Abstract
Early in animal development many cells are conditionally specified based on observations that those cells can be directed toward alternate fates. The endomesoderm is so named because early specification produces cells that often have been observed to simultaneously express both early endoderm and mesoderm transcription factors. Experiments with these cells demonstrate that their progeny can directed entirely toward endoderm or mesoderm, whereas normally they establish both germ layers. This review examines the mechanisms that initiate the conditional endomesoderm state, its metastability, and the mechanisms that resolve that state into definitive endoderm and mesoderm.
Keywords: conditional specification, regulative development, endoderm, mesoderm, nematode, hemichordate, tunicate, zebrafish, frog, sea urchin
Conditional specification has fascinated developmental biologists for more than a century. Early embryologists discovered “regulative development” based on the demonstrated ability of isolated blastomeres to rescue an entire embryo in some cases. Cells of the sea urchin embryo, for example, isolated at the 2-cell or 4-cell stage, regulate and replace the missing parts to produce a normal half-sized or quarter-sized larva (Driesch, 1892). Over time, experiments with many different embryos and cell types eventually led to the proposed existence of a conditionally specified state (as opposed to a committed state). This conditionality can be tested: if a cell or a group of cells is isolated or transferred to an ectopic location in the embryo, the outcome is a measure of their conditionality. If those cells continue to develop toward their original fate only, the cells were committed at the time of transfer. However, if the cells divert to an alternative fate(s), then at the time they were isolated those cells were conditionally specified. Importantly, the experiment also indicates that the environment surrounding the conditionally specified cell provides the necessary information to direct its fate.
Endomesoderm, by definition, is conditionally specified, and this state is broadly distributed in embryos across the animal kingdom. The duration of the endomesoderm state varies greatly, its onset and its resolution into the definitive endoderm and mesoderm also varies mechanistically, but there are a number of properties that are conserved in this process. In most embryos the endoderm vs mesoderm fates are influenced non-autonomously through cell-cell interactions, signals, or other environmental inputs. To gain an impression of how conditional specification works in embryos, this review examines the means by which cells enter the endomesoderm state and the mechanisms leading to eventual fate commitment.
Conditional specification of endomesoderm is distributed broadly in the animal kingdom.
In the nematode Caenorhabditis elegans, the EMS cell at the 4-cell stage is fated to produce both endoderm and mesoderm descendant cells (Fig. 1). So, for a short time period the EMS cell embodies an endomesoderm status in the rapidly developing worm. Shortly after its emergence, at second cleavage, the EMS cell begins receiving a Wnt signal from its posterior neighbor, the P2 cell, which will contribute to the segregation of the endoderm and mesoderm fates. By third cleavage, this Wnt signal causes an asymmetric increase in SYS-1(β-catenin) accumulation in the most posterior daughter cell, the E cell. This causes a reduction of repressive nuclear POP-1 (TCF) activity in the E daughter cell, while repressive POP-1 remains at a highly active in the MS daughter cell (Park and Priess, 2003). The high concentration of POP-1 represses expression of end1 and end3 in the MS cell, thereby inhibiting endoderm establishment in the mesoderm-fated MS cell. POP-1 in the E cell is bound by β-catenin thereby converting the POP-1 repressor into an activator and directing that cell toward endoderm specification through transcriptional activation of end1 and end3 (Maduro and Rothman, 2002; Owraghi et al., 2010). End1 and End3, the early activated genes in the E cell, are Gata transcription factors and serve as pioneers that activate downstream transcription factors in the endoderm lineage. The high level of POP-1 repression in the MS cell represses the endoderm fate, allowing maternal Skn-1 to activate med1 and med2, both pioneering Gata factors, that activate downstream mesodermal genes (Lowry et al., 2009; Owraghi et al., 2010). These early regulatory steps thus produce a very short endomesoderm status. The brevity of these earliest regulatory steps is facilitated by maternal components that activate zygotic expression of different Gata factors in the E and MS cells and these drive endoderm and mesoderm specification, respectively.
Figure 1:
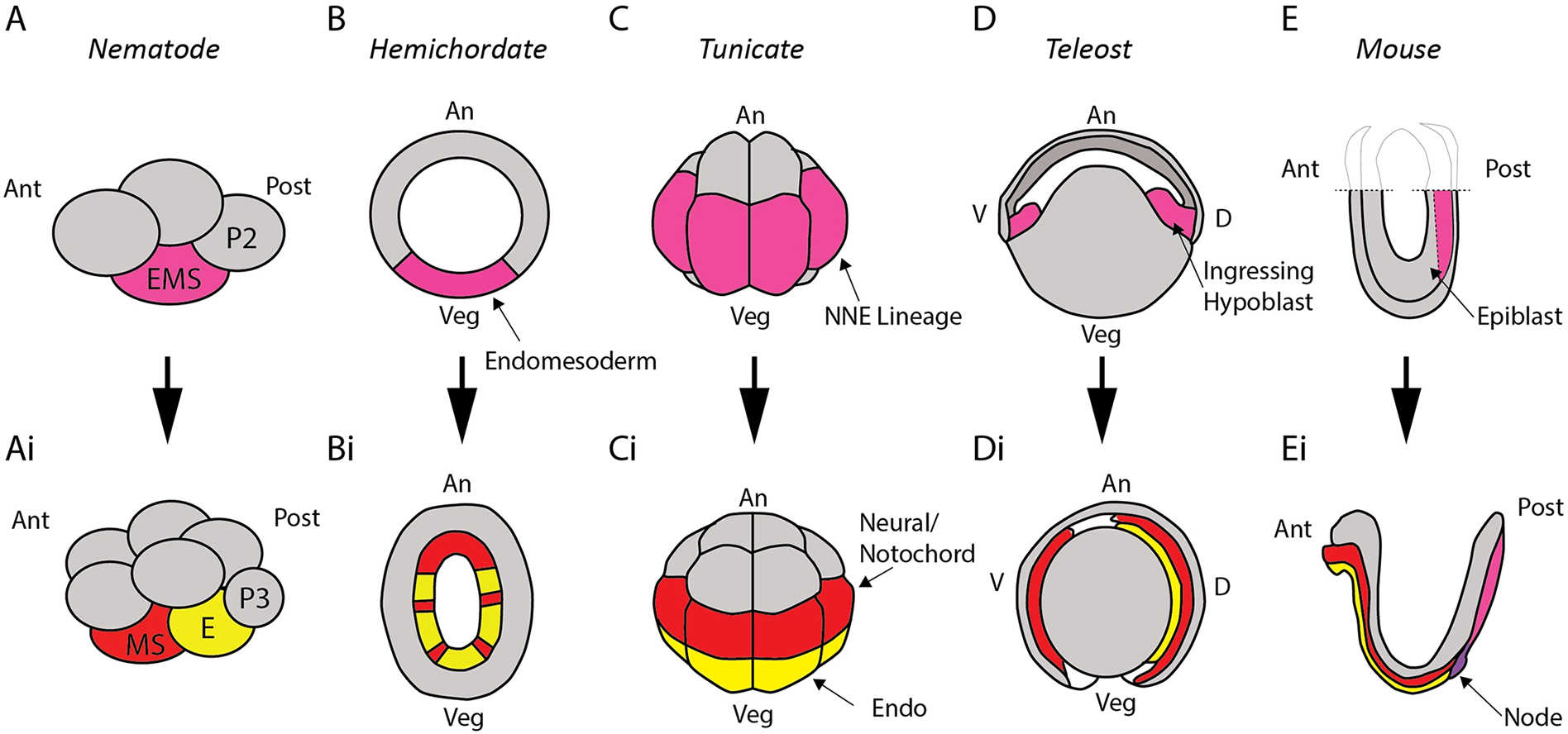
Conditionally specified endomesoderm (or mesendoderm) shown in magenta across a selection of metazoan embryos as it resolves into endodermal lineages (yellow) and mesodermal lineages (red). In nematode embryos (A), the EMS cell gives rise to the MS and E cells (Ai), which specify mesoderm and endoderm respectively. In developing hemichordates (B), a conditionally specified endomesoderm later resolves into endoderm and mesoderm after gastrulation (Bi). Tunicate embryos at the 16-cell stage (C) exhibit high levels of nuclear β-catenin in the NNE lineage before it resolves into endoderm and neural-notochord (i.e. ectoderm-mesoderm) lineages (Ci). In fish embryos (D), during epiboly, ingressing hypoblast cells give rise to either endoderm or mesoderm (Di), suggesting a conditionally specified fate earlier. Mouse embryo epiblast (E) also exhibits cells with a potential to form endoderm or mesoderm (Ei), fates that become committed as cells pass through the primitive streak.
