Abstract
For over 30 years, the association between the Hawaiian bobtail squid, Euprymna scolopes, and the bioluminescent bacterium, Vibrio fischeri, has been studied as a model system for the colonization of animal epithelia by symbiotic bacteria. The squid–vibrio light-organ system provides the exquisite resolution only possible with the study of a binary partnership. The impact of this relationship on the partners’ biology has been broadly characterized, including their ecology and evolutionary biology as well as the underlying molecular mechanisms of symbiotic dynamics. Much has been learned about the factors that foster initial light-organ colonization, and more recently about the maturation and long-term maintenance of the association. This Review synthesizes the results of recent research on the light-organ association and also describes the development of new horizons for E. scolopes as a model organism that promises to inform biology and biomedicine about the basic nature of host–microorganism interactions.
Introduction
In the 19th century naturalist Anton de Bary coined the term symbiosis and defined this phenomenon as a specific and stable relationship between two different species regardless of the impact of the association on partner fitness (for example, mutualism [G], parasitism [G] (or pathogenesis) and commensalism [G] )1. The state of symbiosis is therefore context dependent, and there is a growing trend to avoid categorizing microbial species based on terms such as ‘pathogens’, ‘mutualists’ or ‘commensals’ (Ref. 2). Animal and plant hosts often form symbioses with microbial partners, such as bacteria, archaea, eukaryotic microorganisms, or any combination of these clades. The field of symbiosis has undergone a renaissance in recent years, and a growing number of biologists and biomedical researchers recognize that the microbiota [G] and microbiomes of animal hosts, which are widespread in nature, fundamentally influence host health and disease, including development, immune system function, metabolism and even behavior3. Advances in this field have been enabled by novel technologies, including new methods of nucleic acid sequencing, refined imaging, and the development of multi-omics [G] approaches. Coupled with these tools, the development of powerful experimental model systems is transforming our understanding of the form and function of symbiotic systems4,5.
Model associations offer the opportunity to manipulate the partnerships experimentally to reveal the mechanisms underlying their establishment and maintenance4,5. One such symbiosis is between the Hawaiian bobtail squid, Euprymna scolopes, and the marine bacterium Vibrio fischeri, which produces light the host uses as camouflage in a nocturnal behavior6,7 called counterillumination that disrupts the host’s silhouette6,7. This association, studied intensely since 1989, provides insights into how symbiotic bacteria influence all aspects of host biology, including evolution and ecology as well as the cellular, biochemical and molecular features of the partners that promote the functioning as a holobiont [G] (also known as metagorganism). The research community that studies E. scolopes symbioses embraces the opportunity to work with the natural genetic variation presented by wild-caught animals. Individual egg clutches typically have hundreds of eggs, from which subsets of juveniles hatch simultaneously at dusk. As such, a large number of replicates within a treatment provides statistical power to experiments, and the inherent genetic variation within and between mated pairs and egg-clutches can be characterized to define those features that are conserved across the existing natural variation of the host. In essence, what is conserved across the genetic landscape is likely to be critical for the form and function of the symbiosis.
The squid–vibrio partnership is highly specific with only V. fischeri colonizing a specialized light organ, which develops from the rudiment of the embryonic hind gut-ink sac complex of the host 8,9 (Fig. 1). The symbiosis is established in each host generation via colonization by environmental cells of V. fischeri, which make up less than 0.1% of the bacterioplankton in the host habitats10. A number of host- and symbiont-mediated mechanisms ensure specificity (Fig. 2), and in the absence of V. fischeri cells in the environment, the light organ remains uncolonized6. A hallmark of the squid–vibrio symbiosis has been the ability to characterize experimentally the colonization process over the first hours to days following the hatching of the host, as well as the ability to manipulate V. fischeri genetically, which has provided insights into influences on the host that specifically result from genetic modification of the symbiont.
Figure 1: The Hawaiian bobtail squid as a model host for studying symbiosis.
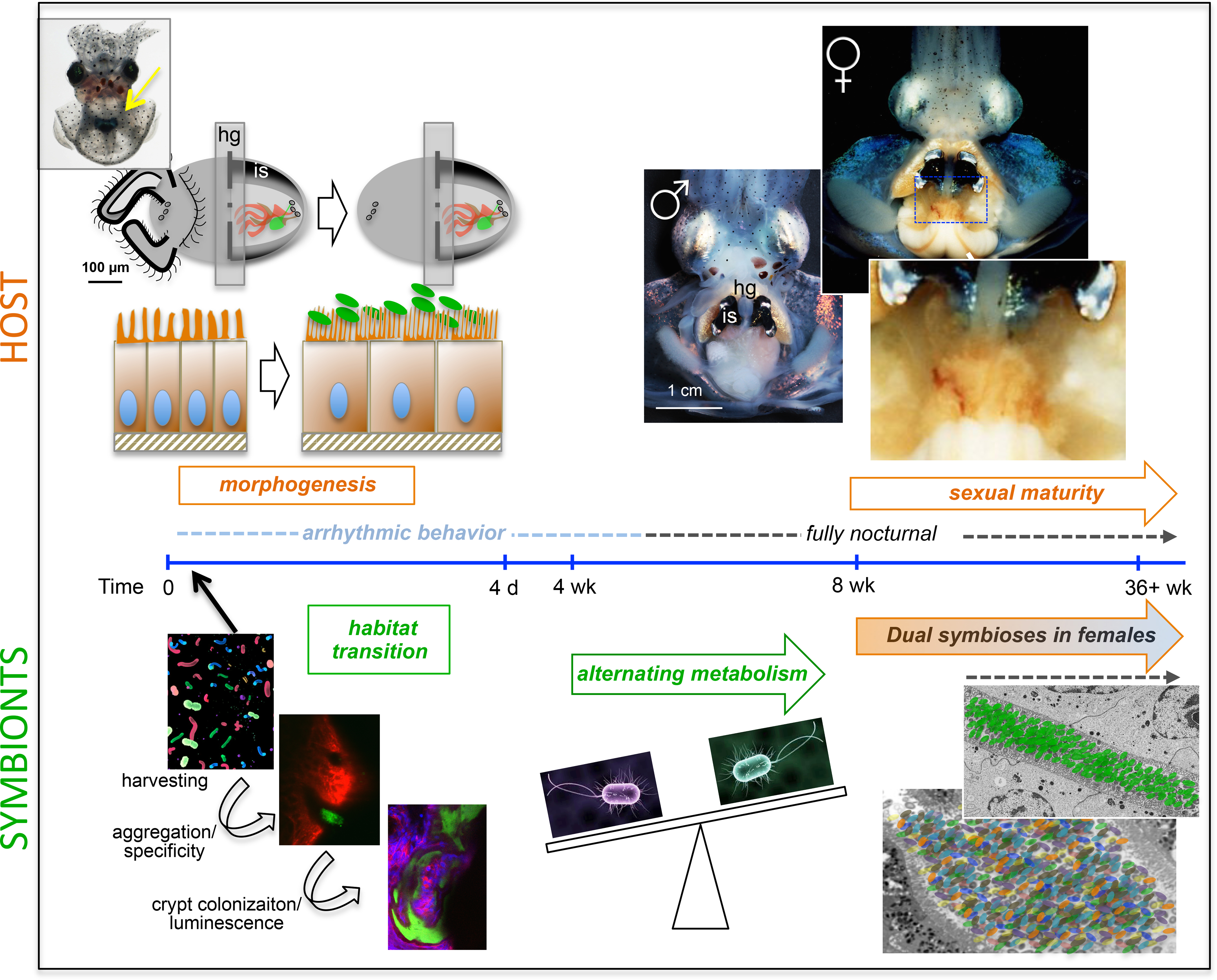
The light organ symbiosis in Euprymna scolopes involves a series of developmental events that ensure successful colonization by Vibrio fischeri and the long-term maintenance of the association. At hatching, juvenile squid (upper left) have a nascent light organ with a superficial ciliated epithelium containing “appendages” that help recruit environmental V. fischeri. Three pores on either side of the light organ lead to epithelium-lined crypts of distinct sizes (crypt 1, peach; crypt 2, brown; crypt, 3 green). Colonization occurs within hours after hatching as V. fischeri undergoes habitat transition from a free-living bacterium found in the bacterioplankton to aggregating in host mucus, the first site of specificity (lower left). Aggregation is followed by migration through the pores and colonization of the crypts where bioluminescence is induced (lower panel left; an inoculum larger than normal was used to visualize the aggregating cells in relation to the host tissues). Colonization leads to light organ morphogenesis over the first 4 days of the association. Cellular changes include epithelial swelling and an increase in microvillar density (upper panel left) such that the crypt spaces of fully colonized hosts contain a dense population of V. fisheri in direct contact with host epithelial cells (lower right). Over a period of approximately four weeks, the light organ symbiosis matures and host behavior changes from being arrhythmic to becoming fully nocturnal. A diel rhythm of alternating symbiont metabolism is also established during which the symbiont switches between glycerol phosphate respiration and chitin fermentation in the day and night respectively (lower right). This alternating metabolism helps facilitate luminescence (i.e., the light organ become acidic at night which increases the availability of oxygen used in light production). Sexual dimorphism is evident after E. scolopes reaches maturity at approximately 8 weeks after hatching. Female squid have a second symbiotic organ, the accessory nidamental gland (ANG) that houses a simple bacterial consortium (upper right; see Box 3). Image credits: juvenile squid, adapted from REF13, male squid; photo credit, William Omerod; copyright M. McFal-Ngai, female squid: adapted from REF64; Transmission electron micrograph courtesy of Mary Montgomery.
Figure 2: Update to the winnowing model of colonization.

