Abstract
Cephalic phase insulin release (CPIR) is a transient pulse of insulin that occurs within minutes of stimulation from foods or food-related stimuli. Despite decades of research on CPIR in humans, the body of literature surrounding this phenomenon is controversial due in part to contradictory findings between studies. This has slowed progress towards understanding the sensory and neural basis of the response, as well as its overall relevance to health. This review aims to examine up-to-date knowledge in CPIR research and identify sources of CPIR variability in humans in an effort to guide future research. The review starts by defining CPIR and discussing its presumed functional roles in glucose homeostasis and feeding behavior. Next, the types of stimuli that have been reported to elicit CPIR, as well as the sensory and neural mechanisms underlying the response in rodents and humans are discussed, and areas where knowledge is limited are identified. Finally, factors that may contribute to the observed variability of CPIR in humans are examined, including experimental design, test procedure, and individual characteristics. Overall, oral stimulation appears to be important for eliciting CPIR, especially when combined with other sensory modalities (vision, olfaction, somatosensation). While differences in experimental design and testing procedure likely explain some of the observed inter- and intra-study variability, individual differences also appear to play an important role. Understanding sources of these individual differences in CPIR will be key for establishing its health relevance.
Keywords: carbohydrates, cephalic phase insulin release, learning, nutrient conditioning, salivary amylase, sensory mechanism
1. Introduction
Cephalic phase responses (CPRs) are reflex responses to nutritional stimuli, which are elicited by stimulation of sensory systems in the head and oropharynx (1,2). These responses include increased salivation, digestive activity (e.g., gastric acid secretion, gastric motility and emptying), thermogenesis, and hormone release (e.g. insulin, glucagon, leptin, and ghrelin secretion) (3–6). Of those responses, cephalic phase insulin release (CPIR) is the most well-studied, with a substantial bulk of research dating back multiple decades (7–11). Nonetheless, the sensory and neural mechanisms underlying CPIR have yet to be fully elucidated. Notably, progress towards understanding these mechanisms has been impeded by inconsistent definitions of CPIR (12), conflicting results across studies (6,13), and difficulties capturing CPIR in humans (14,15) owing in part to a generally small effect size. These outcomes have generated skepticism surrounding the existence and relevance of CPIR in human health (12), despite substantial documentation of CPIR (9,16–19) and its health relevance in animals (19–22).
Recently, independent groups have performed two systematic reviews (12,23) and a meta-analysis (24) on cephalic phase responses. The meta-analysis, including 77 studies with a total of 748 participants, concluded that food-related stimuli are effective at eliciting CPIR in humans (24). Similarly, the systematic review by Skvortsova and others (23) reported that out of the 37 studies measured CPIR, two-thirds showed a significant response. Meanwhile, the systematic review by Lasschuijt and others (12) concluded that, based on 119 experimental conditions across 46 studies and a total of 775 participants, CPIR is small compared to spontaneous insulin fluctuations, bringing into question its physiological relevance. It should be noted, however, that these reviews included studies that used a diverse range of stimuli (e.g., water, NaCl, sugars, complex foods) and a variety of testing protocols (e.g., viewing or thinking about food, tasting but not swallowing a food/food-related stimulus, or ingesting a food/food-related stimulus). This diversity of protocols could have reduced the overall effect sizes reported.
The first objective of this review is to summarize recent developments in the mechanistic understanding of CPIR, primarily using findings from work in rodent models. These potential mechanisms will be consolidated with evidence from humans, offering a translational perspective onto the mechanisms underlying human CPIR. The second objective is to examine inconsistencies in methodology used in human CPIR studies in an effort to identify sources of variability across studies, which will help guide future work in the area. Recognizing that individuals can vary substantially in CPIR magnitude within the same study, factors that could explain this individual variability are also discussed.
This review begins with a definition of CPIR and how it presents in humans and rodents, followed by an overview of its roles in postprandial glucose control and feeding behavior. Next, the stimuli that are known to elicit CPIR are discussed, giving special attention to carbohydrates because of their relevance to insulin release. The contribution of gustatory inputs to evoking CPIR is then considered in rodents and humans, and current understandings of the neural and endocrine mechanisms underlying the response are reviewed. Finally, factors that modulate CPIR expression in humans are examined as a means to explain inconsistencies between studies and assist with guiding design of future experiments.
2. Fundamentals of CPIR
CPIR is a rapid, preabsorptive release of insulin following stimulation from food-related cues (e.g., sight, smell, taste). How it presents in humans and rodents, along with research regarding its proposed functional significance to health is described below.
2.1. Time course and magnitude of CPIR
The consumption of food typically stimulates two phases of insulin secretion, which differ in time course, magnitude, and by the mechanisms leading to their release. The initial phase is relatively small in magnitude and reflects CPIR. It typically reaches its maximum within 2–5 minutes of initiating stimulation, and subsides within 8–10 minutes; this pattern is particularly apparent when CPIR is measured in the absence of nutrient absorption (15,25–28). The second phase of insulin secretion occurs during and after nutrient absorption. It stimulates more extensive insulin secretion than CPIR, and reaches its maximum 20–60 minutes after the start of a meal, depending on meal size.
2.2. Function of CPIR
The collective function of CPRs is to help alleviate the physiologic and metabolic challenges of meals by preparing the body to digest, absorb, and metabolize nutrients efficiently (2,13,29). CPIR, specifically, has been suggested to function primarily in glucose control, but has also been studied for its potential role in influencing feeding behavior.
Given the relatively small magnitude of CPIR in humans, especially given there are naturally occurring insulin fluctuations, questions have been raised about its biological relevance. Two lines of evidence support the hypothesis that CPIR improves blood glucose control. First, oral stimulation by food-related stimuli enhances glucose clearance from the blood in mice (19), rats (21), and humans (30) once nutrients are ingested, and it has been inferred that CPIR mediates this effect. In mice, administering glucose orally (via normal ingestion) has been observed to result in substantially better glucose tolerance than when glucose is administered intragastrically (via oral gavage) (19). Similarly, in humans, some researchers have observed that pairing intragastric glucose infusion with oral stimulation in the form of a modified sham feed (MSF) protocol—wherein subjects taste, chew, and expectorate a food without swallowing—resulted in improved glucose tolerance over conditions implementing intragastric glucose infusion alone (30,31). Nonetheless, stronger evidence for the role of CPIR in glucose control comes from human studies where CPIR has been experimentally manipulated. For instance, when CPIR was blocked by administering trimethaphan (i.e., a ganglionic antagonist that blocks neural influences of islet function by impairing neurotransmission across autonomic ganglia), subjects displayed higher peak blood glucose and delayed postprandial clearance of glucose from the blood (Figure 1) (32). Another study found that blocking CPIR through administration of somatostatin (i.e., a hormone that inhibits somatotropin secretion from the hypothalamus and insulin secretion from the pancreas) also impaired glucose tolerance (33).
Figure 1.
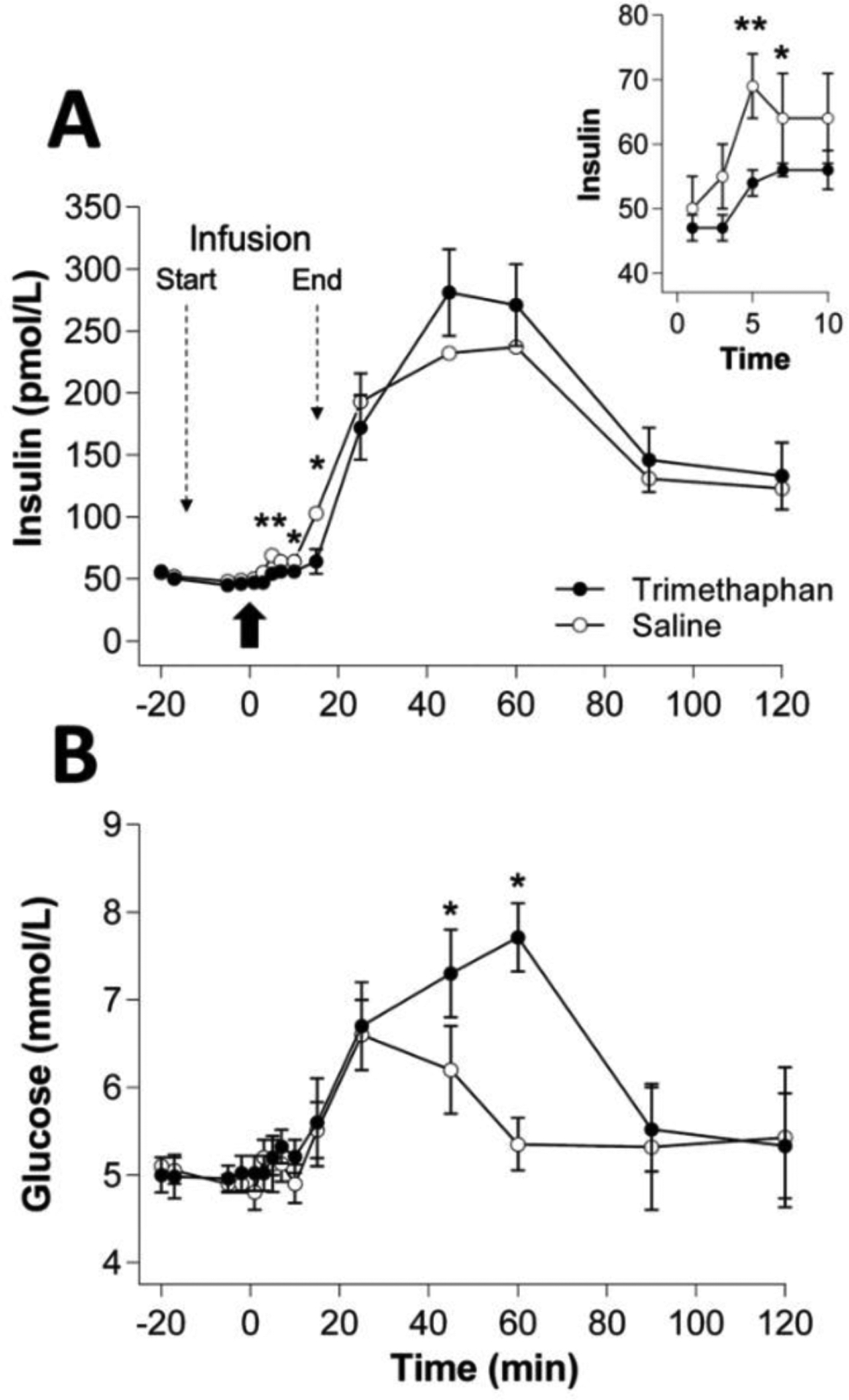
(A) Serum insulin and (B) plasma glucose levels (means ± SE) in 6 subjects following either a 30-min intravenous infusion (−15 min to + 15 min) of trimethaphan (a ganglionic antagonist) or saline. At time 0, a standardized meal was served (bold arrow). Insert shows insulin levels during the CPIR period (0–10 min). *, ** indicate differences between groups at p < 0.05 and p < 0.01, respectively, by analysis of variance. The data used to construct this figure were taken from (32).