An earlier study with C. elegans on the timing of endoderm cell fate decision demonstrates the temporal sequence of this regulation. Goldstein (1992) separated the EMS cell from the signaling P2 cell at different timepoints after completion of second cleavage. Approximately 5–6 min of cell contact (signaling) between the P2 and EMS cell, after completion of second cleavage, was necessary before a then isolated EMS cell produced an E cell at the next division. Since reception of a canonical Wnt signal results in nuclear accumulation of β-catenin, it is presumed that 5–6 minutes into the 4-cell stage provides the necessary time for enough β-catenin accumulation to effect the response in the E cell. A later study determined when the EMS cell loses its ability to respond to the P2 signal. The EMS cell was isolated before the signal from P2 was received, and subsequently was recombined with the P2 cell at different timepoints. The competence of the EMS cell to respond to the P2 Wnt signal was lost about 3 min before the subsequent division (third cleavage) (Goldstein, 1995). Given that the cleavage cycle at that time is about 15 minutes, the EMS cell is thus competent to receive the inducing signal for approximately 12 minutes (Goldstein, 1995). However, Goldstein (1995) also showed that the P2 cell continues to signal after the EMS cell divides into an E and a MS cell, highlighting that signal reception can continue for several minutes into third cleavage assuring accumulation of enough nuclear β-catenin to specify the E blastomere. In this way the conditionality of the EMS cell declines as the different Gata factors become activated in the E and the MS cell.
In hemichordates, Wnt signaling is also involved in the conditional status of the endomesoderm. In these animals, Wnt signaling induces the establishment of this conditional state rather than its separation (Darras et al., 2011) (Fig. 1). FoxA (an endoderm marker) and zic (a mesoderm marker) are co-expressed by the endomesoderm cells for a period of time, demonstrating the existence of that conditional endomesoderm state. Later, during gastrulation, a FGF8/17/18 signal, issued from the ectoderm, induces some of the endomesoderm cells to become mesoderm (Green et al., 2013). Augmented FGF8/17/18 causes the entire endomesoderm to be specified as mesoderm, while knockdown of FGF8/17/18 results in the expression of endoderm markers only (Green et al., 2013). Thus, the endomesoderm of hemichordates is conditionally specified from early cleavage until sometime during gastrulation. Current data however do not reveal how that conditionality is maintained or what happens normally in endomesoderm cells that do not receive the FGF8/17/18 signal, and presumably become endoderm. Further, while early Wnt signaling plays a role in the initiation of endomesoderm, the details of this process are still incomplete.
In the urochordate Ciona intestinalis, a tunicate, mesendoderm (here, as with each of the model embryos covered, we use the naming convention for either mesendoderm or endomesoderm, used in that model system) establishment again is triggered by Wnt signaling. This takes place at the 16-cell stage in four vegetal cells, the NNE lineage (Fig. 1). Subsequently, one cell division later, the vegetal-most progeny of these cells exhibit continued nuclear accumulation of β-catenin and become endoderm, while the animal progeny of these cells lose nuclear accumulation of β-catenin and become neural/notochord (i.e. ectoderm/mesoderm) (Hudson et al., 2013; Imai et al., 2016; Oda-Ishii et al., 2016). At the 16-cell stage, the mesendoderm cells express foxA, foxD, and fgf9/16/20 and knockdown experiments demonstrate that all three genes are necessary for the transient mesendoderm state (Hudson et al., 2016). The short-lived mesendoderm state in C. intestinalis is similar to the EMS cell in C. elegans, in that the mesendoderm and EMS cells display a very short period of conditional specification. In addition, in both animals, the short-lived mesendoderm state deploys the same mechanism to exit the conditional state, since in the tunicate β-catenin binds to TCF7 target sites as a co-activator to initiate Gata factor expression in the cells fated to be endoderm. Coincidentally, β-catenin blocks the binding of the Gata.A factor onto the enhancer of a mesoderm specifying gene zic-r-b (Hudson et al., 2013, Imai et al., 2016). This dual effect hence leads to the establishment of endoderm. In the animal progeny of the NNE cells the absence of β-catenin enables activation of the Gata.A factor and development of mesoderm. Thus, in this tunicate it appears that β-catenin is involved both in specifying mesendoderm and also in the later separation of mesendoderm progeny into either endoderm or mesoderm, with asymmetrically positive β-catenin signaling favoring the endodermal fate. There is also evidence that asymmetric Nodal signaling is involved in the separation of endoderm and mesoderm (Shi and Levine, 2008). Daughters of the mesendoderm that receive Nodal inputs from bordering cells become mesoderm, the Nodal signal resulting in inhibition of MAPK signal transduction thereby allowing activation of mesoderm genes in these cells (Shi and Levine, 2008).
Lineage studies in zebrafish also reveal a mesendoderm population of cells during epiboly (Warga and Nusslein-Volhard, 1999; Kikuchi et al., 2004; Kimmel and Warga, 1988) (Fig. 1). When single cells were labeled at the margin of the gastrulating zebrafish, the progeny of some of those cells gave rise to endoderm while other progeny of that labeled cell became mesoderm, and this was true if a single cell was initially labeled as late as 40% epiboly. If a single cell was first labeled later than 40% epiboly, all progeny of that labeled cell became either mesoderm or endoderm, thus indicating that after 40% epiboly a mesendoderm state was no longer present. The presence of a mesendoderm state was further supported recently through single cell RNA-seq studies, which show that the trajectory of the ectoderm first diverges from a mesendoderm population before the progeny of this population then diverges into mesoderm and endoderm after a relatively brief dual state (Farrell et al., 2018; Wagner et al., 2018). Induction of mesendoderm in zebrafish, as in other vertebrates examined, requires β-cateninGF β-catenin signaling (Rodaway et al., 1999), with two genes, cyclops and squint, both Nodal related genes (Feldman et al., 1998) providing the inputs. Several transcription factors necessary for specification of both mesoderm and endoderm are activated by this induction and co-expressed in the same cells (Poulain and Lepage, 2002). Thus, at least for a short while, a mesendoderm gene regulatory network operates in some cells at the margin of the blastoderm. Shortly thereafter, as epiboly continues, the mesendoderm state diverges, and this has been attributed in part to FGF and Delta-Notch signaling (Kikuchi et al., 2004; van Boxtel et al., 2018). A recent study indicates that this separation involves an incoherent feed forward regulatory loop such that Nodal activates expression of fgf and dusp4. The marginal mesendoderm cells that express a higher level of dusp4 repress FGF signal transduction and become endoderm. Mesendoderm cells more distant from the margin retain FGF signaling due to a low level of dusp4 expression and as a consequence these more distant cells become mesoderm (van Boxtel et al., 2018). This signaling and regulatory sequence begins at the dome stage and is completed by 50% epiboly. Thus, the conditional specification of mesendoderm lasts for only about 1.5 hours, though for individual cells the timing may vary since the conditionality is not synchronous for all cells in the population.
The early stages of amphibian development include an overlap between the endodermal and mesodermal domains leading to an area often referred to as the mesendoderm (Charney et al., 2017). Early cut and paste experiments demonstrated that amphibian embryos are remarkably regulative, and these were highlighted by the famous organizer induction experiments by Spemann and Mangold, (1924). At the molecular level, a compilation of genes expressed during early mesendoderm specification suggests that this regulatory state lasts from mid-blastula transition (MBT) until about stage 10 (hence between 4 to about 11 hours post-fertilization). Perturbations of a number of those genes, expressed during this time frame, shift either mesoderm to endoderm or in the opposite direction (Henry and Melton, 1998; Zorn et al., 1999; White et al., 2002; Chiu et al., 2014; Shivdasani, 2002). It isn’t clear whether mesendoderm cells exist that express transcription factors of both fates, or whether the networks are separately mesoderm or endoderm from the beginning, but retain a level of conditionality enabling external inputs to easily shift the networks toward the alternative fate. Vegetal cells isolated prior to the start of gastrulation and inserted into the blastocoel of host embryos do not demonstrate commitment to endoderm until the early gastrula stage (about stage 10.5) (Wylie et al., 1987). Thus, the extended period of regulative development is a period prior to gastrulation during which the mesendoderm is conditional, regardless of whether the GRN leans toward an endoderm or mesoderm fate. Nodal and VegT signals, from the underlying normally-fated endoderm, induce mesoderm, though it has been noted that Nodal also plays a dual role to specify endoderm (Loose and Patient, 2004; Zhang and Klymkowsky, 2007). In addition, genes activated that lead toward commitment of either endoderm or mesoderm include Sox, Gata, and Fox family of transcription factors, all considered to be pioneer factors (Iwafuchi-Doi and Zaret, 2016; Iwafuchi et al., 2020; Zaret, 2020; Zaret and Carroll, 2011; Takahashi and Yamanaka, 2006). A valuable asset of research on endomesoderm in Xenopus is the large number of regulatory genes that are already known, including a rich knowledge of GRN contributions (Charney et al., 2017; Loose and Patient, 2004).
