Abstract
Introduction:
Preterm birth remains the leading cause of perinatal morbidity and mortality worldwide. Preterm birth is preceded by spontaneous preterm labor, which is commonly associated with sterile intra-amniotic inflammation; yet, no approved treatment exists for this clinical condition. Corticosteroids are the standard of care to improve neonatal outcomes in women at risk of preterm birth. Herein, we first validated our model of alarmin-induced preterm birth. Next, we investigated whether treatment with betamethasone could prevent preterm birth resulting from sterile intra-amniotic inflammation in mice.
Methods:
Under ultrasound guidance, the first cohort of dams received an intra-amniotic injection of the alarmin high-mobility group box-1 (HMGB1, n=10) or phosphate-buffered saline (PBS, n=9) as controls. Next, a second cohort of dams received HMGB1 intra-amniotically and were subcutaneously treated with betamethasone (n=15) or vehicle (n=15). Dams were observed until delivery, and perinatal outcomes were observed.
Results:
Intra-amniotic HMGB1 reduced gestational length (p=0.04), inducing preterm birth in 40% (4/10) of cases, of which 100% (4/4) were categorized as late preterm births. Importantly, treatment with betamethasone extended the gestational length (p=0.02), thereby reducing preterm birth by 26.6% [from 33.3% (5/15) to 6.7% (1/15)]. Treatment with betamethasone did not worsen the rate of neonatal mortality induced by HMGB1 or alter weight gain in the first three weeks of life.
Conclusion:
Treatment with betamethasone prevents preterm birth induced by the alarmin HMGB1. This study supports the potential utility of betamethasone for treating women with sterile intra-amniotic inflammation.
Keywords: Alarmins, Antenatal corticosteroids, HMGB1, Preterm labor, Prematurity, Pregnancy
INTRODUCTION
Preterm birth remains one of the foremost causes of perinatal morbidity and mortality globally [1–4]. Preterm birth occurs after spontaneous preterm labor in approximately 70% of cases [5]. While several etiologies have been described for the syndrome of spontaneous preterm labor [6], the only well-established causal link is intra-amniotic infection and/or inflammation [7–21]. While intra-amniotic infection is caused by microbes invading the amniotic cavity [8, 9, 11, 22–37], it is now well-accepted that sterile intra-amniotic inflammation is triggered by non-microbial stimuli [35, 38–46].
Sterile inflammation is driven by damage-associated molecular patterns (DAMPs) or alarmins [47–51], which include high-mobility group box-1 (HMGB1) [52, 53], interleukin (IL)-1α [54], heat-shock protein 70 (HSP70) [55], and S100 calcium-binding protein (S100B) [56]. Importantly, sterile intra-amniotic inflammation is commonly diagnosed in women who underwent spontaneous preterm labor with intact membranes [40]. Moreover, women who had high concentrations of HMGB1 in amniotic fluid and sterile intra-amniotic inflammation underwent earlier delivery than women with low concentrations of this alarmin [40]. In tandem with clinical observations, murine studies demonstrated that the intra-amniotic injection of HMGB1, S100B, or IL-1α causes preterm delivery [15, 21, 57], thereby establishing a causal link between intra-amniotic inflammation triggered by alarmins and spontaneous preterm birth. However, to date there are no approved treatment regimens for patients with sterile intra-amniotic inflammation.
The administration of antenatal corticosteroids such as betamethasone has become the standard of care for women at risk of delivering preterm within one week, regardless of the underlying cause [58]. Corticosteroids are recommended as they have been shown to accelerate fetal organ maturation, not only diminishing the rate of Respiratory Distress Syndrome and intraventricular hemorrhage but, more importantly, reducing the risk of perinatal and neonatal death [59]. Notably, pre-treatment with betamethasone has also been shown to delay delivery and reduce amniotic fluid cytokine concentrations using an in vivo model of preterm birth caused by systemic endotoxin administration [60]. However, the effects of betamethasone in the clinical setting of sterile intra-amniotic inflammation have not been evaluated.
In the current study, we first validated our established in vivo model of sterile intra-amniotic inflammation induced by the administration of the alarmin HMGB1 under ultrasound guidance. Using this model, we examined the potential utility of betamethasone treatment for preventing preterm birth.
METHODS
Mice
C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in the vivarium of our institution. All mice were maintained under a circadian cycle (light:dark = 12:12 h). Females (8-12 weeks old) were mated with males of demonstrated fertility. Female mice were checked every morning between 8:00 - 9:00 a.m. for the appearance of a vaginal plug, which designated 0.5 days post coitum (dpc). Dams were then housed separately from the males and their weights were checked daily. An increase in weight of ≥2 grams by 12.5 dpc confirmed pregnancy. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) (Protocol No. 18-03-0584).
Intra-amniotic administration of HMGB1
The intra-amniotic administration of HMGB1 was performed as previously described [57]. Briefly, dams were anesthetized on 14.5 dpc by inhalation of 2% isoflurane [Fluriso™ (Isoflurane, USP) Vetone Boise, ID, USA] and 1–2 L/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.5–2% isoflurane and 1.5–2 L/min of oxygen. Mice were positioned on a heating pad and stabilized with adhesive tape. Fur was removed from the abdomen and thorax by applying Nair cream (Church & Dwight Co., Inc., Ewing, NJ, USA). Body temperature was maintained at 37 ± 1°C as indicated by rectal thermometer (VisualSonics, Toronto, ON, Canada), and respiratory and heart rates were monitored by electrodes embedded in the heating pad. The ultrasound transducer was placed in a mechanical holder, and was slowly moved towards the abdomen. Ultrasound-guided intra-amniotic injection was performed in each amniotic sac using recombinant human HMGB1 (Biolegend, San Diego, CA, USA) dissolved in sterile 1X phosphate-buffered saline (PBS; Life Technologies, Grand Island, NY, USA) at a concentration of 9 ng/100 μL using a 30-G needle (BD PrecisionGlide Needle; Becton Dickinson, Franklin Lakes, NJ, USA) (n = 10). Controls were injected with 100 μL of PBS alone (n = 9). Color Doppler ultrasound was used to identify the “injection jet sign” [61], which confirmed that the fluid was injected inside of the amniotic cavity (Figure 1A). Following the ultrasound, dams were positioned under a heat lamp for recovery. Afterwards, dams were monitored via video camera until delivery to evaluate pregnancy outcomes.
