for which PWD received more medications than PWOD. For CNS medications, PWD received on average 2.0 medications compared to 0.46 in PWOD; greater medication use among PWD compared to PWOD within this category included (but was not limited to) antidepressants, anxiolytics, sedatives, antiepileptics, and antipsychotics. The Figure depicts the contribution by medication category to overall medication use among all visits involving PWD and PWOD, showing that PWD received a greater absolute number of medications on average as well as relative increases compared to PWOD across multiple medication categories.
Table 2:
Medication use in people with versus without dementia, adjusted for age, sex, and comorbidity burden
| Medication Category |
Mean number of medications in use per visit | Probability of visit with at least 1 prescribed medication in use | ||
|---|---|---|---|---|
| Persons with dementia | Persons without dementia | Persons with dementia | Persons without dementia | |
| Central Nervous System | 2.0 +/− 0.10 | 0.46 +/− 0.02 | 85% (0.81-0.90) | 27% (0.25-0.28) |
| --Cholinesterase inhibitors | 0.57 +/− 0.04 | - | 56% (0.50-0.63) | - |
| --Antidepressants | 0.42 +/− 0.04 | 0.15 +/− 0.01 | 34% (0.28-0.40) | 11% (0.10-0.12) |
| --Anxiolytics, sedatives, hypnotics | 0.26 +/− 0.04 | 0.15 +/− 0.01 | 22% (0.14-0.30) | 12% (0.11-0.14) |
| --Antiepileptics | 0.24 +/− 0.04 | 0.07 +/− 0.004 | 19% (0.12-0.27) | 6% (0.05-0.07) |
| --Memantine | 0.23 +/− 0.03 | - | 23% (0.17-0.29) | - |
| --Antipsychotics | 0.10 +/− 0.02 | 0.01 +/− 0.002 | 9% (0.05-0.12) | 1% (0.008-0.01) |
| Cardiovascular | 2.0 +/− 0.12 | 1.7 +/− 0.05 | 73% (0.66-0.79) | 56% (0.53-0.60) |
| Vitamins & Supplements | 1.1 +/− 0.09 | 0.76 +/− 0.03 | 51% (0.45-0.57) | 33% (0.31-0.35) |
| Other | 0.88 +/− 0.09 | 0.76 +/− 0.03 | 46% (0.39-0.52) | 42% (0.40-0.45) |
| Gastrointestinal | 0.66 +/− 0.08 | 0.40 +/− 0.02 | 41% (0.31-0.50) | 26% (0.24-0.29) |
| Analgesic | 0.51 +/− 0.06 | 0.30 +/− 0.01 | 33% (0.27-0.39) | 22% (0.20-0.23) |
| --Opioids | 0.25 +/− 0.04 | 0.17 +/− 0.01 | 20% (0.14-0.26) | 15% (0.13-0.16) |
| Diabetes | 0.32 +/− 0.07 | 0.22 +/− 0.01 | 14% (0.10-0.19) | 10% (0.09-0.11) |
| Hormone/Metabolic | 0.31 +/− 0.03 | 0.33 +/− 0.02 | 26% (0.21-0.31) | 26% (0.24-0.28) |
| Respiratory | 0.27 +/− 0.04 | 0.25 +/− 0.01 | 17% (0.11-0.22) | 14% (0.13-0.15) |
| Genitourinary | 0.27 +/− 0.04 | 0.15 +/− 0.007 | 19% (0.11-0.27) | 9% (0.08-0.10) |
| Coagulation Modifiers | 0.24 +/− 0.04 | 0.16 +/− 0.008 | 18% (0.14-0.22) | 13% (0.11-0.14) |
Results reflect the predicted mean number of prescription and non-prescription medications in use per visit and the predicted probabilities of taking at least one medication by medication category for a sample patient whose age, sex, and comorbidity count correspond to average values observed among PWD. Selected subgroups for the central nervous system and analgesic categories are provided; these do not represent all possible medication subgroups within those groups. All results are adjusted for weights and survey design to produce nationally representative estimates. Standard errors are presented for means and 95% confidence intervals are presented for percentages. Non-parametric Wilcoxon signed-rank tests support the predominance of higher mean number of medications in use per visit (p=0.007) and higher predicted probabilities of taking at least one medication by category (p<0.001) among PWD compared to PWOD.