A second proposed function of CPIR relates to its role in feeding behavior (2,29). Cephalic stimulation is thought to trigger early CPRs that help subjects accommodate meals; this is because CPRs stimulate digestive processes and accelerate the removal of nutrients from the blood, which minimizes disruptions in homeostasis (2,29). Subsequently, continued cephalic stimulation evokes additional CPRs, which are thought to activate satiation signals (i.e., sensory-specific satiety) and aid in meal termination (2). Insulin is one of the two main glucose regulatory hormones, and has functions in controlling appetite (34). However, there is no direct evidence at this time that CPIR modulates appetite or satiety. While one study reported that CPIR magnitude increased with higher prospective consumption ratings (15), a relationship between CPIR and other feeding behaviors (e.g., hunger, motivation to eat, satiety) has not yet been well supported in humans (10,15,35).
3. Stimuli that elicit CPIR
Food-related cues have been reported to elicit CPIR [see tables in (12) and (23) for comprehensive lists of stimuli used]. This section examines the efficacy of these cues as CPIR stimuli, and discusses the importance of multisensory inputs for eliciting CPIR in humans. Particular focus will be given to oral stimuli, including sugars and nonnutritive sweeteners, starch-derived complex carbohydrates, and mixed-nutrient foods, which together represent a majority of the stimuli used in published CPIR research.
3.1. Food-related cues
Food-related cues include both common sensory cues, such as the sight, smell, taste, and texture of food, as well as contextual cues, such as the thought of food, time of day, and specific location where food is consumed (Figure 2). While the impact of cues related to the expectation of food (i.e., timing and location) on CPIR has been studied primarily in rodents [see Table 2 in (23) for summary], work in humans has generally focused on responses to sensory cues [but see (36,37)].
Figure 2.
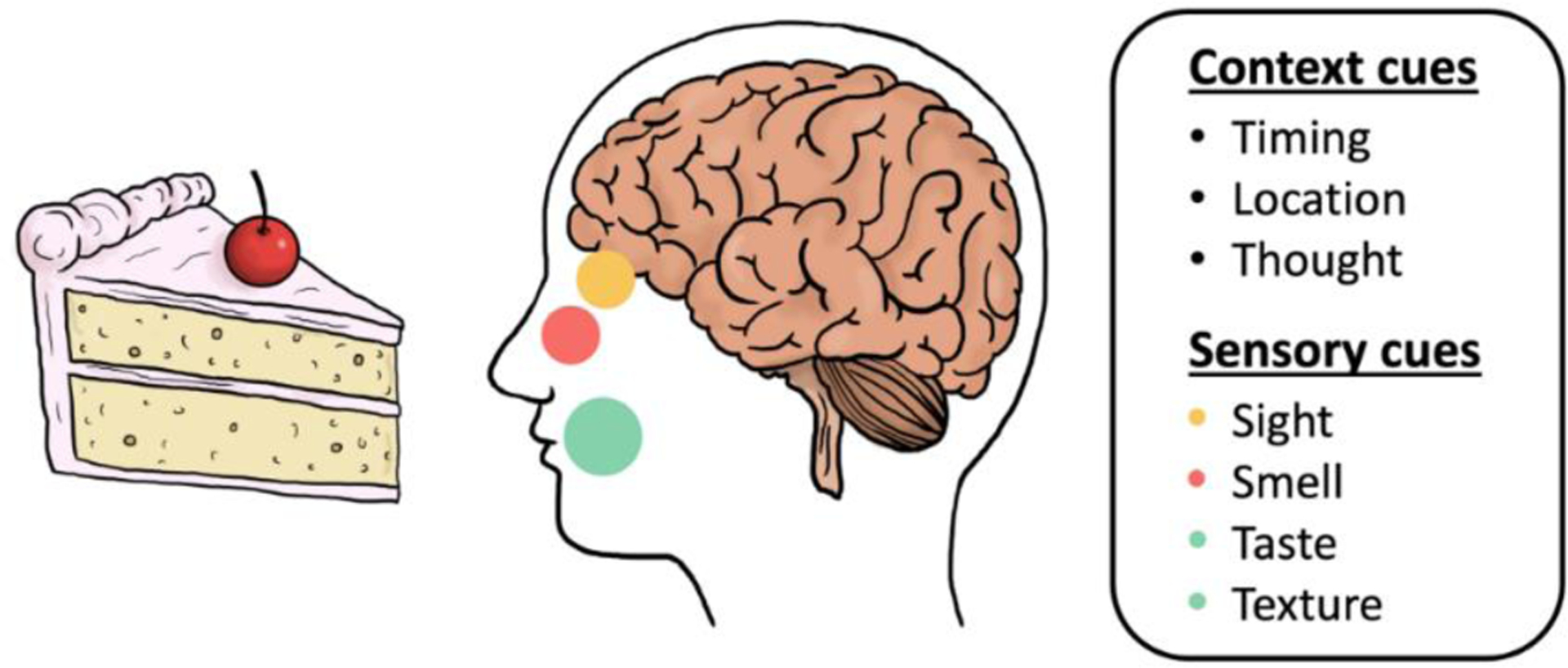
Food-related cues that have been linked to CPIR, including those related to the context of the food consumption (i.e., context cues), and those perceived by the cephalic senses (i.e., sensory cues).
3.2. Sensory cues
While the specific contribution of the different types of food-related sensory cues to CPIR remains unclear, teasing out the impacts of these cues is a common goal of human CPIR research. This has been done by presenting stimuli that selectively target a limited number of sensory inputs, and determining whether a CPIR ensues [e.g., (15,26,38,39)]. Only two studies have attempted to measure CPIR in response to different sensory modalities [vision, olfaction, or both (39); olfaction, or olfaction and gustation (i.e., eating) (38)] within the same subject group. However, neither study observed CPIR under any condition. Some studies have also attempted to measure CPIR following stimulation from visual and olfactory cues (e.g., placing a warm cinnamon roll in front of subjects) (35,39–46). Reports of significant CPIR following stimulation by these cues, however, are inconsistent and often differ between subject groupings [e.g., significant CPIR in obese only or anorexic only (40,42,43)]. Nevertheless, there have been cases of human studies using simplified taste stimuli—such as tastants dissolved in water (25–27,47)—that have elicited a CPIR, suggesting oral stimulation may play a unique role in evoking the response. It is recognized, though, that the effect size of the CPIR following these isolated stimuli in humans is particularly small. In theory, the integration of multiple sensory inputs, including those from visual, olfactory, gustatory, and trigeminal systems, should produce a larger magnitude of CPIR (32,48–50). This idea is supported by a recent meta-analysis, reporting that these sensory inputs can act in an additive manner in humans—that is, the greater number of sensory inputs, the greater the CPIR magnitude (24). These combined inputs are thought to more accurately mimic the oronasal stimulation that occurs during the ingestion of food during a meal.
3.3. Oral stimuli
Oral stimulation, whether using simplified (e.g., a tastant in water) or complex stimuli (i.e., a real food), has been the most common means of stimulus delivery in CPIR research. This section discusses rodent and human studies that have investigated the role of oral stimulation in CPIR, with a particular focus on sugars, sweeteners, and starch-based carbohydrates.
3.3.1. Taste quality
It seems that some but not all classical “taste” stimuli elicit CPIR. Two studies [rat (51), human (26)] measured CPIR to aqueous stimuli representative of each taste quality (i.e., sweet, salty, sour, bitter, and umami). Both studies reported a significant CPIR only within the sweet taste condition, suggesting that other taste qualities may have less impact on the response. It should be noted, however, that one study reported CPIR in response to oral stimulation with monosodium glutamate in rats (52).
3.3.2. Taste vs. nutritional content
It is unclear whether the nutritional content of a stimulus, as opposed to its taste quality, is critical to evoke CPIR. When it comes to sweeteners, some (i.e., sugars) provide calories whereas others (e.g., sucralose, saccharin) do not. The latter compounds are thus collectively referred to as nonnutritive sweeteners. Outside of sweeteners, other studies have explored CPIR in response to complex carbohydrates, as well as mixed nutrient foods.
3.3.2.1. Sugars
Sugars are commonly used as stimuli in CPIR research. One study in rats (9) reported that glucose reliably elicited CPIR, but that other sugars (e.g., sucrose, fructose, galactose and mannose) were ineffective to elicit CPIR. In contrast, another study found that sucrose was an effective stimulus in rats (51). Recent experiments revealed that free glucose is the only effective elicitor of CPIR in naïve mice (see section 4.1 for details) (18,19). In humans, numerous studies have reported that both glucose (27,53) and sucrose (15,25,26,47) evoke CPIR. However, some human studies have failed to record significant CPIR to these sugars (14,54–56). It should be recognized, however, that the latter studies did not have positive CPIR controls, or only found CPIR using a stimulus of a different form (i.e., a complex food) (57). Therefore, the lack of CPIR may reflect limitations of the experimental procedure. CPIR responses to fructose or other non-glucose-containing sugars (e.g., galactose) have not yet been measured in humans.
3.3.2.2. Nonnutritive sweeteners
In rats, two studies reported that CPIR is evoked by saccharin (22,58). However, other studies in rats (9) and mice (18) reported that saccharin was not sufficient to stimulate CPIR in the animals. A lack of CPIR was also reported in mice following stimulation with sucralose, acesulfame K, and SC45647 (18). In humans, two studies have reported small but statistically significant CPIR from baseline following stimulation with saccharin (26) and sucralose (15). Meanwhile, Härtel et al. (47) found that a number of nonnutritive sweeteners, including saccharin, aspartame, acesulfame K, and cyclamate did not evoke CPIR, while sucrose did. Similarly, other human studies have reported no CPIR to stimuli containing nonnutritive sweeteners (14,54–57,59), although unlike Härtel et al., these studies had no positive CPIR controls. It is possible that the inconsistent results in humans and rats reflect differences in prior diet-induced CPIR conditioning (see section 4.3).
3.3.2.3. Starch and its derivatives
When starch and its derivatives (i.e., glucose polymers; including maltooligo- and maltopoly-saccharides [MOS and MPS, respectively]) enter to the oral cavity, digestion occurs via catalytic action of salivary α-amylase (see Figure 3). This action eventually generates shorter, sweet-tasting end products including maltose and maltotriose (60,61), which are further digested by other α-glucosidases in taste cells to produce free glucose (62,63).
Figure 3.