In birds and mammals, mesendoderm formation is initiated in the epiblast (Tsakiridis et al., 2014) and by the time the cells enter the primitive streak both endoderm and mesoderm appear to be largely committed toward their separate fates (Nowotschin et al., 2019; Nowotschin and Hadjantonakis, 2020) (Fig. 1). Studies on embryonic stem cell progression have been used to model the molecular path toward endoderm and mesoderm fates of epiblast cells. The pluripotent state is activated by Nodal family members and is maintained by expression of Nanog (Vallier et al., 2009). Once the primitive streak is established the expression of several transcription factors has been reported to be essential both for the entry of mesendoderm progeny through the primitive streak and for the fate restrictions of these cells (Acloque et al., 2011; Stryjewska et al., 2017). Curiously two such transcription factors, Snail and Zeb2, are also known regulators of epithelial mesenchymal transition (Cano et al., 2000; Taube et al., 2010).
From the brief sampling of the literature, a survey across the animal kingdom indicates that a conditional state of endomesoderm exists for many embryos, even if for a very brief period of time. Establishment of that state frequently involves Wnt signaling, and in vertebrates Nodal signaling is the most common signal used, although in vertebrates there is evidence that an earlier Wnt/β-catenin signal may also initiate the specification sequence (Larabell et al., 1997; Miller and Moon, 1997). Experiments in several organisms have demonstrated that once a cell is conditionally specified as endomesoderm, perturbations can push this cell entirely toward endoderm or entirely toward mesoderm. Further, once the endoderm and mesoderm fates are achieved, conditionality tends to be quickly terminated. A common feature of that termination is the activation of Gata and Fox factors, both known to be pioneer transcription factors that open closed chromatin regions (Zaret and Carroll, 2011). FoxA, a gene involved in early specification of endoderm in many embryos throughout the animal kingdom, is a good example of a pioneer transcription factor as it is known to play an important role in opening chromatin to allow access to enhancer sites that then drive the expression of endoderm specification genes (Iwafuchi-Doi and Zaret, 2016).
Developmental plasticity in the sea urchin embryo
The regulative capacities of sea urchin embryos are well known and have been studied for well over 100 years. Following the pioneering observations of Driesch (Driesch, 1892), the remarkable experiments of Hörstadius (1939) provided seminal insights into the regulatory capacity of these embryos. Hörstadius isolated and recombined many different embryo fragments. These showed the regulative capacity of cells, their inductive properties including temporal competence, and a differential loss of plasticity as differentiated cell types emerged. These early findings set the stage for later cellular and molecular studies that began late in the twentieth century. But even before the molecular explosion of information, those classic studies provided a number of clues about endomesoderm. Hörstadius showed that mesomeres (the cells originating in the animal hemisphere during early cleavage), if isolated after third cleavage could only produce ectoderm, while endomesoderm originated in the vegetal half embryo (Hörstadius, 1939). Experimentally, however, Henry et al. (1989) showed that the position of the third cleavage plane actually matters in this determination. Indeed, if that cleavage plane occurs below the equator of the embryo, isolated animal halves now produce endoderm (in addition to ectoderm) with some frequency. This indicates that the maternal information, localized in the vegetal half-embryo is a major contributor to endomesoderm.
A later study asked whether endoderm or mesoderm cells, once in the archenteron, were irreversibly committed. At late gastrulation, pieces of the archenteron were removed and in response the embryos replaced the missing tissues (McClay and Logan, 1996). The replacement came from adjacent cell populations; i.e. if just the midgut was removed, cells of the foregut and hindgut shifted to the identity of midgut cells and re-established the correct proportionality of the three gut parts. Likewise, if the non-skeletal mesoderm (NSM) cells were removed, presumptive endoderm replaced the NSM cells. In still other studies, it was further shown that if the skeletogenic mesoderm cells were removed, they were replaced by NSM cells (Ettensohn and McClay, 1988).
These experiments raised a different question however. Was this demonstrated plasticity a reflection of conditional specification, or was the replacement observed actually a demonstration of regeneration? And what is the difference between the two processes? To address this issue, animal halves (cells above the equator of the spherical embryo) were isolated at different times after fertilization and their capacity to replace vegetal tissues (i.e. endoderm and mesoderm) was examined (Cheng et al., 2014). If isolated at any time from the 16-cell to the hatched blastula (HB) stage, the animal caps produce only ectoderm, suggesting that by fourth cleavage, those cells already lose their regulative properties. Transplantation of one or up to four micromeres (the vegetal-most cells of the embryo) onto animal caps induced the development of a second axis and of endomesoderm (Cheng et al., 2014; Croce et al., 2011; Horstadius, 1939; Ransick and Davidson, 1993), demonstrating that a transitory regulative ability of the animal halves does exist. However, that inductive competence ends after 4th cleavage. (Ransick and Davidson, 1993; Cheng et al., 2014). Subsequently, after a long refractory period, if animal caps are isolated following the beginning of gastrulation, they are capable of replacing endoderm, with no additional input (Cheng et al., 2014). Morpholinos to either Hox11/13b or FoxA, two early endoderm specification factors, prevented the gastrula-stage animal halves from producing endoderm, indicating that replacement of endoderm likely involves similar pathways as the original specification of endoderm. But is that a demonstration of conditionality? These data were interpreted to indicate that the early regulative ability of germ layers likely owes its plasticity, at least in part, to conditional specification, while the late replacement ability is likely due to the onset of a regenerative capacity through cell-reprogramming. Whether the two properties, conditional specification and regenerative capacity, share aspects of their molecular machinery, is unknown.
Interestingly, skeletogenic mesoderm and non-skeletal mesenchyme (NSM) cells demonstrate properties similar to those just described. The micromeres, precursors of the skeletogenic mesoderm cells (also known as Primary Mesenchyme Cells (PMCs)), are known to be specified autonomously almost from the time of their origin at the 16-cell stage. Indeed, if 16-cell stage micromeres are isolated and put into culture, or transplanted to ectopic positions in the embryo, these cells autonomously produce skeletogenic mesoderm cells and these cells only (Okazaki, 1975), hence corroborating an early committed state for these cells. In addition, these cells never replace any other cell type in embryos depleted of any other germ layer. If they are forced to overexpress an NSM specifier, such as Gcm, prior to the 16-cell stage they can differentiate as NSM, but that forced expression has to occur before the seminal specification event, the so-called double repression gate, that initiates skeletal cell specification (Damle and Davidson, 2012; Oliveri et al., 2003; Revilla-i-Domingo et al., 2007).
While the micromeres are specified autonomously, if they are removed from the embryo at the 16-cell stage, NSM cells reprogram to replace them, however not immediately. The replacement doesn’t take place until gastrulation (Cheng et al., 2014), outlining again, the existence of a long refractory period. Thus, in early cleavage the skeletogenic mesoderm cells as well as the animal pole cells depart from conditionality quite quickly, and much later in development the animal pole cells and the NSM cells are capable of cell fate changes following an extended refractory period. These cell fate change capacities, launched at gastrulation, are likely due to the onset of a regenerative ability to reprogram the cells. While the causal mechanisms behind that reprogramming property are still not understood, they are likely distinct from the mechanisms that maintain conditionality. By contrast, the endomesoderm appears early in cleavage and experiments demonstrate that it retains conditionality for an extended period of time relative to the ectoderm and skeletogenic cells.
Conditionally specified endomesoderm in the sea urchin
Over the past 20+ years a detailed gene regulatory network (GRN) has been constructed for early sea urchin development. Cells arising from the vegetal half of the embryo are specified as endomesoderm for a period of time before separating into the distinct endoderm and mesoderm lineages. The GRN underlying these events is supported by an extensive series of experiments and in some cases, detailed cis-regulatory analyses, thereby providing a number of insights into the onset, maintenance, and termination of the conditional endomesodermal state. The GRN that is assembled during the conditional period includes expression of mRNAs that will ultimately become part of either the mesoderm or endoderm GRNs. The GRN that persists to become endoderm or mesoderm depends on the input each cell receives from its local environment.