Figure: Mean Number Of Medications Per Visit By Medication Category.
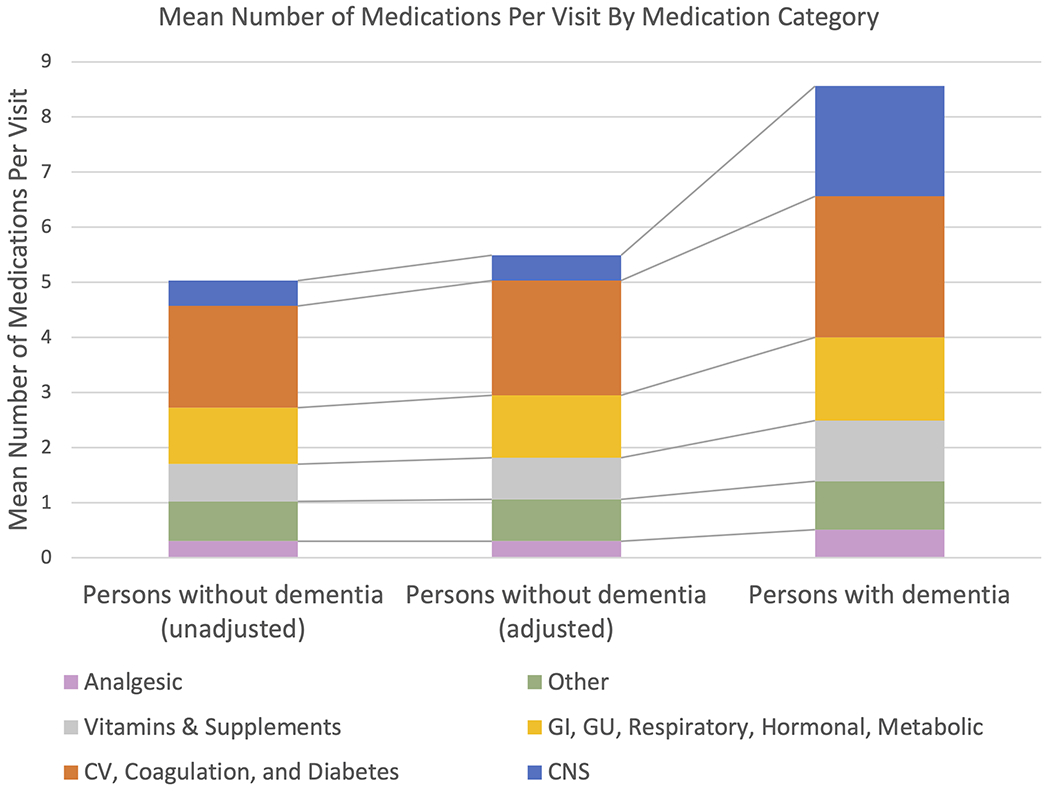
The second column represents the mean number of medications per visit among PWOD adjusted to the observed average age, sex, comorbidity count of PWD. All values are adjusted by survey weights to provide national estimates. CV: cardiovascular; GI: gastrointestinal; GU: genitourinary; CNS: central nervous system.