Initial colonization of the light organ involves a number of biochemical and biomechanical mechanisms to establish the partnership between the Hawaiian bobtail squid, Euprymna scolopes, and the bioluminescent bacterium, Vibrio fischeri. The habitat transition of V. fischeri from the bacterioplankton to light organ symbiont involves several features that help facilitate colonization. The nascent light organ is made up of ciliated fields (inset; 1/2 of a light organ as shown by a scanning electron micrograph) that are composed of metachronally and randomly beating cilia that create microcurrents (arrows) that help to focus bacteria-sized particles above three pores on either side of the light organ in a shelter zone. The host increases expression of endochitinase and secretes mucus that contains a number of biochemical factors including chitobiose, a symbiont chemoattractant and a suite of host immunity factors that may serve to inhibit other bacteria. This unique microenvironment selects for V. fischeri while preventing colonization by non-symbiotic bacteria. V. fischeri cells that enter the light organ must traverse a unique biogeography that includes ciliated ducts, an antechamber and a bottleneck. A single V. fischeri cell (or on occasion several cells) migrate into each of the three crypts where they become nonmotile and divide and grow until a cell density is reached that enables the induction of quorum sensing and light production (approximately 9–12 h). Colonization also initiates cellular changes in the host, including the induction of haemocyte migration into the host ciliated fields along with apoptosis and regression of the ciliated field four days after colonization. lipopolysaccharide binding protein, LBP; bacteriocidal permeability-increasing protein, BPI; nitric oxide synthase, NOS; nitric oxide; NO, peptidoglycan recognition protein 2, PGRP2. Image credit: REF117
In 2004, 15 years after the first work on the light organ system, we reviewed the state of knowledge of the initial colonization in the squid–vibrio association11. We described what had been learned in those early years about a ‘winnowing’ process by which V. fischeri outcompetes other environmental bacteria through a series of interactions between the host and the co-evolved symbiont. In the years since, a collective effort from a growing number of laboratories has revealed many of the mechanisms that contribute to not only the initiation of the association (Fig. 2), but also those that mediate long-term maintenance through the ontogeny of the host. With the tremendous growth in the field, it would be lengthy to cover, even in broad strokes, what has been learned about this partnership from the vantage point of both host and squid. Thus, in this Review, we address the strides that have been made in recent years in the study of bobtail squid symbiosis from the host viewpoint; specifically, we explore how light organ colonization influences early development and maturation of the host (Fig. 1). Our article is intended to complement the companion Review (see Visick, Stabb and Ruby12), which details advances over this same period in understanding the association from the perspective of the host’s luminous partner V fischeri.
E. scolopes as a symbiosis model host
The bobtail squids and their associations with bacteria are naturally occurring binary (one host and one microbial species) symbioses that offer robust experimental systems (Box 1) complementary to other more complex symbiosis models, such as hydra, drosophila, zebrafish and mice5,13. Its symbiotic associations exemplify a very common type of host–microorganism alliance in animals; that is acquired anew each generation from the environment and characterized by the co-evolved microbial partners residing extracellularly along the apical surfaces of epithelial tissues14. The nocturnal squid host can easily be collected from the wild at night when it emerges to forage and can be raised through the complete life cycle15. Being less active than many other cephalopod species due to their benthic nature and limited diurnal activity, these animals are easily maintained in captivity with natural or artificial seawater and adapt well to modified zebrafish or Xenopus aquaria. Captive females mate and lay serial clutches of eggs year-round, and a single egg clutch can yield hundreds of juveniles that hatch after a ~20-d embryonic period. Over 50,000 juveniles can be produced in the laboratory over a year by maintaining a population of ~12 females and ~6 males.
Box 1 |. Key features and advantages of the Euprymna scolopes–Vibrio fischeri association.
The host E. scolopes
easily obtained from the field
is fecund year-round
no parental care of eggs
hatchlings live on yolk reserves and therefore do not need to be fed for colonization experiments
small juveniles, entire animal viewable by confocal microscopy
has capacity to be raised in the laboratory aposymbiotically (in natural seawater without symbionts)
with effort, can be reared through multiple generations in the laboratory
full genome sequence available
omics of the light organ possible both within and between clutches; that is, at high and low genetic diversity
The symbiont V. fischeri
free–living stage readily accessible
culturable on minimal or defined media
genetically tractable
displays symbiosis–relevant strain variation
dozens of full genome sequences available
extracellular along apical surface of host epithelia
physiologically well characterized; for example, quorum sensing
related to important human pathogenic species
The E. scolopes–V. fischeri partnership
ecologically obligate (aposymbiotic hosts not observed in the wild)
binary and/or experimentally tractable
natural mode of infection easily reconstituted in the laboratory
symbioses not nutritional; hosts remain healthy without symbionts in the laboratory
large number of juvenile hosts for experiments; robust statistics
short time frame of initiation and early development
luminous, non-invasive measure of symbiotic state
maturation coupled to immunity and partner physiology
has a complex daily rhythm
antibiotics, fluorochromes, inhibitors and activators easily administered
amenable to the study of space biology and microgravity experiments122,123
The morphology of the light organ fosters emission of symbiont luminescence from the ventral surface of the squid host; it is hypothesized that the luminescence is used as camouflage to avoid predation5. As such, the authors have confirmed that the host is not physiologically compromised and requires no special care when maintained in the aposymbiotic state in the laboratory; that is exposed to natural seawater without sufficient symbionts for colonization. Further, assessment and manipulation of the host symbiotic state can be carried out by measuring the light emitted by the light organ6. The relationship between luminescence output and symbiont number can be empirically defined for a given strain6,17; different strains of V. fischeri have different luminescent outputs in vivo18. Thus, host light emission not only defines the presence or absence of symbiosis by wild-type or light-producing mutants, but also enables an estimate of the relative degree of colonization in the light organ.
Several other features make the squid a compelling experimental subject. The ability to raise animals under aposymbiotic conditions throughout their life cycle has enabled researchers to determine critical features of the associations, such as the phases of symbiosis development and their associated morphological, physiological, biochemical and molecular signatures. The symbionts interface with two types of polarized epithelia, specifically ciliated and microvillous6,8,19, which are the two most common types of epithelial mucosal surfaces associated with host–microorganism interactions across the animal kingdom. Because the light organ epithelial tissues are exposed to the environment via the mantle cavity, the responses of the system to pharmaceuticals (for example, concentration and time of the response to exposure) are similar to what is observed in mammalian cell culture. In addition, the light organ can be quickly cured of its symbionts using low-dose antibiotics for a period of hours to days, depending on life stage; such experimental treatment enables the identification of reversible and irreversible symbiont-induced changes in the host organ9,19–21.
The squid–vibrio association is also amenable to many different observational and experimental methods and techniques (Box 1 and Table 1). For example, in the light-organ symbiosis, because the newly hatched juvenile is ~2 mm in total length, one can observe and analyze the colonization process as the symbionts are harvested from the ambient seawater during the first hours after the juvenile emerges from the egg22. Once V. fischeri cells have been preferentially recruited onto the ciliated light-organ surface, the symbionts migrate into the tissues and traverse several distinct biomechanical and biochemical environments across an ~150-μm microbiogeographical landscape11,23,24. They reach their final destination of the microvillous crypt spaces by ~9 h (Fig 2), although the timing of this can vary with different strains of V. fischeri. The transparency of this entire pathway permits real-time observation of the spatiotemporal relationship of the symbionts to host tissues over the trajectory of the colonization process. Perhaps one of the most powerful attributes of the light organ association is that it is a model with well-developed genetic tools in the bacterial partner (see Visick, Stabb and Ruby12). As such, unlike symbioses with bacterial consortia, the squid–vibrio model, similar to the legume–rhizobia association, is amenable to genetic manipulation in 50% of the partners (see below).
Table 1 |.
Advances in techniques used to study the symbiotic associations of Euprymna scolopes
| Technique | Tissues or cells analysed | Applications | References |
|---|---|---|---|
| Molecular | |||
| Whole genome | Genomes of host and 62 Vibrio fischeri strains, including ES114 and MJ11; 13 D and S strains from V. fischeri; 16 ANG symbiont strains of E. scolopes | Discovery of novel genes important for symbiosis and host range; phylogenetic comparison of ANG strains and biosynthetic potential; identification of symbiosis-related host genes | 18,38,58,105–109 |
| Microarrays | V. fischeri and light organ | Comparisons of global gene expression in host and symbiont (13,982 ESTs) | 64,110,111 |
| RNA sequencing | Light organ and V. fischeri | Identification of host and symbiont gene expression during the first hours to days of the association | 35,59,112 |
| NanoString (nCounter Analysis) | E. scolopes and V. fischeri | Resolution of multiple genes with as few as 10 juvenile animals | 59 |
| Shotgun proteomics | Light-organ exudate and haemocytes | Identification of host and symbiont proteins in exudate and first description of innate immune-related proteins from haemocytes | 86,113 |
| Quantitative proteomics (iTRAQ and spectral counting) | Haemocytes (symbiotic and cured) | Identified quantitative differences in protein abundance in haemocytes | 87 |
| 16S amplicon sequencing | ANG bacteria | Characterization of the bacterial community in ANG and associated eggs | 114 |
| Metagenomics | ANG | 16S rRNA gene diversity of the consortium | 115 |
| INSeq | V. fischeri | Discovery of colonization determinants | 116 |
| Gene editing | Euprymna spp. | Knockout of host genes | a |
| Cellular | |||
| Confocal microscopy | E. scolopes and V. fischeri | Live cell imaging of colonization; ultrastructure; cytochemistry; immunofluorescence | 19,22,23,28,41,84 |
| Transmission electron microscopy | E. scolopes and V. fischeri | Characterization of ultrastructure | 6,17,115 |
| HCR FISH | Light organ and V. fischeri | Cellular localization of gene expression in host and symbiont in time and space | 47 |
| In situ hybridization | Developing E. scolopes embryos | Allowed visualization of HOX gene expression in developing embryos | 96 |
| Imaging mass spectrometry | V. fischeri and host light organ | Imaging of small molecules in light organ | 117,118 |
| Physiological | |||
| Molecular networking | Bacteria residing in the ANG and eggs | Chemical identification and modelling of secondary metabolites | 119 |
| Model-enabled gene search | V. fischeri | Identification of metabolic function pathways | 120 |
| Metabolomics | E. scolopes | Identification of host metabolites | 78 |
| NanoSIMS | E. scolopes and V. fischeri | Transfer and localization of metabolites | 49 |
ANG, accessory nidamental gland; EST, expressed sequence tag; HCR FISH, hybridization chain reaction fluorescence in situ hybridization; INSeq, insertion sequencing; iTRAQ, isobaric tags for relative and absolute quantification; NanoSIMS, nano secondary ion mass spectrometry.
C. Albertin and J. Rosenthal, personal communication.
The winnowing and early development
Recruitment of the symbiont.
In the past 15 years, researchers studying the squid–vibrio symbiosis have learned that the winnowing, from the species-rich bacterioplankton to the exclusively colonizing V. fischeri cells, is a complex biomechanical and biochemical process. Long cilia on the outer surfaces of the juvenile light-organ ‘appendages’ beat metachronally, a behavior that focuses bacteria-sized particles and host-shed mucus to an area above the three pores on each side of the organ25 (Fig. 2). Along the inner surface and radiating out from the base of the appendages are short, randomly beating cilia whose motion may mix the rich array of host immunity factors, principally antimicrobials, that are present in the chitin-rich mucus, as well as factors that are exported from the aggregating V. fischeri cells25–31.