To understand the onset of the conditionality, it is necessary to review a few of the early cleavage events. An unequal fourth cleavage results in four micromeres at the vegetal pole of the embryo and four macromeres above them. The next cleavage of the micromeres also is unequal and produces small and large micromeres with the large micromeres fated to be the skeletogenic mesoderm cells and the small micromeres fated to contribute to, or be, the primordial germ cells (Fresques et al., 2016; Juliano et al., 2010; Yajima and Wessel, 2012). As soon as the micromeres appear they accumulate nuclear β-catenin and activate expression of pmar1 (Logan et al., 1999; Oliveri et al., 2002; Oliveri et al., 2003). Pmar1 is a repressor that rapidly represses activation of a second repressor, hesC (Revilla-i-Domingo et al., 2007). One consequence of the hesC repression is that by 5th cleavage the micromeres initiate expression of delta (Sweet et al., 2002). As this occurs, the eight macromeres at 5th cleavage, the cells just above the micromeres (Fig. 2), exhibit nuclear accumulation of β-catenin, a necessary step in the activation of endoderm (Logan et al., 1999; Wikramanayake et al., 1998; Emily-Fenouil et al., 1998). At the same time, Delta, produced by the micromeres, begins signaling to those eight adjacent macromeres, each of which expresses maternal Notch (Sherwood and McClay, 1999), and the consequence is activation of gcm, a mesoderm-specific transcription factor (Ransick, et al., 2002). Meanwhile, Wnt signaling results in those same cells expressing eve, and perhaps other endoderm transcription factors {Peter, 2010 #8462}. Thus, by the end of the 5th cleavage to the early 6th cleavage the eight Veg2 macromeres have become endomesoderm and express marker genes of both germ layers. Perturbation experiments showed that augmentation of β-catenin throughout the embryo causes all cells to become endoderm, while inhibition of β-catenin accumulation results in a completely animalized embryo that expresses ectoderm markers only (Emily-Fenouil et al., 1998; Logan et al., 1999; Wikramanayake et al., 1998). Later studies with a GFP tagged form of β-catenin further showed that the β-catenin is actively destroyed in the animal hemisphere (Weitzel et al., 2004), while its initial accumulation, at least in the micromere nuclei, appears to be due, not to a Wnt signal, but to absence of Axin from these cells (Sun et al., 2021). Axin normally promotes destruction of β-catenin to prevent its accumulation (Zeng et al., 1997), so this early accumulation of β-catenin likely occurs in the absence of a Wnt signaling input. Whether that mechanism also is involved in the increased nuclear β-catenin in macromere nuclei at 5th cleavage, or whether that accumulation is due to expression of Wnt8 by micromeres at 4th cleavage (Wikramanayake et al., 2004), isn’t known. Overexpression of Wnt8 leads to an excess of endomesoderm, while knockdown of Wnt8 expression eliminates or greatly reduces endomesoderm specification (Wikramanayake et al., 2004). These observations led to the hypothesis that Wnt8 signaling either activates or augments β-catenin, depending on the amount of Axin present.
Figure 2:
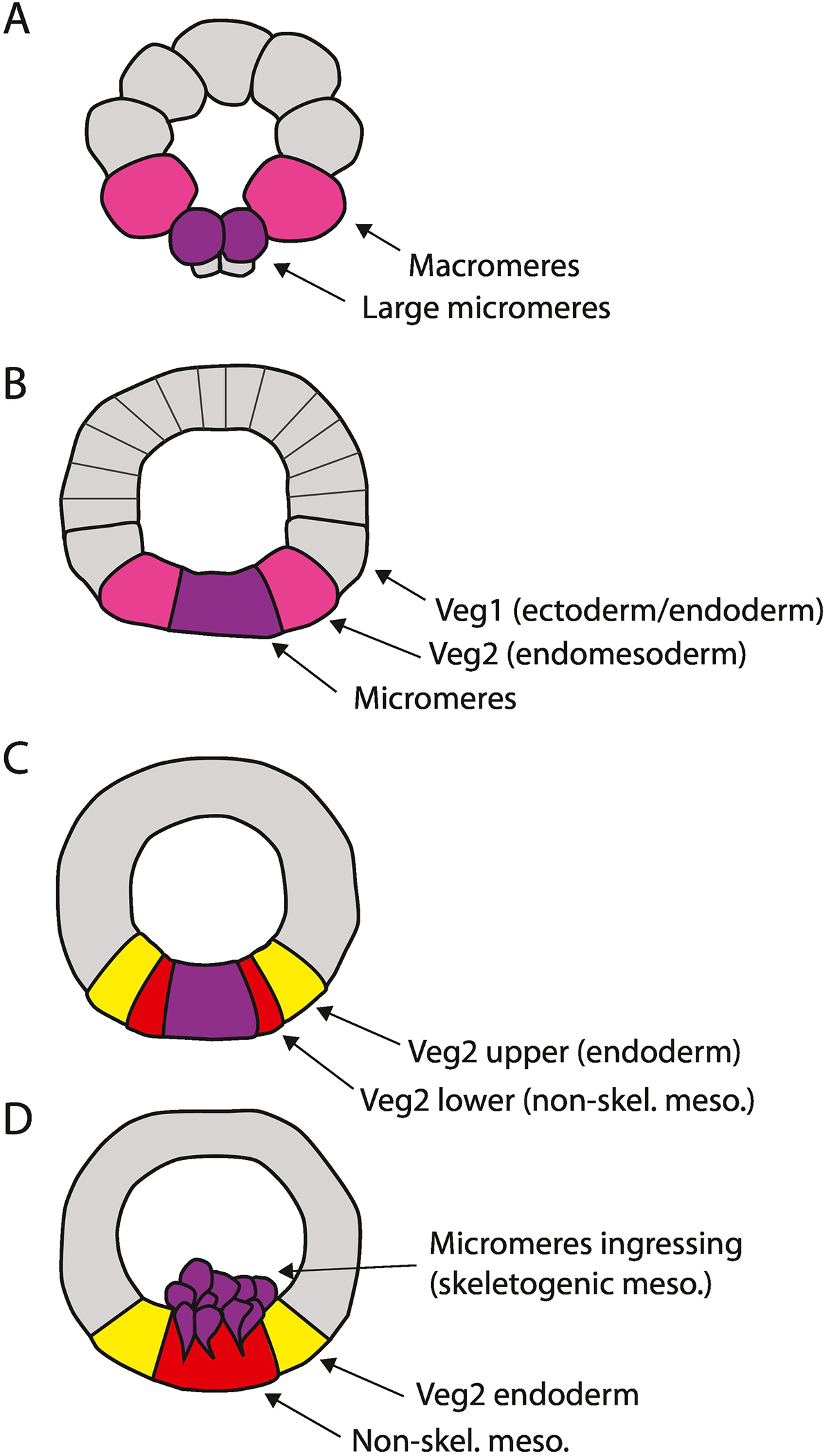
Conditional specification of endomesoderm in the sea urchin. At the 32-cell stage (A) the large micromeres (purple) first activate delta expression. The neighboring macromeres (magenta) display a high level of nuclear β-catenin that, along with the Delta input, initiates a conditionally specified endomesoderm. These signals persist through at least the 8th cleavage. (C) Eighth cleavage divides Veg2 cells into two tiers. The anterior progeny (the Veg2 upper tier) lose contact with the micromeres and therefore with the Delta signal thereby losing their mesodermal potential, while the Veg2 lower cells remain in contact with the Delta producing micromeres and adopt a mesodermal fate. (D) At the beginning of gastrulation, the micromeres undergo an epithelial-mesenchymal transition to become the PMCs, and the non-skeletal mesoderm will soon initiate invagination of the archenteron, followed by invagination of the Veg2 endoderm and finally the Veg1 endoderm.
Perturbation experiments with Delta demonstrate that any macromere contacting micromeres initiates expression of the mesodermal transcription factor gcm (Croce and McClay, 2010), and gcm is a direct target of Notch signal transduction through SuH (Ransick and Davidson, 2006; Ransick et al., 2002). Thus, the Wnt pathway and the Delta-Notch pathway combine to activate endomesoderm starting late in 5th cleavage. In the sea urchin Paracentrotus lividus, similar experiments showed that the transmembrane receptor Frizzled1/2/7, likely activated by the ligand Wnt6, triggers the nuclear accumulation of β-catenin specifically in the macromeres at fifth cleavage (Lhomond et al., 2012), supporting the hypothesis that β-catenin nuclearization in the macromeres is driven by a Wnt signal. The Frizzled1/2/7 signaling was further established as necessary for the early expression of some endomesoderm genes, such as wnt8 and blimp1, in the vegetal progeny of the macromeres as well as subsequently for the expression of early endoderm genes, such as foxA, in their descendants (Lhomond et al., 2012). Thus, as seen in embryos from many other phyla, canonical Wnt signaling appears to establish the top of the endomesoderm gene regulatory network in the sea urchin and to subsequently promote endoderm development.