Highly anticholinergic and/or highly sedating medication use
Table 3 shows the average number of highly anticholinergic and highly sedating medications prescribed at visits involving PWD and PWOD. At least one highly anticholinergic medication was prescribed in 16% of visits involving PWD compared to 8% of visits involving PWOD (p<0.001, Supplementary Table S1). In adjusted analyses, PWD attending outpatient visits had 1.9-fold (95% CI, 1.3-2.6) greater odds of receiving at least one highly anticholinergic medication compared to PWOD. At least one highly sedating medication was prescribed in 35% of visits involving PWD compared to 16% of visits involving PWOD (p<0.001). PWD attending outpatient visits had 2.5-fold (95% CI, 1.6-3.9) greater odds of receiving at least one highly sedating medication compared to PWOD. Table 4 summarizes prescribing information regarding the top five categories of highly anticholinergic or sedating medications among PWD, including benzodiazepines, gabapentinoids, antipsychotics, urinary antispasmodics, and antihistamines.
Table 3:
Mean number of highly anticholinergic and sedating medications among persons with versus without dementia
| Category | Persons with dementia 29.0 million visits (weighted) |
Persons without dementia 780 million visits (weighted) | p value for comparison |
|---|---|---|---|
| Number of highly sedating medications | 0.47 +/− 0.08 | 0.19 +/− 0.01 | p=0.001 |
| Number of highly anticholinergic medications | 0.17 +/− 0.03 | 0.09 +/− 0.004 | p<0.001 |
| Number of highly anticholinergic and sedating medications | 0.64 +/− 0.09 | 0.28 +/− 0.01 | p<0.001 |
Means are presented with standard errors. The unweighted sample included 918 visits for PWD and 26,543 visits for PWOD.
Table 4:
Most commonly prescribed highly anticholinergic or highly sedating medications among persons with dementia attending outpatient visits
| Medication category | Average number of medications prescribed per visit | Number of unique outpatient visits from 2014-2016 in which medication was recorded as continued or newly prescribed |
|---|---|---|
| Benzodiazepines | 0.17 +/− 0.04 | 5,013,092 |
| Gabapentinoids | 0.17 +/− 0.04 | 4,878,901 |
| Antipsychotics | 0.07 +/− 0.01 | 2,054,807 |
| Urinary antispasmodics | 0.07 +/− 0.02 | 2,029,820 |
| Antihistamines | 0.04 +/− 0.01 | 1,123,114 |
Means are presented with standard errors. The table includes any medication groups or specific medications among the most commonly prescribed highly anticholinergic or sedating medications in which the relative standard error was less than 30% and therefore was considered reliable per NCHS guidance. Results are adjusted for weights and survey design to produce nationally representative estimates.
Sensitivity analyses
Sensitivity analyses supported the main results. In analyses involving primary care visits alone (which included 362 of the original 918 people with dementia) and in those excluding PWD identified only through anti-dementia medication use (which included 558 of the original 918 people with dementia), PWD had significantly higher odds of polypharmacy, driven by medications from multiple categories, as well as receipt of at least one highly anticholinergic or sedating medication compared to PWOD. The magnitude of the differences was slightly attenuated (Supplementary Tables S2–S5). In analyses with PWD identified by a diagnosis of dementia on the encounter form alone, 38% (95% CI, 0.31-0.46) were prescribed a cholinesterase inhibitor and 19% (95% CI, 0.14-0.25) were prescribed memantine.
DISCUSSION
In this nationally representative cross-sectional study, a large proportion of older adults with dementia attending outpatient visits were affected by polypharmacy, driven not only by central nervous system drugs but by a broad array of medication categories. Alarmingly, older adults with dementia had high rates of use of highly anticholinergic and sedating medications, a problematic finding given well-documented adverse cognitive and other health-related effects associated with these medications and limited evidence for benefit. However, these medications only contributed a small fraction to the overall medication burden experienced by PWD. Our findings suggest that older adults with dementia attending outpatient visits in the US receive a wide array of medications that may be inconsistent with goals of care, associated with adverse outcomes, and representative of potential targets for deprescribing interventions.