Under laboratory conditions similar to the natural environment, V. fischeri cells are recruited and these cells attach to the host cilia32 as aggregates, where they signal a change in host gene expression across the entire organ33; the number of aggregating V. fischeri cells varies with strain34. In the host, genes encoding antimicrobials are upregulated, as are those encoding a chitinase33 (Fig. 2). The data suggest that this activity has two priming functions in the mucus: to create a biochemical environment that selects for V. fischeri and prepares the bacterial cells for the increased antimicrobial environment of host tissues; and to breakdown the polymeric squid-generated chitin into chitobiose, which is the chemoattractant that draws would-be symbionts into host tissues35. Much study has gone into the characterization of a V. fischeri secreted exopolysaccharide and regulation of its production, which is essential for efficient colonization, as is the ability to chemotax normally (see Visick, Stabb and Ruby12).
The journey into host tissues and settling into a long-term symbiosis.
After pausing in the aggregates, V. fischeri cells then release from the superficial ciliated epithelium and move into one of six pores, through a duct, and into a broadened antechamber, the medial edge of which has a narrow bottleneck that leads to one of the six independent crypts13. On occasion more than one V. fischeri recruit can enter each crypt space. However, on average and irrespective of the inoculum size, a single V. fischeri cell, which has traversed the narrow bottleneck, enters each crypt space and grows clonally36,37. Through quorum signaling, luminescence is induced at about 12 h following inoculation of the seawater38. Analysis of the bottleneck region by confocal microscopy revealed that this is a highly dynamic region of the light organ. It undergoes a substantial constriction after the V. fischeri inoculum enters the crypts, which spatially restrict the symbiont populations over the day; during the daily venting of ~90% of the symbionts at dawn the bottleneck briefly reopens24.
Shortly after crypt colonization, the symbionts signal the cell-death mediated decommissioning of the superficial ciliated surface that has potentiated symbiont colonization20,39. This process is induced by V. fischeri microorganism-associated molecular patterns (MAMPs) that are derivatives of the cell envelope (Box 2), as well as by light production39,40. As the ciliated cells die, the activity of a matrix metalloproteinase and a cathepsin drives disruption of their basement membrane and their eventual detachment from neighboring cells29,41. The symbionts affect this morphogenesis remotely, that is, from inside the crypt spaces42. Haemocyte infiltration and changes in host gene expression are essential for this process43,44, which suggests that these blood-borne cells may be the messengers. Finally, the presence of the symbionts in the crypts shuts down mucus shedding from the ciliated surface19.
Box 2 |. Signalling and microorganism-associated molecular patterns.
Host-microorganism associations are often mediated through the recognition of microorganism-associated molecular patterns (MAMPs) by host pattern recognition receptors (PRRs). Many such molecular interactions occur in the squid-vibrio symbiosis. For example, peptidoglycan (PGN) and lipopolysaccharide (LPS) are required for initiating host mucus secretion and apoptosis, respectively, in the nascent light organ19,41. A key study demonstrated that a derivative of peptidoglycan (tracheal cytotoxin (TCT)) along with LPS trigger morphogenesis of the juvenile light organ42. Even though the structure of TCT is identical in the non-pathogenic symbiont Vibrio fischeri and the human pathogens Neisseria gonorrhoeae and Bordetefla pertussis, it does not lead to virulence in the squid and is instead a signalling MAMP that is part of normal developmental pathways. Other findings showed that modification of the lipid A portion of LPS in V. fischeri is important for signalling to the host 27,124. V. fischeri also releases LPS and outer membrane vesicles (OMVs) from flagella ‘50,51. These OMVs can trigger development of the juvenile light organ independently of TCT44. For the host, studies have revealed several PRRs along with a conserved nuclear factor-tcB (NF-tcB) signalling pathway86,110,125. These PRRs include five peptidoglycan recognition proteins (PGRPs), several Toll-like receptors (TLRs), LPS-binding proteins (LBPs), two galectins and four bactericidal permeability-increasing proteins (BPIs)26,28,80,81,86,87,110,125. Some of these PRRs are expressed in specific tissues and cell types and change localization after colonization. For example, PGRPl is present in the nuclei of host epithelial cells, but is lost in those cells later in development80. V. fischeri mutants that are defective in TCT release do not induce loss of PGRPl, suggesting that this MAMP can directly influence localization of this host PRR. PGRP2 is secreted into the light-organ crypt spaces and in mucus shed from the ciliated epithelium during initiation of the association28. Many of these PGRPs also have amidase activity28,86, thus potentially enabling these PRRs to regulate MAMPs.
The presence of the symbionts also affects the tissues along the pathway of colonization as well as those of the crypts. Both the duct and bottleneck constrict, and changes in gene expression occur along this migration path45. The epithelial cells lining the crypts, which interact directly with the symbionts, swell and increase the density of microvilli along their apical surfaces, a response that is also controlled by the MAMP lipopolysaccharide (LPS) 46. Within the crypts, the symbiont population is exposed to many of the same antimicrobials as those that mediate initial colonization26–31,33,47,48, which might shape or control the V. fischeri population.
Studies of the mechanisms of communication between host and symbiont have suggested both interactions at the symbiotic partner cell surfaces as well as uptake of symbiont products into host cells. A recent study that used quantitative ion microprobe imaging via Nano secondary ion mass spectrometry (NanoSIMS) showed that biomolecules from V. fischeri are taken up directly by the light-organ crypt epithelium, and become enriched in the euchromatin and nucleoli of these cells49. The delivery mechanism of symbiont products is either by diffusion of molecules from the symbiont to the host cells or by host-cell uptake of outer membrane vesicles (OMVs) produced and released by the bacteria37, 45. The ability of host cells to readily take up molecules generated by V. fischeri could be one mechanism by which symbiont signaling leads to some of the downstream cellular changes observed in the host. For example, a recent study demonstrated the abundant trafficking of a small RNA of V. fischeri, SsrA, into host cells50. This symbiont product profoundly affects the host’s ability to form a normal symbiosis. Continuing studies of the role of OMV cargo promise to provide a clear picture of transport of bacterial biomolecules into host cells. Finally, within 12 h after colonization, the resident symbionts also induce the onset of a profound daily rhythm in the host51 (Fig. 3; see below).
Figure 3: The diel rhythm of the host–symbiont association highlights major differences between juvenile and adult stages.

A. Within the first four weeks after hatching, the host transitions to a fully nocturnal active period with a functional symbiosis that allows for the camouflage behavior (counterillumination) and a diurnal quiescent period where the host buries in the sand. Symbiont density is also regulated on a diel cycle where 95% of the symbionts are expelled each morning at dawn followed by a period of regrowth so that the host has a full complement of V. fischeri to engage in counterillumination via bioluminescence at night. b. Host and symbiont gene expression are regulated as part of these critical diel and circadian rhythms that drive the cell biology and biochemistry of the light to facilitate the production of light when the host is active at night. The symbiont diel population and corresponding luminescence rhythms are established early in the association as are some critical host pathways including changes in microvillar density, and genes that help regulate circadian rhythms and immunity. By four weeks major cellular and physiological shifts occur in the light organ over the day/night cycle as indicated by changes in genes/proteins involved with osmolarity, oxidative stress, actin rearrangement, circadian rhythms, and haemocyte trafficking. This haemocyte migration into the crypts at night helps deliver chitin to the symbionts; a process involved with the symbiont undergoing a major metabolic shift, using the anaerobic respiration of glycerol phosphate during the day and fermentation of host-derived chitin at night that helps facilitate effective luminescence by acidifying the crypts and releasing oxygen from bound haemocyanin.
In the absence of the symbiont.
Most of the above-mentioned host immune and developmental phenotypes are defective in animals colonized by a non-bioluminescent (dark) mutant of V. fischeri, reflecting the fact that light production is the principal commodity that the host derives from the symbiont52. Morphological and molecular studies of the light organ have demonstrated that it shows remarkable convergence with the eye, including luminescence perception with the same gene products (for example, opsin, retinochrome and visual G proteins) that control environmental light perception in the retina 53–56 (see also, description of findings from sequencing of the host genome below). Further, a recent study57 that described the influence of light-organ colonization on the gene expression of remote tissues revealed that symbiosis induces changes in the transcriptome of the eye; studies with symbionts defective in light production showed that all changes in gene expression in the eye are dependent on the production of symbiont luminescence in the light organ. This correlation perhaps reflects the critical coordination between the eye and light organ that mediates effective counterillumination, the antipredatory behavior of the host57.
Finally, it should be noted that the vast majority of work on the early stages of light organ symbiosis has used a single strain, V. fischeri ES114, as the colonizing symbiont. Recent studies have shown that symbiont strain variation can affect the processes of initiation34,58; therefore, like many other facets of the system, studying strain variation offers new opportunities (see Visick, Stabb and Ruby12).
Symbiosis maintenance
The dynamic daily rhythms.
Over the past decade, it has become clear that microorganisms have a profound influence on animal daily rhythms that can affect everything from metabolism to immune response59,60. The squid–vibrio system has been one of the principal experimental models for the exploration of this phenomenon (for example, see Refs. 61,62). Much of the work on the squid–vibrio system over the past three decades focused on the characterization of the early events of symbiosis, and the long-term maintenance of the association remained poorly understood. However, during the past 10 years, research on the system has demonstrated that one principal component of maintenance is the development of a profound daily rhythm of the association that begins with the onset of the symbiosis38 and continues throughout the life of the host62 (Fig 3). This daily rhythm has two components, a diel feature (requiring a light cue each day for host response) and a circadian portion (a recurring endogenous cycling). In the former, the animal expels much of the crypt contents each day in response to the dawn light cue63,64, including 70 – 95% of the symbiont population. As mentioned above, this daily expulsion is concurrent with a widening of the bottleneck that helps facilitate movement of the symbionts out of the crypts and into the antechamber and ducts in preparation for venting; the bottleneck then constricts again within 2 h after venting24, confining the renewed cohort of symbionts that repopulate the crypts. In addition to the expelled symbionts, the vented exudate contains host cell debris and haemocytes that were present in the matrix that surrounds the bacterial cells within the crypts64. The later circadian component is reflected in cycling of behaviors and associated gene expression that occurs in anticipation of the light and dark cues62. This daily venting has at least three purposes: seeding the environment with viable V. fischeri that are symbiosis-competent and can colonize the next generation of bobtail squid65,66; providing a way to renew the otherwise non-growing crypt population62; and perhaps providing a mechanism to sanction or remove underperforming or cheating symbionts, such as dark mutants that do not produce light67,68. Early observations of these phenomena prompted in-depth studies that have characterized aspects of the molecular, cellular and physiological events occurring over the day–night cycle of the symbiosis.