At sixth cleavage, an equatorial division separates the macromeres into an upper tier of Veg1 cells and a lower tier of Veg2 cells (Fig. 2). Starting at this stage and onward, the Veg2 cells remain in contact with the Delta-positive micromeres, while the Veg1 cells lose contact with Delta. From that point forward, the Veg1 cells continue specification toward endoderm, and also, depending on relative inheritance of maternal information (due to the position of the third cleavage plane), toward ectoderm (Logan and McClay, 1997). The Veg2 cells, continue contact with the delta expressing micromeres and this maintains expression of mesodermal genes in these cells. The Veg2 cells also continue to respond to Wnt signaling through β-catenin and TCF/lef to maintain early endoderm specification by out-competing Groucho repression of TCF/lef (Range et al., 2005). As a consequence, between 6th and 8th cleavage the Veg2 endomesoderm cells express both early endoderm genes such as foxA and hox11/13b, and early mesoderm genes such as gcm and gatae (Croce and McClay 2010; Peter and Davidson, 2010; Lhomond et al., 2012; Materna et al., 2013), thereby demonstrating the endomesodermal state of these cells through this time period
A number of experiments reveal the conditionality of that endomesoderm state during this time. Increased Wnt signaling increases endoderm (Logan et al., 1999; Wikramanayake et al., 1998; Wikramanayake et al., 2004; Lhomond et al., 2012), while increased Notch signaling expands mesoderm at the expense of endoderm (Sherwood and McClay, 1999). Decreased Notch signaling results in endoderm expansion at the expense of mesoderm (Sherwood and McClay, 1999), and decreased Wnt signaling diminishes both mesoderm and endoderm specification (Logan et al., 1999; Wikramanayake et al., 1998; Lhomond et al., 2012). Thus, the dual signaling from Wnt and from Delta appears to maintain the conditional state of endomesoderm for at least from 6th cleavage (60-cell stage) to 8th cleavage. Experiments showed that Delta signaling must be continuous in order to maintain the mesoderm gene expression in the endomesoderm (Croce and McClay, 2010), and a continuing Wnt signal likely is necessary to maintain expression of the endoderm genes during this time.
At 8th cleavage, an equatorial cell division separates the Veg2 cells into two distinct cell tiers, the lower Veg2 tier and the upper Veg2 tier (Fig. 2). The upper Veg2 cells, farther away from the vegetal pole, lose contact with the Delta-producing micromeres and extinguish mesoderm marker gene expression, while they maintain expression of the endoderm markers (Croce and McClay, 2010; Peter and Davidson, 2010). If a Delta-expressing micromere is introduced ectopically to continue the contact with the upper Veg2 cells beyond the 8th cleavage, the upper Veg2 cells in contact with that ectopic cell continue to be endomesoderm and become mesoderm (Croce and McClay, 2010) (Fig.3). That experiment thus begged the question as to how long was the Delta-Notch signal necessary before cells commit to mesoderm. To address this question, a series of transplantation experiments used a fluorescently tagged micromere that was ectopically placed between the Veg1 and Veg2 cells at 6th cleavage (60-cell stage) before removing it later. When left in that position, at least until to the HB stage (9 hours post-fertilization), both upper and lower Veg2 cells, remaining in contact with a Delta-producing micromere, continued to express gcm beyond 8th cleavage (Fig. 3). In the experiment the ectopically placed micromere was later removed to reveal when the Veg2 cell was able to continue toward mesoderm differentiation in the absence of Delta. If the micromere was removed between 7th and 8th cleavage (~6.5 hours post-fertilization), the upper Veg2 cells that had been expressing gcm for one or two cell cleavages, extinguished gcm expression and became endoderm. On the other hand, if the fluorescently tagged ectopic micromere was removed about an hour after 8th cleavage (that is to say after about 3 hours of continuous contact with Delta), the upper Veg2 cells differentiated as mesoderm (Croce and McClay, 2010). The explanation for these results is understood through experiments showing establishment of a feedback regulatory circuit between the Delta-Notch signal and Gcm (Materna et al., 2013; Ransick and Davidson, 2012). Gcm expression is first seen early in 6th cleavage. Under continuing Delta signaling, Gcm activates gatae expression. If Delta signaling continues even longer, Gatae activates expression of six1/2. If Delta signaling lasts even longer, Six1/2 accumulates and feeds back to maintain active gcm expression. That feedback activation of gcm expression removes the requirement for continuing Delta signaling, and now establishes a mesodermal differentiation trajectory. This at least partially explains how the conditionality is terminated for cells that will become mesoderm. Additionally, it has been hypothesized that Notch signaling represses hox11/13b expression in endomesoderm thereby reducing the likelihood that Veg2 cells become endoderm (Sethi et al., 2012). Since it is thought that the endodermal contribution to endomesoderm is maintained by Wnt signaling, Sethi, et al., (2012) also showed that Notch signaling reduced the expression of Wnt1, a reduction that may divert cells increasingly toward the mesodermal fate.
Figure 3:
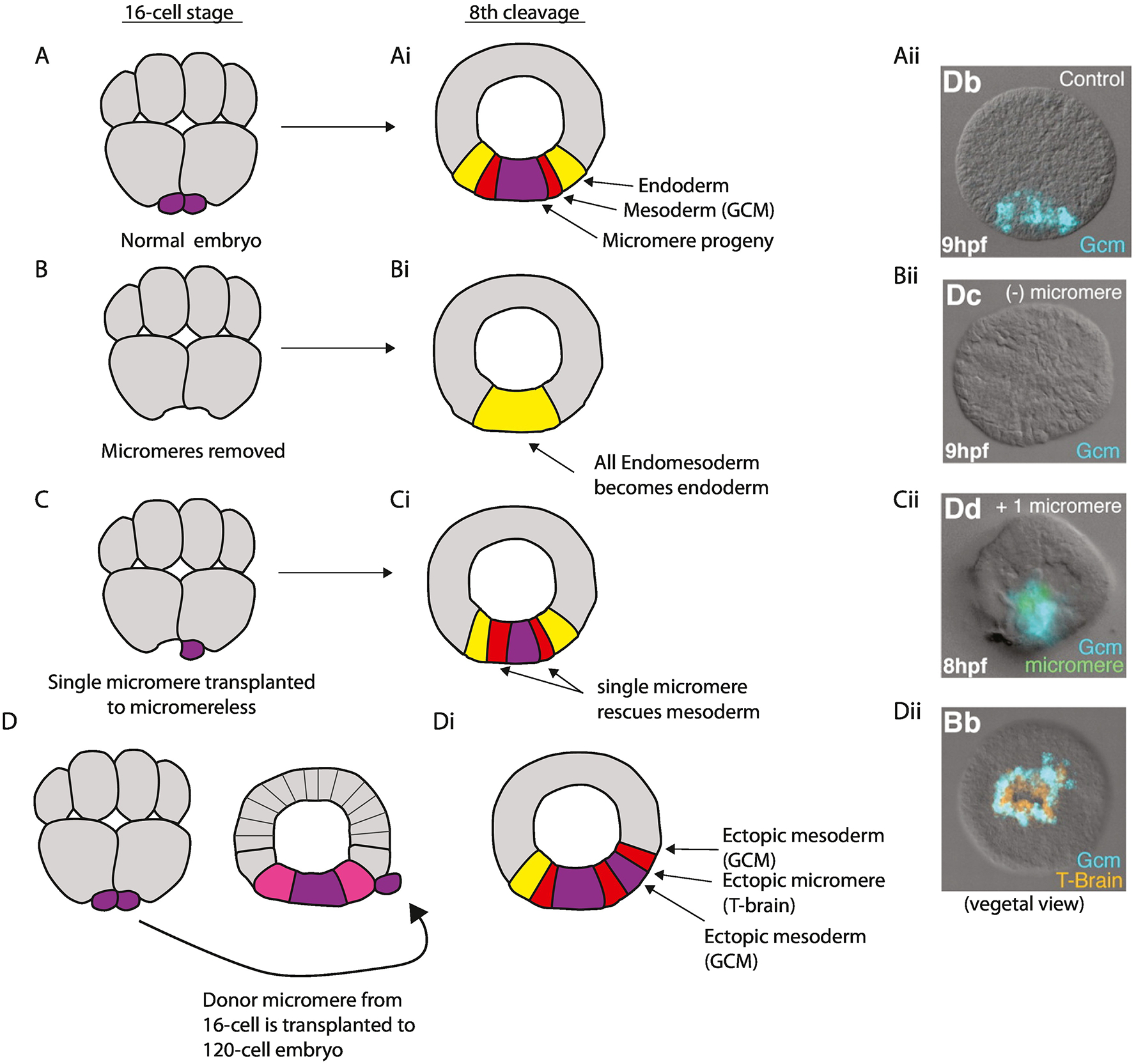
Delta-Notch signaling from the micromeres establishes mesoderm. Micromeres first appear at the 16-cell stage (A), activate delta expression at the 32-cell stage and begin expressing Delta at the 60-cell stage (Croce and McClay, 2010). (Ai) This signaling induces mesoderm gene expression in the adjacent endomesoderm (Veg2) cell layer (Ransick et al., 2002; Croce and McClay, 2010; Peter and Davidson, 2010). (Aii) Gcm, a mesoderm marker in the endomesoderm, is expressed at hatched blastula (HB) (reproduced from Croce and McClay, 2010). (B) If the micromeres are surgically removed at the 16-cell stage all endomesoderm is specified as endoderm only (Bi) because Delta is absent. (Bii) Consequently gcm is not expressed. (C) A single labeled micromere reintroduced at 16-cell stage in an otherwise micromere deficient embryo is sufficient to rescue mesoderm development and gcm expression (Ci, Cii). (D) If a micromere from a 16-cell stage embryo is transplanted to an ectopic location of a 60-cell stage wild-type embryo between the Veg1 and Veg2 layers that ectopic micromere induces ectopic mesoderm in the adjacent Veg2 lower and Veg2 upper tissues (Di, Dii). Embryos Aii, Bii, Cii and Dii are at the HB stage (8 to 9 hours post-fertilization). They are all in lateral view with the animal pole to the top, except the embryo in Dii that is rotated to view the embryo from the vegetal pole to show the ring of mesoderm expressing gcm surrounding the endogenous and ectopic micromeres, which otherwise express T-brain, a micromere-descendant marker.