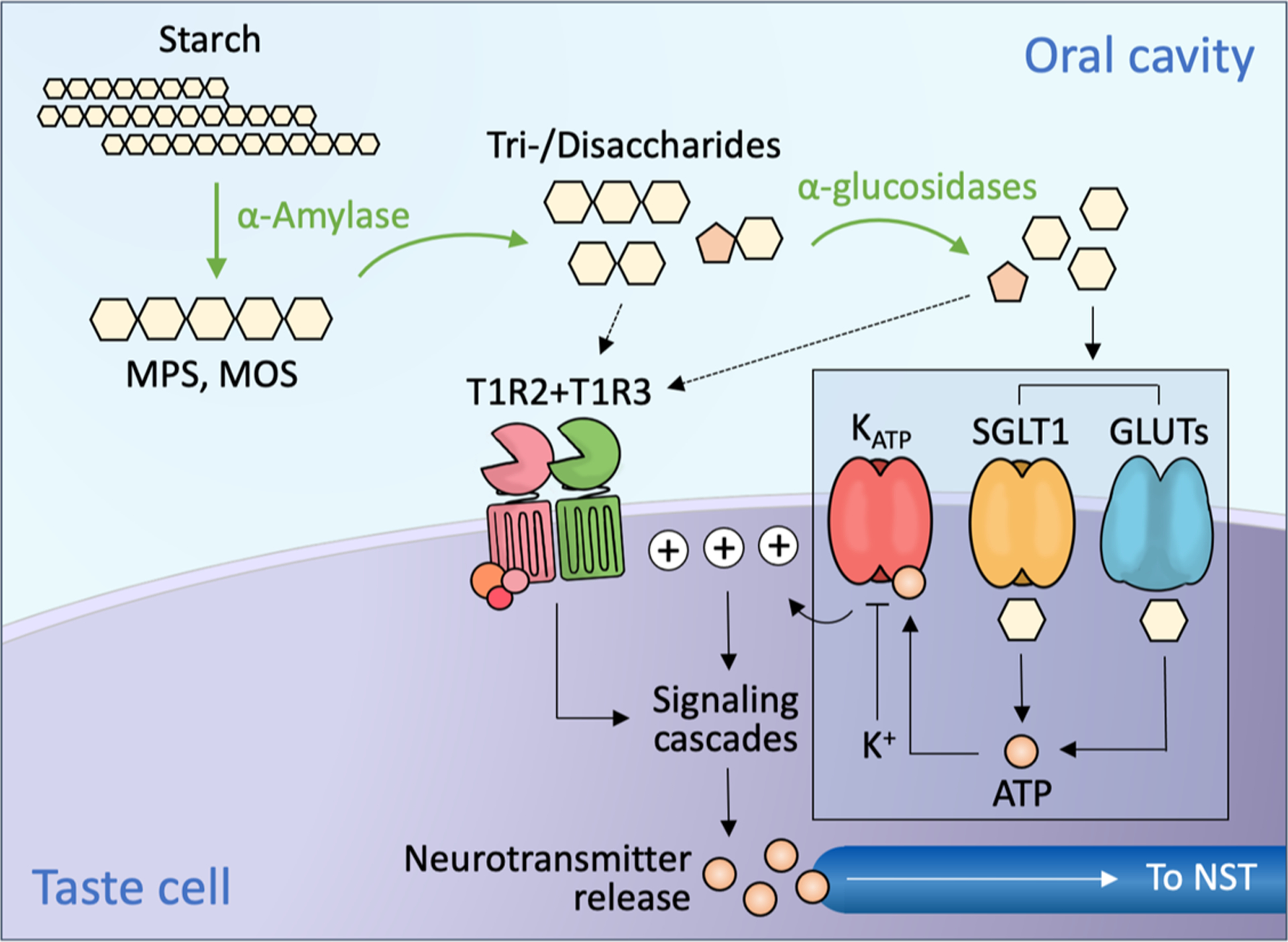
In the oral cavity, salivary α-amylase catalyzes the hydrolysis of starch to produce maltopolysaccharides (MPS) and maltooligosaccharides (MOS), then the subsequent end products maltotriose and maltose. These saccharides, as well as other digestible sugars from the diet, are further hydrolyzed to monosaccharides by taste cell-expressed α-glucosidases. Sugars are thought to activate at least two distinct signaling pathways. One pathway is activated by the binding of sugars or nonnutritive sweeteners to a G protein-coupled receptor, T1R2+T1R3; this in turn activates the inositol trisphosphate (IP3) signaling cascade, resulting in neurotransmitter (ATP) release. Another pathway (inset), identified in mice, is thought to be activated when glucose diffuses through SGLT1 or one of the GLUTs in the cell membrane, and is then metabolized, yielding ATP. The ATP binds to KATP channels and blocks K+ efflux; this causes membrane depolarization and neurotransmitter release, which sends signals to the nucleus of the solitary tract (NST) in the brainstem. These two pathways are thought to operate in distinct populations of taste cells (64).
A few studies have investigated the impact of isolated starch-based stimuli on CPIR. In mice, a solution containing maltodextrin (i.e., a processed starch product comprising MOS and MPS mixtures) was found to stimulate the response (18). Importantly, however, CPIR was not captured in the mice when oral enzymatic digestion was blocked, suggesting that free glucose could have been responsible for the observed CPIR (18). In humans, investigators have attempted to test starch or processed glucose polymer stimuli (polydextrose or maltodextrins) presented as solutions or tablets, but did not observe a significant CPIR (14,26,54,59). Note, however, that two of these studies did not observe CPIR under any stimulus condition (14,54). One study measured CPIR to a starch hydrolysate (i.e., maltodextrin) solution and found a significant response only in individuals with high α-amylase activity (see 5.2.3 for additional discussion) (53). Importantly, the form of starch (e.g., as raw starch, cooked starch, or processed starch) could have a significant impact on the extent of oral digestion [see (60)], but this has not yet been studied. It follows that the role that starch-derived oligomers and polymers contribute towards CPIR warrants further investigation.
3.3.2.4. Mixed-nutrient foods
Some mixed-nutrient stimuli such as muffins and gelatin-based desserts containing sweeteners (e.g., aspartame, sucrose) and fat have been demonstrated to be strong elicitors of CPIR in humans (10,65,66). There have also been reports of stimuli that contain starch-based carbohydrates and other macronutrients (e.g., peanut butter sandwich, savory tarts, pizza) stimulating CPIR (67–69). Yet, there are other reports of milk-based pudding desserts (70), yogurt (71), dark chocolate (38), and cake (72) failing to stimulate CPIR. Null findings following stimulation with these items is particularly interesting, given that they represent real foods that stimulate multiple sensory modalities. Likely, the mixed results from these studies could be due to a combination of individual differences in conditioned responses to the food items (see section 4.3), experimental design (see section 5.1), or α-amylase activity (see section 5.2).
4. Mechanisms underlying CPIR
Of the inputs thought to contribute to CPIR, gustatory stimulation has been of great interest given its specific role in nutrient sensing. Although the gustatory mechanisms underlying CPIR in humans have not been studied directly, there has been substantial progress towards understanding how CPIR is stimulated in rodents. Here, the gustatory and neural mechanisms thought to be involved in CPIR are discussed. In addition, the contribution of learning to CPIR, and how this could potentially explain responses to other modes of sensory stimulation, is covered.
4.1. Role of the gustatory system in eliciting CPIR
Given the reported ability of sugars to elicit CPIR (see section 3.3.2), it is logical to hypothesize that the sweet taste receptor, T1R2+T1R3 (73,74) mediates the response (see Figure 3). However, two lines of recent evidence contradict this possibility, at least in mice. First, the T1R2+T1R3 sweet taste receptor binds to many different types of caloric and noncaloric sweeteners (74), but only free glucose elicits CPIR in naïve mice (18). Second, T1R3 knockout (KO) mice exhibit impaired behavioral attraction to sugars, but normal CPIR to glucose (19). These findings suggest that CPIR in mice is mediated by a T1R2+TR3-independent mechanism.
Recently, the sodium-glucose transporter (SGLT1), glucose transporters (GLUTs), and an ATP-gated K+ channel have been reported to be expressed and functional in mouse taste cells (64,75–77) (see Figure 3). There is evidence that knocking out the KATP channel eliminates CPIR, and that pharmacological modulation of channel activity can produce predicable increases or decreases in CPIR (18). In addition, recordings from individual taste neurons in the chorda tympani nerve indicate that some mouse taste cells respond to glucose via a T1R2+T1R3-independent signaling pathway (78). Apparently, glucose is transported into these taste cells by SGLT1 and GLUTs, and then metabolized to yield ATP, which in turn closes the KATP channel (see Figure 3). By blocking efflux of K+ from the taste cell, the taste cell could depolarize and stimulate neurotransmitter release.
The hypothesis that the KATP channel is part of the peripheral taste signaling pathway for CPIR is supported by mouse studies showing that free glucose is necessary to evoke the response (17,18). For example, Glendinning et al. (18) compared CPIR responses from glucose and glucose-containing stimuli (sucrose, maltose, and the maltodextrin product Polycose) to those from non-glucose-containing stimuli (fructose, saccharin, sucralose, AceK, and sugar analog SC45647) and found that only glucose and the glucose-containing stimuli evoked CPIR (Figure 4A). To explain how the glucose-containing stimuli elicited CPIR, recall that multiple enzymes are present in the oral cavity that catalyze the hydrolysis of starch-based oligomers and polymers, as well as digestible disaccharides, to generate free glucose (Figure 3). When the same glucose-containing stimuli were mixed with acarbose, an α-glucosidase inhibitor, which would prevent the release of free glucose from the polymeric carbohydrates, the mice no longer elicited CPIR (Figure 4B).
Figure 4.
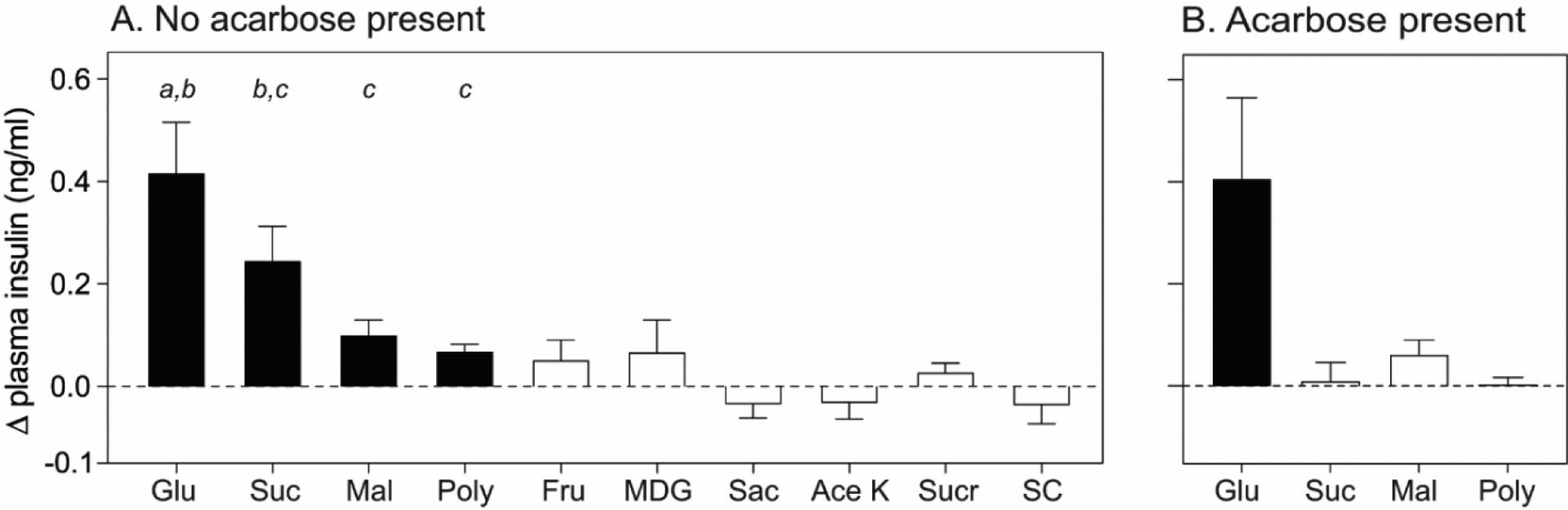
(A) Some but not all sweeteners elicit CPIR in B6 mice. CPIR was defined as a significant increase in Δ plasma insulin relative to baseline within 5 min of initiating licking, based on a one-sample t-test (p < 0.05). Closed and open bars indicate taste stimuli that elicited vs. did not elicit CPIR, respectively. Means (± SE) of CPIR magnitude are compared across glucose, sucrose, maltose and Polycose with a Tukey’s test. The means that differ significantly from one another lack a shared letter (p < 0.05). (B) Impact of acarbose (5mM) on the ability of glucose-containing carbohydrates to elicit CPIR. Glu (1M glucose), Suc (1M sucrose), Mal (1M maltose), Poly (32% Polycose), Fru (1M fructose), MDG (1M alpha-methyl-d-glucopyranoside), Sac (38mM sodium saccharin), Ace K (100mM acesulfame potassium), Sucr (30mM sucralose) and SC (3mM SC45647). Portions of this figure were previously published in (17,18).
