Abstract
In the event of a radiological attack or accident, it is more likely that the absorbed radiation dose will be heterogeneous, rather than uniformly distributed throughout the body. This type of uneven dose distribution is known as partial-body irradiation (PBI). Partial exposure of the vital organs, specifically the highly radiosensitive intestines, may cause death, if the injury is significant and the post-exposure recovery is considerably compromised. Here we investigated the recovery rate and extent of recovery from PBI-induced intestinal damage in large animals. Rhesus macaques (Macaca mulatta) were randomly divided into four groups: sham-irradiated (0 Gy), 8 Gy PBI, 11 Gy PBI and 14 Gy PBI. A single dose of ionizing radiation was delivered in the abdominal region using a uniform bilateral anteroposterior and posteroanterior technique. Irradiated animals were scheduled for euthanasia on days 10, 28 or 60 postirradiation, and sham-irradiated animals on day 60. Intestinal structural injuries were assessed via crypt depth, villus height, and mucosal surface length in the four different intestinal regions (duodenum, proximal jejunum, distal jejunum and ileum) using H&E staining. Higher radiation doses corresponded with more injury at 10 days post-PBI and a faster recovery rate. However, at 60 days post-PBI, damage was still evident in all regions of the intestine. The proximal and distal ends (duodenum and ileum, respectively) sustained less damage and recovered more fully than the jejunum.
INTRODUCTION
Partial-body irradiation (PBI), or non-uniform irradiation to the body, may occur in the event of a mass casualty situation involving radioactive and/or nuclear materials or during cancer radiotherapy. Although there has been no incidence of radiological terrorism to date, several radiological accidents have been reported in various professional settings, including industries, hospitals and nuclear power plants, where victims received an inhomogeneous radiation dose (1). The Chernobyl tragedy in 1986 and the Fukushima nuclear power plant accident in 2011 are notable examples of disastrous nuclear accidents, which affected a large number of people and contaminated soil, water, and the atmosphere with radioactive elements (2, 3). Moreover, the local radioactive fallout spread through contaminated air and water to neighboring countries threatening their health and safety as well (4, 5). Lack of knowledge and education, and inappropriate handling of radioactive sources have also caused a significant number of accidents involving PBI to individuals throughout the world (6). Moreover, there exists the potential for PBI resulting from the criminal use of radioactive substances for poisoning (7).
Regardless of the source, the biological consequences of PBI are not limited to the exposed area of the body. Vital organs outside the radiation field can also be adversely affected because of radiation-induced bystander effects, which may eventually lead to fatal multi-organ failure (8). PBI causes varying degrees of adverse effects based on the dose, quality and duration of exposure, as well as the medical condition, age, gender, exposed area, and the organ type of the victim. Importantly, not all organs are equally radiosensitive; it is well established that hematopoietic and gastrointestinal systems are highly sensitive to ionizing radiation.
To study the effects of PBI on hematopoietic and gastrointestinal systems, two models have been adopted in laboratory settings: 1. Mixing of different fractions of irradiated and nonirradiated hematopoietic cells; 2. Partial blocking of bone marrow cells ranging from 2.5% to 40% during total-body irradiation (TBI) (8, 9). Studies by various groups have shown that TBI with a certain portion of bone marrow shielding disrupts the structural integrity of the intestine in non-human primates (NHPs) (8–17). However, the mechanism underlying postirradiation morphological changes in the intestine is not well understood. It is thought that epithelial cell apoptosis results in crypt involution, shortening of villus height, and breakdown of the epithelial barrier, making the gut permeable to luminal microbes. Microbial invasion can also occur due to postirradiation alterations in the expression of intestinal tight-junction–related proteins, which are essential for maintaining gut permeability (18, 19). Using a NHP model, we have recently demonstrated that TBI negatively affects tight-junction proteins in a region-specific manner (18). Invading microbes activate macrophages and mesenchymal cells in the microenvironment and trigger acute inflammation, which may subsequently lead to stricture formation in the intestine (20). Concurrently, radiation-induced apoptosis of vascular endothelial cells allows immune cells to leave the circulatory system and enter into the damaged tissue where immune cells further activate inflammatory responses. Endothelial damage can also promote edema, tissue ischemia and hypoxia in the various intestinal compartments (21). As a result, additional pathological cascades are triggered, including but not limited to, inflammation, coagulation, oxidative stress and fibrotic changes. Inflamed intestinal tissues often become severely hypoxic; this can adversely affect the intestinal structural integrity by impairing redox homeostasis, activating inflammatory signaling pathways, and downregulating the expression of tight-junction proteins (22). These data clearly indicate that radiation-induced structural changes in the intestine involve a highly complex pathophysiology.
Notably, to our knowledge, there has been no published study addressing how PBI effects on structural integrity systematically differ among the four segments across radiation dose and time in NHPs. A recently published study using an NHP model showed that lethal doses of TBI with approximately 5% bone marrow shielding (BM5) triggered a persistent impairment in both mucosal barrier function and restitution in the small intestine and colon (13). In addition, the authors observed persistent upregulation of pro-inflammatory markers in the jejunum and colon after TBI-BM5 (13). These data clearly indicate that partial exposure, which is most likely to occur in a mass casualty radiation event, has the potential to exert persistent, undesirable changes in the intestine. To systematically address the issue, we administered PBI at various doses to NHPs and harvested intestinal tissue at different postirradiation time intervals to assess the structural modifications in the duodenum, proximal jejunum, distal jejunum, and ileum. We observed substantial structural injury in all four intestinal segments after PBI at an early time point; however, the severity and recovery of damage depended on the dose, time interval, and the region of intestine receiving exposure.
MATERIALS AND METHODS
Animal Grouping, Euthanasia Schedule, Irradiation Method and Supportive Care
Animal housing and care.