It has been proposed that the accumulation of FoxA in presumptive endoderm cells is required to exclude mesoderm by repressing gcm (Oliveri et al., 2006), and that accumulation of Gcm causes repression of foxA expression (Peter and Davidson, 2009). However, cis regulatory analyses of the foxA enhancer does not show the presence of a Gcm binding site (de-Leon and Davidson, 2010), nor does analysis of the gcm enhancer show the presence of a FoxA binding site (de-Leon and Davidson, 2010), so this previously proposed reciprocal repression, if present, is indirect.
Furthermore, the mechanism through which endoderm cells lose their endomesoderm conditionality is not fully resolved. It could be by default (simply loss of Delta input while continuing to receive a Wnt input), or there could be an active mechanism directing endodermal commitment. What is known is that immediately following the loss of direct Delta signaling at 8th cleavage, the upper Veg2 cells begin expressing the brachyury (bra) transcription factor (Croce et al., 2001; Gross and McClay, 2001). Cis-regulatory studies indicate that Hox11/13b, Otx, and Blimp1, all factors expressed in the endomesoderm, have an input into the regulatory region of bra (Peter and Davidson, 2010). Bra, in turn, binds to the enhancer of foxA (de-Leon and Davidson, 2010). FoxA is expressed in endomesoderm earlier than endodermal bra, but at a low level. If bra expression is perturbed, foxA expression is retained at that low expression level. When bra expression is unperturbed, however, foxA expression increases after 8th cleavage to a high level and gcm expression is extinguished (Oliveri et al., 2006). The high level of foxA expression also requires continuing expression of Hox11/13b, otx and blimp1, as well as β-catenin-TCF activity and the expression of their common target bra (de-Leon and Davidson, 2010; Oliveri et al., 2006; Peter and Davidson, 2010). Thus, it is likely that the loss of conditionality of the endoderm-fated cells is due to the dual loss of Delta signaling and the increased expression of foxA, with FoxA acting as a pioneer transcription factor to open chromatin sites for further endoderm specification, while perhaps simultaneously, directly or indirectly inhibiting gcm.
A recent single cell RNA-seq analysis provides further insights into the endomesoderm state (Massri, 2021). Cells were sequenced at 18 time points through the first 24 hours of Lytechinus variegatus development. A bioinformatic approach (Waddington Optimal Transport) revealed the molecular trajectory of the lineages. That analysis provided a view of the endomesoderm trajectory, and the later separation of endoderm and mesoderm. As expected, cells expressing bra diverged toward endoderm, while cells expressing gcm diverged toward mesoderm after the HB stage. The divergence, however, was not synchronous. Some cells expressed only endoderm or mesoderm markers soon after the HB stage, but many cells continued to express both endoderm and mesoderm markers for at least an additional 4 hours (Massri, 2021). These data indicate that conditionality ends asynchronously and perhaps inputs other than retention or loss of Delta-Notch signaling have an influence on resolution of the endomesodermal state.
Conclusions
Conditionality of the endomesoderm across the animal kingdom reveals a number of shared properties that lead to that state, properties of the conditionality itself, and properties that commit cells to subsequent fates. The Wnt signaling pathway is almost universally used to help initiate endomesoderm specification with Nodal signaling also used in many deuterostomes. The duration of conditionality is variable, from minutes to a number of hours. Where it has been studied in detail, that conditionality ends asynchronously with some cells retaining a conditionally specified state up to hours longer than other cells in the same organism. Resolution of the endomesoderm state is almost always due to asymmetric reception or loss of a signal. And in reaching definitive mesoderm and endoderm, pioneer transcription factors are thought to open chromatin, thereby initiating the gene regulatory sub networks of the two distinct germ layers. Often, when exiting the endomesoderm state, transcriptional repressors actively repress network components of the opposite germ layer.
The sea urchin endomesoderm GRN exhibits most of these shared properties. The endomesoderm state is regulated by a metastable network that can be pushed in either direction, endoderm or mesoderm, by perturbation of relatively few factors. The mesodermal state is increasingly stabilized by acquisition of a feedback loop established in sea urchins by Gcm, Gatae, and Six1/2. The endodermal state is increasingly stabilized by loss of Delta-Notch signaling, and an increase in Brachyury-directed foxA expression which represses the mesoderm circuitry and opens downstream endodermal GRN targets. Some features of the sea urchin endomesoderm network appear however to be unique to echinoderms. For example, Delta-Notch signaling is a prominent component of mesoderm specification in the sea urchin while it is used less frequently in other deuterostomes.
It is unclear if there is a selective pressure on the existence of conditionally specified states during embryonic development. There could be many answers to this question, though one prominent idea is that conditionality provides a way for the embryo to adjust proportionality. With conditional specification it isn’t necessary to program each and every cell for an exact fate. Rather, with later signaling the embryo can dictate via signaling the correct proportion of mesoderm and endoderm to the embryo. As mentioned earlier in this review, the third cleavage of the sea urchin embryo is variable in providing a larger or smaller amount of cytoplasm to the future endomesoderm. The ability to adjust the endomesoderm outcome through signaling, and the outcome of the Veg1 cells that contribute to endoderm plus a variable amount of ectoderm, gives the embryo the plasticity it needs to achieve a correctly proportioned larva.
The endomesoderm of the sea urchin embryo is only one example of a conditionally specified tissue. There are many instances during development in which cells within a germ layer later diverge into distinct fates. Perturbations prior to such a divergence often show that conditionality exists by pushing the cell fate in one direction or the other. Thus, there is a progression of conditional states of gene regulatory networks throughout development and the endomesoderm is simply an early example of many such metastable states in building a metazoan.
Highlights.
A number of embryos deploy conditional specification of endomesoderm prior to divergence into definitive mesoderm and endoderm.
This review discusses experiments that reveal how embryos enter this state and how the conditional state is resolved.
Acknowledgements:
The authors thank the McClay, Croce and Warner labs for their input. We appreciate clarifications from Clare Hudson and Chris Lowe.