It is suspected that a similar mechanism could be present in humans, given that numerous studies using glucose-based stimuli have reported significant CPIR (see 3.3.2). Nonetheless, this hypothesis is inconsistent with reports of nonnutritive sweeteners eliciting CPIR in humans (see 3.3.2.2). It is possible that the ability of nonnutritive sweeteners to elicit CPIR stems from a dietary conditioning process (see section 4.3).
4.2. Neural mechanisms underlying CPIR
The primary mechanism for stimulating insulin release is called glucose-induced insulin secretion (GIIS) (79). It is initiated when glucose diffuses into beta cells from the blood. There, glucose is rapidly metabolized and produces a concentration-dependent increase in ATP. As the ratio of intracellular ATP to ADP increases, the normal efflux of potassium through KATP channels in the membrane of beta cells is blocked, raising the membrane potential. When the membrane potential exceeds a threshold voltage, a cascade of intracellular signaling events is initiated, resulting in secretion of stored insulin.
GIIS can be augmented by neural input from the brain. In both rodents and humans, food-related sensory inputs are relayed by cranial nerves to sensory processing areas in the brainstem. Specifically, afferent fibers originating from peripheral sensory cells (e.g., taste cells) terminate in the nucleus of the solitary tract (NST), located in the medulla oblongata. This information is subsequently relayed to circuits in the dorsal motor nucleus of the vagus (DMNX) (11,80). Efferent signals from the DMNX are then carried through cholinergic fibers in the vagus nerve (81). In rodents, some of these fibers make direct contacts with beta cells in pancreatic islets. Stimulation of these fibers causes the release of acetylcholine (ACh), which binds to muscarinic receptors on the surface of beta cells (82). In humans, the neural mechanisms that stimulate beta cells are less clear because the pancreatic islets appear to have sparse innervation, and the primary source of ACh appears to be the alpha cells [for review, see (83)]. The binding of ACh to muscarinic receptors on beta cells is thought to activate a KATP-independent depolarizing current (84). This ACh current does not need to be large because circulating glucose concentrations, even prior to a meal, keep the membrane potential of beta cells near threshold (79). Accordingly, when a subject encounters food-related cues, the resulting ACh current is thought to raise the membrane potential above threshold and trigger CPIR.
There are several lines of evidence supporting the necessity of neural input for eliciting CPIR. For example, vagotomy in rodents (20,85) or administration of a muscarinic receptor antagonist (atropine) in humans can block CPIR (27,32,43). Further evidence comes from rats whose beta cells had been destroyed by streptozotocin treatment. When the same animals subsequently received intrahepatic islet isografts, which were presumably not innervated, they exhibited robust insulin secretion in response to elevations in blood glucose, but no CPIR in response to oral stimulation with glucose or saccharin (58).
4.3. Role of learning
While some physiological and behavioral responses to food-related cues [e.g., salivation and aversive facial expressions in response to sour taste (86,87)] are exhibited in the absence of learning, others are learned through association (88). For example, insulin release can be conditioned to time of day in rats subjected to scheduled meals (89,90). It is also possible that responses to stimuli may be modified by associating sensory features of a stimulus with its post-ingestive outcomes. For example, frequent consumption of calorically-dense foods that are flavored with nonnutritive sweeteners (e.g., saccharin) could, in theory, condition metabolic responses (e.g., insulin release) to the sweet taste (91,92). Likewise, it is also possible that regular use of nonnutritive sweeteners, especially if they are consumed in the absence of other foods that contain significant calories, could eventually break the association between sweet taste and incoming calories (93). These types of conditioning may underlie the inconsistent reports of CPIR following stimulation with non-glucose-containing sweeteners. Note that this hypothesis assumes that CPIRs are elicited exclusively by glucose-containing sugars in the absence of conditioning (see section 4.1). Conditioning could also explain reports that the sight or smell of food alone [e.g., (35,42), see section 3.2] can elicit CPIR. Notably, some CPRs, including increased salivation and gastric acid secretion, seem to be particularly sensitive to the sight and thought of food (94–96), likened to the work of Pavlov in the early 20th century (13). At this point, the extent to which CPIR can be conditioned remains under investigation. If CPIR can in fact be conditioned to many different types of food-related stimuli, then it is possible that CPIR magnitude in individuals could vary as a function of diet history.
Testing rodents naïve to sweeteners provides a means to examine the roles of nutrient conditioning on the CPIR. There are two studies in rodents showing that CPIR to sweet-tasting stimuli can be eliminated after pairing the taste solution with a noxious stimulus (i.e., lithium chloride) (97,98). Meanwhile, Berthoud and others (22) studied whether the CPIR to a saccharin-water stimulus could extinguish over 15 trials in rats, since the animals would come to associate saccharin (a low-calorie sweetener) with minimal post-ingestive consequences; however, the authors found no trial-dependent reduction in CPIR magnitude, suggesting that it is resistant to extinction.
Given the diversity of flavors that people experience across their lifetime, conditioning CPIR to a novel flavor stimulus is challenging (13). For instance, Dhillon and others (15) examined whether they could condition a CPIR to a nonnutritive sweetener in overweight or obese subjects, who were not regular nonnutritive sweetener users. After two weeks of training on the stimulus, the authors reported that learning had no effect on the CPIR response. They could not be certain, however, that the conditioning process was not confounded by prior dietary experiences. Nevertheless, conditioning individuals to elicit CPIR in a specific context seems to be more achievable. For example, Bellisle and others (8) performed a set of conditioning experiments wherein subjects attended multiple training visits with test meals (cocktail-size open sandwiches). In one of the experiments, individuals were randomly subjected at one of their visits to a tease condition (i.e., a test meal was anticipated but not served) after attending one or more (unspecified) trainings. During this condition, 3 of the 4 subjects still produced a significant CPIR, suggesting that the CPIR was conditioned to the experimental situation. In another experiment, all subjects produced an anticipatory CPIR to no food presentation, after completing 4 training sessions during which food had been presented. Importantly, this study also included a condition with naïve subjects who had never eaten in the experimental situation, and confirmed that those individuals did not produce a CPIR without food. This study, therefore, provides evidence that CPIR in humans may be conditioned to context cues (Figure 2) under specific conditions.
5. Factors impacting CPIR variability in humans
One of the most puzzling features of human CPIR is that its expression and magnitude varies greatly across studies. Some studies observe a robust CPIR in either all (66) or a subset (10,15,44,53) of the subjects, while other studies report no significant CPIR overall for the subject group (14,56,59). How can this variability in CPIR across human studies be explained? Focusing specifically on human research, this section begins by discussing external factors that could add variability to research findings, including experimental design and testing procedure. Next, biological characteristics that may impact the natural variability in CPIR between subjects are considered.
5.1. Experimental design and testing procedure
Procedure standardization could assist in limiting variability in the degree of CPIR observed within and across studies. Factors related to stimulus properties, method and duration of sensory stimulation, blood sampling, and documenting CPIR data seem to be critical to explain why some studies reported robust CPIR while others reported no CPIR.
5.1.1. Stimulus properties
There are numerous components to a sensory stimulus that could modulate its ability to elicit CPIR. Below, the importance of these stimulus properties are discussed. Because studies have not systematically examined the importance of visual or olfactory stimuli, the discussion focuses on oral stimuli.
5.1.1.1. Liquid vs. solid stimuli and other textural properties
The textural properties of a stimulus could impact the degree to which it evokes CPIR. For example, despite several reports of liquid stimuli eliciting CPIR (15,26,53), some research groups have concluded that liquids are in general a poor model system because they elicit CPIR unreliably (54,57,59,99,100). The low magnitude or lack of measurable CPIR in some of these cases could be explained by insufficient tactile stimulation. For example, in the studies by Smeets et al. (54) and Spetter et al. (100), subjects were instructed to sip and swallow a liquid stimulus through a tube (note that these studies were intentionally mimicking beverage consumption through a straw). The lack of CPIR reported in those studies support the concern that beverages may not provide sufficient stimulation to evoke normal metabolic or satiety responses (101). Indeed, studies that implemented more extensive oral stimulation with a liquid [e.g., asking subjects to swish the liquid in their mouth after sipping (15,26)] reported a more reliable CPIR response. Therefore, liquids may be capable of stimulating CPIR in humans; however, they may require more extensive oral manipulation to be effective.
Solid stimuli may be more effective elicitors of CPIR. Two recent studies compared CPIR after tasting liquid and solid samples formulated with the same target stimulus [see (15,55)]. Dhillon et al. (15) measured CPIR to two sweeteners (sucrose and sucralose) in solid and liquid form following the same presentation protocol (multiple 15-second interval MSF) and found that the subjects who exhibited a CPIR had a greater magnitude response following stimulation with the solid than liquid form. Similarly, Lasschuijt et al. (55) explored whether the degree of stimulus firmness impacts CPIR by creating gel-based stimuli with softer and harder textures. Although the authors reported no significant CPIR for the combined subject group, a sub-analysis of responsive insulin curves showed that the model food with a hard texture resulted in insulin concentrations that were 1.2 times greater than for the model food with a soft texture. In line with these findings, the systematic review performed by Wiedemann et al. (24) found that solid stimuli significantly enhance effect size of CPIR. Because solid stimuli require more chewing and oral manipulation, they may increase the duration and complexity of sensory stimulation.
5.1.1.2. Intensity of stimulus
The intensity of the test stimulus would be particularly important if CPIR is dose-dependent. Two studies have looked at this directly (27,55). One reported that 555 mM (10% w/v) glucose, applied to the tongue tip on a piece of saturated gauze, was sufficient to stimulate CPIR, but 277 mM (5% w/v) glucose was not. The same study found that two higher concentrations (1110 mM and 2220 mM glucose stimuli, equating to 20% and 40% w/v, respectively) evoked greater CPIR than the 555 mM stimulus. Another study (55) measured CPIR using gel-based stimuli that differed in sweetness (17 mm difference rated on a 100 mm visual analogue scale). While no CPIR was reported when all subjects were considered, a sub-analysis of the subjects that exhibited an apparent CPIR revealed higher plasma insulin levels in response to the sweeter stimulus at 5 minutes. A related issue is that some studies did not control the amount of stimulus tasted or consumed prior to measuring CPIR [e.g., (8,102)]. In these cases, it is challenging to determine whether the variability of CPIR reflects differences in oral stimulation between individuals. The best approach would therefore be to control the intensity, duration, and volume of stimulus experienced by each subject.