Figure 1. Intra-amniotic injection of HMGB1 guided by ultrasound.
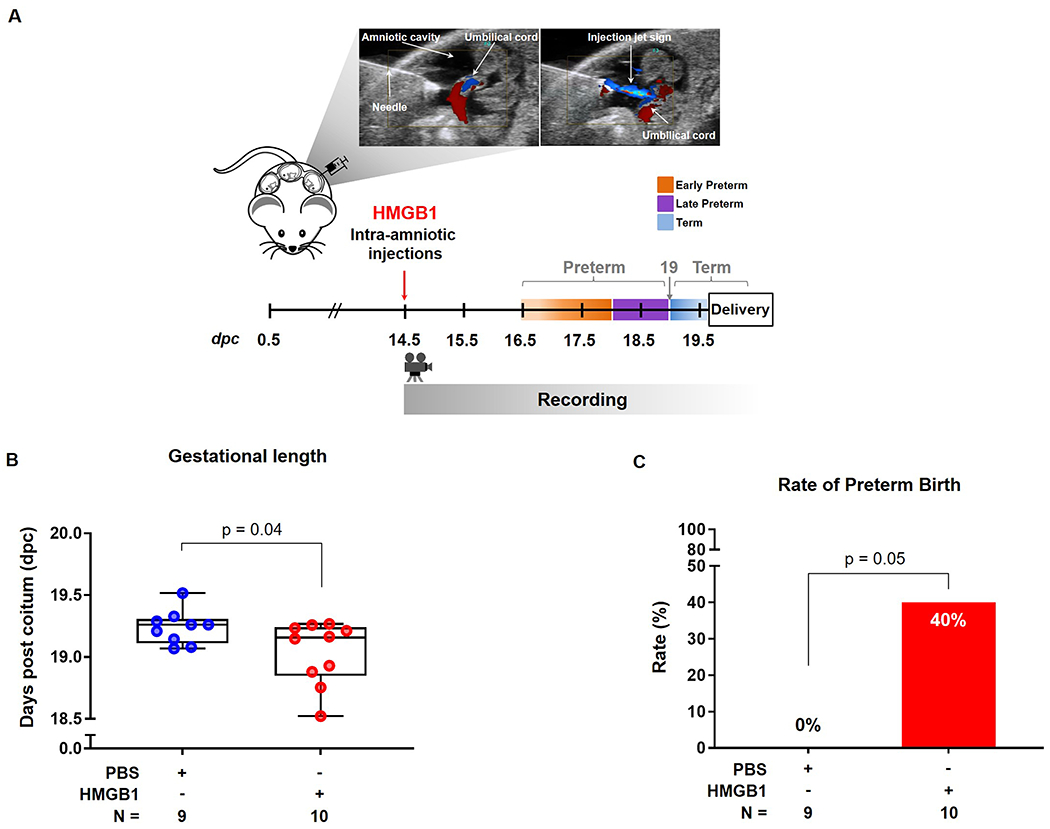
(A) Experimental design: Dams were intra-amniotically injected with HMGB1 (9 ng/100 μL) or PBS (100 μL) on 14.5 days post coitum (dpc) and observed until birth. Color Doppler was used to identify the “injection jet sign.” (B) Gestational length of dams injected with vehicle (blue dots) or HMGB1 (red dots) (n = 9-10 each). Data are shown as boxplots where the midline represents the median, boxes represent interquartile range, and whiskers represent the minimum/maximum range. (C) Preterm birth rate (<19 dpc) for dams that received vehicle (blue dots) or HMGB1 (red dots) (n = 9-10 each).
Betamethasone treatment of mice intra-amniotically injected with HMGB1
Dams received an intra-amniotic injection of HMGB1 in each amniotic sac on 14.5 dpc under ultrasound guidance. Dams were then treated subcutaneously with either 0.1 mg/100 μL of Betamethasone Sodium Phosphate and Betamethasone Acetate Injectable Suspension (American Regent Inc. Shirley, NY, USA; n = 15) diluted in vehicle control [0.9% sodium chloride injection (saline), Hospira, Lake Forest, IL, USA] or vehicle control alone (n = 15) at 12 hours (h) and 36 h after the intra-amniotic injection of HMGB1. Dams were then observed via video camera until delivery to evaluate pregnancy and neonatal outcomes. The betamethasone dosage was chosen based on prior reports indicating that 0.1 mg of betamethasone induced lung maturation in mice and was equivalent to the two 12 mg doses used in pregnant women [60, 62]. The latter dose and schedule is used as the standard of care in pregnant women who are predicted to be at risk of preterm delivery within one week [58].
Video monitoring and pregnancy and neonatal outcomes
Pregnancy parameters, which included the preterm birth rate and neonatal mortality rate were recorded using a video camera (Sony Corporation, Tokyo, Japan). The gestational length was determined as the time period from the observation of the vaginal plug until the delivery of the first pup. Preterm birth was defined as delivery <19.0 dpc, and this rate was calculated as the percentage of dams delivering <19.0 dpc among the total number of injected dams. Late preterm birth was defined as delivery between 18.0 - 19.0 dpc, and early preterm birth as delivery <18.0 dpc (Figure 1A). The neonatal weights were reported as the mean weight of all neonates from a single dam (litter). The neonatal mortality rate for each litter was calculated using the number of dead delivered pups among the total litter size. Neonates were observed until three weeks postpartum to evaluate neonatal weight and survival.