A total of 40 (20 males and 20 females) rhesus macaques (Macaca mulatta) of Chinese origin were used for this study. Animals were 3–5 years old and weighed between 3.3 kg and 6.2 kg. Animals were housed individually in cages that complied with the Animal Welfare Act and recommendations set forth in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). The animal room environment was controlled (temperature and relative humidity between 188C and 298C, and 30% and 70%, respectively). An automatic lighting system was set to provide a 12:12 h light-dark schedule. Animals were offered LabDiet® Fiber-Plus® Monkey Diet 5049 biscuits twice daily (PMI Nutrition International, St. Louis, MO). Fresh drinking water was provided ad libitum. Produce treats were routinely provided to all animals. Treats given during of the study excluded citrus, nuts, seeds, potatoes, popcorn and other similarly hard or sharp items. Acceptable treats included, but were not limited to, banana, apple, cucumber, grape, squash, pumpkin and pear. All animals were fasted overnight prior to irradiation.
Animal experiments were conducted by SNBL USA, Ltd. (Everett, WA), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, has an Animal Welfare Assurance issued by the Office of Laboratory Animal Welfare, is registered with the U.S. Department of Agriculture, and has an Institutional Animal Care and Use Committee responsible for compliance with applicable laws and regulations concerning the humane care and use of laboratory animals.
Irradiation procedure.
Parameters were established for animals to receive PBI at the prescribed doses using a Varian Clinact® 21EX linear accelerator (LINAC; Palo Alto, CA). Animals were irradiated with LINAC-derived 6-MV photons at a dose rate at midline of 0.75 ± 0.05 Gy/min using a uniform bilateral anteroposterior (AP) and posteroanterior (PA) technique with approximately 50% dose contribution from both the AP and PA beams. The average photon energy was 2 MV. Diode dosimetry was performed on every animal to estimate dose at midline. Chest measurements at the level of the umbilicus were obtained prior to irradiation and used to calculate midline tissue dose. Animals were administered an antiemetic (ondansetron HCl, 1.0 mg/kg) by intramuscular (IM) injection 15–90 min prior to irradiation. The gantry angle was 90° and the collimator angle was 90°. The field size was 20 × 20 cm, and the source-to-midline distance was 230 cm. PBI was conducted with anesthetized animals positioned in an upright vertical position and restrained to keep the arms outside the radiation field. A Plexiglast plate was placed between the animal and the beam. The LINAC was collimated to define a field of 20 × 40 cm, which limited the exposure to half the field aperture. The crosshair of the LINAC light field was aligned with the bottom of the last rib for each animal, such that the entire area above the point was shielded, and the entire area below that point was exposed. Based on approximate bone marrow distribution in humans, ~50% of the bone marrow was expected to be spared with this radiation setup, as no parts of the skull, scapula, clavicles, sternum, ribs, or cervical and thoracic vertebrae were in the radiation field. Real-time in vivo dosimetry was performed on every animal to estimate the dose at midline by placing the dosimeter on the entrance side of the beam and calculating the dose to the midline using a Sun Nuclear In-Vivo diode (Melbourne, FL). Moreover, nanoDote optically stimulated luminescence (OSL) dosimeters were placed only for the first few animals to ensure the radiation shadow dose was appropriately shielded. The off-axis doses outside of the irradiated area have been measured in phantoms. Results are consistent with the AAPM Task Group TG-158 report (Measurement and calculation of doses outside treatment volume).
Animal groupings and euthanasia schedule.
Forty rhesus macaques were randomized into four radiation dose groups. The sham-irradiated group (n = 4) was treated identically to the exposed group but received no radiation (0 Gy group). The three remaining groups (each with 12 animals) received a single dose of LINAC photon abdominal irradiation (8, 11 or 14 Gy). Both male and female animals were used in each group. Sham-irradiated animals were euthanized on day 60; animals within each irradiated group were randomly assigned to undergo euthanasia on days 10, 28 (or 31) or 60 postirradiation. However, to reduce terminal distress, moribund animals were euthanized before the scheduled date (unscheduled euthanasia) for autopsy and that day was recorded. Table 1 shows details of the study design and numbers of animals assigned to different postirradiation time points.
TABLE 1.
Study Design Showing, Radiation Doses, Animal Numbers and Postirradiation Scheduled Tissue Harvest Time (Male/Female)
| PBI (Gy) | Number of animals (male/female) | Postirradiation tissue harvest times (male/female) |
||
|---|---|---|---|---|
| Day 10 | Day 28 | Day 60 | ||
| 0 | 2/2 | - | - | 2/2 |
| 8 | 6/6 | 2/2 | 2/2 | 2/2 |
| 11 | 6/6 | 2/2 | 2/2 | 2/2 |
| 14 | 6/6 | 2/2 | 1/1 | 3/3 |
Supportive care.
All animals were provided standardized supportive care consisting of nutritional and fluid support, as well as antibiotic, analgesic, antiemetic and antidiarrheal medications. Nutritional and fluid support included provision of fresh produce throughout the study, softened primate diet once daily on postirradiation days 5 through 30 in addition to regular biscuits, and provision of diluted Gatorade ice cubes twice daily on postirradiation days 5 through 30. All animals were offered oral antibiotic (enrofloxacin 5 mg/kg) once daily beginning on postirradiation day 5 and continuing through day 30. Animals refusing oral administration for two consecutive treatments received enrofloxacin by IM injection for the duration of the treatment period. All animals were offered oral antidiarrheal (loperamide HCl, approximately one half of a 2-mg tablet) twice daily beginning on postirradiation day 3 and continuing through day 30. All animals were offered analgesic support on postirradiation days 5 through 30 in the form of tramadol (approximately one fourth of a 50-mg tablet) orally, twice a day. Animals refusing oral administration of tramadol for two consecutive treatments received buprenorphine (0.01 mg/kg) by IM injection twice a day for the duration of the treatment period. Animals received antiemetic treatment (ondansetron HCl, 1.0 mg/kg IM, once or twice daily) as needed for control of multiple episodes of emesis or profuse emesis.
Euthanasia criteria.
Clearly defined criteria were established and consistently followed for moribund euthanasia. Absolute criteria included the following: indication of unrelieved pain or distress despite administration of two consecutive increased doses of buprenorphine (0.02 mg/kg, IM injection); recumbent in the cage for at least 15 min, or non-responsive to touch; respiratory distress; uncontrolled hemorrhage from any orifice; or severe dehydration. Combination criteria for euthanasia included two or more of the following: tachypnea (≥60 breaths per min); loss of body weight ≥25% of baseline for two consecutive days; observation of severe injury or condition; hyperthermia (rectal temperature ≥41 °C); hypothermia (rectal temperature ≤35 °C); or complete anorexia for 48 h.