5.1.1.3. Palatability of stimulus
Palatability appears to be relevant for CPRs [e.g., salivary and gastric activity; (7,50)]. The importance of stimulus palatability in eliciting CPRs was originally hypothesized by Powley (7), and has been supported by taste aversion data from animals. For example, food adulterated with quinine did not stimulate CPIR in rats, but the same food plain or with added sweetener did (103). In light of these findings, palatability is often treated as a critical factor in human CPIR research [e.g., see (2,29)], but this issue is controversial.
Two studies found that CPIR occurred more frequently following exposure to highly palatable foods vs. less palatable foods (8,69). Nonetheless, some subjects in these studies still expressed CPIR in cases when the stimulus was of lower palatability. Additionally, in one of these studies (69), the texture of the stimulus covaried with palatability between the conditions (solid foods vs. foods blended to a paste), making the findings difficult to interpret. In a separate study, LeBlanc and others (104) concluded that palatability, rather than nutrient composition, is the primary driver of CPIR. They based this inference on the finding that palatable foods with high carbohydrate (sugar pie), high fat (whipped cream) or high protein (beef steak, cod fillet) all elicited CPIR. It is notable, however, that palatability ratings were not explicitly measured. However, other studies have reported that stimulus palatability does not play a significant role in CPIR magnitude (10,26,35,57,102,105). Overall, the importance of palatability to elicit CPIR in humans requires further investigation.
5.1.2. Method and duration of oral stimulation
The method of oral stimulation varies widely across studies. Tasting, but not ingesting a food or food-like stimulus (i.e., modified sham feeding, or MSF), is one approach commonly used for CPIR studies because it provides oral sensory stimulation but limits gastrointestinal stimulation. Within these MSF designs, studies have either implemented a passive tasting procedure [i.e., placing a saturated piece of gauze on the tongue; (27)], or a more active tasting procedure [i.e., chewing/swishing, followed by spitting (26,28,55)]. However, it is probable that oral stimulation, particularly coupled with ingestion, is the optimal method of stimulus delivery because it is both most comparable to real eating situations, and provides maximal sensory stimulation (49,57). Multiple studies have used this approach [e.g., (54,100,106)]. Importantly, this study design necessitates concurrent monitoring of other metabolites (e.g., glucose) during the cephalic phase to separate any insulin released due to nutrient absorption.
The duration of sensory stimulation also differs substantially between studies. Whereas some studies implemented a relatively short stimulation period [i.e., ≤ 2 minutes (10,26,57,65)], others implemented a relatively long stimulation period, some of which even extended beyond 10 minutes [e.g., (15,53)]. Longer stimulation periods could increase the magnitude of the resultant CPIR. For instance, Teff et al. (57) showed that 3 minutes of MSF produced a significantly larger CPIR than 1 minute of MSF. Investigators have also varied the number of stimulation periods. For example, some studies included a single period of sensory stimulation [e.g., (26)] while others asked subjects to perform the stimulation procedure multiple times [e.g. repeat MSF for 15 second intervals over 14 minutes; (15,53,55)]. Others investigators did not control the duration of sensory stimulation and allowed subjects to consume the stimulus at their own pace [e.g.,(106,107)]. While such a protocol is useful for increasing the relevance of the results to real eating situations, it could also lead to substantial variability in the observed responses across studies.
5.1.3. Fasting before testing
Although food deprivation (i.e., fasting) may influence CPIR magnitude (108), this variable is not controlled consistently across studies. Some investigators fasted subjects overnight [e.g., (26,32,56,99)], whereas others did so for 3–4 hours [e.g., (8,14,55,69,102)]. Given that the metabolic changes associated with a meal can last up to five hours (109), it is generally recommended to implement a longer fasting period prior to measuring CPIR in order to limit the influence of nutrients and metabolic processes from previous meals. With this being said, prolonged periods of fasting are not always typical of some modern diets. To address this concern, it would be worthwhile to systematically investigate the impact of fasting duration on CPIR magnitude.
5.1.4. Blood sampling procedure
Given the transience of CPIR, it is essential that blood samples are taken at appropriate time points and with adequate frequency. These practices are critical during both the pre- and post-stimulation periods.
5.1.4.1. Resting period
Insulin secretion has been demonstrated to be impacted by brief periods of stress (110), and it has been specifically reported that serum insulin levels can increase following catheterization. For instance, one study reported that serum insulin levels increased by 0.9 mIU/L about 14 minutes after catheter placement in the absence of oral stimulation (15). Implementing a sufficient resting period prior to baseline sampling could therefore limit this effect (see Figure 5). Per the systematic review by Lasschuijt et al. (108), which included both CPIR and cephalic phase pancreatic polypeptide release studies, the average acclimatization time between cannula insertion and the first drawn blood sample was 39±49 min, with about one-third of those studies reporting a 30 min acclimatization period.
Figure 5.
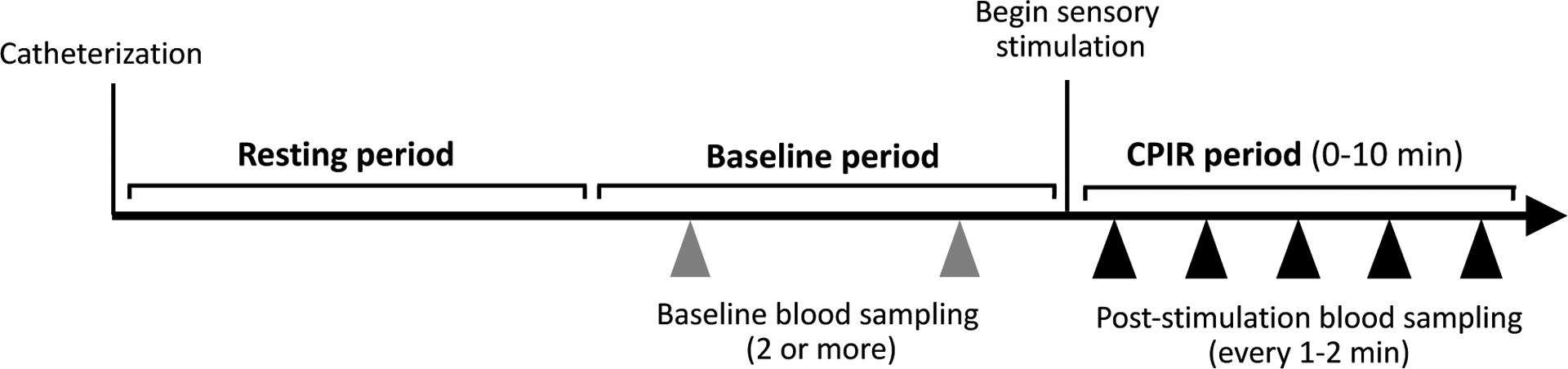
Recommendations for blood sampling protocol. Following catheterization, a resting period is recommended to allow insulin and other metabolite levels to stabilize. Multiple baseline blood samples should be collected for more accurate evaluation of fasting insulin concentration. Following sensory stimulation, blood samples should be collected within 2 minutes, and at least every 1–2 minutes throughout the remainder of the CPIR period. Arrows represent blood draws.
5.1.4.2. Baseline insulin sampling
Sufficient baseline sampling (see Figure 5) is particularly critical since most studies typically define CPIR as an increase in insulin concentration relative to baseline. Notably, resting insulin has been reported to undergo spontaneous fluctuations. This is important to consider because fluctuations of substantial magnitude could conceal a lower magnitude CPIR, or conversely, be incorrectly interpreted as a CPIR. Reports on the time course and magnitude of spontaneous insulin fluctuations tend to be highly variable between studies. Cyclical variation in insulin secretion has been reported to follow periods ranging from 7 to 31 minutes (8,69,111,112), although from most of these cited studies, the average cycle duration was about 12–13 min (14,111,112). Spontaneous fluctuations in serum insulin levels have been reported to be relatively small in some studies [e.g., ~0.2 to 3.6 μU/mL; (14,69)] and relatively large in others [up to 15 μU/mL; (111)]. Also, most of these studies report cyclical variation in insulin secretion in only a subset of subjects. For example, Lang et al. (112) and Lucas et al. (69) found, in respective order, that 25% and 65% of subjects exhibited significant insulin cycles. In contrast, some studies report that insulin levels show very low variability between multiple baseline timepoints [e.g., (45)]. While the explanation for this variation awaits future studies, it follows that adequate baseline sampling would help researchers control for this potential confound. However, a specific recommendation for the duration of baseline sampling has not been defined; published studies have included baseline periods between 5 and 45 min.
5.1.4.3. Blood sampling timeline and frequency
Following the sensory stimulation period, beginning blood sampling late, or with insufficient frequency, could result in underestimating the amplitude of the CPIR to the stimulus, or bypassing the response altogether. For example, some studies report that CPIR reaches its peak 2 (15), 3 (27,57), or 4 (10,67) minutes after sensory stimulation, but other studies did not begin blood sampling until 5 minutes post-stimulation, and continued sampling at relatively large intervals [i.e., 5 minutes; (25,38,45,47,70)]. Other studies that did not begin blood sampling until 9 minutes (113) or 15 minutes (114,115) after sensory stimulation likely missed the cephalic phase period. Sampling blood rapidly after the onset of sensory stimulation (within at least 2 min) and performing frequent blood sampling (e.g., every 1 to 2 min) during the CPIR period would allow the most accurate estimates of insulin release (see Figure 5).
5.1.5. Documenting CPIR
Currently, there is no established criteria for what constitutes a significant CPIR. For instance, it has been defined as a positive increase in insulin from mean baseline at a specified timepoint (15), a difference in positive AUC for the baseline vs cephalic phase period (105), or as a positive increase greater than two standard deviations of spontaneous insulin fluctuations (69). Given these variable definitions, it is likely that a lack of significant CPIR reported by some researchers could qualify as a significant response by others, and vice versa. These somewhat arbitrary CPIR designations make study findings difficult to compare. Some studies take an additional step to distinguish groups of individuals based on the presence or magnitude of CPIR and use these groupings for further analyses [e.g., (15,44)].
Studies of CPIR in humans have reported substantial individual variation. In several cases, the subjects that produced an apparent CPIR were classified as “responders” while the ones that did not produced a CPIR were classified as “non-responders” (15,55). Attempts to distinguish responders and non-responders using sensory stimulation protocols have been largely inconclusive [e.g., (15,55,66)]. It is possible that the responder/non-responder distinction represents a false dichotomy, and the so-called non-responders may exhibit CPIR of a low magnitude that is difficult to capture. This latter viewpoint could explain why mice, which express relatively large CPIRs, appear to exhibit CPIRs more reliably (6). To address the apparent variability of CPIR magnitude between humans, it may be preferable to present data from each subject separately, and avoid simply reporting group means. In other words, combining CPIR profiles across all subjects could produce blunted average values. On the other hand, considering the CPIR data on an individual level could provide helpful insights into potential groupings of subjects.