This study adds to a growing literature regarding prescribing practices—including polypharmacy as well as potentially inappropriate prescribing—affecting community-dwelling PWD in the US and abroad. Several international studies with nationally representative data have examined these issues. Our findings of a point prevalence of polypharmacy of 72% among PWD and 44% among PWOD exceed figures in a 2018 Danish registry study34 that reported more frequent polypharmacy (≥5 medications) among community-dwelling PWD (55%) compared to PWOD (34%) in Denmark. Recently, a cohort study in England found a 73% prevalence of potentially inappropriate prescribing (defined by the Screening Tool of Older Persons’ Prescriptions V2) among PWD from a representative primary care population, which was linked to important health outcomes including falls and all-cause mortality over one year.35 To date, nationally representative data regarding overall prescribing practices among community-dwelling PWD in the US are more sparse, a key motivating factor for our study.9 A retrospective cohort study of an integrated delivery system in one US state found a prevalence of polypharmacy (≥5 medications) of 67% among PWD with at least 2 additional chronic medical conditions.15 A longitudinal study using National Alzheimer’s Coordinating Center data36 found that the number of overall medications and odds of exposure to PIMs increased annually for both PWD and matched controls without dementia before and after the diagnosis of dementia. This study also found that compared with matched controls without dementia, PWD were less likely to utilize PIMs over time. However, the study population was not representative of the US population, thus limiting the generalizability of the results. In contrast, one of the primary strengths of NAMCS is its ability to represent office-based physicians’ practice and prescribing patterns at a national level.28
By providing a broad view of various medication categories (including but not limited to CNS medications) contributing to overall medication usage among PWD, our study complements a recent study by Maust et al. investigating the prevalence of CNS-active polypharmacy, defined as receipt of three or more CNS agents including opioids, among PWD in the US.19 In their nationally representative study, which was bolstered by categorization of medication exposure in Medicare claims in 2018, 13.9% of older adults with dementia were exposed to CNS-active polypharmacy for longer than 30 consecutive days. We found that CNS medications as a category were prescribed more frequently and in greater numbers among PWD compared to PWOD, again highlighting the prominent role of CNS-active polypharmacy in this population. In addition, we found that PWD were more likely than PWOD to receive highly anticholinergic and sedating medications, a worrisome finding given the attendant risks of worsening cognition and other ADEs associated with these medications.15,20,22,37 This finding likely reflects the pharmacological management of the behavioral and psychological symptoms of dementia (BPSD). Due to the complex and varied causes of BPSD as well as the fact that nonpharmacological approaches to BPSD are recommended before pharmacological therapies29,38,39, it is difficult to discern the appropriateness of these medications in PWD attending outpatient visits. Nevertheless, evidence of broad usage of highly anticholinergic and sedating medications including antipsychotics and benzodiazepines among PWD is suggestive of an ongoing need to optimize nonpharmacological management and prescribing in this population.
A central finding of our study is that CNS medications explain only part of the picture of polypharmacy among PWD in the US. Indeed, there were many other medication categories contributing to polypharmacy in this population, as evidenced by a higher mean number of medications prescribed from almost all medication categories in PWD compared to PWOD after adjustment for age and comorbidity burden. In our study, CV medications were prescribed more frequently and in greater numbers in PWD compared to PWOD, even when adjusting for age and comorbidity burden. Notably, and potentially unbeknownst to some providers, CV medications have been shown to contribute substantially to anticholinergic burden in people with cognitive impairment and cardiac conditions.15,40 Other medication categories including vitamins and dietary supplements, which have been linked with important and overlooked drug-drug interactions,41 as well as GI-related and analgesic medications, were very common among PWD. The finding regarding analgesic medications is of interest given prior research that raised concern about potential undertreatment of pain among PWD43; our findings are more in line with a Swedish population-based study of PWD from both community and institutional settings that found PWD to be equally or more likely to use a variety of analgesic medications compared to PWOD.42 Exclusion of PWD from many trials and the resulting paucity of data supporting many therapeutic decisions among PWD22,43 figure into complex decision-making and challenging weighing of risks and benefits in this population. Medications from all of these categories should be examined carefully in clinical practice for appropriateness or potential harms.