Collection of Different Regions of Intestinal Tissue
Upon euthanasia, intestinal tissue collection was performed under the supervision of an ACVP board certified pathologist. Four different regions of small intestine (duodenum, proximal jejunum, distal jejunum, and ileum) were procured and fixed in 10% formalin, processed to paraffin blocks, sectioned, and stained with hematoxylin and eosin for histological and morphometric studies.
Assessment of Intestinal Structural Injury
Computer-assisted Image-Pro® Premier software (Meyer Instruments Inc., Houston, TX) was used to measure mucosal surface length (MSL), crypt depth (CD) and villus height (VH). For each animal, MSL, CD and VH measurements were performed under 4× magnification in five areas of each of the four small intestine segments. The average of the measurements from the five areas was used for statistical analyses for each parameter and segment.
Statistical Analysis
Each animal provided a panel of 12 measures: MSL, CD and VH from each of the four regions (duodenum, proximal jejunum, distal jejunum and ileum). The values within a panel are correlated because they come from the same animal. We thus used mixed effects regression models to estimate recovery rates (i.e., slope parameter on euthanasia day) and final recovery (i.e., intercept parameters). We compared recovery rates and final recovery among the regions within the regression model. We considered two regression models: unstructured and structured. The unstructured regression model assumed the recovery rates among the radiation doses were unrelated, and similarly for final recovery parameters. The structured regression model assumed recovery rates and final recovery were linearly related to radiation dose. In both models, data from sham-irradiated animals were used to estimate "full recovery", i.e., the value to which final recovery parameters were compare. Comparisons were made with contrasts within the regression models. Sex of the animal was not included as a factor in these two regressions. No sample size or power calculations were made in the original protocol.
All analyses were conducted with the MIXED procedure in SAS/ STAT software, version 9.4, SAS System for Windows (SAS Institute Inc., Cary, NC). We estimated 95% confidence intervals in lieu of P values wherever possible; confidence intervals that do not include 0 indicate significance at the 0.05 level. The source data and SAS code are available upon request.
RESULTS
We investigated the degree of structural changes and extent of recovery in the four regions of the small intestine over the period of 60 days post-PBI at various doses. No unscheduled euthanasia was performed for the animals that received 0 or 8 Gy PBI. Two animals that received 11 Gy PBI were euthanized early as a result of their severely debilitated health condition: one on day 7 and the other on day 54. Eight animals that received 14 Gy PBI did not survive until their scheduled euthanasia day: seven were euthanized early as a result of moribund condition (three on day 7 and one each on days 8, 10, 24 and 30), and one was found dead on day 10. Histological data from the latter animal was not included in analyses.
Clinical observations postirradiation included findings commonly associated with radiation exposure, including episodic loose or liquid stools, occasional emesis, decreased activity level, alopecia and hunched posture. A decrease in food consumption was apparent in all irradiated animals, beginning around day 4 postirradiation. In general, animals that received 11 Gy PBI resumed normal food consumption by day 18 postirradiation; normal food consumption resumed in all 14 Gy PBI animals by day 21, although occasional low food consumption was recorded for animals that survived to day 60 postirradiation. With few exceptions, normal food consumption in animals euthanized for moribund condition did not resume prior to meeting the euthanasia criteria.
Clinical pathological analyses detected mild alterations that are considered consistent with radiation exposure with preservation of bone marrow due to significant shielding. The most consistent hematological finding for the majority of 11 Gy PBI animals was a decrease in lymphocyte count; this was initially noted on day 3 and persisted through days 6, 8 and 10. The decreases in lymphocyte counts were frequently associated with decreased eosinophil counts and occasionally a mild neutropenia. Body temperature was recorded for animals in acclimation and then at variable time points postirradiation as necessary to assess declining condition. All irradiated groups experienced decreases in body temperature over the first 10–14 days, with increasingly faster declines for the higher radiation doses; this is based on all animals. Body temperatures of less than 35°C were recorded for 14 of the 18 animals meeting the criteria for moribund euthanasia. For those animals that survived to their scheduled euthanasia date, body temperatures increased slowly but still tended to remain between 1.3 to 4.3 percentage points lower than baseline levels by the time of euthanasia (results not shown). Hyperthermia and sepsis were not observed in any of the animals.
Compared to intestines of sham-irradiated animals 60 days post-PBI, structural damage due to a range of PBI doses over 60 days was estimated based on three attributes, i.e., crypt depth (CD), villus height (VH) and mucosal surface length (MSL), within the four different intestinal segments: duodenum, proximal jejunum, distal jejunum, and ileum. Representative images of all four intestinal segments after either sham irradiation at day 60 or PBI at three different doses on day 10 are shown in Fig. 1.
FIG. 1.