5.2. Individual characteristics
A number of factors have been explored to explain the CPIR variability across subjects. They include sex, BMI, resting blood glucose levels, salivary α-amylase activity as well as dietary pattern.
5.2.1. Sex
Differences in CPIR responses between sexes have been suggested (65,116). However, outside of one study reporting that overweight females had a CPIR peak significantly greater than overweight males (35), additional evidence for a distinction has not been reported (26,102). Recently, Wiedemann et al. (24) concluded in their meta-analysis that sex had no significant influence on CPIR.
5.2.2. BMI
There are claims that the magnitude of CPIR is exaggerated in humans with obesity. Some human studies have supported these claims, finding that subjects who are overweight or obese exhibited greater CPIR compared to lean subjects. The CPIR was reported in terms of its overall magnitude (43), its maximal peak height (35), or as a trend towards a higher maximal insulin peak (36). In contrast, other studies have reported a lack of CPIR in subjects with obesity (42,45), although one of these studies (45) did not include lean controls and did not begin measuring insulin until 5 minutes post-stimulus (see section 5.1.4.3). A study conducted by Teff et al. (65) found that, while the magnitude of CPIR was greater in obese vs. lean subjects when insulin data were expressed as absolute differences from baseline, this relationship disappeared when insulin data were expressed as a percentage of baseline. In fact, in the latter analysis, there was an overall trend of attenuated CPIR in subjects with obesity. In that study, a greater magnitude CPIR was only observed in individuals with obesity that also had elevated fasting insulin (65). It should be noted that CPIR expression and magnitude has been reported to vary even within subjects that are overweight or obese (15,44).
5.2.3. Salivary α-amylase activity
Salivary α-amylase level is another factor that could contribute to CPIR variability. Although the oral processing period of food is short (typically < 30 sec), there is evidence that considerable starch hydrolysis takes place within this timeframe (60,117). Importantly, salivary α- amylase activity differs substantially across the human population (118,119), which could theoretically allow for variable amounts of hydrolysis products to be generated across different individuals. Only one study has explored the relationship between salivary α-amylase and CPIR. In that study (53), subjects with both low and high α-amylase showed CPIR to ingesting glucose, but only subjects with high α-amylase activity showed CPIR in response to ingesting a maltodextrin solution. Findings from this study may imply that results of other studies using starch-based model stimuli [e.g., (26,54)] or complex foods containing cooked starch [e.g., (30,66,69)] could have been influenced by individual variation in salivary α-amylase activity.
5.2.4. Dietary pattern
A positive association between level of dietary restraint and magnitude of CPIR has been reported in lean (35,105) and overweight subjects (35). Interestingly, Broberg & Bernstein (40) found that anorexic women—a population characterized by extreme dietary restriction—showed CPIR following visual and olfactory stimulation while control females showed no CPIR. In contrast, no relationship has been found between dietary restraint and CPIR in subjects with obesity (44). Together, this suggests that CPIR magnitude may reflect degree of dietary restraint, but only in non-obese individuals. More broadly speaking, prior dietary experience (e.g., high vs. low carbohydrate diets) may impact CPIR magnitude, considering the potential role that nutrient conditioning may play in the response (see section 4.3).
6. Conclusions
Tremendous effort has been made to understand the sensory, neural, and endocrine mechanisms underlying CPIR and its implications to human health. A flurry of recent work has discovered that a glucose-based signaling pathway is both expressed and functional in the taste cells of mice, bringing new insights on how tasting a food containing glucose-based carbohydrates could elicit CPIR. A key challenge is to confirm whether a similar pathway exists in the human gustatory system, and uncover its role in eliciting CPIR. A recent report also suggests that multiple sensory inputs can act in an additive manner to elicit CPIR in humans. The extent to which the glucose-based signaling pathway vs. other routes of sensory stimulation (i.e., visual, olfactory, gustatory, and trigeminal) contributes to CPIR remains to be clarified.
Another critical point in human CPIR research is identifying the sources of variability between and within studies. While inter-study variability can often be explained by differences in experimental design and testing procedure, less is known about why some individuals show robust CPIR and others do not. We have identified three factors that appear to contribute to this individual variability. First, a substantial portion of secreted insulin is rapidly degraded by the liver, and the degree of this degradation varies between individuals, populations and disease states (120). For instance, 50–70% of secreted insulin is cleared from the bloodstream during its first pass through the liver of healthy human subjects (121). This degradation tends to decrease in patients with obesity (122) [but see (123)] and those with glucose intolerance (124), and decreases precipitously with the onset of fatty liver disease (125) or cirrhosis (126). Second, based on findings of Mandel and Breslin (53), subjects with low α-amylase levels could be less likely to exhibit CPIR to a starch-based complex carbohydrate stimulus. However, this hypothesis would only apply to foods that contain complex carbohydrates. Finally, differences in prior diet history could play a significant role in modulating an individual’s response to certain stimuli—e.g., some individuals could have conditioned a CPIR to specific food-related chemicals (e.g., nonnutritive sweeteners). For the future CPIR research, understanding the sources of individual differences would be critical. Such information could help establish the importance of CPIR in human health and further contribute to the concept of precision nutrition (127), a recent initiative from the National Institutes of Health.
Research Highlights.
This review presents current knowledge on mechanisms underlying CPIR.
Stimuli that elicit CPIR are discussed.
Potential sources of variability between and within studies are examined.
Funding
This work was supported in part by grant R01DC017555 (JL) from the NIH/NIDCD. AJP is an ARCS (Achievement Rewards for College Scientists) Scholar.
Abbreviations
- ACh
acetylcholine
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- CPR
cephalic phase response
- CPIR
cephalic phase insulin release
- DMNX
dorsal motor nucleus of the vagus
- GIIS
glucose-induced insulin secretion
- GLUT
glucose transporter
- MOS
maltooligosaccharide
- MPS
maltopolysaccharide
- MSF
modified sham feed
- NST
nucleus of the solitary tract
- SGLT-1
sodium glucose transporter-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflicts of interest. The content is solely the responsibility of the authors and does not represent the official views of the NIH.
References
- 1.Brand JG, Cagan RH, Naim M. Chemical Senses in the Release of Gastric and Pancreatic Secretions. Annu Rev Nutr. 1982July1;2(1):249–76. [DOI] [PubMed] [Google Scholar]
- 2.Smeets PA, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutr Rev. 2010November1;68(11):643–55. [DOI] [PubMed] [Google Scholar]
- 3.Mattes RD. Nutritional implications of the cephalic-phase salivary response. Appetite. 2000April1;34(2):177–83. [DOI] [PubMed] [Google Scholar]
- 4.Katschinski M Nutritional implications of cephalic phase gastrointestinal responses. Appetite. 2000;34(2):189–96. [DOI] [PubMed] [Google Scholar]
- 5.LeBlanc J Nutritional implications of cephalic phase thermogenic responses. Appetite. 2000;34(2):214–6. [DOI] [PubMed] [Google Scholar]
- 6.Teff K Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000April1;34(2):206–13. [DOI] [PubMed] [Google Scholar]
- 7.Powley TL. The ventromedial hypothalamic syndrome, satiety, and a cephalic phase hypothesis. Psychol Rev. 1977;84(1):89–126. [PubMed] [Google Scholar]
- 8.Bellisle F, Louis-Sylvestre J, Demozay F, Blazy D, Le Magnen J. Cephalic phase of insulin secretion and food stimulation in humans: a new perspective. Am J Physiol-Endocrinol Metab. 1985;249(6):E639–45. [DOI] [PubMed] [Google Scholar]
- 9.Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. Am J Physiol-Regul Integr Comp Physiol. 1984Jan 1;246(1):R88–95. [DOI] [PubMed] [Google Scholar]
- 10.Teff K, Mattes RD, Engelman K. Cephalic phase insulin release in normal weight males: verification and reliability. Am J Physiol-Endocrinol Metab. 1991;261(4):E430–6. [DOI] [PubMed] [Google Scholar]
- 11.Powley TL. Vagal circuitry mediating cephalic-phase responses to food. Appetite. 2000April1;34(2):184–8. [DOI] [PubMed] [Google Scholar]
- 12.Lasschuijt MP, Mars M, de Graaf C, Smeets PAM. Endocrine Cephalic Phase Responses to Food Cues: A Systematic Review. Adv Nutr. 2020September1;11(5):1364–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teff K How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol Behav. 2011April18;103(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdallah L, Chabert M, Louis-Sylvestre J. Cephalic phase responses to sweet taste. Am J Clin Nutr. 1997March1;65(3):737–43. [DOI] [PubMed] [Google Scholar]
- 15.Dhillon J, Lee JY, Mattes RD. The cephalic phase insulin response to nutritive and low-calorie sweeteners in solid and beverage form. Physiol Behav. 2017November1;181:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berthoud H-R, Jeanrenaud B. Sham feeding-induced cephalic phase insulin release in the rat. Am J Physiol-Endocrinol Metab. 1982Apr 1;242(4):E280–5. [DOI] [PubMed] [Google Scholar]
- 17.Glendinning JI, Lubitz GS, Shelling S. Taste of glucose elicits cephalic-phase insulin release in mice. Proc SSIB 2017 Annu Meet. 2018August1;192:200–5. [DOI] [PubMed] [Google Scholar]
- 18.Glendinning JI, Frim YG, Hochman A, Lubitz GS, Basile AJ, Sclafani A. Glucose elicits cephalic-phase insulin release in mice by activating KATP channels in taste cells. Am J Physiol-Regul Integr Comp Physiol. 2017January27;312(4):R597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glendinning JI, Stano S, Holter M, Azenkot T, Goldman O, Margolskee RF, et al. Sugar-induced cephalic-phase insulin release is mediated by a T1r2+T1r3-independent taste transduction pathway in mice. Am J Physiol-Regul Integr Comp Physiol. 2015July8;309(5):R552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis-Sylvestre J Preabsorptive insulin release and hypoglycemia in rats. Am J Physiol-Leg Content. 1976;230(1):56–60. [DOI] [PubMed] [Google Scholar]