Setting the early rhythms.
In the juvenile host, the daily rhythm is simpler than that of the mature adult animals. These two life stages share the dawn venting, which in both is accompanied by an effacement of the microvilli from the apical surfaces of the crypt epithelial cells. Over the following hours, the apical surfaces of these cells repolarize and the bacterial cells that remain behind after venting proliferate to fill the crypt spaces. Earlier studies of the crypt epithelia in juveniles showed that symbiosis induces an increase in microvillar density21. Microscopy analyses of the juvenile light organ over the course of this daily rhythm demonstrated that crypt epithelia of both apo- and symbiotic juveniles efface (effacement occurs in response to the light cue at dawn and not colonization)46. Whereas the microvilli of aposymbiotic animals repolarize to the same density, the presence of the symbionts induces an increase in microvillar density with each day, such that, by day 4, the bacterial cells are surrounded by host microvilli21, increasing the surface area between host and symbiont cells. Recent experimental studies of this phenomenon demonstrated that the symbiont MAMP LPS is responsible for this increase in microvilli46. Also, over the day–night cycle in both juvenile and adult animals, the expression of the host cry gene cycles51. The cry genes encode a blue-light receptor protein that is a component of the molecular clock of animal circadian rhythms, and typically their transcription is highest at peak ambient light regimes. The light-organ cry isoform, escry1, cycles with the same timing as symbiont luminescence, peaking in the evening hours. This finding was consistent with early work on the system that showed that per-cell luminescence of symbionts in the organ cycles out of phase with environmental light; that is, it is brightest at night when the animal requires symbiont luminescence for its behaviors69. Experiments with juvenile animals demonstrated that the presence of light-producing symbionts is essential for escry1 cycling51.
In juvenile and/or adult light organs, a set of antimicrobial genes are also regulated on the day–night cycle, for example, those encoding PGRP2 (peptidoglycan recognition protein 2)28, AP (alkaline phosphatase)27, galaxin31, cathepsin L29, complement protein C3 (Ref. 62), and the squid’s blood pigment haemocyanin, which has a carboxy terminal peptide with antimicrobial activity30.
Two of these proteins, PGRP2 and AP, which detoxify the peptidoglycan monomer (also called ‘tracheal cytotoxin’ (TCT)) and LPS of the symbionts, respectively, are not detectable in the early hours of the symbiosis. These bacterial ‘toxins’ (Box 2) are the agents mediating the induction of light-organ morphogenesis, which is irreversibly triggered at 12 h, so their detoxification before that point would compromise the normal symbiosis-induced developmental program. Thereafter, the genes that encode these proteins cycle over the day, and the proteins can always be detected in the light-organ crypt spaces. However, they are not always active; in the adult, but not juvenile animals, the crypt spaces change pH over the day–night cycle61. The pH optima of both PGRP2 and AP are at ~8 and, whereas the adult host cells would be protected by these proteins during the day when the pH is near neutral, the crypt acidifies in the late night, which would diminish the activity of PGRP2 and AP in the hours preceding the dawn light cue for venting, rendering the host cells susceptible to perturbation by the intact MAMPs.
Maturation of the rhythms.
Daily rhythms associated with the symbiosis are more pronounced and complex in the adult, in which they were first discovered in a study of the daily patterns of gene expression62. This analysis showed that both the host and the symbiont transcriptomes fluctuate, an oscillation that is reflected in a cellular restructuring of the host crypt epithelia, which is much more extensive in the adults; and a change in symbiont metabolism over the day–night cycle. These studies characterized the ʻcentral coreʻ of the light organ, which comprises both the symbiont cells and the epithelial tissues that support them, at four time points over the day: 0400 h, when the light organ crypts are full of symbionts (just before the dawn expulsion); 1000 h, when the animal is quiescent and the remaining symbiont cells grow to repopulate the crypts; 1600 h, in the hours before the host emerges from the sand to use symbiont bioluminescence during foraging; and 2200 h, when animal activity and symbiont luminescence are at their peak levels. These data revealed that gene expression changes in the host are most pronounced before and after dawn, around the time of the above-mentioned restructuring and repolarization that is associated with expulsion of the symbionts. Among the host genes regulated are all those recognized as encoding cytoskeletal proteins; at 0400 h, expression of these genes is up relative to 2200 h and, by the 1000 h, expression is down relative to 0400 h. Concomitant ultrastructural analyses revealed that animals at 0400 h have highly disrupted epithelial cells, i.e., the microvilli are effaced from the apical surfaces, the tight junctions are absent or abbreviated, and portions of the cells have blebbed into the crypt spaces; by 1000 h, the cells repolarize. Given that effacement is triggered by a light cue in juvenile animals46, the increase in expression of cytoskeletal genes in adults at 0400 h may be anticipatory to the venting event at dawn.
Regulated similarly to the host cytoskeletal genes are several genes encoding antimicrobial proteins, which may be protecting the animal from invasion of other tissues by V. fischeri when the integrity of the crypt epithelia is compromised. Large changes in symbiont gene expression follow those of the host. Bacterial activity over the day–night cycle has consequences for the host. Notably, as mentioned, the crypts acidify in the hours before venting. Haemocytes from the host circulatory system migrate into the light organ where a certain number of these cells lyse, releasing chitin stores that the symbionts metabolize by fermentation33. A recent study demonstrated that movement of the haemocytes into the crypts is regulated by a cytokine, macrophage migration inhibitory factor (MIF)70. This immune protein is abundant in the symbiotic tissues during the day, perhaps to deter migration of the haemocytes into those regions, and is in low abundance at night, enabling haemocytes to traffic into the crypts to release their chitin stores. As such this cytokine is controlling the provision of nutrients to the bacterial symbionts. Further, the major oxygen-binding respiratory protein of the squid, haemocyanin, traffics into the crypt spaces where it would offload its oxygen more readily under the acidic conditions, thereby providing the symbionts the oxygen that is critical for the luminescence reaction30. After the effacement of the host cells, the symbionts increase expression of the genes associated with anaerobic respiration of glycerol phosphate51, a pH neutral process, and the crypt loses its acidic nature. The data support the model that the bacteria are using membranes of the host animal for their regrowth in the crypts, as the membrane signature of the symbionts in the crypts includes fatty acids only produced by the host animal51. Once the membranes are depleted by symbiont growth and the host cells repolarize, the genes associated with use of membranes for nutrition decrease in expression. Then, the symbionts express the genes associated with chitin fermentation, an acidifying metabolism, which suggests that the host is supplying the symbionts with this polymer as the subsequent nutrient source.
Beyond the daily rhythm
Advances in husbandry.
Characterization of long-term development has been facilitated by major strides in husbandry efforts to raise E. scolopes reliably to sexual maturity15,71,72. These advances have enabled researchers to move beyond either the first 4–5 days when the light organ association is experimentally initiated, or the use of field-caught adults, and ask: how symbiosis influences long-term development of the host; and what are the mechanisms by which a stable association is maintained. Although the presence or absence of accessory tissues (i.e., lens, reflector, and filters) does not seem to depend on light-organ colonization, a number of physiological, biochemical and molecular changes occur concomitantly with a transition of the animal from the juvenile to mature state3,15,57,73. One of the most obvious transitions is in host behavior; after four weeks, the animals transition between two distinct patterns: an active nocturnal period and a quiescent diurnal period15,71. The ability to raise the animals has also enabled the study of the window of colonization.
Although in the environment colonization is likely to occur within hours after hatching, in the laboratory setting, the host can be receptive to colonization by V. fischeri for at least four weeks after hatching. Earlier studies using symbiotic juveniles found that if colonized hosts are treated with antibiotics within five days to remove the symbionts, then the light organ can be recolonized19,20. However, if the light organ is colonized by the symbiont for at least 5 days followed by curing, the host enters a refractory state and subsequent colonization does not occur15 normally. This refractory period seems to be refined to restrict further colonization by other V. fischeri cells. By contrast, animals that are colonized for up to 10 days by a dark V. fischeri mutant that is defective in light production (Δlux) can be recolonized, which suggests that the host has the ability to restrict strains that are under-producing the currency of the association (that is, light). Morphologically, the superficial ciliated epithelium of aposymbiotic animals (Fig. 1) persists for up to a month but undergoes different degrees of regression, perhaps due to constant exposure to low levels of exogenous MAMPs15,73 produced by the nonsymbiotic bacterioplankton. Despite a loss of the ciliated epithelium, aposymbiotic animals can generally be colonized within the first month with high densities (>5000 cells/ml) of V. fischeri in the environment. Taken together, work with juvenile and adult hosts suggests that, although the superficial ciliated epithelium facilitates colonization, it is not essential for the colonization process.
Other morphological developmental events include a change in pore number on the surface of the light organ73. Aposymbiotic animals maintain three independent pores on the surface, whereas the pores of symbiotic animals coalesce into a single pore by four weeks. Animals colonized with a V. fischeri Δlux mutant show an intermediate morphology73. Analysis from this same study concluded that, compared with symbiotic animals, the average length and density of cilia associated with the superficial epithelium is greater in animal raised aposymbiotically. A comparison of 4-week old aposymbiotic or wild-type colonized animals, with animals colonized by the Δlux strain, also revealed that the epithelial cells of the light organ crypts change from the columnar morphology characteristic of aposymbiotic or Δlux-colonized animals, to cuboidal in wild-type colonized animals73. These observations are consistent with work in juvenile hosts that showed an increase in crypt epithelial cell volume and microvillar density within the first 24 h after colonization21. Therefore, these early symbiont-induced phenotypes persist from the juvenile into the adult stages.
A comparison of light organ gene expression among aposymbiotic, wild-type colonized and Δlux-colonized animals after 4 weeks supports the morphological changes noted above73. For example, the expression of genes associated with osmotic regulation and fluid uptake is increased in symbiotic animals, consistent with the observation of an increase in crypt-cell swelling after colonization. Genes associated with signaling are overrepresented in symbiotic animals, whereas those associated with a response to oxidative stress remained unchanged or were poorly expressed. Common immune pathways also seemed unchanged between aposymbiotic and symbiotic animals after 4 weeks. Together, these data suggest that the host undergoes a transition that facilitates a persistent accommodation of the symbiont albeit dynamic on a daily basis.