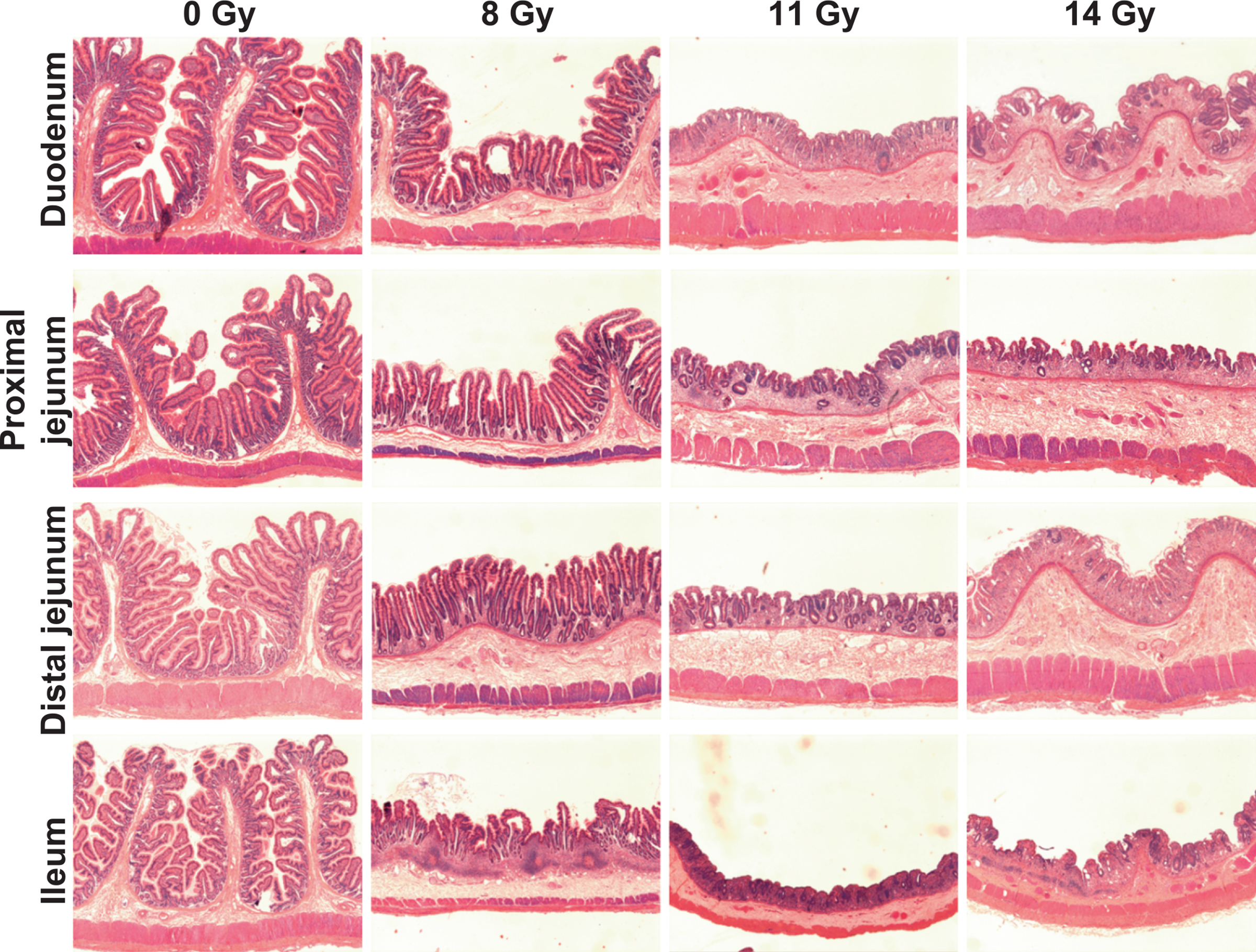
Representative photomicrographs showing loss in structural integrity of different intestinal compartments of rhesus macaques on day 10 post-PBI, day 60 after sham irradiation (0 Gy).
The scale of measurement differed substantially among the three attributes, with the overall mean ± standard deviation (SD) of CD being 77 ± 40 μm, VH being 238 ± 103 μm, and MSL being 8,208 ± 3,566 μm. To draw conclusions across the three attributes, we standardized the values of each attribute to have a mean of 0 and SD of 1; this standardization was done for each attribute across all segments within an animal and across all animals. The standardized measures allowed us to meaningfully average over the three attributes when comparing radiation effects among regions, but we also present results for each attribute on the original scale in the figures.
Regarding the two regression models, unstructured and structured, described in Materials and Methods, the unstructured model estimated a regression for each of the 36 combinations of radiation dose, region and attribute (3 PBIs × 4 regions × 3 attributes); the covariate in these regressions was time of euthanasia relative to day 60, the final day of the study. Importantly, the unstructured model treated radiation dose as a categorical variable; that is, it disregarded the quantitative nature of radiation dose. Figure 2 shows the estimated regression lines from the unstructured model. The structured model estimated a regression for each of the 12 combinations of region and attribute (4 regions × 3 attributes); the covariate in these regressions was also time of euthanasia relative to day 60. Importantly, the intercept and slope coefficients were linearly related to radiation dose; that is, this model used the quantitative nature of radiation dose. For example, suppose the structured model estimates the recovery rate (i.e., the slope) for crypt depths in the duodenum to be 0.02 SD/week/Gy, then the estimated recovery rate for the 8 Gy PBI animals is 0.16 SD/week, and for the 14 Gy PBI animals is 0.28 SD/week. Similarly suppose the final recovery at 60 days (i.e., the intercept) is estimated to be 0.1 SD/Gy lower than sham; then the estimated final recovery for 8 Gy PBI animals is 0.8 SD lower than sham, and for 14 Gy PBI animals is 1.4 SD lower than sham. Figure 3 shows regression lines from the structured model.
FIG. 2.

Estimated regression lines from the unstructured model. Gold lines indicate sham means, assumed constant across time; black dotted, dashed, and solid lines represent animals that received 8, 11 and 14 Gy irradiation, respectively. The model provides regressions for each attribute (crypt depth, villus height, mucosal surface length) within each region (duodenum, proximal jejunum, distal jejunum, ileum). The data are from 39 animals. Figure 4 displays the values of the recovery rates (slopes) and final recovery (intercepts), along with their 95% confidence intervals.
FIG. 3.