- 21.Steffens A Influence of the oral cavity on insulin release in the rat. Am J Physiol-Leg Content. 1976May1;230(5):1411–5. [DOI] [PubMed] [Google Scholar]
- 22.Berthoud H-R, Bereiter DA, Trimble ER, Siegel EG, Jeanrenaud B. Cephalic phase, reflex insulin secretion neuroanatomical and physiological characterization. Diabetologia. 1981March1;20(1):393–401. [PubMed] [Google Scholar]
- 23.Skvortsova A, Veldhuijzen DS, Kloosterman IEM, Pacheco-López G, Evers AWM. Food anticipatory hormonal responses: A systematic review of animal and human studies. Neurosci Biobehav Rev. 2021July1;126:447–64. [DOI] [PubMed] [Google Scholar]
- 24.Wiedemann SJ, Rachid L, Illigens B, Böni-Schnetzler M, Donath MY. Evidence for cephalic phase insulin release in humans: A systematic review and meta-analysis. Appetite. 2020;104792. [DOI] [PubMed] [Google Scholar]
- 25.Dušková M, Macourek M, Šrámková M, Hill M, Stárka L. The role of taste in cephalic phase of insulin secretion. Prague Med Rep. 2013;114(4):222–30. [DOI] [PubMed] [Google Scholar]
- 26.Just T, Pau HW, Engel U, Hummel T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite. 2008November1;51(3):622–7. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki M, Sakaguchi T. Effects of D-glucose anomers on sweetness taste and insulin release in man. Brain Res Bull. 1986August1;17(2):271–4. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Hsu WH, Hollis JH. Modified sham feeding of foods with different macronutrient compositions differentially influences cephalic change of insulin, ghrelin, and NMR-based metabolomic profiles. Physiol Behav. 2014;135:135–42. [DOI] [PubMed] [Google Scholar]
- 29.Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: Cephalic phase responses. Appetite. 2008March1;50(2):194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teff K, Engelman K. Oral sensory stimulation improves glucose tolerance in humans: effects on insulin, C-peptide, and glucagon. Am J Physiol-Regul Integr Comp Physiol. 1996June1;270(6):R1371–9. [DOI] [PubMed] [Google Scholar]
- 31.Lorentzen M, Madsbad S, Kehlet H, Tronier B. Effect of sham-feeding on glucose tolerance and insulin secretion. Acta Endocrinol (Copenh). 1987January1;115(1):84–6. [DOI] [PubMed] [Google Scholar]
- 32.Ahrén B, Holst JJ. The Cephalic Insulin Response to Meal Ingestion in Humans Is Dependent on Both Cholinergic and Noncholinergic Mechanisms and Is Important for Postprandial Glycemia. Diabetes. 2001May1;50(5):1030. [DOI] [PubMed] [Google Scholar]
- 33.Calles-Escandon J, Robbins DC. Loss of Early Phase of Insulin Release in Humans Impairs Glucose Tolerance and Blunts Thermic Effect of Glucose. Diabetes. 1987October1;36(10):1167. [DOI] [PubMed] [Google Scholar]
- 34.Pliquett RU, Führer D, Falk S, Zysset S, von Cramon DY, Stumvoll M. The effects of insulin on the central nervous system-focus on appetite regulation. Horm Metab Res. 2006;38(07):442–6. [DOI] [PubMed] [Google Scholar]
- 35.Simon C, Schlienger JL, Sapin R, Imler M. Cephalic phase insulin secretion in relation to food presentation in normal and overweight subjects. Physiol Behav. 1986January1;36(3):465–9. [DOI] [PubMed] [Google Scholar]
- 36.Johnson WG, Wildman HE. Influence of external and covert food stimuli on insulin secretion in obese and normal persons. Behav Neurosci. 1983;97(6):1025–8. [DOI] [PubMed] [Google Scholar]
- 37.Goldfine ID, Abraira C, Gruenewald D, Goldstein MS. Plasma Insulin Levels During Imaginary Food Ingestion Under Hypnosis. Proc Soc Exp Biol Med. 1970January1;133(1):274–6. [DOI] [PubMed] [Google Scholar]
- 38.Massolt ET, van Haard PM, Rehfeld JF, Posthuma EF, van der Veer E, Schweitzer DH. Appetite suppression through smelling of dark chocolate correlates with changes in ghrelin in young women. Regul Pept. 2010April9;161(1):81–6. [DOI] [PubMed] [Google Scholar]
- 39.Morricone L, Bombonato M, Cattaneo AG, Enrini R, Lugari R, Zandomenighi R, et al. Food-related sensory stimuli are able to promote pancreatic polypeptide elevation without evident cephalic phase insulin secretion in human obesity. Horm Metab Res. 2000;32(06):240–5. [DOI] [PubMed] [Google Scholar]
- 40.Broberg DJ, Bernstein IL. Cephalic insulin release in anorexic women. Physiol Behav. 1989May1;45(5):871–4. [DOI] [PubMed] [Google Scholar]
- 41.Broberg DJ, Bernstein IL. Preabsorptive insulin release in bulimic women and chronic dieters. Appetite. 1989December1;13(3):161–9. [DOI] [PubMed] [Google Scholar]
- 42.Osuna JI, Pages I, Motiño MA, Rodriguez E, Osorio C. Cephalic phase of insulin secretion in obese women. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 1986July;18(7):473–5. [DOI] [PubMed] [Google Scholar]
- 43.Sjöström L, Garellick G, Krotkiewski M, Luyckx A. Peripheral insulin in response to the sight and smell of food. Metab - Clin Exp. 1980October1;29(10):901–9. [DOI] [PubMed] [Google Scholar]
- 44.Karhunen LJ, Lappalainen RI, Niskanen LK, Turpeinen AK, Uusitupa MIJ. Determinants of the cephalic-phase insulin response in obese nondiabetic subjects. Metabolism. 1996February1;45(2):168–73. [DOI] [PubMed] [Google Scholar]
- 45.Parra-Covarrubias A, Rivera-Rodriguez I, Almaraz-Ugalde A. Cephalic Phase of Insulin Secretion in Obese Adolescents. Diabetes. 1971December1;20(12):800–2. [DOI] [PubMed] [Google Scholar]
- 46.Sahakian BJ, Lean MEJ, Robbins TW, James WPT. Salivation and insulin secretion in response to food in non-obese men and women. Appetite. 1981September1;2(3):209–16. [DOI] [PubMed] [Google Scholar]
- 47.Härtel B, Graubaum H, Schneider B. The influence of sweetener solutions on the secretion of insulin and the blood glucose level. Ernährungsumschau. 1993;40(4):152–5. [Google Scholar]
- 48.Buss C, Kraemer-Aguiar LG, Maranhão PA, Marinho C, Maria das Graças C, Wiernsperger N, et al. Novel findings in the cephalic phase of digestion: a role for microcirculation? Physiol Behav. 2012;105(4):1082–7. [DOI] [PubMed] [Google Scholar]
- 49.Zafra MA, Molina F, Puerto A. The neural/cephalic phase reflexes in the physiology of nutrition. Neurosci Biobehav Rev. 2006January1;30(7):1032–44. [DOI] [PubMed] [Google Scholar]
- 50.Mattes RD. Physiologic Responses to Sensory Stimulation by Food: Nutritional Implications. J Am Diet Assoc. 1997April1;97(4):406–13. [DOI] [PubMed] [Google Scholar]
- 51.Tonosaki K, Hori Y, Shimizu Y, Tonosaki K. Relationships between insulin release and taste. Biomed Res. 2007;28(2):79–83. [DOI] [PubMed] [Google Scholar]
- 52.Niijima A, Togiyama T, Adachi A. Cephalic-phase insulin release induced by taste stimulus of monosodium glutamate (umami taste). Physiol Behav. 1990;48(6):905–8. [DOI] [PubMed] [Google Scholar]
- 53.Mandel AL, Breslin PAS. High Endogenous Salivary Amylase Activity Is Associated with Improved Glycemic Homeostasis following Starch Ingestion in Adults. J Nutr. 2012May1;142(5):853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr. 2005November1;82(5):1011–6. [DOI] [PubMed] [Google Scholar]
- 55.Lasschuijt MP, Mars M, de Graaf C, Smeets PAM. Exacting Responses: Lack of Endocrine Cephalic Phase Responses Upon Oro-Sensory Exposure. Front Endocrinol [Internet]. 2018. [cited 2020 Dec 24];9. Available from: https://www.frontiersin.org/articles/10.3389/fendo.2018.00332/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cedernaes J, Lampola L, Axelsson EK, Liethof L, Hassanzadeh S, Yeganeh A, et al. A single night of partial sleep loss impairs fasting insulin sensitivity but does not affect cephalic phase insulin release in young men. J Sleep Res. 2016;25(1):5–10. [DOI] [PubMed] [Google Scholar]
- 57.Teff K, Devine J, Engelman K. Sweet taste: effect on cephalic phase insulin release in men. Physiol Behav. 1995;57(6):1089–95. [DOI] [PubMed] [Google Scholar]
- 58.Berthoud H-R, Trimble ER, Siegel EG, Bereiter DA, Jeanrenaud B. Cephalic-phase insulin secretion in normal and pancreatic islet-transplanted rats. Am J Physiol-Endocrinol Metab. 1980;238(4):E336–40. [DOI] [PubMed] [Google Scholar]
- 59.Ford HE, Peters V, Martin NM, Sleeth ML, Ghatei MA, Frost GS, et al. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur J Clin Nutr. 2011;65(4):508–13. [DOI] [PubMed] [Google Scholar]
- 60.Lapis TJ, Penner MH, Balto AS, Lim J. Oral digestion and perception of starch: Effects of cooking, tasting time, and salivary α-amylase activity. Chem Senses. 2017;42(8):635–45. [DOI] [PubMed] [Google Scholar]
- 61.Pullicin AJ, Penner MH, Lim J. Human taste detection of glucose oligomers with low degree of polymerization. PLOS ONE. 2017Aug 29;12(8):e0183008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sim L, Quezada-Calvillo R, Sterchi EE, Nichols BL, Rose DR. Human Intestinal Maltase–Glucoamylase: Crystal Structure of the N-Terminal Catalytic Subunit and Basis of Inhibition and Substrate Specificity. J Mol Biol. 2008January18;375(3):782–92. [DOI] [PubMed] [Google Scholar]
- 63.Sukumaran SK, Yee KK, Iwata S, Kotha R, Quezada-Calvillo R, Nichols BL, et al. Taste cell-expressed α-glucosidase enzymes contribute to gustatory responses to disaccharides. Proc Natl Acad Sci U S A. 2016May24;113(21):6035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yasumatsu K, Ohkuri T, Yoshida R, Iwata S, Margolskee RF, Ninomiya Y. Sodium-glucose cotransporter 1 as a sugar taste sensor in mouse tongue. Acta Physiol. 2020;230(4):e13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teff K, Mattes RD, Engelman K, Mattern J. Cephalic-phase insulin in obese and normal-weight men: Relation to postprandial insulin. Metabolism. 1993December1;42(12):1600–8. [DOI] [PubMed] [Google Scholar]
- 66.Eliasson B, Rawshani A, Axelsen M, Hammarstedt A, Smith U. Cephalic phase of insulin secretion in response to a meal is unrelated to family history of type 2 diabetes. PLOS ONE. 2017March13;12(3):e0173654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teff K, Levin BE, Engelman K. Oral sensory stimulation in men: effects on insulin, C-peptide, and catecholamines. Am J Physiol-Regul Integr Comp Physiol. 1993December1;265(6):R1223–30. [DOI] [PubMed] [Google Scholar]
- 68.Robertson MD, Jackson KG, Williams CM, Fielding BA, Frayn KN. Prolonged effects of modified sham feeding on energy substrate mobilization. Am J Clin Nutr. 2001January1;73(1):111–7. [DOI] [PubMed] [Google Scholar]
- 69.Lucas F, Bellisle F, Di Maio A. Spontaneous insulin fluctuations and the preabsorptive insulin response to food ingestion in humans. Physiol Behav. 1987;40(5):631–6. [DOI] [PubMed] [Google Scholar]