Funding sources:
Support was provided by NIH RO1-HD14483 (to DRM), by CNRS-INSB DBM254552 (to JCC), and by NIH R15 GM139113-01A1 (to JFW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acloque H, Ocana OH, Matheu A, Rizzoti K, Wise C, Lovell-Badge R, Nieto MA, 2011. Reciprocal repression between Sox3 and snail transcription factors defines embryonic territories at gastrulation. Dev Cell 21, 546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA, 2000. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2, 76–83. [DOI] [PubMed] [Google Scholar]
- Charney RM, Paraiso KD, Blitz IL, Cho KWY, 2017. A gene regulatory program controlling early Xenopus mesendoderm formation: Network conservation and motifs. Semin Cell Dev Biol 66, 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Lyons DC, Socolar JE, McClay DR, 2014. Delayed transition to new cell fates during cellular reprogramming. Dev Biol 391, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WT, Charney Le R, Blitz IL, Fish MB, Li Y, Biesinger J, Xie X, Cho KW, 2014. Genome-wide view of TGFbeta/Foxh1 regulation of the early mesendoderm program. Development 141, 4537–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce J, Lhomond G, Lozano JC, Gache C, 2001. ske-T, a T-box gene expressed in the skeletogenic mesenchyme lineage of the sea urchin embryo. Mech Dev 107, 159–162. [DOI] [PubMed] [Google Scholar]
- Croce J, Range R, Wu SY, Miranda E, Lhomond G, Peng JC, Lepage T, McClay DR, 2011. Wnt6 activates endoderm in the sea urchin gene regulatory network. Development 138, 3297–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce JC, McClay DR, 2010. Dynamics of Delta/Notch signaling on endomesoderm segregation in the sea urchin embryo. Development 137, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle SS, Davidson EH, 2012. Synthetic in vivo validation of gene network circuitry. Proc Natl Acad Sci U S A 109, 1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras S, Gerhart J, Terasaki M, Kirschner M, Lowe CJ, 2011. beta-catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development (Cambridge, England) 138, 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de-Leon SB, Davidson EH, 2010. Information processing at the foxa node of the sea urchin endomesoderm specification network. Proc Natl Acad Sci U S A 107, 10103–10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesch H, 1892. The potency of the first two cleavage cells in echinoderm development. Experimental production of partial and double formations. In: Willier BH and Oppenheimer JM (eds), Foundations of Experimental Embryology (1974) Hafner, New York. [Google Scholar]
- Emily-Fenouil F, Ghiglione C, Lhomond G, Lepage T, Gache C, 1998. GSK3beta/shaggy mediates patterning along the animal-vegetal axis of the sea urchin embryo. Development 125, 2489–2498. [DOI] [PubMed] [Google Scholar]
- Ettensohn CA, McClay DR, 1988. Cell lineage conversion in the sea urchin embryo. Dev Biol 125, 396–409. [DOI] [PubMed] [Google Scholar]
- Farrell JA, Wang Y, Riesenfeld SJ, Shekhar K, Regev A, Schier AF, 2018. Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS, 1998. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395, 181–185. [DOI] [PubMed] [Google Scholar]
- Fresques T, Swartz SZ, Juliano C, Morino Y, Kikuchi M, Akasaka K, Wada H, Yajima M, Wessel GM, 2016. The diversity of nanos expression in echinoderm embryos supports different mechanisms in germ cell specification. Evol Dev 18, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, 1992. Induction of gut in Caenorhabditis elegans embryos. Nature 357, 255–257. [DOI] [PubMed] [Google Scholar]
- Goldstein B, 1995. Cell contacts orient some cell division axes in the Caenorhabditis elegans embryo. J Cell Biol 129, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Norris RP, Terasaki M, Lowe CJ, 2013. FGF signaling induces mesoderm in the hemichordate Saccoglossus kowalevskii. Development (Cambridge, England) 140, 1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JM, McClay DR, 2001. The role of Brachyury (T) during gastrulation movements in the sea urchin Lytechinus variegatus. Dev Biol 239, 132–147. [DOI] [PubMed] [Google Scholar]
- Henry GL, Melton DA, 1998. Mixer, a homeobox gene required for endoderm development. Science 281, 91–96. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Amemiya S, Wray GA, Raff RA, 1989. Early inductive interactions are involved in restricting cell fates of mesomeres in sea urchin embryos. Developmental Biology 136, 140–153. [DOI] [PubMed] [Google Scholar]
- Horstadius S, 1939. The mechanics of sea urchin development as studied by operative methods. Biological Review 14, 132–179. [Google Scholar]
- Hudson C, Kawai N, Negishi T, Yasuo H, 2013. beta-Catenin-driven binary fate specification segregates germ layers in ascidian embryos. Curr Biol 23, 491–495. [DOI] [PubMed] [Google Scholar]
- Hudson C, Sirour C, Yasuo H, 2016. Co-expression of Foxa.a, Foxd and Fgf9/16/20 defines a transient mesendoderm regulatory state in ascidian embryos. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai KS, Hudson C, Oda-Ishii I, Yasuo H, Satou Y, 2016. Antagonism between beta-catenin and Gata.a sequentially segregates the germ layers of ascidian embryos. Development 143, 4167–4172. [DOI] [PubMed] [Google Scholar]
- Iwafuchi M, Cuesta I, Donahue G, Takenaka N, Osipovich AB, Magnuson MA, Roder H, Seeholzer SH, Santisteban P, Zaret KS, 2020. Gene network transitions in embryos depend upon interactions between a pioneer transcription factor and core histones. Nat Genet 52, 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M, Zaret KS, 2016. Cell fate control by pioneer transcription factors. Development 143, 1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano CE, Swartz SZ, Wessel GM, A conserved germline multipotency program. Development 137, 4113–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Verkade H, Reiter JF, Kim CH, Chitnis AB, Kuroiwa A, Stainier DY, 2004. Notch signaling can regulate endoderm formation in zebrafish. Dev Dyn 229, 756–762. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, 1988. Cell lineage and developmental potential of cells in the zebrafish embryo. Trends in Genetics 4(3), 68–74. [DOI] [PubMed] [Google Scholar]
- Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT, 1997. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol 136, 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhomond G, McClay DR, Gache C, Croce JC, 2012. Frizzled1/2/7 signaling directs beta-catenin nuclearisation and initiates endoderm specification in macromeres during sea urchin embryogenesis. Development 139, 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Miller JR, Ferkowicz MJ, McClay DR, 1999. Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 126, 345–357. [DOI] [PubMed] [Google Scholar]
- Loose M, Patient R, 2004. A genetic regulatory network for Xenopus mesendoderm formation. Dev Biol 271, 467–478. [DOI] [PubMed] [Google Scholar]
- Lowry JA, Gamsjaeger R, Thong SY, Hung W, Kwan AH, Broitman-Maduro G, Matthews JM, Maduro M, Mackay JP, 2009. Structural analysis of MED-1 reveals unexpected diversity in the mechanism of DNA recognition by GATA-type zinc finger domains. J Biol Chem 284, 5827–5835. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Rothman JH, 2002. Making worm guts: the gene regulatory network of the Caenorhabditis elegans endoderm. Dev Biol 246, 68–85. [DOI] [PubMed] [Google Scholar]
- Massri AJ, Greenstreet L, Afanassiev A, Berrio Escobar A, Wray GM, Schiebinger G, McClay DR, 2021. Developmental Single-cell transcriptomics in the Lytechinus variegatus Sea Urchin Embryo. bioRxiv 2020.11.12.380675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materna SC, Ransick A, Li E, Davidson EH, 2013. Diversification of oral and aboral mesodermal regulatory states in pregastrular sea urchin embryos. Dev Biol 375, 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClay DR, Logan CY, 1996. Regulative capacity of the archenteron during gastrulation in the sea urchin. Development 122, 607–616. [DOI] [PubMed] [Google Scholar]
- Miller JR, Moon RT, 1997. Analysis of the signaling activities of localization mutants of beta- catenin during axis specification in Xenopus. J Cell Biol 139, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotschin S, Hadjantonakis AK, 2020. Guts and gastrulation: Emergence and convergence of endoderm in the mouse embryo. Curr Top Dev Biol 136, 429–454. [DOI] [PubMed] [Google Scholar]