Light organ colonization influences systemic host gene expression.
The long-term effects of light-organ colonization also influence gene expression in remote tissues, specifically the eyes and gills57.The influence of microbiomes on remote tissues is currently a highly active area of research; for example, the gut–brain axis in mammals74. In the squid–vibrio association, the eye was chosen as a study subject because it shows a remarkable convergence with the light organ, from morphology to biochemistry53–55,75, presumably because both organ types function to modulate light. By contrast, the gill tissue was selected for comparison because it has an immune function in squids and their close relatives57, and responds to bacterial infection. Experiments with the Δlux mutant of V. fischeri showed that most of the regulated light-organ genes respond to symbiont light production rather than the presence of the symbiont itself. Both eye and gill tissue also exhibit changes in gene expression as a result of light-organ colonization, but almost no overlap was observed in responsive genes among the three organs. Amazingly, the eye lost its entire response to symbiosis when the host was colonized by a dark mutant of V. fischeri, demonstrating that the response of the eye is exclusively due to the symbiont’s bioluminescence. By contrast, the gene expression patterns in the gill in response to colonization was the same in the wild-type or Δlux V. fischeri, demonstrating that the gill responds to the presence of bacteria in the light organ, rather than the symbiont’s light production. Change in many of the genes that are regulated on a daily rhythm persists as the host matures from juvenile to adult57. A recent study that analyzed the metabolome [G] of adult hemolymph showed that light-organ colonization influenced approximately 25% of the metabolites present in the blood of adult males, and that the levels of these molecules fluctuated on a diel cycle76. Future studies will help to uncover the systemic effects of bacterial colonization on an animal host.
The effect of symbiosis on innate immunity
Symbiosis with microorganisms can influence a number of developmental pathways in eukaryotes, including that of the immune system. The ability to either maintain animals aposymbiotic or use antibiotics to cure the host has revealed a number of ways in which V. fischeri influences both cellular and acellular components of the hosts immune system during development of the association. In particular, symbiosis influences the production of reactive oxygen and nitrogen species48,77, components of the complement pathway47, antimicrobial proteins like haemocyanin30, peptidoglycan recognition proteins28,78, and proteases and LPS-binding proteins79.
One other advantage of E. scolopes as a model host is that it relies solely on an innate immune system, and that it has only one type of circulating immune cell, a macrophage-like haemocyte (Box 1). Therefore, understanding the influence of symbiosis on cellular immunity is not confounded by responses of an adaptive immune system or of multiple blood cell types. Like other haemocytes of invertebrates, these cells have macrophage-like properties and are capable of binding and phagocytosing bacteria and other microorganisms80–82. Because bobtail squid have a closed circulatory system, and the light organ is highly vascularized, haemocytes can traverse through the surrounding superficial epithelia and enter the crypt spaces in both juveniles and adults43,64.
Several studies have focused on the ability of haemocytes to differentiate between V. fischeri and other non-symbiotic species with regard to binding and phagocytosis. Immediately after hatching, juvenile haemocytes display differential phagocytosis between V. fischeri and non-symbiotic Vibrio harveyi83. Despite binding to both bacterial species equally, only V. harveyi is engulfed. The ability of haemocytes to bind bacteria increases over the first 4 days after hatching independent of the colonization state of the light organ83. However, as the host ages, the haemocytes seem to undergo maturation, leading to differential binding and phagocytosis depending on light-organ colonization state. Haemocytes from adult animals bind fewer cells of V. fischeri compared to non-symbiotic V. harveyi and Photobacterium leiognathi 82. Although all bound bacteria are also phagocytosed haemocytes from aposymbiotically raised adult animals bind more, but phagocytose substantially fewer V. fischeri cells; thus, the haemocyte response of hosts raised without the symbiont more resembles that of juvenile squid83. However, symbiotically raised and wild-caught animals bind fewer V. fischeri, yet phagocytose more bacteria overall83. Further, curing the adult light organ with antibiotics alters the binding of haemocytes to V. fischeri, which leads to an increase in binding and phagocytosis to a level similar to that of V. harveyi 82. Taken together, these juvenile and adult studies suggest that initial haemocyte recognition of the symbiont occurs principally at the level of phagocytosis, whereas haemocyte-binding dynamics are more important later in development.
The mechanisms behind this immune maturation are unknown, but transcriptomic and proteomic studies of haemocytes from symbiotic and cured hosts have revealed potential targets that may be involved in the recognition of bacteria, including pattern recognition receptors (PRRs) such as peptidoglycan recognition protein (PGRP5) and galectins84–86. In cephalopods haemocytes develop in the white body, and gene expression analysis in a related sepiolid squid species supports the notion that this organ is an active site for hematopoiesis87. Future analyses will focus on whether colonization of the light organ by V. fischeri influences hematopoiesis remotely, leading to downstream effects on haemocyte binding and phagocytosis. V. fischeri is likely to also exhibit mechanisms to actively avoid haemocyte recognition, as a strain with a mutation in an outer membrane porin (OmpU)-encoding gene binds statistically significantly more to haemocytes, and in a manner similar to the non-symbiotic species V. harveyi and P. leiognathi 82.
Conclusions and future directions
The squid–vibrio symbiosis is one of a growing number of model associations that have revealed general principles to help inform basic biology and biomedicine about the form and function of host–microorganism interactions (reviewed in Ref. 14). The use of models has been essential for the study of highly complex features of animals and plants, such as developmental and neurobiology, revealing patterns of evolutionary conservation and diversification. Every model association brings its unique strengths to microbiome [G] research. As human symbioses involve complex consortia, highly valuable to biomedicine are studies of both simple and complex consortia, typically in invertebrates and vertebrates, respectively. However, studying the role of all partners in a natural, coevolved system is currently only possible with binary or simple consortial symbioses. The oldest and best understood of the binary relationships is the symbiosis between leguminous plants and rhizobia bacteria, which have provided foundational knowledge for the field88.
From the outset of research on the squid–vibrio system, the highly conserved nature of interkingdom interactions was demonstrated with comparisons drawn between the squid–bacteria and legume–bacteria symbioses89. For decades the legume–rhizobia symbioses provided the only genetic model for studying symbiotic associations. Similar to the squid–vibrio system, genetics was only available in the bacterial partners for the early studies of plant associations. However, in the past 20 years, genetics have been developed in the host plant, rendering these symbioses the only available models with high quality genetics in all partners90. Development of genetics is on the horizon for the squid host, which promises to provide an analogous system and strong complement to the legume–rhizobia associations. Nonetheless, with genetics largely restricted to the microbial partner, the squid–vibrio symbiosis has provided valuable insights into the basic nature of animal symbioses, helping to broaden our conceptual framework of the field. Particularly valuable for biomedicine, the squid–vibrio system offers the opportunity to explore the interaction of extracellular Gram-negative bacteria with epithelial surfaces, the principal type of host–microorganism relationship in humans91. For example, work from the squid–vibrio association demonstrated the importance of MAMPs in signaling to animal hosts, a phenomenon that is critically important in both mutualistic and pathogenic associations40 (Box 2). In this benchmark paper, the authors changed the lexicon from ‘pathogen-associated…’ to ‘microorganism-associated molecular patterns’ (that is, from ‘PAMPs’ to ‘MAMPs’), with the recognition that these molecules are general symbiont cues of host–bacteria communication, independent of the type of association. Similarly, the squid–vibrio system has also been at the forefront of studies of symbiont-driven daily rhythms, a phenomenon that recent studies have shown are also important in humans and other mammals92. As presented in the companion review12, many discoveries of V. fischeri have also contributed to our general understanding of the biomedical ramifications of animal–bacteria associations. Beyond the field of symbiosis, E. scolopes and related sepiolid species are emerging model organisms for the study of other areas of biology, including cephalopod genomics56,93, the evolution of developmental mechanisms94–97 and behavior98–101.
Over the past thirty years, the squid–vibrio association has revealed mechanisms by which environmentally transmitted symbioses are established and maintained. The experimental tractability of the association and the ability to manipulate the partners has helped elucidate the intricate molecular conversation between the partners. One exciting new area of research in E. scolopes is the study of a second symbiosis; a bacterial consortium found in the accessory nidamental gland (ANG) of female hosts (Box 3). Although the mechanisms of colonization in the ANG association are not yet as well understood as those of the light organ, the presence of two experimentally tractable symbiotic systems in the same genome-defined host now enables the identification and comparison of common and divergent mechanisms of colonization and maintenance in both binary and consortial associations. New advancements in ‘-omics’, microscopy and animal husbandry have also moved this field forward, facilitating a greater understanding of the specific features of these associations (Table 1).
Box 3 |. The symbiosis of the accessory nidamental gland.
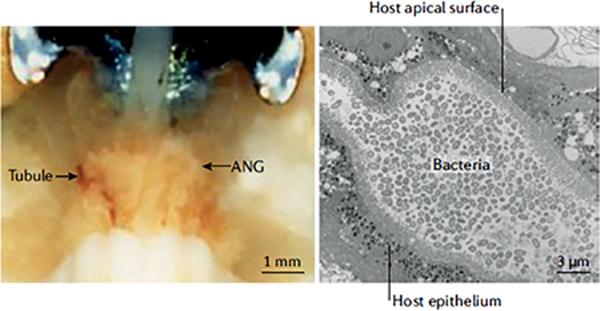
Although for more than 30 years research on symbiosis in Euprymna scolopes has focused principally on the binary light-organ partnership, a second association involving a bacterial consortium and the accessory nidamental gland (ANG) of female animals is now being intensely investigated. The ANG is an organ common to several cephalopods (see the figure, left panel), and comprises epithelium- lined tubules that, in contrast to the light organ, harbour a low complexity consortium (see the figure, right panel). In E. scoiopes, this consortium is dominated by culturable roseobacters (Alphaproteobacteria), as yet uncultivated Verrucomicrobia and some culturable Flavobacteriia and Gammaproteobacteria species114,115,126“. Approximately 50 core operational taxonomic units (OTUs) belonging to these taxa have been described from wild-caught populations off E. scoiopes114“, representing approximately 80% of the ANG community. Each tubule of the ANG is dominated by a specific bacterial taxon115, but the nature of this segregation is not understood. It may suggest that specific tubule microenvironments promote the growth and selection of d ifferent types of symbiont, or there are founder effects (that is, the microorganism that colonizes the space first proliferates and remains the dominant competitive species in that space). Haemocytes also traffic into the ANG tubules, although whether they have a similar role as in the light organ has not been determined115. As with the light-organ symbiosis, the ANG symbionts are environmentally transmitted at each host generation114,127. The cephalopod ANG develops approximately 1–2 months after hatching and is poised to recruit bacteria from the environment127”. Whether, as compared with the light-organ association, E. scoiopes uses similar mechanisms to ensure specificity of ANG colonization remains to be determined.