- 70.Mennella I, Fogliano V, Vitaglione P. Salivary lipase and α-amylase activities are higher in overweight than in normal weight subjects: Influences on dietary behavior. Food Res Int. 2014December1;66:463–8. [Google Scholar]
- 71.Bello NT, Coughlin JW, Redgrave GW, Moran TH, Guarda AS. Oral sensory and cephalic hormonal responses to fat and non-fat liquids in bulimia nervosa. Physiol Behav. 2010;99(5):611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crystal SR, Teff K. Tasting fat: cephalic phase hormonal responses and food intake in restrained and unrestrained eaters. Physiol Behav. 2006;89(2):213–20. [DOI] [PubMed] [Google Scholar]
- 73.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci. 2002April2;99(7):4692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DuBois GE. Molecular mechanism of sweetness sensation. Physiol Behav. 2016October1;164:453–63. [DOI] [PubMed] [Google Scholar]
- 75.Merigo F, Benati D, Cristofoletti M, Osculati F, Sbarbati A. Glucose transporters are expressed in taste receptor cells. J Anat. 2011August;219(2):243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res. 2011August;345(2):243–52. [DOI] [PubMed] [Google Scholar]
- 77.Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci. 2011March29;108(13):5431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ninomiya Y, Yasumatsu K, Iwata S, Yoshida R. Signal detection and transmission pathways for sugars and fatty acids in the mouse peripheral taste system. 2020 Meeting of the International Society for Olfaction and Taste; 2020; Portland, OR. [Google Scholar]
- 79.Rorsman P, Ashcroft FM. Pancreatic β-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol Rev. 2017December6;98(1):117–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baker E, Lui F. Neuroanatomy, Vagal Nerve Nuclei. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 [cited 2021 May 2]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK545209/ [PubMed] [Google Scholar]
- 81.Babic T, Travagli RA. Neural control of the pancreas. Pancreapedia Exocrine Pancreas Knowl Base. 2016; [Google Scholar]
- 82.Gilon P, Henquin J-C. Mechanisms and Physiological Significance of the Cholinergic Control of Pancreatic β-Cell Function. Endocr Rev. 2001October1;22(5):565–604. [DOI] [PubMed] [Google Scholar]
- 83.Moede T, Leibiger IB, Berggren P-O. Alpha cell regulation of beta cell function. Diabetologia. 2020;63(10):2064–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Henquin J-C. Do Pancreatic β Cells “Taste” Nutrients to Secrete Insulin? Sci Signal. 2012August28;5(239):pe36. [DOI] [PubMed] [Google Scholar]
- 85.Berthoud H-R, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am J Physiol-Regul Integr Comp Physiol. 1990;258(2):R523–30. [DOI] [PubMed] [Google Scholar]
- 86.Spielman AI. Interaction of saliva and taste. J Dent Res. 1990;69(3):838–43. [DOI] [PubMed] [Google Scholar]
- 87.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25(1):53–74. [DOI] [PubMed] [Google Scholar]
- 88.Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110(1–2):175–82. [DOI] [PubMed] [Google Scholar]
- 89.Woods SC, Vasselli JR, Kaestner E, Szakmary GA, Milburn P, Vitiello MV. Conditioned insulin secretion and meal feeding in rats. J Comp Physiol Psychol. 1977;91(1):128. [DOI] [PubMed] [Google Scholar]
- 90.Strubbe JH. Parasympathetic involvement in rapid meal-associated conditioned insulin secretion in the rat. Am J Physiol-Regul Integr Comp Physiol. 1992;263(3):R615–8. [DOI] [PubMed] [Google Scholar]
- 91.Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tucker RM, Tan S-Y. Do non-nutritive sweeteners influence acute glucose homeostasis in humans? A systematic review. Physiol Behav. 2017;182:17–26. [DOI] [PubMed] [Google Scholar]
- 93.Burke MV, Small DM. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol Behav. 2015December1;152:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wooley SC, Wooley OW. Salivation to the sight and thought of food: a new measure of appetite. Psychosom Med. 1973; [DOI] [PubMed] [Google Scholar]
- 95.Rogers PJ, Hill AJ. Breakdown of dietary restraint following mere exposure to food stimuli: interrelationships between restraint, hunger, salivation, and food intake. Addict Behav. 1989;14(4):387–97. [DOI] [PubMed] [Google Scholar]
- 96.Keesman M, Aarts H, Vermeent S, Häfner M, Papies EK. Consumption simulations induce salivation to food cues. PLoS One. 2016;11(11):e0165449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ninomiya Y, Hellekant G, Higashi T, Kawamura S, Okamura T, Funakoshi M. Preabsorptive insulin responses to sweet tasting stimuli in the rat. Jpn J Oral Biol. 1988;30(1):121–5. [DOI] [PubMed] [Google Scholar]
- 98.Berridge K, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. J Comp Physiol Psychol. 1981;95(3):363. [DOI] [PubMed] [Google Scholar]
- 99.Bruce David, Storlien LH, Furler SM, Chisholm DJ. Cephalic phase metabolic responses in normal weight adults. Metabolism. 1987;36(8):721–5. [DOI] [PubMed] [Google Scholar]
- 100.Spetter MS, Mars M, Viergever MA, de Graaf C, Smeets PA. Taste matters–effects of bypassing oral stimulation on hormone and appetite responses. Physiol Behav. 2014;137:9–17. [DOI] [PubMed] [Google Scholar]
- 101.de Graaf C Texture and satiation: the role of oro-sensory exposure time. Physiol Behav. 2012;107(4):496–501. [DOI] [PubMed] [Google Scholar]
- 102.Bellisle F, Louis-Sylvestre J, Demozay F, Blazy D, Le Magnen J. Reflex insulin response associated to food intake in human subjects. Physiol Behav. 1983October1;31(4):515–21. [DOI] [PubMed] [Google Scholar]
- 103.Louis-Sylvestre J, Le Magnen J. Palatability and preabsorptive insulin release. Neurosci Biobehav Rev. 1980;4:43–6. [DOI] [PubMed] [Google Scholar]
- 104.LeBlanc J, Soucy J, Nadeau A. Early insulin and glucagon responses to different food items. Horm Metab Res. 1996;28(06):276–9. [DOI] [PubMed] [Google Scholar]
- 105.Teff K, Engelman K. Palatability and dietary restraint: Effect on cephalic phase insulin release in women. Physiol Behav. 1996August1;60(2):567–73. [DOI] [PubMed] [Google Scholar]
- 106.Morey S, Shafat A, Clegg ME. Oral versus intubated feeding and the effect on glycaemic and insulinaemic responses, gastric emptying and satiety. Appetite. 2016;96:598–603. [DOI] [PubMed] [Google Scholar]
- 107.LeBlanc J, Soucy J, Lalanne M, Nadeau A. Effects of a protein meal on plasma amino acids, glucose and insulin in control and non insulin dependent diabetes mellitus. Nutr Res. 1998;18(3):433–45. [Google Scholar]
- 108.Lasschuijt MP, Mars M, de Graaf C, Smeets PAM. How oro-sensory exposure and eating rate affect satiation and associated endocrine responses—a randomized trial. Am J Clin Nutr. 2020June1;111(6):1137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stipanuk MH, Caudill MA. Biochemical, Physiological, and Molecular Aspects of Human Nutrition - E-Book. Elsevier Health Sciences; 2018. 1109 p. [Google Scholar]
- 110.Alvarez MA, Portilla L, Gonzalez R, Ezcurra E. Insulin response to a short stress period. Psychoneuroendocrinology. 1989;14(3):241–4. [DOI] [PubMed] [Google Scholar]
- 111.Hansen BC, Jen K-LC, Pek SB, Wolfe RA. Rapid oscillations in plasma insulin, glucagon, and glucose in obese and normal weight humans. J Clin Endocrinol Metab. 1982;54(4):785–92. [DOI] [PubMed] [Google Scholar]
- 112.Lang DA, Matthews DR, Burnett M, Ward GM, Turner RC. Pulsatile, synchronous basal insulin and glucagon secretion in man. Diabetes. 1982;31(1):22–6. [DOI] [PubMed] [Google Scholar]
- 113.Kashima H, Eguchi K, Miyamoto K, Fujimoto M, Endo MY, Aso-Someya N, et al. Suppression of Oral Sweet Taste Sensation with Gymnema sylvestre Affects Postprandial Gastrointestinal Blood Flow and Gastric Emptying in Humans. Chem Senses. 2017May1;42(4):295–302. [DOI] [PubMed] [Google Scholar]
- 114.Taylor IL, Feldman M. Effect of Cephalic-Vagal Stimulation on Insulin, Gastric Inhibitory Polypeptide, and Pancreatic Polypeptide Release in Humans*. J Clin Endocrinol Metab. 1982December1;55(6):1114–7. [DOI] [PubMed] [Google Scholar]
- 115.Konturek SJ, Swierczek J, Kwiecień N, Obtutowicz W, Dobrzańska M, Kopp B, et al. Gastric secretory and plasma hormonal responses to sham-feeding of varying duration in patients with duodenal ulcer. Gut. 1981December1;22(12):1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stockhorst U, Steingrüber H-J, Scherbaum WA. Classically conditioned responses following repeated insulin and glucose administration in humans. Behav Brain Res. 2000;110(1–2):143–59. [DOI] [PubMed] [Google Scholar]
- 117.Hoebler C, Karinthi A, Devaux M-F, Guillon F, Gallant DJG, Bouchet B, et al. Physical and chemical transformations of cereal food during oral digestion in human subjects. Br J Nutr. 1998;80(5):429–36. [DOI] [PubMed] [Google Scholar]
- 118.Mandel AL, Peyrot des Gachons C, Plank KL, Alarcon S, Breslin PAS. Individual differences in AMY1 gene copy number, salivary α-amylase levels, and the perception of oral starch. PloS One. 2010October13;5(10):e13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peyrot des Gachons C, Breslin PAS. Salivary Amylase: Digestion and Metabolic Syndrome. Curr Diab Rep. 2016October;16(10):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee CC, Haffner SM, Wagenknecht LE, Lorenzo C, Norris JM, Bergman RN, et al. Insulin Clearance and the Incidence of Type 2 Diabetes in Hispanics and African Americans. Diabetes Care. 2013April1;36(4):901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saccá L, Orofino G, Petrone A, Vigorito C. Direct Assessment of Splanchnic Uptake and Metabolic Effects of Human and Porcine Insulin*. J Clin Endocrinol Metab. 1984August1;59(2):191–6. [DOI] [PubMed] [Google Scholar]
- 122.Polonsky KS, Given BD, Hirsch L, Shapiro ET, Tillil H, Beebe C, et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988February1;81(2):435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim MK, Reaven GM, Chen Y-DI, Kim E, Kim SH. Hyperinsulinemia in individuals with obesity: Role of insulin clearance. Obesity. 2015December1;23(12):2430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism. 1983May1;32(5):438–46. [DOI] [PubMed] [Google Scholar]
- 125.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki–Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135(1):122–30. [DOI] [PubMed] [Google Scholar]
- 126.Letiexhe MR, Scheen AJ, Gerard PL, Bastens BH, Pirotte J, Belaiche J, et al. Insulin secretion, clearance, and action on glucose metabolism in cirrhotic patients. J Clin Endocrinol Metab. 1993;77(5):1263–8. [DOI] [PubMed] [Google Scholar]
- 127.Kaiser J NIH’s ‘precision nutrition’ bet aims for individualized diets. Science. 2021February5;371(6529):552–552. [DOI] [PubMed] [Google Scholar]