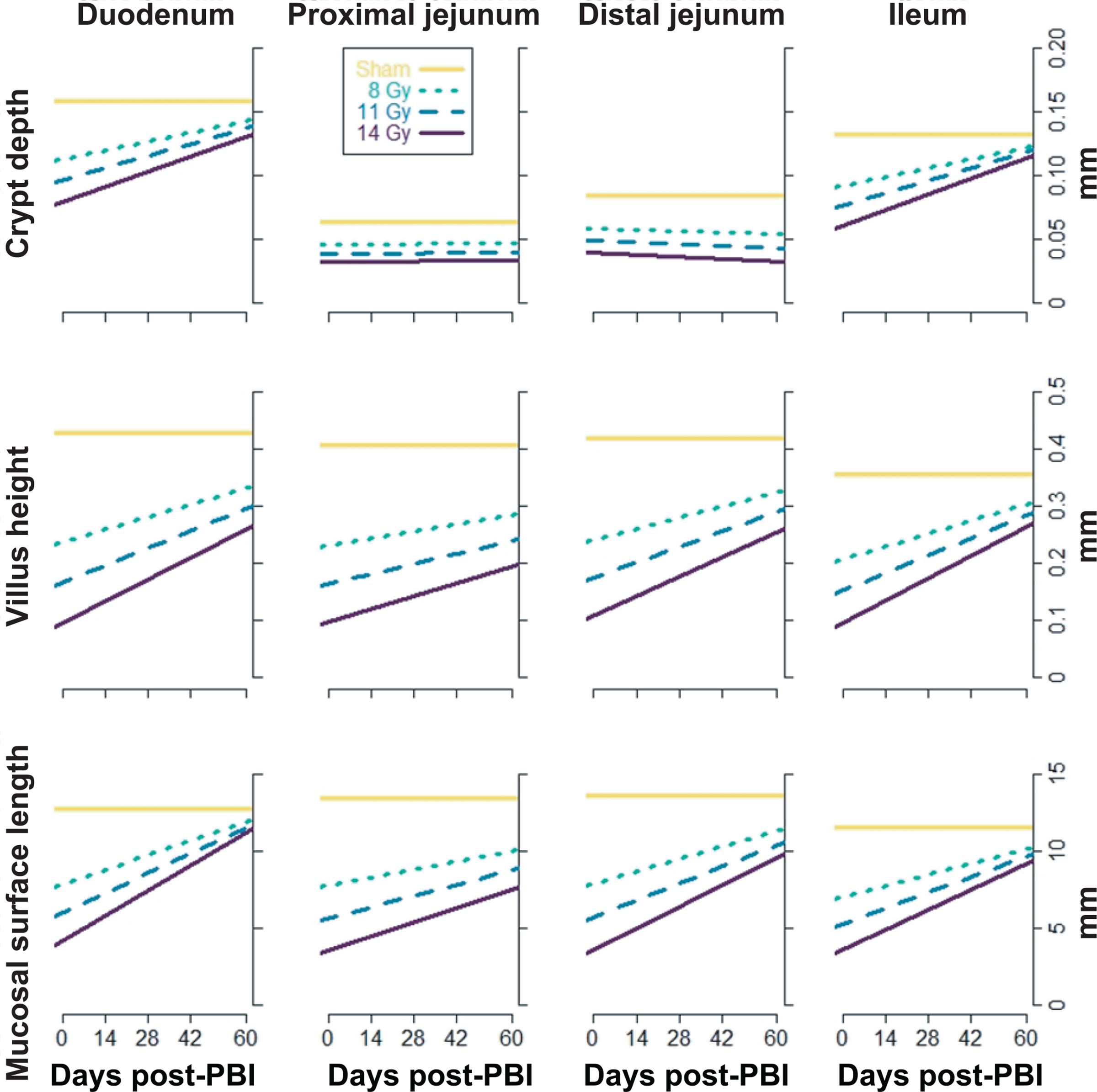
Estimated regression lines from the structured model. Gold lines indicate sham means, assumed constant across time; black dotted, dashed, and solid lines represent animals that received 8, 11 and 14 Gy irradiation, respectively. The model provides regressions for each attribute (crypt depth, villus height, mucosal surface length) within each region (duodenum, proximal jejunum, distal jejunum, ileum). The data analyzed in the model are from 39 animals. Figure 4 displays the values of the recovery rates (slopes) and final recovery (intercepts), along with their 95% confidence intervals.
Furthermore, we made overall comparisons between pairs of regions averaging over the three radiation doses in the unstructured and in the structured models.
Recovery Rates
Recovery rates describe how quickly the four intestinal sections recovered from (unobserved) maximal damage induced by acute PBI. The slopes on euthanasia day estimate the recovery rate. Overall, each of the four sections were at or near their worst damage (i.e., most decreased from sham levels) around day 10, i.e., the first scheduled euthanasia day, and demonstrated recovery through the end of the experiment, at day 60. However, both sections of the jejunum recovered more slowly than the duodenum and ileum; this was corroborated by the unstructured and structured models (Fig. 4A).
FIG. 4.

Panel A: Estimated recovery rates (slopes) and their 95% confidence intervals. Panel B: Estimated final recovery (intercepts) and their 95% confidence intervals. Gold lines are sham means, assumed constant across time, black lines are estimates from the structured model, and gray lines are estimates from the unstructured model. Panels show, from top to bottom, crypt depths, villus heights and mucosal surface lengths. For the structured model, the slope is estimated for the 11 Gy dose; the other two doses are proportional. For the unstructured model, diamonds represent 8 Gy, triangles 11 Gy, and squares 14 Gy. Confidence intervals that do not contain 0 (horizontal orange line) are significantly different from 0. Positive slopes indicate recovery and negative slopes indicate deterioration. No adjustments were made for multiple comparisons. Data are from 39 animals.
Compared to the duodenum, the dose-averaged recovery rate of the proximal jejunum was slower by 0.083 SD/week (95% CI: 0.040, 0.126) as estimated by the unstructured model, and by 0.085 SD/week (95% CI: 0.031, 0.138) as estimated by the structured model. The dose-averaged recovery rate of the distal jejunum was lower than that of duodenum by 0.054 SD/week (95% CI: 0.006, 0.101) in the unstructured model, and by 0.058 SD/week (95% CI: 0.013, 0.104) in the structured model.
Compared to the ileum, the dose-averaged recovery rate of the proximal jejunum was lower by 0.088 SD/week (95% CI: 0.046, 0.131) in the unstructured model, and by 0.074 SD/week (95% CI: 0.024, 0.124) in the structured model. The dose-averaged recovery rate of the distal jejunum was lower than that of the ileum by 0.059 SD/week (95% CI: 0.021, 0.098) in the unstructured model, and by 0.048 SD/ week (95% CI: 0.003, 0.093) in the structured model.
Final Recovery
Final recovery was defined as the state of the four intestinal sections on the final day of the experiment, i.e., day 60. Final recovery was quantified by the intercepts in the regression models. Overall, the duodenum recovered most closely to sham levels at day 60, followed by the ileum; both sections of the jejunum showed less recovery than the distal ends of the intestine (Fig. 4B). However, the statistical evidence that the jejunum had worse final recovery than the duodenum and ileum was not strong. The unstructured model found the proximal jejunum to have a statistically lower level of recovery than the duodenum, whereas the structured model found both sections of the jejunum to have a statistically lower level of recovery than the ileum.
