Abstract
Ketogenic diets (KD) are high in fat and low in carbohydrates, forcing cells to utilize mitochondrial fatty acid oxidation for energy production. Since cancer cells demonstrate increased mitochondrial oxidative stress relative to normal cells, we hypothesized that a KD may selectively enhance metabolic oxidative stress in head and neck cancer cells, sensitizing them to radiation and platinum-based chemotherapy without causing increased toxicity in surrounding normal tissues. This hypothesis was tested in preclinical murine xenografts and in a phase 1 clinical trial (NCT01975766). In this study, mice bearing human head and neck cancer xenografts (FaDu) were fed either standard mouse chow or KetoCalt KD (90% fat, 8% carbohydrate, 2% protein) and exposed to ionizing radiation. Tumors were harvested from mice to test for glutathione, a biomarker of oxidative stress. In parallel, patients with locally advanced head and neck cancer were enrolled in a phase 1 clinical trial where they consumed KD and received radiation with concurrent platinum-based chemotherapy. Subjects consumed KetoCal KD via percutaneous endoscopic gastrostomy (PEG) tube and were also allowed to orally consume water, sugar-free drinks, and foods approved by a dietitian. Oxidative stress markers including protein carbonyls and total glutathione were assessed in patient blood samples both pre-KD and while consuming the KD. Mice bearing FaDu xenografts that received radiation and KD demonstrated a slight improvement in tumor growth rate and survival compared to mice that received radiation alone; however a variation in responses was seen dependent on the fatty acid composition of the diet. In the phase 1 clinical trial, a total of twelve patients were enrolled in the study. Four patients completed five weeks of the KD as per protocol (with variance in compliance). Eight patients did not tolerate the diet with concurrent radiation and platinum-chemotherapy (5 were patient decision and 3 were removed from study due to toxicity). The median number of days consuming a KD in patients who did not complete the study was 5.5 (range: 2–8 days). Reasons for discontinuation included “stress of diet compliance” (1 patient), grade 2 nausea (3 patients), and grade 3 fatigue (1 patient). Three patients were removed from the trial due to dose-limiting toxicities including: grade 4 hyperuricemia (2 patients) and grade 3 acute pancreatitis (1 patient). Median weight loss was 2.95% for the KD-tolerant group and 7.92% for patients who did not tolerate the diet. In conclusion, the ketogenic diet shows promise as a treatment combined with radiation in preclinical mouse head and neck cancer xenografts. A phase 1 clinical trial evaluating the safety and tolerability of KD demonstrated difficulty with diet compliance when combined with standard-of careradiation therapy and cisplatin chemotherapy.
INTRODUCTION
Despite advances in cancer therapy in the past 30 years, head and neck squamous cell carcinoma (HNSCC) continues to cause significant morbidity and mortality. A majority of patients with HNSCC present with advanced-stage disease and have significantly worse clinical outcomes (1). The standard of care for locoregionally advanced HNSCC includes a combination of radical surgical resection, therapeutic radiation, and platinum-based chemotherapy cisplatin (Cis). However, Cis is associated with significant treatment toxicities and is not suitable for patients with impaired kidney function or poor performance status. Thus, there remains a need for complementary approaches that can selectively enhance the therapeutic effects of radiation in HNSCC patients.
Malignant cells are known to have high levels of glucose uptake, glycolysis and pentose phosphate activity even in the presence of oxygen (2–6). Cancer cells have alterations in their mitochondrial structure and function resulting in increased levels of reactive oxygen species such as O2•− and H2O2 relative to normal cells (7, 8). Data suggest that the increased glycolysis seen in cancer cells represents a response to protect against increased hydroperoxide-mediated oxidative stress caused by altered mitochondrial metabolism (7, 9). Strategies to exploit the increased mitochondrial metabolic oxidative stress in cancer cells may selectively enhance the effects of radiation and chemotherapy. The current study targets mitochondrial metabolism using a highly ketogenic diet (KD).
A classical KD consists of approximately 90% fat, 8% protein and 2% carbohydrate and is a well-documented therapy for intractable epilepsy (10). The KD’s relative safety has been demonstrated in pediatric and adult populations (11–13). The KD has been shown to reduce tumor growth and improve survival in a variety of xenograft models including malignant glioma, gastric, colon and prostate cancers (14–17). Furthermore, KDs potentiate the effect of standard anticancer agents in malignant glioma models (18, 19). We previously showed that a KD increases radiation and chemotherapy sensitivity in pancreas and non-small cell lung cancer (NSCLC) xenograft models via a mechanism that increases cancer cell oxidative stress (20–22). As a result of these preclinical data, there has been a great deal of interest in using KD as an adjunct cancer treatment in the clinical setting. Several studies with limited patient numbers have been published, and reported that a KD is safe in patients, but diet adherence varied greatly (18, 23–27), reviewed by Shingler et al. (28). Two phase 1 clinical trials conducted at the University of Iowa assessing the safety of a KD in NSCLC and pancreatic cancer patients receiving concurrent chemoradiation showed that while the diet is generally safe, compliance with the KD is poor; patients discontinued the KD predominantly due to difficulties with unpleasant taste, nausea and vomiting (21). We hypothesized that these patient-reported issues could be circumvented if the KD is administered via a PEG tube. Because some HNSCC patients receive upfront PEG tube placement, we hypothesized this patient population might have improved KD compliance compared to NSCLC and pancreatic cancer patients. A phase I trial (NCT01975766) was developed to test if KD combined with standard radiation and concurrent chemotherapy for HNSCC patients who had upfront PEG tube placement is feasible and safe.
MATERIALS AND METHODS
Cell and Culture Conditions
EGFR-positive FaDu HNSCC were obtained from the American Type Culture Collection (ATCC®, Gaithersburg, MD) and maintained in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (FBS) (HyClone™ Laboratories, Logan, UT) without antibiotics. Cells were passaged a maximum of 15 times.
Tumor Xenograft Growth
Athymic-nu/nu female mice aged 4 to 6 weeks were purchased from Envigo RMS Inc. (Indianapolis, IN). Mice were housed in the Animal Care Facility at the University of Iowa and all procedures were approved by the University of Iowa Institutional Animal Care and Use Committee and conformed to NIH guidelines. Three million FaDu tumor cells were injected subcutaneously into the right upper leg as described elsewhere (29).
Ketogenic Diet
The mice in the KD groups were fed ad libitum either the original or the new formula KetoCal vanilla powder (Nutricia North America, Gaithersburg, MD). See Table 1 for the diet’s lipid composition. These diets both have a ketogenic ratio (fats: proteins and carbohydrates) of 4:1 (energy distribution: fat 88.7%, carbohydrate 3.1%, protein 8.2% and fiber 1.5%) but differ in the kind of lipids that compose the fat component (Table 1). KetoCal was prepared as a paste on a culture dish lid by adding water (water:KetoCal ratio of 1:2) then placed upside down in the food hopper and attached to the cage lid to ensure animal access. Control mice were fed a standard rodent diet (NIH-31 modified 21% fat, 54% carbohydrate, and 25% protein).
TABLE 1.