Our study has several limitations. NAMCS does not provide granular information regarding the chronicity, severity, or expected underlying pathology of dementia. Additionally, it is likely that dementia was underdiagnosed or under-coded, and individuals with mild dementia may not be reflected in these data.44,45,46 Location of residence is not available in NAMCS. As with prior studies, we expect that most persons attending outpatient visits sampled in NAMCS were community-dwelling.47 It is possible that some sampled visits do not represent an entirely accurate list of medications, due to factors including time constraints, incomplete records, or multiple prescribers. Additionally, the source of medication reconciliation is unknown; it is possible that bias could be introduced in the comparison of medication usage between PWD and PWOD depending on whether patients, family members, or caregivers reported or confirmed medications listed in the sampled physician’s medication record. Medication lists reflect both continued or newly prescribed medications at each sampled outpatient visit, and it is not possible to determine longitudinal changes or decisions to discontinue medications. Finally, our measure of polypharmacy included both regular and as-needed medications, which cannot be distinguished in NAMCS; this could have led to an overestimation of actual medication exposure, which may be better captured using other data sources.19 Despite these potential limitations, NAMCS has been used historically in many studies20,27,28 to provide national estimates of outpatient prescribing practices in the US.
In conclusion, in a nationally representative sample of outpatient visits in the US, polypharmacy was much more prevalent among community-dwelling older adults with dementia than those without dementia. This vulnerable population was exposed not only to strongly anticholinergic and sedating medications, but also to a wide array of medication categories. Further research into clinical and health system interventions to optimize prescribing practices among PWD should be cross-cutting, multidisciplinary, and target a broad array of medications.
Supporting Information (attached files):
Supplementary Methods: Highly Anticholinergic or Sedating Medications
Supplementary Table S1: Regression analyses describing polypharmacy and highly anticholinergic and sedating medication prescribing among persons with dementia
Supplementary Table S2: Regression analyses describing polypharmacy and highly anticholinergic and sedating medication prescribing among persons with dementia (primary care visits only)
Supplementary Table S3: Medication use in people with versus without dementia, adjusted for age, sex, and comorbidity burden (primary care visits only)
Supplementary Table S4: Regression analyses describing polypharmacy and highly anticholinergic and sedating medication prescribing among PWD (removing anti-dementia medication from PWD definition)
Supplementary Table S5: Medication use in people with versus without dementia, adjusted for age, sex, and comorbidity burden (removing anti-dementia medication from PWD definition)
Supplementary Material
KEY POINTS.
Adults with dementia attending outpatient visits had high rates of polypharmacy.
Polypharmacy was driven by many drug types including cardiac and central nervous system drugs.
WHY DOES THIS MATTER?
Addressing polypharmacy among community-dwelling adults with dementia may require targeting multiple medication categories.
Acknowledgments:
Funding:
Dr. Growdon was supported by the National Institute on Aging, T32-AG000212. Dr. Yaffe was supported by the National Institute on Aging, K24AG031155.
Dr. Steinman was supported by the National Institute on Aging (K24AG049057, R24AG064025, and P30AG044281).
Sponsor’s role:
The funders had no role or influence in the design and conduct of the study; collection, management, analysis, and interpretation of the data; decision to publish; or preparation, review, or approval of the manuscript.
Footnotes
Conflict of interest: The authors have no conflicts.
Conference submissions: This project was accepted in abstract form for the national meetings of the Society of General Internal Medicine (poster, 4/23/2021) and the American Geriatrics Society (Presidential ePoster Session, 5/13/2021).