Compared to the duodenum, dose-averaged final recovery (at 60 days) in the proximal jejunum was lower by 0.567 SD (95% CI: 0.145, 0.989) in the unstructured model, and by 0.451 SD (95% CI: -0.044, 0.947) in the structured model. The dose-averaged final recovery of the distal jejunum was also lower than that of duodenum by 0.417 SD (95% CI: – 0.046, 0.880) in the unstructured model, and by 0.323 SD (95% CI: –0.099, 0.745) in the structured model. Comparing the duodenum between PBI and sham animals, the dose-averaged final recovery was lower for irradiated duodenums than for sham-irradiated duodenums by 0.399 SD (95% CI: –0.185, 0.982) in the unstructured model and by 0.729 SD (95% CI: 0.133, 1.325) in the structured model. Thus, irradiated duodenums had not recovered to sham levels 60 days postirradiation, let alone irradiated jejuna (Fig. 4B).
Compared to the ileum, the dose-averaged recovery of the proximal jejunum was lower by 0.357 SD (95% CI: –0.065, 0.779) in the unstructured model, and by 0.648 SD (95% CI: 0.174, 1.123) in the structured model. The dose-averaged final recovery in the distal jejunum was also estimated to be lower than that of the ileum by 0.208 SD (95% CI: –0.171, 0.586) in the unstructured model and by 0.520 SD (95% CI: 0.097, 0.944) in the structured model. Comparing the ilea between PBI and sham-irradiated animals, the dose-averaged final recovery was lower for irradiated ilea by 0.609 SD (95% CI: 0.123, 1.094) in the unstructured model and by 0.532 SD (95% CI: 0.067, 0.996) in the structured model. Thus, irradiated ilea also had not recovered to sham levels 60 days postirradiation (Fig. 4B).
DISCUSSION
The consequences of adverse structural alterations in the small intestine due to PBI from a natural and/or man-made radiological disaster can be widespread, intense and long-lasting (23–26). To reduce the risk associated with PBI-induced gut damage, clear knowledge about the dose- and time-dependent morphometric changes in the intestinal tissue is necessary, and will provide critical information to physicians and researchers in developing strategies to decrease morbidity and mortality in the aftermath of a radiological terrorism/accident event. Moreover, knowledge of radiosensitivity gradients in the small intestine will further aid targeting treatment strategies for PBI-induced structural injury.
Because such experimental studies cannot be performed in humans, we used a rhesus macaque NHP model to assess potential health risks associated with PBI. Use of an NHP model has a number of advantages over other animal models. NHPs and humans share phylogenetic proximity, have highly conserved gene maps and gene orders, have physiological similarities, and most importantly, they are both susceptible to complex diseases, including depression and cognitive impairment (27, 28), which are well-known side effects of radiation-induced intestinal damage. Moreover, NHPs and humans exhibit strong similarities in intestinal physiology, function, immunology and the intestinal microbiome.
The current study determined the dose- and time-dependent structural changes in various parts of the small intestine in NHPs after PBI with approximately 50% bone marrow shielding (PBI/BM50). Studies conducted under TBI with similar supportive care regimens characterized the linear portion of the lethality curve to occur between 6.9 Gy and 7.5 Gy in NHPs (29). This PBI/BM50 study was designed to target doses eliciting measurable changes to the gastrointestinal tract without concurrent hematopoietic-related lethality (i.e., 8 Gy, 11 Gy and 14 Gy). Notably, previously reported studies with NHPs demonstrated that the LD50/60 for TBI and PBI with 5% bone marrow shielding (PBI/BM5) are 7.45 Gy and 11.01 Gy, respectively (8, 29), suggesting that protection of the bone marrow provides a significant survival benefit. Because our current shielding method protects 50% of the bone marrow, we decided to increase the radiation dose up to 14 Gy. Though this study was not designed to estimate LD50 values with good precision, the estimated LD50/10 was 15.4Gy (n = 36), LD50/28 was 13.0 (n = 26), and LD50/60 was 11.6 (n = 18). This PBI/BM50 regimen resulted in 8.3% (1/12) and 58% (7/12) mortality for 11 Gy and 14 Gy, respectively, which is significantly low compared to a previously published PBI/ BM5 study (8), highlighting the role of bone marrow in protection against radiation lethality.
We demonstrated that acute PBI at high, but survivable doses severely compromised the structural integrity of the small intestine 10 days postirradiation, that the initial damage increased with radiation dose and that damage, although improved, was still evident two months postirradiation. Based on postirradiation time points, intestinal damage can be of two types: acute and delayed. Acute symptoms appear within 15 days of irradiation, while delayed symptoms develop after 15 days (30). A study by Jones et al. showed that intestinal recovery in NHPs began 15 days after 11 Gy PBI with 5% bone marrow shielding, evidenced by a steady increase in plasma citrulline level in NHPs (a decrease in plasma citrulline is considered a biomarker of intestinal damage, as citrulline is primarily produced by gut enterocytes) (30). Therefore, we used a 10-day time point to assess the acute effects of radiation and 28- and 60-day time points to assess delayed effects and recovery. Structural damage was at its greatest at our 10-day time point, and increasingly closer to sham levels at the 28-and 60-day time points for all examined PBI doses. Similarly, Jones et al. observed maximum structural damage to the intestine in NHPs on day 9 (after 11 Gy PBI with 5% bone marrow shielding), characterized by extensive reduction in villus height, near-complete loss of crypts and a massive reduction in epithelial surface (30). A study by MacVittie et al. also reported similar disruptions to the morphology of the small intestine in NHPs within 10 days post-PBI with 5% bone marrow shielding (8), which follows a similar trend with our current findings. However, in our studies, approximately 50% of the bone marrow was spared.