Statistical analysis
GraphPad Prism version 8.0.1 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com) was used to conduct statistical analysis. The Fisher’s exact test was used for the rates of preterm birth, the Mann–Whitney U-test was used for gestational length, neonatal mortality, and neonatal weight, and Kaplan–Meier survival curves were used to plot and compare neonatal survival (Mantel–Cox test). A p-value <0.05 was considered statistically significant.
RESULTS
The intra-amniotic injection of HMGB1 causes preterm birth
HMGB1 is one of the most studied alarmins in the amniotic cavity [38, 40, 42, 57, 63–67] and is elevated in amniotic fluid of women with sterile intra-amniotic inflammation [40, 42]. Additionally, we previously demonstrated causality between the intra-amniotic injection of HMGB1 and preterm labor/birth [57]. Given that the bioactivity of HMGB1 is variable [68], our first aim was to validate our previous model of preterm labor/birth caused by HMGB1. Under ultrasound guidance, dams were intra-amniotically injected with 9 ng/100 μL of HMGB1 or 100 μL of PBS on 14.5 dpc and observed until delivery (Figure 1A). This dose of HMGB1 was determined from the pathophysiological amniotic fluid concentrations found in patients with sterile intra-amniotic inflammation [40]. Color Doppler ultrasound was used to identify the “injection jet sign” and confirm that the fluid was injected inside of the amniotic cavity (Figure 1A) [61]. We found that the gestational length of the dams injected with HMGB1 was decreased compared to the gestational length of controls [HMGB1: 19.16 (18.89-19.23) vs. PBS: 19.26 (19.14-19.29) dpc, p = 0.04] (Figure 1B). Specifically, 40% (4/10) of the mice injected with HMGB1 underwent preterm birth (<19.0 dpc), and all (10/10) of the control dams underwent term delivery (≥19.0 dpc) (p = 0.05) (Figure 1C). Notably, all of these preterm births were late preterm (Figure 1B), which is the period when most human preterm births occur [1, 3, 5]. Beyond pregnancy complications, we evaluated the impact of intra-amniotic HMGB1 on the neonates. No differences were found in the mortality at birth between the neonates born to dams that received an intra-amniotic injection of HMGB1 and controls [HMGB1: 37.5% (17.08-41.52) vs. PBS: 25% (14.29-27.5), p = 0.2], which is consistent with our prior study [57]. Thus, the intra-amniotic injection of HMGB1 may result in late preterm birth.
Treatment with betamethasone prevents preterm birth induced by HMGB1
Next, we investigated whether betamethasone, a corticosteroid used as the standard of care in pregnant women at risk of preterm delivery [58], could prevent preterm birth and adverse neonatal outcomes induced by HMGB1. Dams were intra-amniotically injected with HMGB1 on 14.5 dpc and subcutaneously treated with betamethasone 12 h and 36 h after intra-amniotic injection (Figure 2A). Controls were subcutaneously treated with saline. We found that the gestational length of the dams that received HMGB1 was increased after betamethasone treatment compared to the gestational length of dams that received HMGB1 and were treated with the vehicle alone [HMGB1 + Betamethasone: 19.3 (19.17-19.35) vs. HMGB1 + Saline: 19.13 (18.79-19.25) dpc, p = 0.02] (Figure 2B). Specifically, the rate of preterm birth was reduced by 26.6% [from 33.3% (5/15) to 6.7% (1/15), p = 0.08] following betamethasone treatment (Figure 2C). To our knowledge, this is the first demonstration that betamethasone can reduce the rate of preterm birth in the setting of sterile intra-amniotic inflammation.
Figure 2. Betamethasone treatment after intra-amniotic injection of HMGB1.
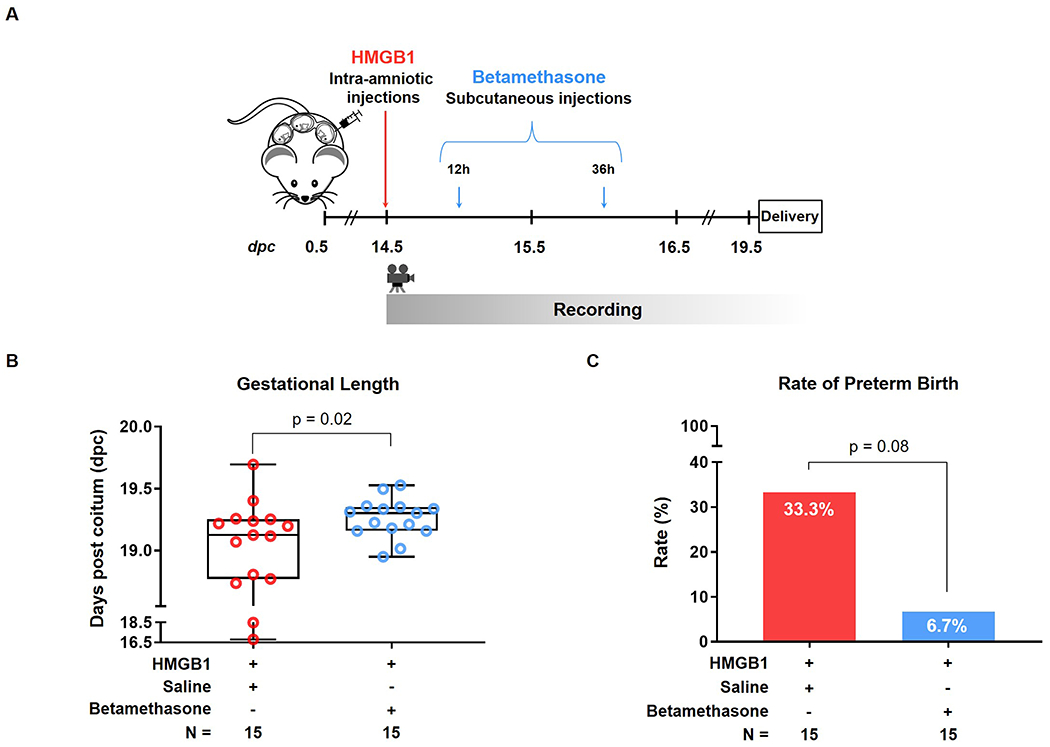
(A) Experimental design: Dams were intra-amniotically injected with HMGB1 and subcutaneously treated with betamethasone (0.1 mg/100 μL) or vehicle control (saline, 100 μL) at 12 and 36 hours (h) after intra-amniotic injection, and monitored until delivery (n = 15 each). (B) Gestational length [shown as days post coitum (dpc)] of dams injected with HMGB1 and treated with vehicle (saline, red dots) or betamethasone (blue dots). Data are shown as boxplots where the midline represents the median, boxes represent interquartile range, and whiskers represent the minimum/maximum range. (C) Preterm birth rate (<19 dpc) in dams that received HMGB1 and were treated with vehicle (saline, red bar) or betamethasone (blue bar).