REFERENCES
- 1.Johnell K Inappropriate Drug Use in People with Cognitive Impairment and Dementia: A Systematic Review. Curr Clin Pharmacol. 2015;10(3):178–184. doi: 10.2174/1574884710666150609154741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman MA Polypharmacy—Time to Get Beyond Numbers. JAMA Intern Med. 2016;176(4):482–483. doi: 10.1001/jamainternmed.2015.8597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assari S, Bazargan M. Race/Ethnicity, Socioeconomic Status, and Polypharmacy among Older Americans. Pharm Basel Switz. 2019;7(2). doi: 10.3390/pharmacy7020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maher RL, Hanlon JT, Hajjar ER. Clinical Consequences of Polypharmacy in Elderly. Expert Opin Drug Saf. 2014;13(1). doi: 10.1517/14740338.2013.827660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott IA, Hilmer SN, Reeve E, et al. Reducing Inappropriate Polypharmacy: The Process of Deprescribing. JAMA Intern Med. 2015;175(5):827–834. doi: 10.1001/jamainternmed.2015.0324 [DOI] [PubMed] [Google Scholar]
- 6.Koller D, Bynum JPW. Dementia in the USA: state variation in prevalence. J Public Health. 2015;37(4):597–604. doi: 10.1093/pubmed/fdu080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivari BS, French ME, McGuire LC. The Public Health Road Map to Respond to the Growing Dementia Crisis. Innov Aging. 2020;4(igz043). doi: 10.1093/geroni/igz043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clague F, Mercer SW, McLean G, Reynish E, Guthrie B. Comorbidity and polypharmacy in people with dementia: insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing. 2017;46(1):33–39. doi: 10.1093/ageing/afw176 [DOI] [PubMed] [Google Scholar]
- 9.Parsons C Polypharmacy and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf. 2017;8(1):31–46. doi: 10.1177/2042098616670798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnjidic D, Hilmer SN, Hartikainen S, et al. Impact of High Risk Drug Use on Hospitalization and Mortality in Older People with and without Alzheimer’s Disease: A National Population Cohort Study. PLOS ONE. 2014;9(1):e83224. doi: 10.1371/journal.pone.0083224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorpe JM, Thorpe CT, Gellad WF, et al. Dual Health Care System Use and High-Risk Prescribing in Patients With Dementia. Ann Intern Med. 2016;166(3):157–163. doi: 10.7326/M16-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayliss EA, Shetterly SM, Drace ML, et al. The OPTIMIZE patient- and family-centered, primary care-based deprescribing intervention for older adults with dementia or mild cognitive impairment and multiple chronic conditions: study protocol for a pragmatic cluster randomized controlled trial. Trials. 2020;21. doi: 10.1186/s13063-020-04482-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi W, Graf E, Hughes L, et al. Older Adults with Dementia and Their Caregivers: Key Indicators from the National Health and Aging Trends Study. The Office of the Assistant Secretary for Planning and Evaluation; 2019. [Google Scholar]
- 14.Lau DT, Mercaldo ND, Harris AT, Trittschuh E, Shega J, Weintraub S. Polypharmacy and Potentially Inappropriate Medication Use among Community-Dwelling Elders with Dementia. Alzheimer Dis Assoc Disord. 2010;24(1):56–63. doi: 10.1097/WAD.0b013e31819d6ec9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green AR, Reifler LM, Boyd CM, Weffald LA, Bayliss EA. Medication profiles of patients with cognitive impairment and high anticholinergic burden. Drugs Aging. 2018;35(3):223–232. doi: 10.1007/s40266-018-0522-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyama A, Steinman M, Ensrud K, Hillier TA, Yaffe K. Ten-Year Trajectory of Potentially Inappropriate Medications in Very Old Women: Importance of Cognitive Status. J Am Geriatr Soc. 2013;61(2):258–263. doi: 10.1111/jgs.