Fatty Acid Composition of KetoCal (Nutricia North America) Original and New Formulas
| Fatty acid composition (g/100 g) |
|||
|---|---|---|---|
| Fatty acid | Common name | Original formulation | New formulation |
|
| |||
| 12:0 | Lauric acid | 0.1 | 0.02 |
| 14:0 | Myristic acid | 0.1 | 0.67 |
| 16:0 | Palmitic acid | 12.1 | 32.5 |
| 16:1 | Palmitoleic acid | 0.01 | |
| 18:0 | Stearic acid | 10.3 | 4.5 |
| 18:1 (cis) | Oleic acid | 25 | 31.4 |
| 18:1 (trans) | Trans-oleic/elaidic acid | 34.3 | |
| 18:2 (cis) | cis-linoleic acid (LA) | 14.5 | 27.44 |
| 18:2 (trans) | trans-Linoleic acid | 1.6 | 0.26 |
| 18:3 (cis) | Alpha linolenic acid (ALA) | 1.2 | 2.8 |
| 18:3 (trans) | trans-alpha linolenic acid | 0.3 | |
| 20:0 | Arachic acid | 0.3 | |
| 20:3 (n-6) | Dihomo gamma linolenic acid | 0.02 | |
| 20:4 (n-6) | Arachidonic acid | 0.18 | |
| 22:0 | Behenic acid | 0.2 | 0.01 |
| 22:6 (n-3) | Docosahexaenoic acid (DHA) | 0.18 | |
| 24:0 | Lignoceric acid | 0.01 | |
Irradiations
Mice in all treatment and sham groups were anesthetized using 87.5 mg/kg ketamine and 12.5 mg/kg xylazine mixture in the radiation facility at the University of Iowa. The mice in the radiation treatment groups were placed in a lead box with only the right leg exposed. Irradiation was performed using a Pantak Therapax DXT 300 X-ray machine operated at 200 kVp with added filtration of 0.35 mm copper and 1.5 mm aluminum, resulting in a beam quality of 0.95 mm copper. Tumors were measured daily using Vernier calipers [volume=(length × 3 width2)/2] and mice were euthanized when tumor length exceeded 1.5 cm in any dimension (euthanasia criteria).
Animal Experimental Design
For data shown in Fig. 1, mice were divided into four treatment groups: Control (n=6); KD (n=5); irradiation alone (n=4); and KD with radiation (n=4). Original formula KetoCal KD was initiated two days before the initiation of irradiation and discontinued the day after the final radiation dose two weeks later. Mice received a total dose of 12 Gy of radiation, comprised of 2 Gy every other weekday, for two weeks. After mice reached euthanasia criteria, the tumor was collected, and a portion homogenized in 5% sulfosalicylic acid, then frozen at −80°C for glutathione analysis. For data shown in Fig. 2, mice were divided into six treatment groups: Standard diet (n = 6); original formula KD (n=6); irradiation alone (n=7); original formula KD with radiation (n = 7); new formula KD (n = 6); or new formula KD with radiation (n = 5). Mice were started on KetoCal KD the day before irradiations were initiated, and continued until sacrifice. Radiation dose of 24 Gy was delivered in four fractions within two weeks followed by 28.8 Gy delivered in 16 fractions in three weeks for a total dose of 52.4 Gy. The final day of irradiation was day 53.
FIG. 1.
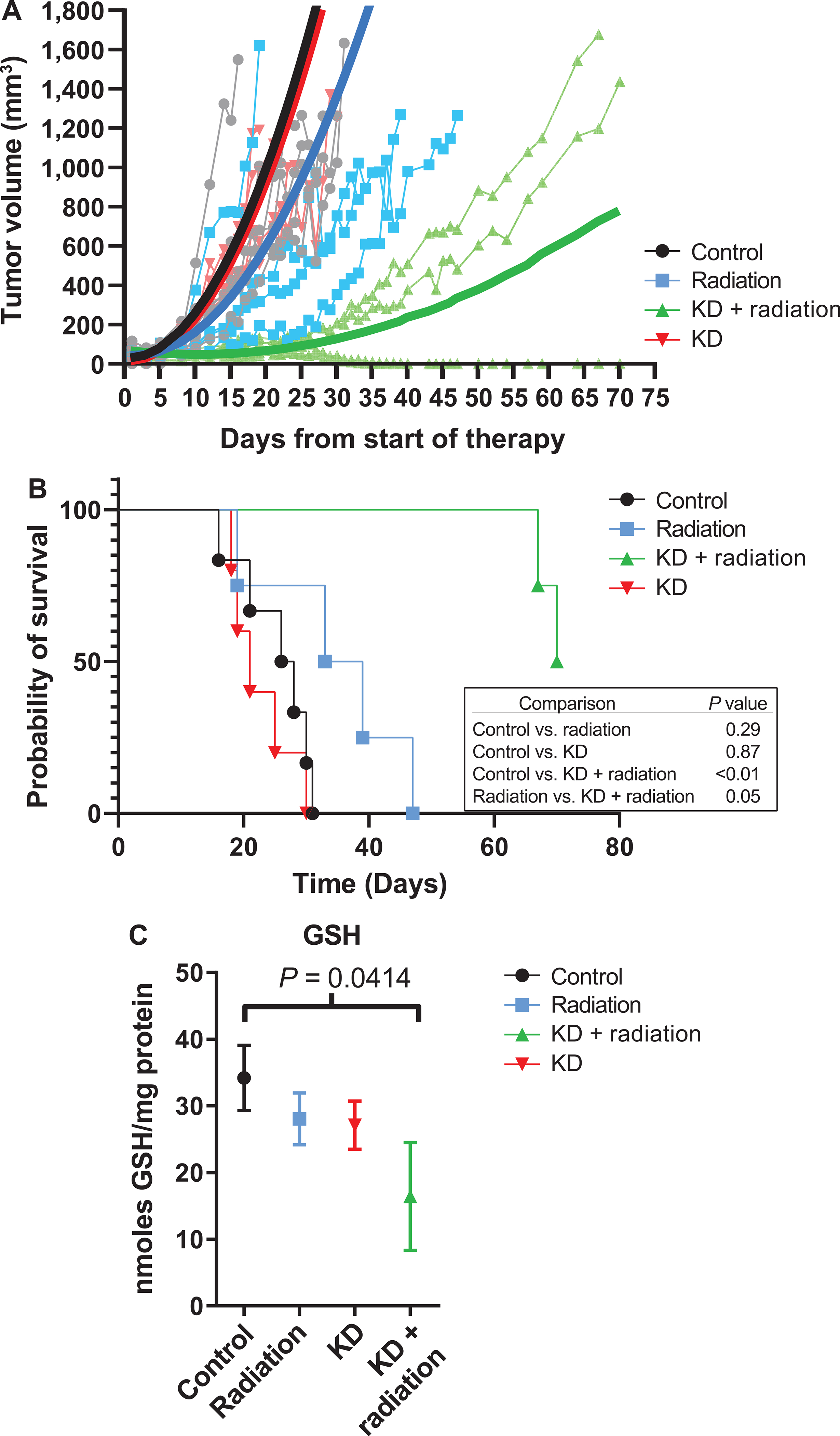
KD (original formulation) is effective in combination with radiation in decreasing tumor growth rate of FADU human head and neck cancer cells in vivo. Mice were fed ad libitum standard rodent chow or KD with or without irradiation, 2 Gy fractions × 3 per week (total 12 Gy). Panel A: The combination KD + radiation significantly decreases average tumor growth rate P=0.00075. Panel B: Kaplan-Meier survival graph resulted in a trend of increased survival in the KD + radiation group compared to radiation alone. Panel C: KD + radiation treatment resulted in a decrease in GSH in xenograft tumors from mice compared to controls. Mean and SEM are shown with significance measured using one-way ANOVA with Fishers LSD.
FIG. 2.