The use of betamethasone in women with high risk of preterm birth has been shown to improve perinatal and neonatal mortality, regardless of the underlying etiology [58]. However, the specific effects of this medication in the subset of women with preterm labor and sterile-intra-amniotic inflammation is unknown. Thus, we next assessed the outcomes of neonates from dams that received HMGB1 and were treated with betamethasone compared to controls. No differences were found in the neonatal mortality rates at week one, two, and three of life (p = 0.6) (Figure 3A). Furthermore, we did not find differences in neonatal weights at week one (p = 0.2), two (p = 0.1), or three (p = 0.4) of life (Figures 3B–D). These results indicate that treatment with betamethasone does not worsen the rate of neonatal mortality induced by HMGB1 or alter the neonatal weight gain.
Figure 3. Neonatal outcomes after betamethasone treatment of HMGB1-injected dams.
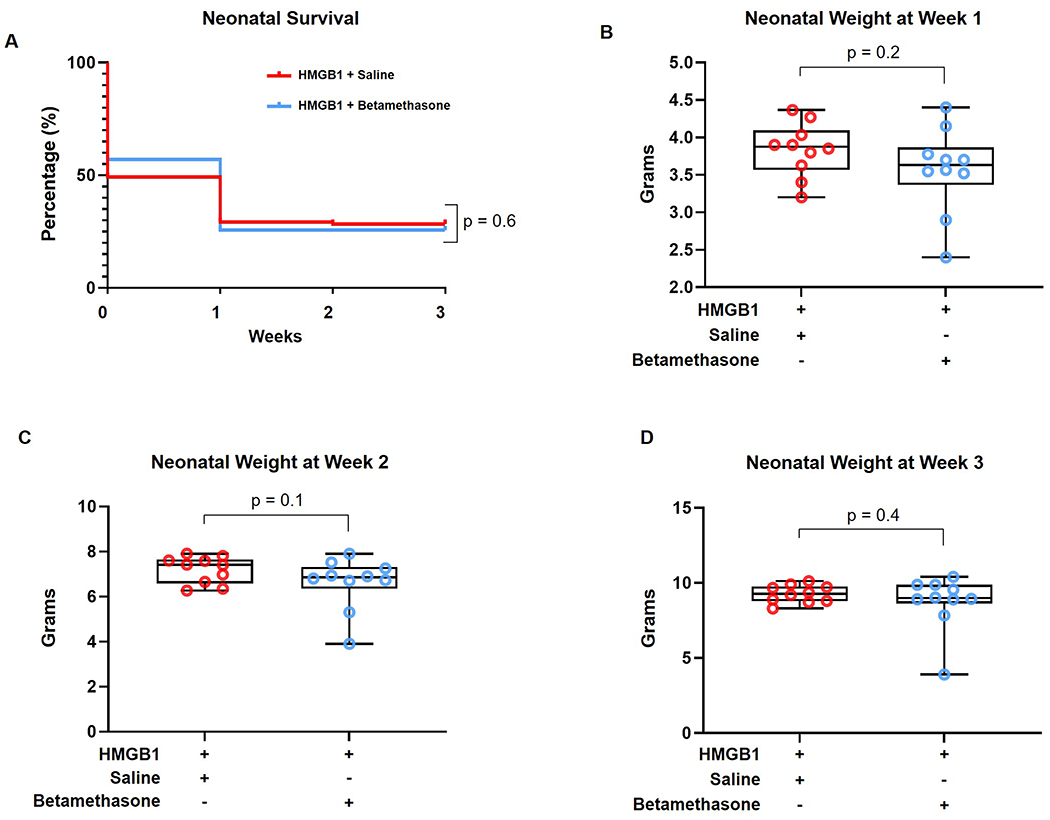
(A) Kaplan-Meier survival curves representing neonatal survival at weeks 1, 2, and 3 postpartum for neonates from dams injected with HMGB1 and treated with vehicle (saline, red line) or betamethasone (blue line) (n = 116 - 120 per group). (B-D) Weights of neonates from dams that received HMGB1 and were treated with vehicle (saline, red dots) or betamethasone (blue dots) at weeks 1 (B), 2 (C), and 3 (D) postpartum (n = 10 litters each). Data are shown as boxplots where the midline represents the median, boxes represent interquartile range, and whiskers represent the minimum/maximum range. Each dot corresponds to the mean weight of all neonates from a single dam (litter).