- Nowotschin S, Setty M, Kuo YY, Liu V, Garg V, Sharma R, Simon CS, Saiz N, Gardner R, Boutet SC, Church DM, Hoodless PA, Hadjantonakis AK, Pe’er D, 2019. The emergent landscape of the mouse gut endoderm at single-cell resolution. Nature 569, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda-Ishii I, Kubo A, Kari W, Suzuki N, Rothbacher U, Satou Y, 2016. A Maternal System Initiating the Zygotic Developmental Program through Combinatorial Repression in the Ascidian Embryo. PLoS Genet 12, e1006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K, 1975. Spicule formation by isolated micromeres of the sea urchin embryo. Amer. Zool 15, 567–581. [Google Scholar]
- Oliveri P, Carrick DM, Davidson EH, 2002. A regulatory gene network that directs micromere specification in the sea urchin embryo. Dev Biol 246, 209–228. [DOI] [PubMed] [Google Scholar]
- Oliveri P, Davidson EH, McClay DR, 2003. Activation of pmar1 controls specification of micromeres in the sea urchin embryo. Dev Biol 258, 32–43. [DOI] [PubMed] [Google Scholar]
- Oliveri P, Tu Q, Davidson EH, 2008. Global regulatory logic for specification of an embryonic cell lineage. Proc Natl Acad Sci U S A 105, 5955–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri P, Walton KD, Davidson EH, McClay DR, 2006. Repression of mesodermal fate by foxa, a key endoderm regulator of the sea urchin embryo. Development 133, 4173–4181. [DOI] [PubMed] [Google Scholar]
- Owraghi M, Broitman-Maduro G, Luu T, Roberson H, Maduro MF, 2010. Roles of the Wnt effector POP-1/TCF in the C. elegans endomesoderm specification gene network. Dev Biol 340, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park FD, Priess JR, 2003. Establishment of POP-1 asymmetry in early C. elegans embryos. Development 130, 3547–3556. [DOI] [PubMed] [Google Scholar]
- Peter IS, Davidson EH, 2009. Modularity and design principles in the sea urchin embryo gene regulatory network. FEBS Lett 583, 3948–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter IS, Davidson EH, 2010. The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev Biol 340, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain M, Lepage T, 2002. Mezzo, a paired-like homeobox protein is an immediate target of Nodal signalling and regulates endoderm specification in zebrafish. Development 129, 4901–4914. [DOI] [PubMed] [Google Scholar]
- Range RC, Venuti JM, McClay DR, 2005. LvGroucho and nuclear beta-catenin functionally compete for Tcf binding to influence activation of the endomesoderm gene regulatory network in the sea urchin embryo. Dev Biol 279, 252–267. [DOI] [PubMed] [Google Scholar]
- Ransick A, Davidson EH, 1993. A complete second gut induced by transplanted micromeres in the sea urchin embryo. Science 259, 1134–1138. [DOI] [PubMed] [Google Scholar]
- Ransick A, Davidson EH, 2006. cis-regulatory processing of Notch signaling input to the sea urchin glial cells missing gene during mesoderm specification. Dev Biol 297, 587–602. [DOI] [PubMed] [Google Scholar]
- Ransick A, Davidson EH, 2012. Cis-regulatory logic driving glial cells missing: self-sustaining circuitry in later embryogenesis. Dev Biol 364, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransick A, Rast JP, Minokawa T, Calestani C, Davidson EH, 2002. New early zygotic regulators expressed in endomesoderm of sea urchin embryos discovered by differential array hybridization. Dev Biol 246, 132–147. [DOI] [PubMed] [Google Scholar]
- Revilla-i-Domingo R, Oliveri P, Davidson EH, 2007. A missing link in the sea urchin embryo gene regulatory network: hesC and the double-negative specification of micromeres. Proc Natl Acad Sci U S A 104, 12383–12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith JC, Patient R, Holder N, 1999. Induction of the mesendoderm in the zebrafish germ ring by yolk cell-derived TGF-beta family signals and discrimination of mesoderm and endoderm by FGF. Development 126, 3067–3078. [DOI] [PubMed] [Google Scholar]
- Sethi AJ, Wikramanayake RM, Angerer RC, Range RC, Angerer LM, 2012. Sequential signaling crosstalk regulates endomesoderm segregation in sea urchin embryos. Science 335, 590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood DR, McClay DR, 1999. LvNotch signaling mediates secondary mesenchyme specification in the sea urchin embryo. Development 126, 1703–1713. [DOI] [PubMed] [Google Scholar]
- Shi W, Levine M, 2008. Ephrin signaling establishes asymmetric cell fates in an endomesoderm lineage of the Ciona embryo. Development 135, 931–940. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, 2002. Molecular regulation of vertebrate early endoderm development. Dev Biol 249, 191–203. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H, 1924. Über Induction von Embryonalanlagen durch Implantation artfremder Organisatoren.. Wilhelm Roux’s Archives 100, 599–638. [Google Scholar]
- Stryjewska A, Dries R, Pieters T, Verstappen G, Conidi A, Coddens K, Francis A, Umans L, van IWF, Berx G, van Grunsven LA, Grosveld FG, Goossens S, Haigh JJ, Huylebroeck D, 2017. Zeb2 Regulates Cell Fate at the Exit from Epiblast State in Mouse Embryonic Stem Cells. Stem Cells 35, 611–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Peng CJ, Wang L, Feng H, Wikramanayake AH, 2021. An early global role for Axin is required for correct patterning of the anterior-posterior axis in the sea urchin embryo. Development 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet HC, Gehring M, Ettensohn CA, 2002. LvDelta is a mesoderm-inducing signal in the sea urchin embryo and can endow blastomeres with organizer-like properties. Development 129, 1945–1955. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S, 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, Hollier BG, Ram PT, Lander ES, Rosen JM, Weinberg RA, Mani SA, 2010. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A 107, 15449–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridis A, Huang Y, Blin G, Skylaki S, Wymeersch F, Osorno R, Economou C, Karagianni E, Zhao S, Lowell S, Wilson V, 2014. Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors. Development 141, 1209–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L, Mendjan S, Brown S, Chng Z, Teo A, Smithers LE, Trotter MW, Cho CH, Martinez A, Rugg-Gunn P, Brons G, Pedersen RA, 2009. Activin/Nodal signalling maintains pluripotency by controlling Nanog expression. Development 136, 1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel AL, Economou AD, Heliot C, Hill CS, 2018. Long-Range Signaling Activation and Local Inhibition Separate the Mesoderm and Endoderm Lineages. Dev Cell 44, 179–191 e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DE, Weinreb C, Collins ZM, Briggs JA, Megason SG, Klein AM, 2018. Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science 360, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warga RM, Nusslein-Volhard C, 1999. Origin and development of the zebrafish endoderm. Development 126, 827–838. [DOI] [PubMed] [Google Scholar]
- Weitzel HE, Illies MR, Byrum CA, Xu R, Wikramanayake AH, Ettensohn CA, 2004. Differential stability of (Aberle et al.)-catenin along the animal-vegetal axis of the sea urchin embryo mediated by dishevelled. Development 131, 2947–2956. [DOI] [PubMed] [Google Scholar]
- White RJ, Sun BI, Sive HL, Smith JC, 2002. Direct and indirect regulation of derriere, a Xenopus mesoderm-inducing factor, by VegT. Development 129, 4867–4876. [DOI] [PubMed] [Google Scholar]
- Wikramanayake AH, Huang L, Klein WH, 1998. beta-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc Natl Acad Sci U S A 95, 9343–9348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikramanayake AH, Peterson R, Chen J, Huang L, Bince JM, McClay DR, Klein WH, 2004. Nuclear beta-catenin-dependent Wnt8 signaling in vegetal cells of the early sea urchin embryo regulates gastrulation and differentiation of endoderm and mesodermal cell lineages. Genesis 39, 194–205. [DOI] [PubMed] [Google Scholar]
- Wylie CC, Snape A, Heasman J, Smith JC, 1987. Vegetal pole cells and commitment to form endoderm in Xenopus laevis. Dev Biol 119, 496–502. [DOI] [PubMed] [Google Scholar]
- Yajima M, Wessel GM, 2012. Autonomy in specification of primordial germ cells and their passive translocation in the sea urchin. Development 139, 3786–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, 2020. Pioneer Transcription Factors Initiating Gene Network Changes. Annual Review of Genetics 54, 367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Carroll JS, 2011. Pioneer transcription factors: establishing competence for gene expression. Genes Dev 25, 2227–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F, 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90, 181–192. [DOI] [PubMed] [Google Scholar]
- Zhang C, Klymkowsky MW, 2007. The Sox axis, Nodal signaling, and germ layer specification. Differentiation 75, 536–545. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Butler K, Gurdon JB, 1999. Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev Biol 209, 282–297. [DOI] [PubMed] [Google Scholar]