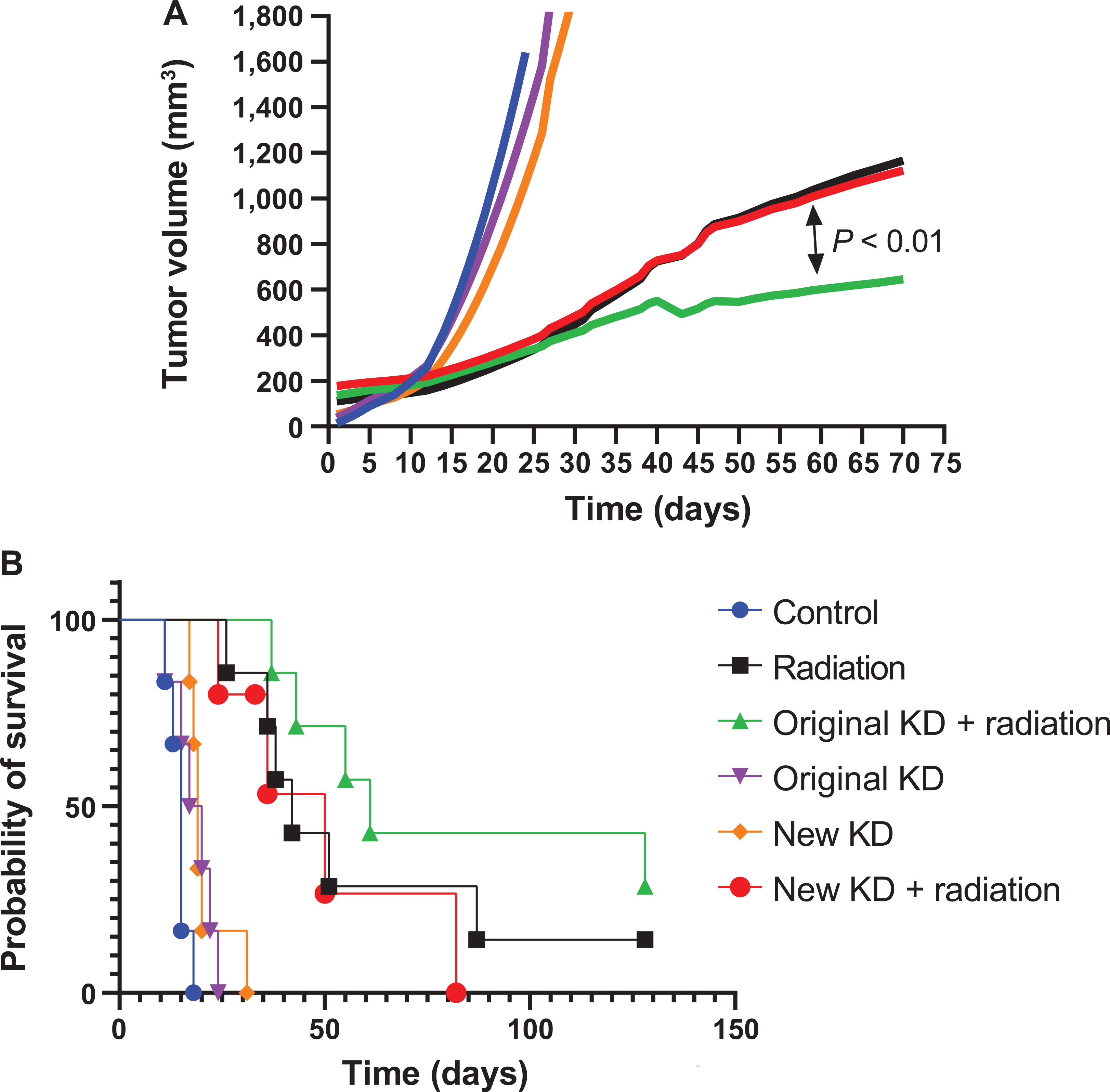
Original formula KD + radiation significantly delayed tumor growth rate compared to radiation alone. FaDu xenografts were grown in nude mice. Mice (6–7 per group) received KD for the entire treatment period (two months) and/or irradiation three times per week (6 Gy×4 fractions then 1.8 Gy×16 fractions, for a total of 52.8 Gy). Mice were sacrificed when death criteria were met (1.5 cm tumor diameter). Panel A: Original formula KD + radiation significantly slowed tumor volume increase compared to radiation alone, P < 0.01, and new formula KD + radiation, P < 0.01. Panel B: Kaplan-Meier survival curves resulted in median survival, in days, of control 15, new KD 19, original KD 19, radiation alone 42, new KD + radiation 50, original KD + radiation 63. Pair-wise group comparisons of Kaplan-Meier survival curves demonstrate that neither the original nor the new formulation KD combined with radiation significantly enhanced survival over radiation alone. P=0.40 and P = 0.58, respectively.
Glutathione Assay
For total glutathione (GSH and GSSG) quantification, samples were lysed in 5% sulfosalicylic acid: approximately 50 mg of mice xenograft tumors in 200 μl, or 50 μl of patient red blood cells in 450 μl. Samples were centrifuged at 5,000 rpm for 5 min and 5–30 μl of the supernatant was used to determine levels of total glutathione by the method described by Anderson (30) and Griffith (31). All values were normalized to the protein content using the BCA method and reported as GSH equivalents versus a standard curve (32). Briefly, a yellow color TNB (5-thio-2-nitrobenzoic acid) generated from GSH reacting with DTNB in the presence of glutathione reductase and NADPH is detected at 412 nm. GSSG was distinguished by adding 20 μl 2-vinylpyridine ethanol mixture to 100 μl of sample, incubating for 30 min, and assayed as described by Griffith (31).
Carbonyl Assay
Protein carbonyl content in subject’s plasma was measured according to a protocol adapted from the Protein Carbonyl Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI). Briefly, 150–200 μl of subject plasma from weekly blood draws was mixed with 800 μl of 2,4 dinitrophenylhydrazine to allow protein carbonyls to form a Schiff base and corresponding hydrazone. The amount of protein hydrazone produced was quantified spectrophotometrically at an absorbance of 360–385, and the carbonyl content was standardized to protein concentration. A standard curve using known quantities of albumin oxidized using vitamin C and iron was used to establish that the samples were in the linear range of the assay. Data were expressed as nmol carbonyl/mg protein.
Statistical Analysis
Analysis of the in vivo mouse data focused on treatment group comparisons of tumor growth and survival. Tumor volume periodic measurement resulted in repeated measurements for each mouse. Linear mixed effects regression models were used to estimate and compare tumor growth curves. The Kaplan-Meier method was used to estimate the survival curves, and groups comparisons were made using the log rank test. All tests were two-sided and carried out at the 5% level of significance using SAS® version 9.4 software (Cary, NC). Statistics were performed by the Biostatistical Core of the Holden Comprehensive Cancer Center.
Clinical Trial
An investigator-initiated protocol adding a KD to standard of care for HNSCC was approved by the University of Iowa Institutional Review Board (Biomedical, IRB-01) and registered on clinicaltrials.gov prior to enrollment of the first subject (NCT01975766). Patients with histologically or cytologically documented squamous cell carcinoma of the oropharynx, larynx or hypopharynx (AJCC stage II, III, IVA or IVb) referred for concurrent chemoradiation (CRT) were invited to participate. Potential participants had to have adequate performance status (KPS > 50) as well as marrow and organ function: leukocyte ≥3,000/mm3, absolute neutrophil count of ≥1,500/mm3, platelets of ≥100,000/mm3, total bilirubin ≤1.5 mg/dL, AST ≤2× the upper limit of normal (ULN), creatinine ≤1.5× ULN (or creatinine clearance ≥60 ml/min/1.73 m3) and an HbA1C ≤8%. Exclusion criteria included prior radiation therapy resulting in treatment field overlap, chronic systemic corticosteroids use, life expectancy of ≤3 months, severe comorbidities, pregnancy, nursing, or active use of other investigational agents. Eligibility was independently confirmed by the Holden Comprehensive Cancer Center’s Data and Safety Monitoring Committee.