Notably, PBI-induced structural damage to the intestine is a highly complex process involving the contribution of various cells and proteins present in the irradiated microenvironment. Damage to or loss of intestinal stem cells (ISCs), imbalance in immune cell populations (i.e., lymphocytes, macrophages, neutrophils and mast cells), and change in matrix proteins [collagen, transforming growth factor beta (TGF-β), connective tissue growth factor (CTGF)] after irradiation all contribute to intestinal structural damage (16). Importantly, structural damage negatively affects the functions of the intestine. Vigneulle et al. showed that alterations in intestinal crypt and villus structure on day 7 after 9.5 Gy total-abdominal X-ray irradiation strongly affected intestinal function by increasing epithelial permeability, decreasing nutrient absorption and inducing diarrhea in NHPs (17). However, the authors observed a partial recovery of intestinal structure and function on day 14 and day 35 (17). Some of the major players in intestinal recovery after radiation exposure are ISCs that reside in the crypts. ISCs can be divided broadly into two categories: 1. Rapidly proliferating radiosensitive columnar Lgr5-expressing stem cells residing at the base of crypts; and 2. Quiescent or slowly proliferating radioresistant Bmi1-expressing stem cells located at positions 3 to 6 above the crypt base (31–33). The Lgr5-expressing stem cells are responsible primarily for maintaining intestinal homeostasis under steady-state conditions, while Bmi1-expressing stem cells trigger intestinal recovery upon radiation damage (34). However, the rate of recovery among different segments of the small intestine after irradiation in NHPs has not been previously reported. Notably, we observed that both the proximal and distal jejunum recovered more slowly than the duodenum and ileum. This disparity in radiosensitivity among the small intestine segments could be due to the differential distribution of ISCs across different regions of tissue. Indeed, Bmi1-expressing cells are most abundant in the duodenum (32), and they could contribute to the faster recovery of the duodenum compared to the jejunum after radiation injury.
Intestinal recovery after irradiation also depends strongly on the amount of bone marrow spared; the more bone marrow protected, the better the intestinal recovery and overall survival. Using a TBI model, Booth et al. showed that the LD50/7 for C57BL/6 mice increased from 10 Gy to 14 Gy after 40% bone marrow shielding during the gastrointestinal acute radiation syndrome (GI-ARS) timescale (9). Similarly, MacVittie et al. reported that the LD50/15 for TBI increased from 11.33 Gy to 11.95 Gy in NHPs when only 5% bone marrow was protected during the GI-ARS timescale (8). Because the bone marrow protects against lethality, transplanting bone marrow cells or administering G-CSF to induce hematopoiesis after irradiation could be important approaches to combat radiation damage (11, 12). However, the efficacy of hematopoietic cell therapy in mitigating GI-ARS is yet to be established.
As we also demonstrated, MacVittie et al. observed partial recovery from PBI-induced intestinal damage at approximately five weeks (precisely 34 days) after exposure, evidenced by an increase in CD and crypt regeneration (8). In addition, they observed increased cellularity in the stroma surrounding the crypts 5 weeks post-PBI (8). These stromal cells, including the endothelial cells (which form the inner lining of intestinal microvasculature) are known to play a critical role in crypt cell regeneration after radiation damage (21). With respect to endothelial cells, a recently published study in NHPs demonstrated that 12 Gy PBI with 2.5% bone marrow sparing significantly decreased the serotonin level in the jejunum and plasma (35). A decrease in serotonin level implies endothelial dysfunction, which enhances intestinal radiation damage (21). The authors also observed significant decrease in plasma citrulline (21). Taken together, it is evident that PBI can inflict long-term structural injury on the intestine by damaging the mucosal epithelial cells or the sub-epithelial stromal cells and may require a prolonged recovery period. Similar to our studies, Booth et al. also observed long-term damage to the murine intestine, including microadenomas, thicker submucosa, shorter crypts and villi, and increased collagen, extending 125 to 200 days post-PBI with 40% bone marrow shielding (9). In Wistar rats that received 12 Gy and 14 Gy irradiation with approximately 20% bone marrow sparing also showed decreased villus height even after 64 and 104 days postirradiation (36). In total, these findings indicate that PBI can exert long-lasting mucosal damage in animals irrespective of species.
CONCLUSION
Overall, the results of this work indicate that the severity of PBI-induced intestinal damage and recovery from that damage depends on radiation dose. We also observed differential radiosensitivity between the four regions of the small intestine, with the jejunum being the most radiosensitive region. Together, our model provides a tool to study GI-ARS after PBI and to design a strategy of intestinal radiation protection in the aftermath of a radiological disaster.
ACKNOWLEDGMENTS
This project was supported by funding from the ASPR, BARDA (contract no. HHSO100201100045C to MH-J), the NIH National Institute of General Medical Sciences (NIGMS grant no. P20 GM109005 to MH-J and RP and NIGMS Collaborative Research Grant no. 2P20GM103429-19 to RP), and an intramural Medical Research Endowment Grant from University of Arkansas for Medical Sciences (UAMS) (to RP). The Science Communication Group at UAMS provided editorial support.