DISCUSSION
Principal findings of the study
In the current study, we established that the intra-amniotic administration of HMGB1 causes late preterm birth. Importantly, treatment with betamethasone, a commonly used corticosteroid in women with high risk of preterm birth, extended gestational length, preventing preterm birth. This corticosteroid neither worsened the neonatal mortality induced by HMGB1 nor altered neonatal weight gain in the first three weeks of life. These findings show the potential utility of betamethasone for the treatment of preterm birth associated with sterile intra-amniotic inflammation.
The alarmin HMGB1 drives sterile intra-amniotic inflammation
HMGB1 is a nuclear protein initially described as an important regulator of transcription and gene expression [52]. Yet, HMGB1 has also been recognized as an alarmin that is secreted by leukocytes in response to inflammatory stimuli [69–71]. HMGB1 represents a prototypical alarmin, given that it meets the proposed criteria for such molecules [49]. Growing evidence has suggested a role for HMGB1 in the induction of sterile intra-amniotic inflammation, which includes the following: 1) the concentrations of HMGB1 were significantly higher in amniotic fluid of patients with sterile intra-amniotic inflammation than in those without [38, 40]; 2) intra-amniotic HMGB1 shortens gestational length in mice, leading to preterm birth [57]; and 3) HMGB1 causes inflammasome-mediated inflammation in the chorioamniotic membranes [66, 72]. Together with the current study, these findings firmly establish HMGB1 as an alarmin that promotes sterile inflammatory responses and preterm birth.
Can sterile intra-amniotic inflammation be treated?
The treatment of sterile inflammation has been challenging. Standard methods for treating sterile inflammatory processes (e.g. gout, rheumatoid arthritis, etc.) include anti-inflammatory drugs such as non-steroidal anti-inflammatory [73] or corticosteroid [74–76] medications. Moreover, treatments that specifically decrease the concentration of danger signals driving sterile inflammation have also been utilized, such as allopurinol, a drug that decreases uric acid in patients with gout [77–79]. However, the treatment of sterile intra-amniotic inflammation is even more challenging, since the majority of drugs used to treat sterile inflammation-related diseases are not approved for use in pregnant women. Specific treatment options have been explored in mice, such as the inhibition of the NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome [15, 21] This pathway is associated with the pathogenesis of sterile intra-amniotic inflammation [15, 21, 80, 81] Specifically, the blockade of the NLRP3 inflammasome was effective in diminishing the rates of preterm birth and neonatal mortality in mice [15]; however, this potential therapy has not been approved for clinical use during pregnancy [15]. Therefore, there is a growing need for drugs that are already approved for use in pregnant women and can be used to effectively treat sterile intra-amniotic inflammation. A pioneer study using catheterized rhesus macaques demonstrated that the uterine contractions caused by the intra-amniotic administration of IL-1β were reduced in macaques treated with indomethacin [82], a tocolytic agent that can also be used to delay preterm labor. Yet, indomethacin is only recommended for use until 32 weeks of gestation due to the risk of ductus arteriosus closure, limiting its utility [83–85]. Subsequently, these investigators showed that treatment with either dexamethasone or IL-10 reduced the uterine activity caused by the intra-amniotic administration of IL-1β in pregnant macaques [86]. Furthermore, recent studies have proposed the use of clarithromycin, an approved antibiotic for use in pregnancy, as part of a potential treatment regimen for intra-amniotic infection/inflammation [87–90]. Indeed, clarithromycin was also shown to be effective for decreasing the severity of intra-amniotic inflammation in patients with sterile intra-amniotic inflammation [90] and improving perinatal outcomes in an animal model of intra-amniotic infection [19]. However, further mechanistic experimentation to evaluate the effectiveness of clarithromycin in the setting of sterile intra-amniotic inflammation is required.
Betamethasone as a treatment for sterile intra-amniotic inflammation
Herein, we demonstrated for the first time that betamethasone restores the normal timing of delivery in a model of HMGB1-induced sterile intra-amniotic inflammation. Corticosteroids, both endogenous and pharmacological, exert their classic anti-inflammatory functions by traversing the plasma membrane of immune cells and binding to cytosolic corticosteroids receptors, which then regulate gene transcription and signal transduction within the inflamed target tissues [91, 92]. Interestingly, corticosteroids can also inhibit the expression and release of alarmins, including HMGB1, under various inflammatory conditions [93–98].