The treatment schema is provided in Fig. 3. Prior to beginning the KD, baseline glucose, ketones, BUN, serum uric acid and fasting whole blood lipid panels were obtained. A prophylactic PEG tube was placed in all patients prior to beginning the KD and initiation of CRT (33). The need for feeding tube placement was agreed upon by both the treating medical oncologist and radiation oncologist. Subjects who participated in the clinical trial were anticipated to have large regions of oral, oropharyngeal and hypopharyngeal mucosa in the high-dose radiation field culminating in a high probability of dysphagia and odynophagia. Patients primarily consumed a KD by PEG tube to achieve a target β-hydroxybutyrate level of ≥0.6 mmol/l (see below for monitoring protocol). Patients also met with a dietitian prior to KD initiation for an overall nutrition assessment and for education about the KD. Patients were instructed on formula mixing (new formulation KetoCal 4:1®) as primary intake of KD. Patients were instructed on KD-appropriate oral intake options to preserve swallow function and/or for comfort and pleasure. Approved items included but were not limited to water and sugar-free beverages, various Atkins® snacks or meals, along with KD home-cooked meals following a 4:1:1 ratio of fats to protein and carbohydrates. Patients continued taking their usual dietary supplements during the study after verifying the lack of carbohydrate content with the dietitian. The dietitian also evaluated each patient’s individual KD for adequacy of nutrient intake and need for further nutrition supplementation throughout the clinical trial period. Supplements included, but were not limited to a multivitamin, additional protein, sodium and fat.
FIG. 3.
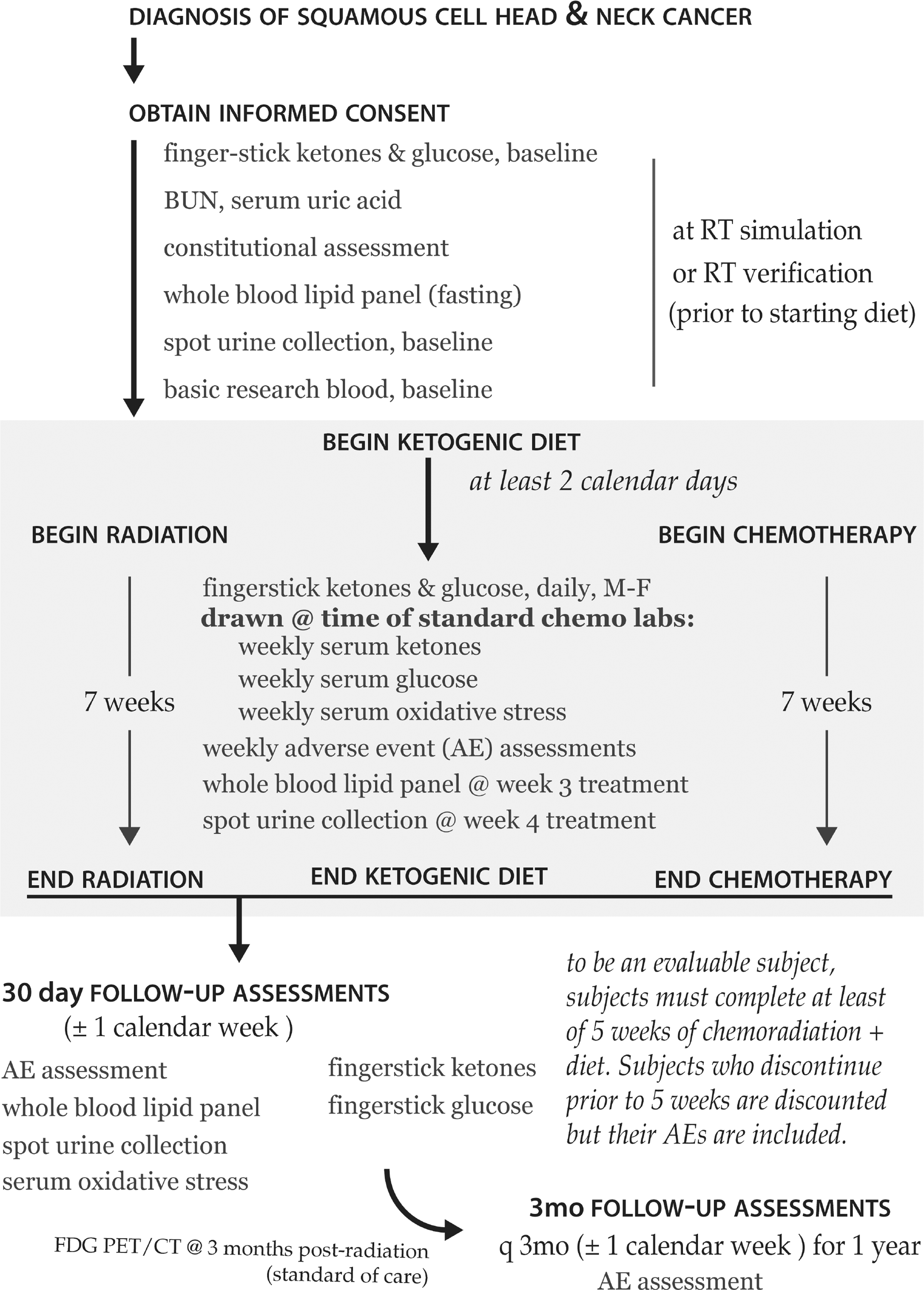
Schematic overview of the KD head and neck cancer clinical protocols. Patients consumed a KD for five weeks while receiving standard-of-care radiation and chemotherapy. Patients were followed for up to one year after completing the KD to assess for subsequent adverse events.
Patients began the KD at least two calendar days prior to beginning CRT. Fingerstick glucose (Accu-chek Aviva or Aviva Plus, Roche Diagnostics, Indianapolis, IN) and ketone measurements using Precision Xtra Blood Glucose and Ketone Monitoring System, were obtained and documented by research nursing on the days of radiation treatment. In addition, weekly ketone values were obtained from venous blood samples utilizing a beta-hydroxybutyrate assay (Stanbio Laboratory, Boerne, TX) and spectrophotometer (Roche Diagnostics). Ketosis was defined as serum β-hydroxybutyrate ≥0.6 mmol/l (3.5 mg/dL). Participants were assessed weekly with physical examinations and standard-of-care laboratory assessments. A repeat fasting lipid panel was obtained three weeks into CRT. Patients were to consume a KD for a period of approximately five weeks while receiving concurrent radiation therapy (66–70 Gy/33–35 fractions) and chemotherapy (Cis 100 mg/m2 every 3 weeks, Cis 40 mg/m2 weekly, Cis 20 mg/m2 and paclitaxel 30 mg/m2 weekly, or carboplatin AUC = 2 per the Calvert formula and paclitaxel 40 mg/m2 weekly) and encouraged to continue KD through the entirety of radiation treatment. Patients were followed every three months for a year for adverse events, which are graded according to Common Terminology Criteria for Adverse Events v.4.0 (CTCAE). Adverse events were initially determined by the treating physician and then reviewed by the study principal investigator (BGA) and medical monitor (DJB). All dose-limiting toxicities (DLT) were reviewed by the UIHC HCCC DSMC. The goal of the phase 1 trial was to assess the safety and tolerability of the KD delivered via a feeding tube combined with CRT and was not powered for efficacy.