During oviposition, bacteria from the ANG are transmitted directly into an egg jelly coat that surrounds the embryo. Full genome sequences have been acquired for several of the ANG symbionts106-109, which affords the opportunity to analyse symbiont genomics, metagenomics and metatranscriptomics of both the ANG bacteria and the colonized eggs. The resulting data have provided insights into the functional features of the association centred on the symbionts providing defence against pathogens and biofouling microorganisms during embryogenesis. Bacteria isolated from the ANG and/or eggs of cephalopods exhibit antibacterial activity in vitro, and antimicrobial compounds have been described from these strains106,107,128. A landmark study in E. scoiopes showed that eggs that are cured of their symbionts through antibiotic treatment are more susceptible to fungal infections119. Bacterial isolates and their organic extracts from the ANG and eggs inhibit these fungi, and secondary metabolites were identified that may have a role in egg defence119. Mechanisms to prevent biofouling and/or pathogenesis in externally laid eggs have been described in other aquatic systems129–131, and the ANG association in E. scoiopes provides an emerging model to study the influences of microbiota in defensive symbioses.
Finally, a study of the whole genome of E. scoiopes provides insight into cephalopod evolution and how symbiosis drives the evolution of organs that house microbiota58. These analyses show that the light organ and ANG are likely to have evolved via different mechanisms. Analysis of the host genome and light-organ transcripts supports findings of previous studies that light-organ physiology and biochemistry are most similar to the eye54. These results, along with previous studies54,55,57,100, suggest that subfunctionalization of genes expressed in the eye had an important role in the evolution of the light organ. Gene expression analysis of tissues from adult hosts showed that the ANG has a high number of E. scolopes-specific transcripts (>35%). Analysis of the genomic regions surrounding these ‘orphan’ genes showed a greater proportion of repetitive-element content compared with genes expressed in other tissues, which suggests a high evolutionary turnover in genomic regulatory regions associated with the ANG. Expression of unique or taxonomically restricted orphan genes is hypothesized to contribute to genetic novelty in other animal groups132,133. The E. scoiopes genome and patterns of the ANG transcriptome support this hypothesis, and future genomic and transcriptomic data sets from additional ANG-containing cephalopods will enable broader comparative approaches that will inform a better understanding of the evolution of this organ. Left panel reprinted with permission from REF.66, University of Chicago Press; right panel reprinted from REF.134, Springer Nature Limited.
These advancements and other emerging techniques present exciting opportunities to expand the use of the Hawaiian bobtail squid as a model organism in several areas of biology and medicine. For the study of both the light organ and ANG associations, the recent completion of the host genome sequence offers exciting avenues for research and tool development in E. scolopes56. This key advance provides the opportunity to define how the blueprint of the animal host shapes and has been shaped by its symbiotic associations. For example, it will now be possible to study gene regulation in females and males in the presence and absence, respectively, of the ANG consortium. Also, the development of CRISPR-Cas9 technology for genetic manipulation of cephalopods is currently underway102, including in Euprymna spp.(Table 1); such an advance will enable researchers to interrogate the function of candidate genes that have been identified by studying natural variation in the host. In addition, many emerging tools are available for the study of the light organ symbiont, V. fischeri (see Visick, Stabb and Ruby12).
Acknowledgements
The authors thank E. Ruby, K. Visick and E. Stabb for helpful comments about the manuscript. Work in the authors’ laboratories has been supported by the National Science Foundation NSF IOS 1557914 to S.V.N.; National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases R37 AI50661 to M.M.-N.; NIH, Office of the Director, R01 OD11024 and GM135254 (M.M.-N.); and National Science Foundation, Integrated NSF Support Promoting Interdisciplinary Research and Education INSPIRE Grant MCB1608744 (to M.M.-N.).
Glossary:
- Mutualism
The fitness of both symbiotic partners is enhanced by the association
- Commensalism
The fitness of one symbiotic partner is enhanced and the other is unaffected
- Parasitism (or pathogenesis)
The fitness of one partner is enhanced and the other is diminished
- Microbiota
Often refers to the group of microorganisms found in a specific habitat, such as a host or biofilm
- Multi-omics
multiple analysis approaches applied to a biological system, e.g., genomics, transcriptomics, proteomics, and metabolomics
- Metabolome
Collective metabolites associated with a given organism(s)
- Microbiome
Often used interchangeably with the term microbiota. However, some researchers use ‘‘microbiome’ to refer to the collective genomes of the microbiota
- Holobiont (also known as metaorganism)
Refers to a host and all of its associated partners
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Microbiology thanks T. Bosch, who co-reviewed with C. Giez, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.de Bary A De la symbiose. Rev Int Sci 3, 301–309 (1879). [Google Scholar]
- 2.Casadevall A & Pirofski LA Ditch the term pathogen. Nature 516, 165–166 (2014). [DOI] [PubMed] [Google Scholar]
- 3.McFall-Ngai M et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. U. S. A. 110, 3229–36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruby EG Symbiotic conversations are revealed under genetic interrogation. Nature Reviews Microbiology 6, 752–762 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas AE Simple animal models for microbiome research. Nat. Rev. Microbiol. 12, 764–775 (2019). [DOI] [PubMed] [Google Scholar]
- 6.McFall-Ngai MJ & Ruby EG Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial mutualism. Science 254, 1491–1494 (1991). [DOI] [PubMed] [Google Scholar]
- 7.Jones BW & Nishiguchi MK Counterillumination in the Hawaiian bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda). Mar. Biol. 144, 1151–1155 (2004). [Google Scholar]
- 8.McFall-Ngai M & Montgomery MK The Anatomy and Morphology of the Adult Bacterial Light Organ of Euprymna scolopes Berry (Cephalopoda:Sepiolidae). Biol. Bull. 179, 332–339 (1990). [DOI] [PubMed] [Google Scholar]
- 9.Montgomery MK & McFall-Ngai M Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development 120, 1719–1729 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Lee KH & Ruby EG Detection of the light organ symbiont, Vibrio fischeri, in Hawaiian seawater by using lux gene probes. Appl. Environ. Microbiol. 58, 942–947 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyholm SV & McFall-Ngai MJ The winnowing: establishing the squid-vibrio symbiosis. Nat. Rev. Microbiol. 2, 632–642 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Visick Karen L., Stabb Eric V., Ruby Edward, R. A lasting symbiosis: how Vibrio fischeri finds a squid partner and persists within its natural host. Nat. Rev. Microbiol. (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McFall-Ngai MJ The Importance of Microbes in Animal Development: Lessons from the Squid-Vibrio Symbiosis. Annu. Rev. Microbiol. 68, 177–194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFall-Ngai M & Bosch TCG Animal development in the microbial world: The power of experimental model systems. Curr. Top. Dev. Biol. (2020). doi: 10.1016/bs.ctdb.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koch EJ, Miyashiro T, McFall-Ngai MJ & Ruby EG Features governing symbiont persistence in the squid-vibrio association. Mol. Ecol. 23, 1624–1634 (2014). Ground-breaking study that helped establish husbandry protocols to study the effects of persistent colonization and biolminescence in the development of the light organ.
- 16. Kerwin AH et al. Shielding the next generation: Symbiotic bacteria from a reproductive organ protect bobtail squid eggs from fungal fouling. MBio 10, (2019). Described for the first time a functional role involving antifungal egg defense for the cephalopod accessory nidamental gland.
- 17.Ruby EG & Asato LM Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 159, 160–167 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Mandel MJ, Wollenberg MS, Stabb EV, Visick KL & Ruby EG A single regulatory gene is sufficient to alter bacterial host range. Nature 458, 215–218 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyholm SV, Deplancke B, Gaskins HR, Apicella MA & McFall-Ngai MJ Roles of Vibrio fischeri and nonsymbiotic bacteria in the dynamics of mucus secretion during symbiont colonization of the Euprymna scolopes light organ. Appl. Environ. Microbiol. 68, 5113–5122 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doino JA & McFall-Ngai MJ A transient exposure to symbiosis-competent bacteria induces light organ morphogenesis in the host squid. Biol. Bull. 182, 4578–86 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Lamarcq LH & McFall-Ngai MJ Induction of a gradual, reversible morphogenesis of its host’s epithelial brush border by Vibrio fischeri. Infect. Immun. 66, 777–785 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyholm SV, Stabb EV, Ruby EG & McFall-Ngai MJ Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. U. S. A. 97, 10231–5 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sycuro LK, Ruby EG & McFall-Ngai M Confocal microscopy of the light organ crypts in juvenile Euprymna scolopes reveals their morphological complexity and dynamic function in symbiosis. J. Morphol. 267, 555–568 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Essock-Burns T et al. Interactions of symbiotic partners drive the development of a complex biogeography in the squid-vibrio symbiosis. MBio 11, e00853–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nawroth JC et al. Motile cilia create fluid-mechanical microhabitats for the active recruitment of the host microbiome. Proc. Natl. Acad. Sci. U. S. A. 114, 9510–9516 (2017). Demonstrated that the ciliated surfaces of the nascent light organ create a fluid-mechanical microhabitat that helps select for V. fischeri during light organ colonization.
- 26.Chen F et al. Bactericidal permeability-increasing proteins shape host-microbe interactions. MBio 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rader BA, Kremer N, Apicella MA, Goldman WE & McFall-Ngai MJ Modulation of symbiont lipid a signaling by host alkaline phosphatases in the squid-vibrio symbiosis. MBio 3, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troll JV et al. Taming the symbiont for coexistence: a host PGRP neutralizes a bacterial symbiont toxin. Environ. Microbiol. 12, 2190–203 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyer SM, Kremer N & McFall-Ngai MJ Involvement of a host Cathepsin L in symbiont-induced cell death. Microbiologyopen 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kremer N et al. The dual nature of haemocyanin in the establishment and persistence of the squid-vibrio symbiosis. Proc. Biol. Sci. 281, 20140504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heath-Heckman EAC et al. Shaping the microenvironment: Evidence for the influence of a host galaxin on symbiont acquisition and maintenance in the squid-vibrio symbiosis. Environ. Microbiol. (2014). doi: 10.1111/1462-2920.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altura MA et al. The first engagement of partners in the Euprymna scolopes-Vibrio fischeri symbiosis is a two-step process initiated by a few environmental symbiont cells. Environ. Microbiol. 15, 2937–2950 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kremer N et al. Initial symbiont contact orchestrates host-organ-wide transcriptional changes that prime tissue colonization. Cell Host Microbe 14, 183–194 (2013). Demonstrated that initial contact with only a few V. fischeri cells leads to changes in host gene expression that promotes light organ colonization.