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pugh MJV, Starner CI, Amuan ME, et al. Exposure to potentially harmful drug-disease interactions among older community-dwelling veterans based on the Healthcare Effectiveness Data and Information Set quality measure: Who is at risk? J Am Geriatr Soc. 2011;59(9):1673–1678. doi: 10.1111/j.1532-5415.2011.03524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorpe JM, Thorpe CT, Kennelty KA, Gellad WF, Schulz R. The Impact of Family Caregivers on Potentially Inappropriate Medication Use in Non-institutionalized Older Adults with Dementia. Am J Geriatr Pharmacother. 2012;10(4):230–241. doi: 10.1016/j.amjopharm.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maust DT, Strominger J, Kim HM, et al. Prevalence of Central Nervous System–Active Polypharmacy Among Older Adults With Dementia in the US. JAMA. 2021;325(10):952. doi: 10.1001/jama.2021.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharya R, Chatterjee S, Carnahan RM, Aparasu RR. Prevalence and Predictors of Anticholinergic Agents in Elderly Outpatients with Dementia. Am J Geriatr Pharmacother. 2011;9(6):434–441. doi: 10.1016/j.amjopharm.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Patel T, Slonim K, Lee L. Use of potentially inappropriate medications among ambulatory home-dwelling elderly patients with dementia: A review of the literature. Can Pharm J Rev Pharm Can. 2017;150(3):169–183. doi: 10.1177/1715163517701770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado J, Bowman K, Clare L. Potentially inappropriate prescribing in dementia: a state-of-the-art review since 2007. BMJ Open. 2020;10(1):e029172. doi: 10.1136/bmjopen-2019-029172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavan AH, Gallagher P. Predicting risk of adverse drug reactions in older adults. Ther Adv Drug Saf. 2016;7(1):11–22. doi: 10.1177/2042098615615472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency Hospitalizations for Adverse Drug Events in Older Americans. N Engl J Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053 [DOI] [PubMed] [Google Scholar]
- 25.NAMCS/NHAMCS - Ambulatory Health Care Data Homepage. Published November10, 2020. Accessed December 2, 2020. https://www.cdc.gov/nchs/ahcd/index.htm
- 26.NAMCS/NHAMCS - Ambulatory Care Drug Database System - Search. Accessed December 15, 2020. https://www2.cdc.gov/drugs/applicationnav1.asp
- 27.Tan ECK, Bell JS, Lu CY, Toh S. National Trends in Outpatient Antihypertensive Prescribing in People with Dementia in the United States. Wucherer D, ed. J Alzheimers Dis. 2016;54(4):1425–1435. doi: 10.3233/JAD-160470 [DOI] [PubMed] [Google Scholar]
- 28.Rhee TG, Choi YC, Ouellet GM, Ross JS. National Prescribing Trends of High-risk Anticholinergic Medications in Older Adults. J Am Geriatr Soc. 2018;66(7):1382–1387. doi: 10.1111/jgs.15357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults: 2019 AGS BEERS CRITERIA® UPDATE EXPERT PANEL. J Am Geriatr Soc. 2019;67(4):674–694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 30.Rudolph JL. The Anticholinergic Risk Scale and Anticholinergic Adverse Effects in Older Persons. Arch Intern Med. 2008;168(5):508. doi: 10.1001/archinternmed.2007.106 [DOI] [PubMed] [Google Scholar]
- 31.Linjakumpu T, Hartikainen S, Klaukka T, Koponen H, Kivelä S-L, Isoaho R. A model to classify the sedative load of drugs. Int J Geriatr Psychiatry. 2003;18(6):542–544. doi: 10.1002/gps.846 [DOI] [PubMed] [Google Scholar]