RESULTS
KD Enhances Radiation Response and Increases Tumor Oxidative Stress in Mouse FaDu Xenografts
Athymic nude mice (4–6 per group) were injected with FaDu HNSCC cells in their right flank and received two weeks of irradiation (12 Gy delivered in 6 fractions at 2 Gy per fraction three times per week) with and without original formula KD (Table 1) starting the day before the first fraction. All mice consuming a KD had a β-hydroxybutyrate >.0.3 mmol/l by the second day of KD initiation (data not shown). We have previously shown that nude mice fed the KD achieve blood β-hydroxybutyrate level of 1.4 ± 0.4 mmol/l by day 3 and maintain blood β-hydroxybutyrate from 0.6–1.8 mmol/l (20). Mice treated with original formula KD and radiation demonstrated a significantly increased survival compared to control or radiation treatment alone (P ≤ 0.01 or 0.05, respectively, Fig. 1B). The median survival days of each group are as follows: control = 27; KD = 21; radiation = 36; and combination treatment, inestimable. However, the decrease in tumor growth rate seen with combined KD and radiation was not significant (control vs. KD with radiation, P = 0.12) (Fig. 1A). To examine the changes in markers of redox stress, the concentrations of total glutathione (GSH) in tumor samples were measured. Tumors from mice administered KD with radiation showed a significant decrease in total glutathione levels (Fig. 1C) compared to controls, suggesting a possible link between decreased levels of total GSH and KD during treatment.
KD Enhancement of Radiation Response in Mouse FaDu Xenografts is Dependent on KD Formulation
The fatty acid composition of the KD was changed by the supplier during these studies. The most notable differences were the removal of trans-oleic acid, increased percentage of palmitic acid, increased linoleic acid, and the addition of docosahexaenoic acid and arachidonic acid (Table 1). Athymic nude mice bearing FaDu xenografts were treated with radiation ± the remains of the original formulation KD to repeat the results seen in Fig. 1. All treatment combinations and diets were well tolerated as demonstrated by a lack of weight loss and general condition of the mice. The radiation treatment regimen was 24 Gy delivered in 4 fractions in two weeks followed by 28.8 Gy delivered in 16 fractions over three weeks. This mouse experiment continued treatment for five weeks to more closely resemble the human experience with a total equivalent dose in 2 Gy fractions of 60 Gy. Blood ketone measurements were obtained in a subset of mice from consuming either the old or new formulation KetoCal during the first two weeks of therapy to confirm ketosis. The average blood β-hydroxybutyrate was 1.1 mmol/l in mice consuming either the original or new formulation diets. Mice administered the original formula KD with radiation had significantly slower tumor growth rate compared to control, irradiation alone, or new formulation diet with radiation (P < 0.01; Fig. 2A). Mice administered the original KD with radiation had a slight but insignificant improvement in survival over mice that received irradiation on standard mouse chow (Fig. 2B). However, mice administered the new formulation KD with radiation did not significantly differ from those that received irradiation alone in either growth rate or survival (Fig. 2A and B), suggesting that the fatty acid composition of the diet may play a pivotal role in treatment outcome when combined with radiation.
Head and Neck Cancer Patients have Difficulty Tolerating a Ketogenic Diet with Concurrent Radiation and Cisplatin Therapy
Twelve head and neck cancer patients were enrolled in a phase 1 clinical study assessing the tolerability of a KD administered via feeding tube combined with CRT (Fig. 3). Patient clinical parameters are listed in Table 2. Four participants completed the KD for five weeks as prescribed. Patients started KD two days prior to receiving CRT and entered ketosis on the first day of CRT as evident by their serum β-hydroxybutyrate ≥0.6 mmol/l. Of the four patients who completed the diet, only patient no. 2 maintained elevated ketones, whereas three patients (nos. 1, 8 and 9) had fluctuations in their ketone values over the treatment period (Fig. 4A). Patients who consumed a KD showed stable blood glucose levels (Fig. 4B). Several CTCAE grade 1–4 events occurred over the course of the trial (Table 3), including nausea, vomiting, diarrhea, constipation, neutropenic fever, fatigue, hyperuricemia and pancreatitis. The median weight loss was 2.95% for the KD group over the course of their CRT (range: −9.32% to 7.53%). Median weight loss for patients who did not tolerate the diet over the entire course of CRT was 7.92% (range: −5.77% to 11.27%). Though not significant, these data support the literature reporting that KD prevents significant weight loss during CRT (34, 35). Lipid panels remained normal in all patients and none of the patients developed ketoacidosis.
TABLE 2.
Characteristics of Head and Neck Patients (n = 12)
| Completed ketogenic diet |
||
|---|---|---|
| Yes | No | |
|
| ||
| Age | ||
| Median | 53 | 57 |
| Range | 50–78 | 40–65 |
| Sex | ||
| Male | 3 | 7 |
| Female | 1 | 1 |
| Karnofsky performance status | ||
| 100 | 2 | 1 |
| 90 | 0 | 4 |
| 80 | 1 | 2 |
| 70 | 1 | 1 |
| Staging Tumor stage |
||
| T1 | 0 | 1 |
| T2 | 1 | 1 |
| T3 | 2 | 2 |
| T4a | 1 | 3 |
| T4b | 0 | 1 |
| Node stage | ||
| N1 | 0 | 2 |
| N2 | 1 | 0 |
| N2b | 1 | 2 |
| N2c | 2 | 4 |
| Metastasis stage | ||
| M0 | 4 | 8 |
FIG. 4.
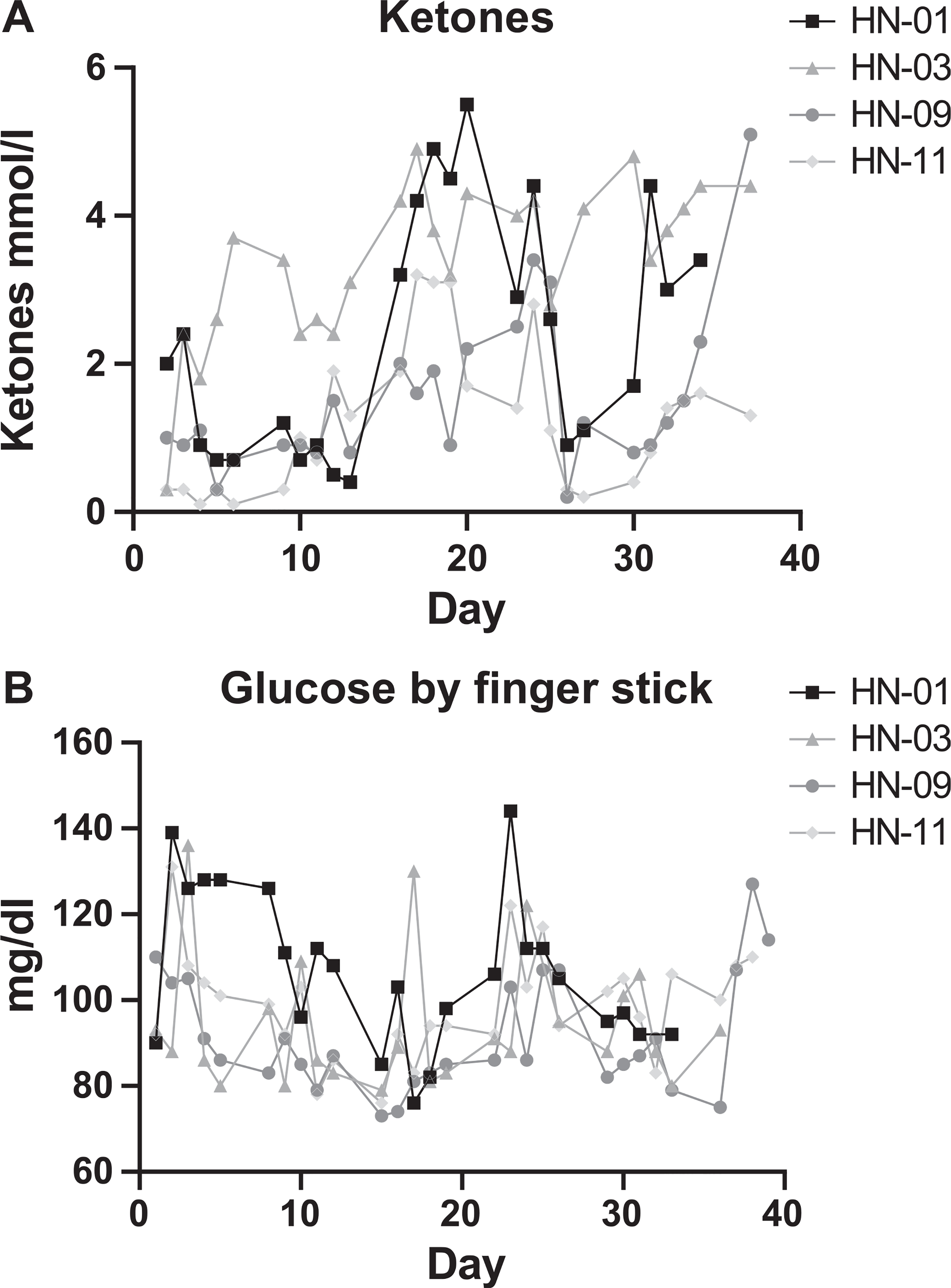
Blood ketones remain elevated and glucose levels were stable in patients who completed the KD (patient ID nos. HN-01, 03, 09 and 11). Patients completing the KD had a reduction in oxidized glutathione compared to similar patents not on the diet. Panels A and B: Blood samples were collected weekly to assess ketone (panel A) and glucose (panel B) levels. Day 0 indicates the day patient began KD. Panel A: Ketosis is defined as blood ketone levels ≥ 0.6 mmol/l. Patients entered ketosis by day 3 of the diet and were able to maintain ketosis most of the time.