Corticosteroids have several different mechanisms of action for the regulation of inflammation, most of which are dependent on the timing of exposure [76]. Prior in vivo studies have shown that treatment with betamethasone did not decrease the inflammatory response taking place in the amniotic fluid [99], chorioamniotic membranes [99], fetal thymus [100], and fetal lungs [101, 102] in sheep with intra-amniotic inflammation induced by endotoxin. Yet, such inflammatory response was diminished when betamethasone was administered prior to the endotoxin stimuli [99–101]. However, in the clinical setting, the administration of corticosteroids before or at the onset of intra-amniotic inflammation is very unlikely. Therefore, our experimental model resembles the clinical scenario and management of sterile intra-amniotic inflammation, and the fact that betamethasone restored the timing of delivery supports its clinical administration in patients with this condition. Furthermore, one possible mechanism by which betamethasone exerts its effects on sterile intra-amniotic inflammation is by dampening PRR signaling pathways [92], thus suppressing production of the inflammatory mediators typically found in high concentrations in amniotic fluid of women with this clinical condition [38, 42, 46, 63]. Additionally, corticosteroids have been shown to attenuate leukocyte migration to damaged tissue sites by inhibiting transcription of adhesion molecules such as SELE (E-selectin), intercellular adhesion molecule (ICAM)-1, and vascular cell adhesion molecule (VCAM)-1 [103, 104], all of which are involved in neutrophil migration to the reproductive tissues [105–107]. We have previously shown that spontaneous term labor is associated with upregulation of VCAM-1 and ICAM-1 in the choriodecidua [108], suggesting that betamethasone could delay the process of labor by preventing leukocyte migration to these tissues [109, 110]. Lastly, corticosteroids have been shown to mediate the polarization of monocytes and macrophages towards an anti-inflammatory phenotype, promoting the phagocytosis of apoptotic cells and debris [111–113]. The latter finding suggests that treatment with betamethasone could prevent aberrant pro-inflammatory (M1) polarization of macrophages at the maternal-fetal interface, which is consistent with prior reports showing that an increased fraction of M1-like macrophages in this compartment is correlated with preterm labor and birth [114]. In this regard, betamethasone may exert its beneficial effects by promoting M2 polarization of monocytes and macrophages. Furthermore, it is most likely that betamethasone has a complex role in regulation of the inflammatory response, and mitigates the consequences of sterile intra-amniotic inflammation through multiple non-exclusive mechanisms. Future studies are warranted to elucidate the mechanisms whereby betamethasone reduces sterile intra-amniotic inflammation and prevents preterm labor and birth.
In our model, treatment with betamethasone did not worsen the rate of neonatal mortality induced by HMGB1 or alter the neonatal weight gain in the first three weeks of life. This finding supports the cautious use of corticosteroids for the prevention of preterm birth. Yet, contrary to what is reported in humans [59, 115], treatment with betamethasone did not improve neonatal survival, which highlights the importance of intensive neonatal care.
It is worth mentioning that a limitation of our model is that a subset of neonates from dams that received an intra-amniotic injection, even with saline, failed to thrive. This is likely due to the invasive nature of an intra-amniotic injection on 14.5 days post coitum, which may have a greater impact on fetal development than that performed on 16.5 days post coitum [13, 15, 16, 19, 21, 116]. Yet, the intra-amniotic administration of alarmins guided by ultrasound allows us to mechanistically study the clinical syndrome of sterile intra-amniotic inflammation.
Conclusion
In this study, we provide evidence that betamethasone, a common medication approved for use during high-risk pregnancies, prevents preterm birth caused by the intra-amniotic injection of the alarmin HMGB1. These findings support the potential utility of this corticosteroid for preventing preterm birth in patients with sterile intra-amniotic inflammation.
List of Abbreviations:
- DAMPs
damage-associated molecular patterns
- dpc
days post coitum
- HMGB1
high-mobility group box-1
- HSP70
heat-shock protein 70
- ICAM
intercellular adhesion molecule
- IL
interleukin
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- PBS
phosphate-buffered saline
- PRR
pattern recognition receptors
- SELE
E-selectin
- S100B
S100 calcium-binding protein
- VCAM
vascular cell adhesion molecule
REFERENCES
- 1.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10Suppl 1:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–40. [DOI] [PubMed] [Google Scholar]
- 3.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AC, Blencowe H, Lawn JE. Small babies, big numbers: global estimates of preterm birth. Lancet Glob Health. 2019;7:e2–e3. [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67:229–37. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–79. [PubMed] [Google Scholar]
- 9.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–24. [DOI] [PubMed] [Google Scholar]
- 10.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. [DOI] [PubMed] [Google Scholar]
- 11.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–76. [DOI] [PubMed] [Google Scholar]
- 12.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, et al. Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front Immunol. 2018;9:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Lopez N, Romero R, Arenas-Hernandez M, Panaitescu B, Garcia-Flores V, Mial TN, et al. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med. 2018;31:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomesdagger. Biol Reprod. 2019;100:1306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, et al. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasomedagger. Biol Reprod. 2019;100:1290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero R, Gomez-Lopez N, Winters AD, Jung E, Shaman M, Bieda J, et al. Evidence that intra-amniotic infections are often the result of an ascending invasion - a molecular microbiological study. J Perinat Med. 2019;47:915–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gultekin-Elbir EE, Ford C, Genc MR. The value of amniotic fluid analysis in patients with suspected clinical chorioamnionitis. J Perinat Med. 2019;47:493–9. [DOI] [PubMed] [Google Scholar]