- 32.Taipale HT, Bell JS, Uusi-Kokko M, Lönnroos E, Sulkava R, Hartikainen S. Sedative Load among Community-Dwelling People Aged 75 Years and Older. Drugs Aging. 2011;28(11):913–925. doi: 10.2165/11597800-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 33.Peklar J, O’Halloran AM, Maidment ID, Henman MC, Kenny RA, Kos M. Sedative Load and Frailty Among Community-Dwelling Population Aged ≥65 Years. JAMDA. 2015;16:282–289. [DOI] [PubMed] [Google Scholar]
- 34.Kristensen RU, Nørgaard A, Jensen-Dahm C, Gasse C, Wimberley T, Waldemar G. Polypharmacy and Potentially Inappropriate Medication in People with Dementia: A Nationwide Study. J Alzheimers Dis. 2018;63(1):383–394. doi: 10.3233/JAD-170905 [DOI] [PubMed] [Google Scholar]
- 35.Delgado J, Jones L, Bradley MC, et al. Potentially inappropriate prescribing in dementia, multi-morbidity and incidence of adverse health outcomes. Age Ageing. 2021;50(2):457–464. doi: 10.1093/ageing/afaa147 [DOI] [PubMed] [Google Scholar]
- 36.Gnjidic D, Agogo GO, Ramsey CM, Moga DC, Allore H. The Impact of Dementia Diagnosis on Patterns of Potentially Inappropriate Medication Use Among Older Adults. J Gerontol A Biol Sci Med Sci. 2018;73(10):1410–1417. doi: 10.1093/gerona/gly078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray SL, Anderson ML, Dublin S, et al. Cumulative Use of Strong Anticholinergic Medications and Incident Dementia. JAMA Intern Med. 2015;175(3):401–407. doi: 10.1001/jamainternmed.2014.7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institute for Health and Care Excellence (UK). Dementia: Assessment, Management and Support for People Living with Dementia and Their Carers. National Institute for Health and Care Excellence (UK); 2018. Accessed January 8, 2021. http://www.ncbi.nlm.nih.gov/books/NBK513207/ [PubMed] [Google Scholar]
- 39.Sink KM, Holden KF, Yaffe K. Pharmacological Treatment of Neuropsychiatric Symptoms of Dementia: A Review of the Evidence. JAMA. 2005;293(5):596. doi: 10.1001/jama.293.5.596 [DOI] [PubMed] [Google Scholar]
- 40.Shaukat A, Habib A, Lane KA, et al. Anticholinergic Medications: An Additional Contributor to Cognitive Impairment in the Heart Failure Population? Drugs Aging. 2014;31(10):749–754. doi: 10.1007/s40266-014-0204-2 [DOI] [PubMed] [Google Scholar]
- 41.Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use Among Older Adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473–482. doi: 10.1001/jamainternmed.2015.8581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haasum Y, Fastbom J, Fratiglioni L, Kåreholt I, Johnell K. Pain Treatment in Elderly Persons With and Without Dementia. Drugs Aging. 2011;28(4):283–293. doi: 10.2165/11587040-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 43.Page A, Etherton-Beer C, Seubert LJ, et al. Medication use to manage comorbidities for people with dementia: a systematic review. J Pharm Pract Res. 2018;48(4):356–367. doi: 10.1002/jppr.1403 [DOI] [Google Scholar]
- 44.Amjad H, Roth DL, Sheehan OC, Lyketsos CG, Wolff JL, Samus QM. Underdiagnosis of Dementia: an Observational Study of Patterns in Diagnosis and Awareness in US Older Adults. J Gen Intern Med. 2018;33(7):1131–1138. doi: 10.1007/s11606-018-4377-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cummings E, Maher R, Showell CM, et al. Hospital Coding of Dementia: Is it Accurate? Health Inf Manag J. 2011;40(3):5–11. doi: 10.1177/183335831104000301 [DOI] [PubMed] [Google Scholar]
- 46.Østbye T, Taylor DH, Clipp EC, Scoyoc LV, Plassman BL. Identification of Dementia: Agreement among National Survey Data, Medicare Claims, and Death Certificates. Health Serv Res. 2008;43(1 Pt 1):313–326. doi: 10.1111/j.1475-6773.2007.00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maneno MK, Lee E, Wutoh AK, et al. National patterns of dementia treatment among elderly ambulatory patients. J Natl Med Assoc. 2006;98(3):430–435. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