TABLE 3.
Summary of Head and Neck Cancer Patient Experience
| Patient ID no. | Cancer site | Initial cTNM stage | Radiation | Chemotherapy | KD (days) | |
|
| ||||||
| 1 | Larynx | T4aN2c | 70 Gy/35 fx | Cisplatin 100 mg/m2 | 38a | |
| 2 | Oropharynx | T3N2 | 70 Gy/35 fx | Carbo/Taxol weekly | 37a | |
| 3 | Oropharynx | T4bN2b | 70 Gy/35 fx | Carbo/Taxol weekly | 6 | |
| 4 | Oropharynx | T2N2c | 70 Gy/35 fx | Cisplatin 20mg/m2 and Taxol weekly | 8 | |
| 5 | Larynx | T3N1 | 70 Gy/35 fx | Cisplatin 100 mg/m2 | 2 | |
| 6 | Oropharynx | T4aN2c | 70 Gy/35 fx | Cisplatin 40 mg/m2 | 3 | |
| 7 | Oropharynx | T1N1 | 70 Gy/35 fx | Cisplatin 100 mg/m2 | 7 | |
| 8 | Oropharynx | T3N2c | 70 Gy/35 fx | Cisplatin 100 mg/m2 | 37a | |
| 9 | Oropharynx | T2N2b | 70 Gy/35 fx | Cisplatin 100 mg/m2 | 38a | |
| 10 | Oral cavity | T4aN2b | 66 Gy/33 fx | Cisplatin 100 mg/m2 | 4 | |
| 11 | Hypopharynx | T3N2c | 70 Gy/35 fx | Cisplatin 100 mg/m2 | 5 | |
| 12 | Hypopharynx | T4aN2c | 66 Gy/33 fx | Cisplatin 100 mg/m2 | 7 | |
|
| ||||||
| Ketosis duration (days) | DLT | Reason for discontinuation | Tumor response | PFS (months) | Overall survival (months)b | Current status |
|
| ||||||
| 19 | Completed | PD (local and distant) | 6.28 | 13.94 | Deceased | |
| 25 | Completed | CR/NED | 32.69 | 48.39 | Alive | |
| N/A | Fatigue grade 3 | CR/NED | 22.7 | 44.22 | Alive | |
| N/A | Hyperuricemia grade 4 | DLT | CR/NED | 25.99 | 43.36 | Alive |
| N/A | Diet intolerance | CR/NED | 18.66 | 34.42 | Alive | |
| N/A | Nausea grade 2, vomiting grade 1, dehydration grade 2 | CR/NED | 17.51 | 33.5 | Alive | |
| N/A | Acute pancreatitis grade 3 | DLT | PD | 5.49 | 33.21 | Alive |
| 24 | Completed | CR/NED | 16.1 | 32.81 | Alive | |
| 25 | Completed | CR/NED | 12.19 | 30.51 | Alive | |
| N/A | Nausea grade 2, vomiting grade 1 | CR/NED | 12.62 | 29.98 | Alive | |
| N/A | Nausea grade 2, vomiting grade 1, bloating grade 2 | CR/NED | 12.55 | 21.21 | Alive | |
| N/A | Hyperuricemia grade 4 | DLT | CR/NED | 12.39 | 27.91 | Alive |
Completed prescribed KD.
Overall survival from date of initiation of primary treatment with radiation.
Abbrevations: KD=ketogenic diet; fx=fractions; DLT=dose limiting toxicity; CR=complete response; NED=no evidence of disease; PD= progressed disease; PFS = progression-free survival.
Eight out of the 12 patients did not tolerate the diet due to either dose-limiting toxicity or voluntary withdrawal from the study due to adverse symptoms. The median number of days consuming a KD in patients who did not complete the study was 5.5 (range: 2–8 days). Reasons for discontinuation included psychological stress of diet (in 1 patient), grade 2 nausea (3 patients), grade 3 nausea (1 patient), grade 3 fatigue (1 patient), and grade 4 hyperuricemia (2 patients). Patient no. 7 developed grade 3 pancreatitis in addition to grade 3 nausea. The trial was temporarily suspended after patient no. 4 developed asymptomatic grade 4 hyperuricemia (12.7 nd/dl). Ketones have been reported to alter the renal handling of urate (36, 37). The protocol was amended to initiate allopurinol and address treatment-related hyperuricemia.
Patients were followed for long-term outcomes; however, this phase 1 trial was not powered to determine statistical differences in overall survival or progression-free survival. The median follow-up after completion of CRT was 13.1 months (range 8.0–26.7 months). Of the 12 patients, 11 were alive with no evidence of disease at last follow-up. Patient no. 1 died with local recurrence and distant disease 12.3 months after completing CRT (Table 2).
Consuming a Ketogenic Diet Does Not Increase Systemic Oxidative Stress Markers
To determine if a KD increased systemic markers of oxidative stress, we compared plasma protein carbonyl content as well as total RBC glutathione levels from patients who completed the KD to samples from head and neck cancer patients receiving standard CRT. Patients consuming a KD showed a trend toward increased protein carbonyl content compared to those receiving standard CRT (Fig. 5C). Interestingly, patients who consumed a KD had no change in total GSH and significantly lower glutathione disulfide (GSSG) in their red blood cells compared to pre-diet levels and to patients receiving standard CRT (Fig. 5A and B). These data suggest that head and neck cancer patients who consume a KD do not have evidence of increased systemic redox stress.
FIG. 5.