- 19.Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD, et al. Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. mBio. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coleman M, Orvis A, Wu TY, Dacanay M, Merillat S, Ogle J, et al. A Broad Spectrum Chemokine Inhibitor Prevents Preterm Labor but Not Microbial Invasion of the Amniotic Cavity or Neonatal Morbidity in a Non-human Primate Model. Front Immunol. 2020;11:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motomura K, Romero R, Garcia-Flores V, Leng Y, Xu Y, Galaz J, et al. The alarmin interleukin-1alpha causes preterm birth through the NLRP3 inflammasome. Mol Hum Reprod. 2020;26:712–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobitt JR, Ledger WJ. Unrecognized amnionitis and prematurity: a preliminary report. J Reprod Med. 1977;19:8–12. [PubMed] [Google Scholar]
- 23.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140:947–52. [DOI] [PubMed] [Google Scholar]
- 24.Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol. 1981;57:483–6. [PubMed] [Google Scholar]
- 25.Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol. 1984;148:739–43. [DOI] [PubMed] [Google Scholar]
- 26.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–84. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci. 1991;622:355–75. [DOI] [PubMed] [Google Scholar]
- 28.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–28. [DOI] [PubMed] [Google Scholar]
- 29.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–7. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15Suppl 2:41–56. [DOI] [PubMed] [Google Scholar]
- 31.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–74. [DOI] [PubMed] [Google Scholar]
- 33.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–9. [DOI] [PubMed] [Google Scholar]
- 34.Monckeberg M, Valdes R, Kusanovic JP, Schepeler M, Nien JK, Pertossi E, et al. Patients with acute cervical insufficiency without intra-amniotic infection/inflammation treated with cerclage have a good prognosis. J Perinat Med. 2019;47:500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Lopez N, Romero R, Maymon E, Kusanovic JP, Panaitescu B, Miller D, et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med. 2019;47:276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh KJ, Romero R, Park JY, Hong JS, Yoon BH. The earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species. J Perinat Med. 2019;47:516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theis KR, Romero R, Motomura K, Galaz J, Winters AD, Pacora P, et al. Microbial burden and inflammasome activation in amniotic fluid of patients with preterm prelabor rupture of membranes. J Perinat Med. 2020;48:115–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213:836 e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, et al. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018;80:e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez-Lopez N, Romero R, Tarca AL, Miller D, Panaitescu B, Schwenkel G, et al. Gasdermin D: Evidence of pyroptosis in spontaneous preterm labor with sterile intra-amniotic inflammation or intra-amniotic infection. Am J Reprod Immunol. 2019;82:e13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peiris HN, Romero R, Vaswani K, Reed S, Gomez-Lopez N, Tarca AL, et al. Preterm labor is characterized by a high abundance of amniotic fluid prostaglandins in patients with intra-amniotic infection or sterile intra-amniotic inflammation. J Matern Fetal Neonatal Med. 2019:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatti G, Romero R, Rice GE, Fitzgerald W, Pacora P, Gomez-Lopez N, et al. Compartmentalized profiling of amniotic fluid cytokines in women with preterm labor. PLoS One. 2020;15:e0227881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matzinger P An innate sense of danger. Semin Immunol. 1998;10:399–415. [DOI] [PubMed] [Google Scholar]
- 48.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–65. [DOI] [PubMed] [Google Scholar]
- 49.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. [DOI] [PubMed] [Google Scholar]
- 50.Lotze MT, Deisseroth A, Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol. 2007;124:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–36. [DOI] [PubMed] [Google Scholar]
- 52.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. [DOI] [PubMed] [Google Scholar]
- 53.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. [DOI] [PubMed] [Google Scholar]
- 54.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–25. [PubMed] [Google Scholar]
- 55.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–42. [DOI] [PubMed] [Google Scholar]
- 56.Donato R S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–68. [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, et al. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol. 2016;75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Committee on Obstetric P Committee Opinion No. 713: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol. 2017;130:e102–e9. [DOI] [PubMed] [Google Scholar]
- 59.McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz WJ 3rd, Christensen HD, Carey JC, Rayburn WF, Gonzalez C. Systemic administration of betamethasone delays endotoxin-induced preterm labor in the murine model. Am J Obstet Gynecol. 2003;188:439–43. [DOI] [PubMed] [Google Scholar]
- 61.Goldschmiedt J, Shilagani C, Patel H. The “injection jet sign”: an innovative method of needle position confirmation during ultrasound guided injections. Skeletal Radiol. 2017;46:559–63. [DOI] [PubMed] [Google Scholar]
- 62.Christensen HD, Sienko AE, Rayburn WF, Gonzalez CL, Coleman FH. A placebo-controlled, blinded comparison between betamethasone and dexamethasone to enhance lung maturation in the fetal mouse. J Soc Gynecol Investig. 1997;4:130–4. [DOI] [PubMed] [Google Scholar]
- 63.Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, et al. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med. 2012;25:558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Regan JK, Kannan PS, Kemp MW, Kramer BW, Newnham JP, Jobe AH, et al. Damage-Associated Molecular Pattern and Fetal Membrane Vascular Injury and Collagen Disorganization in Lipopolysaccharide-Induced Intra-amniotic Inflammation in Fetal Sheep. Reprod Sci. 2016;23:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY). 2016;8:216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod. 2016;95:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Son GH, Kim Y, Lee JJ, Lee KY, Ham H, Song JE, et al. MicroRNA-548 regulates high mobility group box 1 expression in patients with preterm birth and chorioamnionitis. Sci Rep. 2019;9:19746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bertheloot D, Latz E. HMGB1, IL-1alpha, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–16. [DOI] [PubMed] [Google Scholar]
- 71.Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. [DOI] [PubMed] [Google Scholar]
- 72.Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, et al. HMGB1 promotes a p38MAPK associated non-infectious inflammatory response pathway in human fetal membranes. PLoS One. 2014;9:e113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fullerton JN. Use of non-steroidal anti-inflammatory drugs (NSAIDs) as immunomodulatory agents. BMJ. 2013;347:f4984. [DOI] [PubMed] [Google Scholar]