- 34.Koehler S et al. The model squid–vibrio symbiosis provides a window into the impact of strain- and species-level differences during the initial stages of symbiont engagement. Environ. Microbiol. 21, 3269–3283 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandel MJ et al. Squid-derived chitin oligosaccharides are a chemotactic signal during colonization by Vibrio fischeri. Appl. Environ. Microbiol. 78, 4620–4626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bongrand C et al. A genomic comparison of 13 symbiotic Vibrio fischeri isolates from the perspective of their host source and colonization behavior. ISME J. 10, 2907–2917 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wollenberg MS & Ruby EG Population structure of Vibrio fischeri within the light organs of euprymna scolopes squid from two oahu (Hawaii) populations. Appl. Environ. Microbiol. (2009). doi: 10.1128/AEM.01792-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boettcher KJ, Ruby EG & McFall-Ngai MJ Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J. Comp. Physiol. - A Sensory, Neural, Behav. Physiol. (1996). doi: 10.1007/BF00193435 [DOI] [Google Scholar]
- 39.Foster JS, Apicella MA & McFall-Ngai MJ Vibrio fischeri lipopolysaccharide induces developmental apoptosis, but not complete morphogenesis, of the Euprymna scolopes symbiotic light organ. Dev. Biol. 226, 242–54 (2000). [DOI] [PubMed] [Google Scholar]
- 40. Koropatnick TA et al. Microbial factor-mediated development in a host-bacterial mutualism. Science 306, 1186–1188 (2004). Showed that morphogenisis of the light organ in E. scolopes is caused by microbe-associated molecular patterns (MAMPs) from V. fischeri, including tracheal cytotoxin.
- 41.Koropatnick T, Goodson MS, Heath-Heckman EAC & McFall-Ngai M Identifying the cellular mechanisms of symbiont-induced epithelial morphogenesis in the squid-vibrio association. Biol. Bull. 226, 56–68 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aschtgen MS, Wetzel K, Goldman W, Mcfall-Ngai M & Ruby E Vibrio fischeri-derived outer membrane vesicles trigger host development. Cell. Microbiol. 18, 488–499 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koropatnick TA, Kimbell JR & McFall-Ngai MJ Responses of host hemocytes during the initiation of the squid-vibrio symbiosis. Biol. Bull. 212, 29–39 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Kimbell JR & McFall-Ngai MJ Symbiont-Induced Changes in Host Actin during the Onset of a Beneficial Animal-Bacterial Association. Appl. Environ. Microbiol. 70, 1434–1441 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikolakakis K, Lehnert E, McFall-Ngai MJ & Ruby EG Use of hybridization chain reaction-fluorescent in situ hybridization to track gene expression by both partners during initiation of symbiosis. Appl. Environ. Microbiol. 81, 4728–4735 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heath-Heckman EAC, Foster J, Apicella MA, Goldman WE & McFall-Ngai M Environmental cues and symbiont microbe-associated molecular patterns function in concert to drive the daily remodelling of the crypt-cell brush border of the Euprymna scolopes light organ. Cell. Microbiol. 18, 1642–1652 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo MG, Goodson MS & McFall-Ngai M Identification and molecular characterization of a complement C3 molecule in a lophotrochozoan, the Hawaiian bobtail squid Euprymna scolopes. Dev. Comp. Immunol. 33, 69–76 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidson SK, Koropatnick TA, Kossmehl R, Sycuro L & McFall-Ngai MJ NO means ‘yes’ in the squid-vibrio symbiosis: Nitric oxide (NO) during the initial stages of a beneficial association. Cell. Microbiol. 6, 1139–1151 (2004). [DOI] [PubMed] [Google Scholar]
- 49. Cohen SK et al. Tracking the cargo of extracellular symbionts into host tissues with correlated electron microscopy and nanoscale secondary ion mass spectrometry imaging. Cell. Microbiol. 22, e13177 (2020). Using nano secondary ion mass spectrometry (NanoSIMS), demonstrated that abundant products of the extracellular symbiont traffic into the host nucleus and associate with the euchromatin and the nucleolus.
- 50.Moriano-Gutierrez S et al. The noncoding small RNA SsrA is released by Vibrio fischeri and modulates critical host responses. PLoS Biol. 18, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heath-Heckman EACC et al. Bacterial bioluminescence regulates expression of a host cryptochrome gene in the squid-vibrio symbiosis. MBio 4, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McFall-Ngai M, Heath-Heckman EAC, Gillette AA, Peyer SM & Harvie EA The secret languages of coevolved symbioses: Insights from the Euprymna scolopes-Vibrio fischeri symbiosis. Seminars in Immunology 24, 3–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tong D et al. Evidence for light perception in a bioluminescent organ. Proc Natl Acad Sci U S A 106, 9836–9841 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peyer SM, Pankey MS, Oakley TH & McFall-Ngai MJ Eye-specification genes in the bacterial light organ of the bobtail squid Euprymna scolopes, and their expression in response to symbiont cues. Mech. Dev. 131, 111–126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peyer SM, Heath-Heckman EAC & McFall-Ngai MJ Characterization of the cell polarity gene crumbs during the early development and maintenance of the squid–vibrio light organ symbiosis. Dev. Genes Evol. 227, 375–387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Belcaid M et al. Symbiotic organs shaped by distinct modes of genome evolution in cephalopods. Proc. Natl. Acad. Sci. U. S. A. 116, 3030–3035 (2019). Reported the first sequenced cephalopod squid genome and proposed different mechanisms for the evoluiotion of the light organ and accessory nidamental gland in E. scolopes.
- 57. Moriano-Gutierrez S et al. Critical symbiont signals drive both local and systemic changes in diel and developmental host gene expression. Proc. Natl. Acad. Sci. 116, 7990–7999 (2019). Demonstrated that light organ organ colonization and light production leads to systemic changes in gene expression in E. scolopes.
- 58.Bongrand C & Ruby EG Achieving a multi-strain symbiosis: strain behavior and infection dynamics. ISME J. 13, 698–706 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tognini P, Thaiss CA, Elinav E & Sassone-Corsi P Circadian Coordination of Antimicrobial Responses. Cell Host Microbe 22, 185–192 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Bishehsari F, Voigt RM & Keshavarzian A Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nature Reviews Endocrinology (2020). doi: 10.1038/s41574-020-00427-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schwartzman JA et al. The chemistry of negotiation: Rhythmic, glycan-driven acidification in a symbiotic conversation. Proc. Natl. Acad. Sci. U. S. A. 112, 566–71 (2015). Provided compelling evidence that haemocytes traffic into the adult light organ at night and deliver chitin to the symbionts that results in acidification of the microenvironment and promotion of bioluminescence.
- 62. Wier AM et al. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc. Natl. Acad. Sci. U. S. A. 107, 2259–64 (2010). Demonstrated that host and symbiont gene expression is regulated over a day/night cycle and contributes to a daily remodeling of the light organ and symbiont metabolism.
- 63.Graf J & Ruby EG Host-derived amino acids support the proliferation of symbiotic bacteria. Proc. Natl. Acad. Sci. U. S. A. 95, 1818–1822 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nyholm SV & McFall-Ngai MJ Sampling the light-organ microenvironment of Euprymna scolopes: Description of a population of host cells in association with the bacterial symbiont Vibrio fischeri. Biol. Bull. 195, 89–97 (1998). [DOI] [PubMed] [Google Scholar]
- 65.Lee KH & Ruby EG Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl. Environ. Microbiol. 60, 1565–1571 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruby EG & Lee KH The Vibrio fischeri-Euprymna scolopes light organ association: Current ecological paradigms. Appl. Environ. Microbiol. 64, 805–812 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Visick KL, Foster J, Doino J, McFall-Ngai M & Ruby EG Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. (2000). doi: 10.1128/JB.182.16.4578-4586.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Visick KL & Ruby EG Vibrio fischeri and its host: it takes two to tango. Curr. Opin. Microbiol. 9, 632–638 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Boettcher KJ & Ruby EG Detection and quantification of Vibrio fischeri autoinducer from symbiotic squid light organs. J. Bacteriol. (1995). doi: 10.1128/jb.177.4.1053-1058.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koch EJ et al. The cytokine MIF controls daily rhythms of symbiont nutrition in an animal–bacterial association. Proc. Natl. Acad. Sci. U. S. A. 117, 27578–27586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hanlon RT, Claes MF, Ashcraft SE & Dunlap PV Laboratory culture of the sepiolid squid Euprymna scolopes: A model system for bacteria-animal symbiosis. Biol. Bull. 192, 364–374 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Claes MF & Dunlap PV Aposymbiotic culture of the sepiolid squid Euprymna scolopes: Role of the symbiotic bacterium Vibrio fischeri in host animal growth, development, and light organ morphogenesis. J. Exp. Zool. 286, 280–296 (2000). [PubMed] [Google Scholar]
- 73.Kremer N et al. Persistent Interactions with Bacterial Symbionts Direct Mature-Host Cell Morphology and Gene Expression in the Squid-Vibrio Symbiosis. mSystems 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schroeder BO & Bäckhed F Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Montgomery MK & McFall-Ngai MJ The muscle-derived lens of a squid bioluminescent organ is biochemically convergent with the ocular lens. Evidence for recruitment of aldehyde dehydrogenase as a predominant structural protein. J. Biol. Chem. 267, 20999–21003 (1992). [PubMed] [Google Scholar]
- 76.Koch EJ, Moriano-Gutierrez S, Ruby EG, McFall-Ngai MJ & Liebeke M The Impact of Persistent Colonization by Vibrio fischeri on the Metabolome of the Host Squid Euprymna scolopes. J Exp Biol. jeb.212860 (2020). doi: 10.1242/jeb.212860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Altura MA, Stabb E, Goldman W, Apicella M & McFall-Ngai MJ Attenuation of host NO production by MAMPs potentiates development of the host in the squid-vibrio symbiosis. Cell. Microbiol. 13, 527–37 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Troll JV et al. Peptidoglycan induces loss of a nuclear peptidoglycan recognition protein during host tissue development in a beneficial animal-bacterial symbiosis. Cell. Microbiol. 11, 1114–27 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krasity BC et al. Structural and Functional Features of a Developmentally Regulated Lipopolysaccharide-Binding Protein. MBio 6, e01193–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castillo MG, Salazar KA & Joffe NR The immune response of cephalopods from head to foot. Fish Shellfish Immunol. 46, 145–160 (2015). [DOI] [PubMed] [Google Scholar]
- 81.McFall-Ngai M, Nyholm SV & Castillo MG The role of the immune system in the initiation and persistence of the Euprymna scolopes--Vibrio fischeri symbiosis. Semin. Immunol. 22, 48–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nyholm SV, Stewart JJ, Ruby EG & McFall-Ngai MJ Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ. Microbiol. 11, 483–493 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rader B, McAnulty SJ & Nyholm SV Persistent symbiont colonization leads to a maturation of hemocyte response in the Euprymna scolopes/Vibrio fischeri symbiosis. Microbiologyopen 8, (2019). Showed that long-term colonization of the light organ leads to a maturation of the host’s cellular innate immune system that alters hemocyte response to Vibrio fischeri.