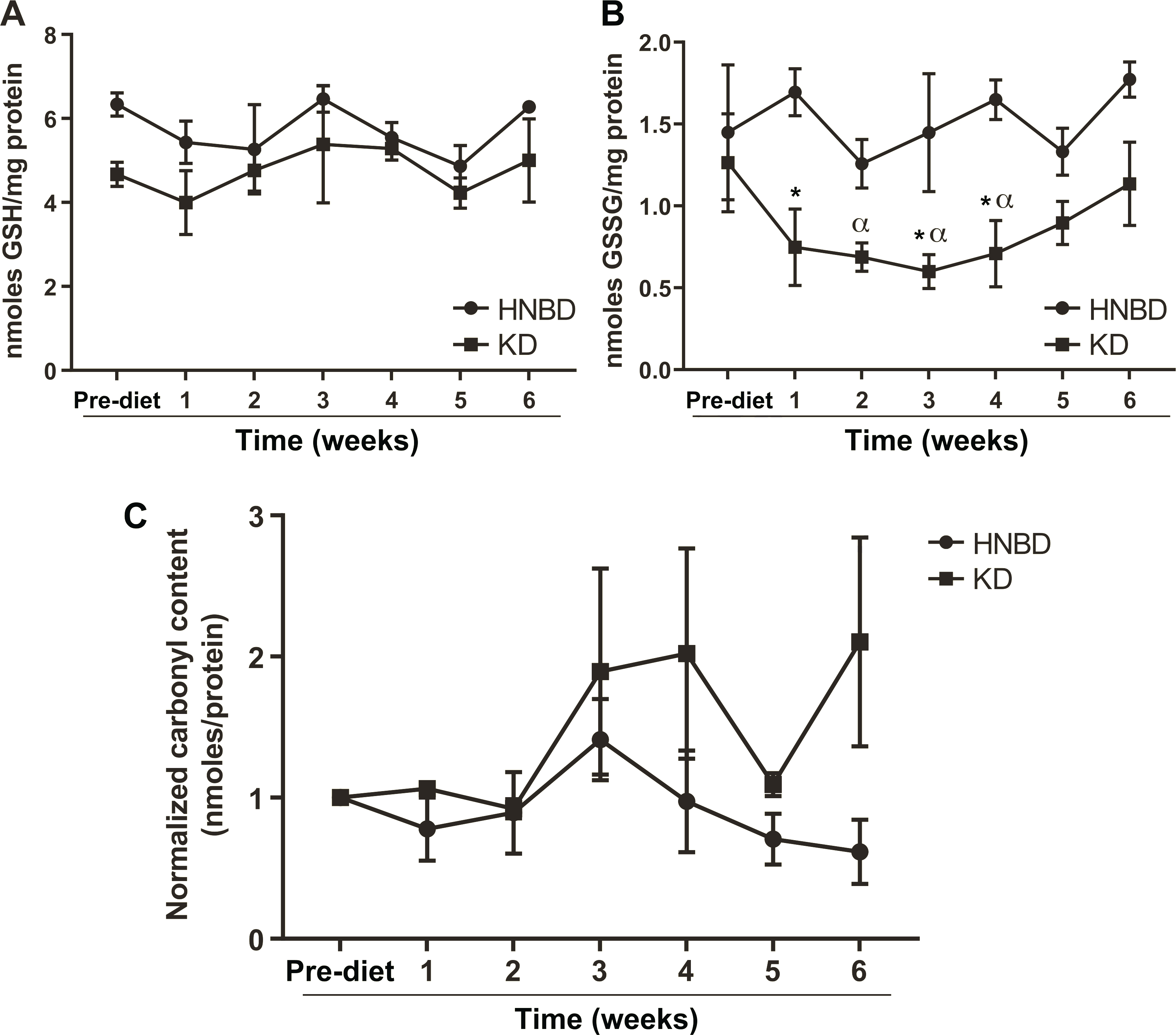
Patients completing the KD had a reduction in oxidized glutathione in red blood cells compared to similar patents not on diet. Panel A: Average total glutathione (GSH) of the four patients who completed the study remained stable throughout the diet. Panel B: There was a significant decrease in oxidized (GSSG) glutathione from pre-diet levels and compared to similar head-and-neck cancer patients (HNBD) not receiving KD. *Significantly different from HNBD. αSignificantly different from pre-diet average two-way ANOVA with Dunnett’s multiple comparison, P < 0.05. Panel C: Carbonyl content of patient serum shows a non-significant trend.
DISCUSSION
Cancer cells are believed to exist in a state of chronic metabolic oxidative stress, which is thought to be mediated by the production of reactive oxygen species including O2•− and H2O2 from disruptions in mitochondrial oxidative metabolism (7). Furthermore, cancer cells adjust their oxidative metabolism by increasing glucose consumption, creating reducing equivalents (NADPH) via the pentose phosphate pathway (7). A KD may theoretically selectively increase the metabolic oxidative stress in cancer cells by generating excess ketone bodies, driving energy production through mitochondrial metabolism, and generating greater steady-state levels of superoxide and hydrogen peroxide. Additionally, increased levels of insulin and insulin-like growth factor associated with elevated blood glucose levels may influence cancer cell growth (38). Thus, reducing blood sugar levels through fasting or KD may slow tumor progression (22, 38). More recently, the KD has gained attention both in the research and the clinical community as an adjunct therapy to standard of care radiation and chemotherapy. Our research team has previously shown in preclinical NSCLC (20) and pancreatic (21) xenograft models that a KD enhanced radiation and chemotherapy efficacy by increasing lipid oxidation in xenograft tumors. Unfortunately, in this previous study, the KD was poorly tolerated by patients in clinical trials. It has been suggested that KD would be a beneficial supportive treatment option in HNSCC (39). Investigation into the potential application of KD in HNSCC was promising since this patient population often requires enteral feeding, which might overcome the poor diet tolerance seen previously (21).
Similar to our previous investigations showing that KD enhanced the radiation response in non-small cell lung cancer (20), our current results demonstrate that KD has the potential to enhance the radiation response in HNSCC. We also provide further preclinical evidence that the KD-mediated enhanced tumor response could in part result from increased cancer cell redox stress as KD combined with radiation treatment decreased the amount of total GSH in tumor cells, suggesting an overall decrease in antioxidant capacity (Fig. 1C). Although previously published investigations demonstrated beneficial anti-cancer effects using KD alone in prostate, gastric and brain cancer (15, 16, 40), our current results, similar to our previously reported investigation, do not demonstrate a therapeutic benefit of using KD alone compared to control (20). Neither the original nor the new formulation KD demonstrate improvement in tumor growth rate or survival compared to control.
Interestingly, in the experiment comparing the original formulation 4:1 KetoCal formula to the new formulation KetoCal only the original formula significantly enhanced radiation response (Fig. 2A and B). A recently published review of the use of (n-3) polyunsaturated fatty acid (PUFA) supplementation as an adjuvant in cancer treatment concluded that although studies frequently show an increase in lipid peroxidation after concomitant treatment with (n-3) PUFA in both tissue cultured cells and tumors, there was great variation in responses depending not only on the PUFA, but also the cancer type (41). One key change in the KetoCal formula is the increased amount of palmitic acid (16:0). Palmitic acid is a substrate for oxidative phosphorylation that theoretically would enhance the production of reactive species by mitochondrial metabolism (42). In addition, the removal of trans 18:1-fatty acids as well as the addition of cis-linoleic (18:2), arachidonic (20:4), and docosahexaenoic (22:6) fatty acids could enhance lipid oxidation by the addition of methylene bridges to lipid content of cell membranes (43). Paradoxically, the new formulation KetoCal was not effective at enhancing radiation response in our model. Overall, this finding suggests that the standardization of KDs across studies is difficult and that subtle changes in the type or ratio of fatty acids may cause conflicting results. In addition, processed diets have potential shortcomings, including the addition of vitamins and emulsifiers, that may affect the gut microbiome and influence treatment response (44).