REFERENCES
- 1.Coeytaux K, Bey E, Christensen D, Glassman ES, Murdock B, Doucet C. Reported radiation overexposure accidents worldwide, 1980–2013: a systematic review. PLoS One 2015; 10:e0118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anzai K, Ban N, Ozawa T, Tokonami S. Fukushima Daiichi Nuclear Power Plant accident: facts, environmental contamination, possible biological effects, and countermeasures. J Clin Biochem Nutr 2012; 50:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medvedev ZA. Ecological aspects of the Chernobyl nuclear plant disaster. Trends Ecol Evol 1986; 1:23–5. [DOI] [PubMed] [Google Scholar]
- 4.Fridman M, Lam AK-Y, Krasko O. Characteristics of young adults of Belarus with post-Chernobyl papillary thyroid carcinoma: a long-term follow-up of patients with early exposure to radiation at the 30th anniversary of the accident. Clin Endocrinol (Oxf) 2016; 85:971–8. [DOI] [PubMed] [Google Scholar]
- 5.McKenzie T, Dulai H. Fukushima-derived radiocesium fallout in Hawaiian soils. J Environ Radioact 2017; 180:106–13. [DOI] [PubMed] [Google Scholar]
- 6.Makkar N, Chandra T, Agrawal P, Bansal H, Singh S, Anand T, et al. Evaluating awareness and practices pertaining to radioactive waste management among scrap dealers in Delhi, India. PLoS One 2014; 9:e91579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller CW, Whitcomb RC, Ansari A, McCurley C, Nemhauser JB, Jones R. Murder by radiation poisoning: implications for public health. Journal Environ Health 2012; 74:8–13. [PubMed] [Google Scholar]
- 8.MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, et al. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys 2012; 103:427–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booth C, Tudor G, Tonge N, Shea-Donohue T, MacVittie TJ. Evidence of delayed gastrointestinal syndrome in high-dose irradiated mice. Health Phys 2012; 103:400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonaka H, Onishi H, Ozaki M, Kuriyama K, Komiyama T, Saito R. Serious gastric perforation after second stereotactic body radiotherapy for peripheral lung cancer that recurred after initial stereotactic body radiotherapy: a case report. J Med Case Rep 2017; 11:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertho J-M, Frick J, Prat M, Demarquay C, Dudoignon N, Trompier F, et al. Comparison of autologous cell therapy and granulocyte-colony stimulating factor (G-CSF) injection vs. G-CSF injection alone for the treatment of acute radiation syndrome in a non-human primate model. Int J Radiat Oncol Biol Phys 2005; 63:911–20. [DOI] [PubMed] [Google Scholar]
- 12.Bertho J-M, Prat M, Frick J, Demarquay C, Gaugler M-H, Dudoignon N, et al. Application of autologous hematopoietic cell therapy to a nonhuman primate model of heterogeneous high-dose irradiation. Radiat Res 2005; 163:557–70. [DOI] [PubMed] [Google Scholar]
- 13.Shea-Donohue T, Fasano A, Zhao A, Notari L, Yan S, Sun R, et al. mechanisms involved in the development of the chronic gastrointestinal syndrome in nonhuman primates after total-body irradiation with bone marrow shielding. Radiat Res 2016; 185:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Accardi MV, Donini O, Rumage A, Ascah A, Haruna J, Pouliot M, et al. Characterization of a partial-body irradiation model with oral cavity shielding in nonhuman primates. Int J Radiat Biol 2020; 96:100–11. [DOI] [PubMed] [Google Scholar]
- 15.MacVittie TJ, Farese AM, Parker GA, Jackson W, Booth C, Tudor GL, et al. The gastrointestinal subsyndrome of the acute radiation syndrome in rhesus macaques: A systematic review of the lethal dose-response relationship with and without medical management. Health Phys 2019; 116:305–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker GA, Li N, Takayama K, Booth C, Tudor GL, Farese AM, et al. Histopathological features of the development of intestine and mesenteric lymph node injury in a nonhuman primate model of partial-body irradiation with minimal bone marrow sparing. Health Phys 2019; 116:426–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vigneulle RM, Rao S, Fasano A, MacVittie TJ. Structural and functional alterations of the gastrointestinal tract following radiation-induced injury in the rhesus monkey. Dig Dis Sci 2002; 47:1480–91. [DOI] [PubMed] [Google Scholar]
- 18.Garg S, Zheng J, Wang J, Authier S, Pouliot M, Hauer-Jensen M. Segmental differences in radiation-induced alterations of tight junction-related proteins in non-human primate jejunum, ileum and colon. Radiat Res 2016; 185:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shukla PK, Gangwar R, Manda B, Meena AS, Yadav N, Szabo E, et al. Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-l-cysteine. Am J Physiol Gastrointest Liver Physiol 2016; 310:G705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut 2007; 56:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol 2007; 13:3047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Sun X, Wu Q, Li H, Li L, Feng J, et al. Disrupted intestinal structure in a rat model of intermittent hypoxia. Mol Med Rep 2016; 13:4407–13. [DOI] [PubMed] [Google Scholar]
- 23.Hatch M, Cardis E. Somatic health effects of Chernobyl: 30 years on. Eur J Epidemiol 2017; 32:1047–54. [DOI] [PubMed] [Google Scholar]
- 24.Nomura S, Blangiardo M, Tsubokura M, Ozaki A, Morita T, Hodgson S. Postnuclear disaster evacuation and chronic health in adults in Fukushima, Japan: a long-term retrospective analysis. BMJ Open 2016; 6:e010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsubokura M, Takita M, Matsumura T, Hara K, Tanimoto T, Kobayashi K, et al. Changes in metabolic profiles after the Great East Japan Earthquake: a retrospective observational study. BMC Public Health 2013; 13:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douple EB, Mabuchi K, Cullings HM, Preston DL, Kodama K, Shimizu Y, et al. Long-term radiation-related health effects in a unique human population: lessons learned from the atomic bomb survivors of Hiroshima and Nagasaki. Disaster Med Public Health Prep 2011; 5:S122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VandeBerg JL, Williams-Blangero S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J Med Primatol 1997; 26:113–9. [DOI] [PubMed] [Google Scholar]
- 28.Uno Y, Uehara S, Yamazaki H. Utility of non-human primates in drug development: Comparison of non-human primate and human drug-metabolizing cytochrome P450 enzymes. Biochem Pharmacol 2016; 121:1–7. [DOI] [PubMed] [Google Scholar]
- 29.MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, et al. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys 2012; 103:411–26. [DOI] [PubMed] [Google Scholar]
- 30.Jones JW, Bennett A, Carter CL, Tudor G, Hankey KG, Farese AM, et al. Citrulline as a biomarker in the non-human primate total- and partial-body irradiation models: Correlation of circulating citrulline to acute and prolonged gastrointestinal injury. Health Phys 2015; 109:440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449:1003–7. [DOI] [PubMed] [Google Scholar]
- 32.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011; 478:255–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim T-H, Escudero S, Shivdasani RA. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A 2012; 109:3932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A 2012; 109:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar P, Wang P, Tudor G, Booth C, Farese AM, MacVittie TJ, et al. Evaluation of plasma biomarker utility for the gastrointestinal acute radiation syndrome in non-human primates after partial body irradiation with minimal bone marrow sparing through correlation with tissue and histological analyses. Health Phys 2020; 119:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boittin F-X, Martigne P, Mayol J-F, Denis J, Raffin F, Coulon D, et al. Experimental quantification of delayed radiation-induced organ damage in highly irradiated rats with bone marrow protection: Effect of radiation dose and organ sensitivity. Health Phys 2015; 109:134–44. [DOI] [PubMed] [Google Scholar]