- 84.Collins AJ, Schleicher TR, Rader BA & Nyholm SV Understanding the role of host hemocytes in a squid/vibrio symbiosis using transcriptomics and proteomics. Front. Immunol. 3, 91 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schleicher TR, VerBerkmoes NC, Shah M & Nyholm SV Colonization state influences the hemocyte proteome in a beneficial squid-vibrio symbiosis. Mol. Cell. Proteomics 13, 2673–2686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McAnulty SJ & Nyholm SV The Role of Hemocytes in the Hawaiian Bobtail Squid, Euprymna scolopes: A Model Organism for Studying Beneficial Host-Microbe Interactions. Front. Microbiol. 7, 2013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salazar KA, Joffe NR, Dinguirard N, Houde P & Castillo MG Transcriptome analysis of the white body of the squid Euprymna tasmanica with emphasis on immune and hematopoietic gene discovery. PLoS One 10, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poole P, Ramachandran V & Terpolilli J Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 16, 291–303 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Hirsch AM & McFall-Ngai MJ Fundamental concepts in symbiotic interactions: Light and dark, day and night, squid and legume. J. Plant Growth Regul. 19, 113–130 (2000). [DOI] [PubMed] [Google Scholar]
- 90.Roy S et al. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 32, 15–41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Proctor LM et al. The Integrative Human Microbiome Project. Nature 569, 641–648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pearson JA, Wong FS & Wen L Crosstalk between circadian rhythms and the microbiota. Immunology 161, 278–290 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanchez G et al. New bobtail squid (Sepiolidae: Sepiolinae) from the Ryukyu islands revealed by molecular and morphological analysis. Commun. Biol. 2, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee PN, Callaerts P, De Couet HG & Martindale MQ Cephalopod Hox genes and the origin of morphological novelties. Nature 424, 1061–1065 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Hartmann B et al. Pax6 in the sepiolid squid Euprymna scolopes: Evidence for a role in eye, sensory organ and brain development. Mech. Dev. 120, 177–183 (2003). [DOI] [PubMed] [Google Scholar]
- 96.Farfán C, Shigeno S, Nödl MT & De Couet HG Developmental expression of apterous/Lhx2/9 in the sepiolid squid Euprymna scolopes supports an ancestral role in neural development. Evol. Dev. 11, 354–362 (2009). [DOI] [PubMed] [Google Scholar]
- 97.Nödl MT, Kerbl A, Walzl MG, Müller GB & de Couet HG The cephalopod arm crown: Appendage formation and differentiation in the Hawaiian bobtail squid Euprymna scolopes. Front. Zool. 13, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crookes WJ et al. Reflectins: The Unusual Proteins of Squid Reflective Tissues. Science (80-. ). 303, 235–238 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Zepeda EA, Veline RJ & Crook RJ Rapid associative learning and stable Long-Term memory in the squid Euprymna scolopes. Biol. Bull. 232, 212–218 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Howard RB, Lopes LN, Lardie CR, Perez PP & Crook RJ Early-life injury produces lifelong neural hyperexcitability, cognitive deficit and altered defensive behaviour in the squid Euprymna scolopes. Philos. Trans. R. Soc. B Biol. Sci 374, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bazarini SN & Crook RJ Environmental estrogen exposure disrupts sensory processing and nociceptive plasticity in the cephalopod Euprymna scolopes. J. Exp. Biol. 223, (2020). [DOI] [PubMed] [Google Scholar]
- 102.Crawford K et al. Highly Efficient Knockout of a Squid Pigmentation Gene. Curr. Biol. 30, 3484–3490.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruby EG et al. Complete genome sequence of Vibrio fischeri: A symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. U. S. A. (2005). doi: 10.1073/pnas.0409900102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gromek SM et al. Leisingera sp. JC1, a bacterial isolate from hawaiian bobtail squid eggs, produces indigoidine and differentially inhibits vibrios. Front. Microbiol. 7, 1342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suria AM et al. Hawaiian Bobtail Squid Symbionts Inhibit Marine Bacteria via Production of Specialized Metabolites, Including New Bromoalterochromides BAC-D/D′. mSphere 5, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Collins AJ & Nyholm SV Draft genome of Phaeobacter gallaeciensis ANG1, a dominant member of the accessory nidamental gland of Euprymna scolopes. J. Bacteriol. 193, 3397–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Collins AJ, Fullmer MS, Gogarten JP & Nyholm SV Comparative genomics of Roseobacter clade bacteria isolated from the accessory nidamental gland of Euprymna scolopes. Front. Microbiol. 6, 123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chun CK et al. An annotated cDNA library of juvenile Euprymna scolopes with and without colonization by the symbiont Vibrio fischeri. BMC Genomics 7, 154 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chun CK et al. Effects of colonization, luminescence, and autoinducer on host transcription during development of the squid-vibrio association. Proc. Natl. Acad. Sci. U. S. A. 105, 11323–8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schleicher TR & Nyholm SV Characterizing the host and symbiont proteomes in the association between the bobtail squid, Euprymna scolopes, and the bacterium, Vibrio fischeri. PLoS One 6, e25649 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kerwin AH & Nyholm SV Symbiotic bacteria associated with a bobtail squid reproductive system are detectable in the environment, and stable in the host and developing eggs. Environ. Microbiol. 19, 1463–1475 (2017). [DOI] [PubMed] [Google Scholar]
- 112.Collins AJ et al. Diversity and partitioning of bacterial populations within the accessory nidamental gland of the squid Euprymna scolopes. Appl. Environ. Microbiol. 78, 4200–4208 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ii JFB et al. Global discovery of colonization determinants in the squid symbiont Vibrio fischeri. Proc. Natl. Acad. Sci. U. S. A. 111, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zink KE, Tarnowski DA, Mandel MJ & Sanchez LM Optimization of a minimal sample preparation protocol for imaging mass spectrometry of unsectioned juvenile invertebrates. J. Mass Spectrom. 55, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zink KE et al. A small molecule coordinates symbiotic behaviors in a host organ. MBio 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan S et al. Model-enabled gene search (MEGS) allows fast and direct discovery of enzymatic and transport gene functions in the marine bacterium Vibrio fischeri. J. Biol. Chem. 292, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McFall-Ngai Margaret and Ruby E. Sepiolids and Vibrios: When First They Meet: Reciprocal interactions between host and symbiont lead to the creation of a complex light-emitting organ. le. Bioscience 48, 257–265 (1998). [Google Scholar]
- 118.Phillips NJ et al. The lipid a from Vibrio fischeri lipopolysaccharide: A unique structure bearing a phosphoglycerol moiety. J. Biol. Chem. 286, 21203–21219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brennan CA et al. A model symbiosis reveals a role for sheathed-flagellum rotation in the release of immunogenic lipopolysaccharide. Elife 2014, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aschtgen MS et al. Rotation of Vibrio fischeri flagella produces outer membrane vesicles that induce host development. J. Bacteriol. 198, 2156–2165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goodson MS et al. Identifying components of the NF-κB pathway in the beneficial Euprymna scolopes-Vibrio fischeri light organ symbiosis. Appl. Environ. Microbiol. 71, 6934–6946 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kerwin AH & Nyholm SV Reproductive System Symbiotic Bacteria Are Conserved between Two Distinct Populations of Euprymna scolopes from Oahu, Hawaii. mSphere 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kaufman MR, Ikeda Y, Patton C, Van Dykhuizen G & Epel D Bacterial symbionts colonize the accessory nidamental gland of the squid Loligo opalescens via horizontal transmission. Biol. Bull. 194, 36–43 (1998). [DOI] [PubMed] [Google Scholar]
- 124.Barbieri E, Barry K, Child A & Wainwrigth N Antimicrobial activity in the microbial community of the accessory-nidamental gland and egg cases of Loligo pealei (Cephalopoda: Loliginidae). in Biological Bulletin 193, 275–276 (1997). [DOI] [PubMed] [Google Scholar]
- 125.Nyholm SV In the beginning: egg–microbe interactions and consequences for animal hosts. Philos. Trans. R. Soc. B Biol. Sci. 375, 20190593 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gil-Turnes MS, Hay ME & Fenical W Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 246, 116–118 (1989). [DOI] [PubMed] [Google Scholar]
- 127.Gil-Turnes MS & Fenical W Embryos of Homarus americanus are protected by epibiotic bacteria. Biol. Bull. 182, 105–108 (1992). [DOI] [PubMed] [Google Scholar]
- 128.Khalturin K, Hemmrich G, Fraune S, Augustin R & Bosch TCG More than just orphans: are taxonomically-restricted genes important in evolution? Trends Genet. 25, 404–413 (2009). [DOI] [PubMed] [Google Scholar]
- 129.Tautz D & Domazet-Lošo T The evolutionary origin of orphan genes. Nature Reviews Genetics 12, 692–702 (2011). [DOI] [PubMed] [Google Scholar]
- 130.Nyholm SV Fiat Lux: The Squid–Vibrio Association as a Model for Understanding Host–Microbe Associations. in“Advances in Environmental Microbiology 2: The Mechanistic Benefits of Microbial Symbionts” Springer Publishing Co; (2016). doi: 10.1007/978-3-319-28068-4_11 [DOI] [Google Scholar]