- 74.Dougherty TF, Schneebeli GL. The use of steroids as anti-inflammatory agents. Ann N Y Acad Sci. 1955;61:328–48. [DOI] [PubMed] [Google Scholar]
- 75.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003;349:1647–55. [DOI] [PubMed] [Google Scholar]
- 78.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci. 2017;24:1382–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R. Inflammasomes: Their Role in Normal and Complicated Pregnancies. J Immunol. 2019;203:2757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sadowsky DW, Haluska GJ, Gravett MG, Witkin SS, Novy MJ. Indomethacin blocks interleukin 1beta-induced myometrial contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2000;183:173–80. [DOI] [PubMed] [Google Scholar]
- 83.Vermillion ST, Scardo JA, Lashus AG, Wiles HB. The effect of indomethacin tocolysis on fetal ductus arteriosus constriction with advancing gestational age. Am J Obstet Gynecol. 1997;177:256–9; discussion 9-61. [DOI] [PubMed] [Google Scholar]
- 84.Macones GA, Marder SJ, Clothier B, Stamilio DM. The controversy surrounding indomethacin for tocolysis. Am J Obstet Gynecol. 2001;184:264–72. [DOI] [PubMed] [Google Scholar]
- 85.American College of O, Gynecologists’ Committee on Practice B-O. Practice Bulletin No. 171: Management of Preterm Labor. Obstet Gynecol. 2016;128:e155–64. [DOI] [PubMed] [Google Scholar]
- 86.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–63. [DOI] [PubMed] [Google Scholar]
- 87.Lee J, Romero R, Kim SM, Chaemsaithong P, Yoon BH. A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J Matern Fetal Neonatal Med. 2016;29:2727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oh KJ, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol. 2019;221:140 e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoon BH, Romero R, Park JY, Oh KJ, Lee J, Conde-Agudelo A, et al. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2019;221:142 e1–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kacerovsky M, Romero R, Stepan M, Stranik J, Maly J, Pliskova L, et al. Antibiotic administration reduces the rate of intraamniotic inflammation in preterm prelabor rupture of the membranes. Am J Obstet Gynecol. 2020;223:114 e1–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beck IM, Vanden Berghe W, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. 2009;30:830–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Snyder DS, Unanue ER. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982;129:1803–5. [PubMed] [Google Scholar]
- 94.Knudsen PJ, Dinarello CA, Strom TB. Glucocorticoids inhibit transcriptional and post-transcriptional expression of interleukin 1 in U937 cells. J Immunol. 1987;139:4129–34. [PubMed] [Google Scholar]
- 95.af Klint E, Grundtman C, Engstrom M, Catrina AI, Makrygiannakis D, Klareskog L, et al. Intraarticular glucocorticoid treatment reduces inflammation in synovial cell infiltrations more efficiently than in synovial blood vessels. Arthritis Rheum. 2005;52:3880–9. [DOI] [PubMed] [Google Scholar]
- 96.Furugen M, Higa F, Hibiya K, Teruya H, Akamine M, Haranaga S, et al. Legionella pneumophila infection induces programmed cell death, caspase activation, and release of high-mobility group box 1 protein in A549 alveolar epithelial cells: inhibition by methyl prednisolone. Respir Res. 2008;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schierbeck H, Wahamaa H, Andersson U, Harris HE. Immunomodulatory drugs regulate HMGB1 release from activated human monocytes. Mol Med. 2010;16:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang YH, Wang PW, Tiao MM, Chou MH, Du YY, Huang CC, et al. Glucocorticoid modulates high-mobility group box 1 expression and Toll-like receptor activation in obstructive jaundice. J Surg Res. 2011;170:e47–55. [DOI] [PubMed] [Google Scholar]
- 99.Wolfe KB, Snyder CC, Gisslen T, Kemp MW, Newnham JP, Kramer BW, et al. Modulation of lipopolysaccharide-induced chorioamnionitis in fetal sheep by maternal betamethasone. Reprod Sci. 2013;20:1447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuypers E, Collins JJ, Jellema RK, Wolfs TG, Kemp MW, Nitsos I, et al. Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids. PLoS One. 2012;7:e38257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuypers E, Collins JJ, Kramer BW, Ofman G, Nitsos I, Pillow JJ, et al. Intra-amniotic LPS and antenatal betamethasone: inflammation and maturation in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2012;302:L380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Visconti K, Senthamaraikannan P, Kemp MW, Saito M, Kramer BW, Newnham JP, et al. Extremely preterm fetal sheep lung responses to antenatal steroids and inflammation. Am J Obstet Gynecol. 2018;218:349 e1–e10. [DOI] [PubMed] [Google Scholar]
- 103.Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992;89:9991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Atsuta J, Plitt J, Bochner BS, Schleimer RP. Inhibition of VCAM-1 expression in human bronchial epithelial cells by glucocorticoids. Am J Respir Cell Mol Biol. 1999;20:643–50. [DOI] [PubMed] [Google Scholar]
- 105.Winkler M, Ruck P, Horny HP, Wehrmann M, Kemp B, Kaiserling E, et al. Expression of cell adhesion molecules by endothelium in the human lower uterine segment during parturition at term. Am J Obstet Gynecol. 1998;178:557–61. [DOI] [PubMed] [Google Scholar]
- 106.Rath W, Winkler M, Kemp B. The importance of extracellular matrix in the induction of preterm delivery. J Perinat Med. 1998;26:437–41. [DOI] [PubMed] [Google Scholar]
- 107.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–36. [PubMed] [Google Scholar]
- 108.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gomez-Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol. 2010;88:625–33. [DOI] [PubMed] [Google Scholar]
- 110.Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, Vadillo-Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204:364 e9–16. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y, Cousin JM, Hughes J, Van Damme J, Seckl JR, Haslett C, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J Immunol. 1999;162:3639–46. [PubMed] [Google Scholar]
- 112.Giles KM, Ross K, Rossi AG, Hotchin NA, Haslett C, Dransfield I. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active Rac. J Immunol. 2001;167:976–86. [DOI] [PubMed] [Google Scholar]
- 113.Ehrchen J, Steinmuller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, et al. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265–74. [DOI] [PubMed] [Google Scholar]
- 114.Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, et al. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol. 2016;196:2476–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25. [PubMed] [Google Scholar]
- 116.Schwenkel G, Romero R, Slutsky R, Motomura K, Hsu CD, Gomez-Lopez N. HSP70: an alarmin that does not induce high rates of preterm birth but does cause adverse neonatal outcomes. J Matern Fetal Neonatal Med. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