We previously reported difficulties for NSCLC and pancreatic cancer patients to adhere to a KD while receiving CRT (21). We hypothesized that compliance may be greater in head and neck cancer patients when a KD is delivered through a PEG tube, removing the difficulty with unpleasant taste. In this phase 1 clinical trial, all 12 patients had a PEG tube placed prior to the start of therapy. Only four out of 12 patients (33%) were able to tolerate five weeks of the KD as prescribed, and patients who did not tolerate the diet discontinued within 8 days. While nausea, fatigue, pancreatitis, and hyperuricemia may all occur with chemotherapy and radiation therapy, the small sample size prevents the attribution of these symptoms to either the KD or standard of care. Historical head and neck cancer patients receiving concurrent radiation and cisplatin have ≥ grade 3 nausea of 13% and vomiting of 11% (45). Prior studies have reported variable KD adherence in cancer patients (18, 24, 25). However, patient sample size is typically small and the disease site is heterogeneous. Outside the context of cancer treatment, a meta-analysis study of 270 adult epileptic patients also demonstrated that adults had difficulty adhering to a KD, with a compliance rate of only 42% (46). With a low baseline adherence rate, it may not be feasible to assess a KD as an adjunctive cancer treatment combined with standard-of-care CRT or biological therapy in a large-scale study.
The current study was a phase 1 clinical trial designed to test the feasibility and safety profile of a KD in conjunction with head and neck CRT and was not powered to detect differences in progression-free survival and overall survival. The median follow-up from completion of CRT was 13.1 months (range 8.0–26.7 months). One of the patients who completed the KD died of local and distant recurrence at 12.3 months, and the remaining patients were alive with no evidence of disease progression at last follow-up. Patients who adhered to the KD maintained stable glucose levels and stable lipid profile. There was no significant difference in weight loss between the KD group and the non-KD group. While a KD is generally safe, significant side effects may occur. Two of the head and neck cancer patients developed grade 4 hyperuricemia and were removed from the trial. A KD has been shown to cause transient hyperuricemia in 26.3% of pediatric patients using a KD as treatment for epilepsy (47). In one study, a KD was shown to cause hyperuricemia in 7 out of 11 cancer patients, although the duration of the KD in that study was as long as 16 weeks and the hyperuricemia was transient (23). We also observed one case of hyperuricemia in our previously published NSCLC study (21). It has been suggested that a high fat diet or a starvation state leads to hyperuricemia secondary to decreased uric acid excretion (48). While the hyperuricemia is likely transient, it is prudent to monitor the uric acid levels in cancer patients on a KD who have a predisposing condition to hyperuricemia or in tumors with a propensity to have tumor lysis effects.
In the current study, we provided additional evidence in a preclinical model supporting the hypothesis that a KD increases cancer cell oxidative stress. Interestingly, there was a significant reduction in the level of glutathione disulfide in red blood cells from patients who consumed a KD with concurrent standard-of-care therapy compared to non-KD standard-of-care patients. This suggests that although local tumor redox balance may be altered during standard CRT, a KD may decrease systemic oxidative stress.
In conclusion, our xenograft model of HNSCC showed that a KD can be an effective treatment in conjunction with standard-of-care anti-cancer therapy. We provided additional evidence that the mechanism by which a KD enhances cancer therapy is via increasing tumor cell oxidative stress. Finally, we had hypothesized that a KD will be more easily tolerated in adult patients when taking the KD via a PEG tube. With only 33% of patients completing the KD, it is considered not tolerable when combined with concurrent radiation and chemotherapy for the treatment of HNSCC.
ACKNOWLEDGMENTS
We thank Anna M. Button and Sarah M. Bell for statistical support from the Biostatistics Core in the Holden Comprehensive Cancer Center, Nutricia North America, Inc. for providing the 4:1 KetoCalt powder, and the Radiation and Free Radical Research Core in the Holden Comprehensive Cancer Center. We would like to acknowledge support from the Radiological Society of North America (RSNA) (grant no. RR1020), the National Institutes of Health/National Cancer Institute (NIH/NCI) (CA139182, CA133114, CA182804, CA161182, CA217797, CA243693, CA0785860), the NIH/ National Institute of Dental & Craniofacial Research (NIDCR) (DE024550). Further support was provided by ASTRO JF2014-1, an institutional research grant 77-004-34 from the American Cancer Society, an Oberley Award from Holden Comprehensive Cancer Center (P30CA086862), the Carver Trust Research Program of Excellence in Redox Biology and Medicine and the Institute for Clinical and Translational Science at the University of Iowa, which is supported by the NIH Clinical and Translational Science Award (CTSA) program (grant no. U54TR001356).
REFERENCES
- 1.Rettig EM, D’Souza G. Epidemiology of head and neck cancer Surg Oncol Clin N Am 2015; 24:379–96. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Rios F, Sanchez-Arago M, Garcia-Garcia E, Ortega AD, Berrendero JR, Pozo-Rodriguez F, et al. Loss of the mitochondrial bioenergetic capacity underlies the glucose avidity of carcinomas. Cancer Res 2007; 67:9013–7. [DOI] [PubMed] [Google Scholar]
- 3.Warburg O On the origin of cancer cells. Science 1956; 123:309–14. [DOI] [PubMed] [Google Scholar]
- 4.Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res 2007; 67:1472–86. [DOI] [PubMed] [Google Scholar]
- 5.Boros LG, Lee PW, Brandes JL, Cascante M, Muscarella P, Schirmer WJ, et al. Nonoxidative pentose phosphate pathways and their direct role in ribose synthesis in tumors: is cancer a disease of cellular glucose metabolism? Med Hypotheses 1998; 50:55–9. [DOI] [PubMed] [Google Scholar]
- 6.Krieg RC, Knuechel R, Schiffmann E, Liotta LA, Petricoin EF 3rd, Herrmann PC. Mitochondrial proteome: cancer-altered metabolism associated with cytochrome c oxidase subunit level variation. Proteomics 2004; 4:2789–95. [DOI] [PubMed] [Google Scholar]
- 7.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J 2009; 418:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, et al. O2(–) and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell 2017; 32:268. [DOI] [PubMed] [Google Scholar]
- 9.Bize IB, Oberley LW, Morris HP. Superoxide dismutase and superoxide radical in Morris hepatomas. Cancer Res 1980; 40:3686–93. [PubMed] [Google Scholar]
- 10.McPherson PA, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J Physiol Biochem 2012; 68:141–51. [DOI] [PubMed] [Google Scholar]
- 11.Seo JH, Lee YM, Lee JS, Kang HC, Kim HD. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios– comparison of 3:1 with 4:1 diet. Epilepsia 2007; 48:801–5. [DOI] [PubMed] [Google Scholar]
- 12.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia 2009; 50:1109–17. [DOI] [PubMed] [Google Scholar]
- 13.Kossoff EH, Rowley H, Sinha SR, Vining EP. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia 2008; 49:316–9. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab 2007; 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate 2008; 68:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 2008; 8:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura K, Tonouchi H, Sasayama A, Ashida K. A ketogenic formula prevents tumor progression and cancer cachexia by attenuating systemic inflammation in colon 26 tumor-bearing mice. Nutrients 2018; 10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieger J, Bahr O, Maurer GD, Hattingen E, Franz K, Brucker D, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol 2014; 44:1843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One 2012; 7:e36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res 2013; 19:3905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zahra A, Fath MA, Opat E, Mapuskar KA, Bhatia SK, Ma DC, et al. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: The University of Iowa experience of two phase 1 clinical trials. Radiat Res 2017; 187:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA, et al. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol 2014; 2:963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan-Shalaby JL, Carrick J, Edinger K, Genovese D, Liman AD, Passero VA, et al. Modified Atkins diet in advanced malignancies – final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr Metab 2016; 13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol 2014; 117:125–31. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab 2011; 8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klement RJ, Champ CE, Kammerer U, Koebrunner PS, Krage K, Schafer G, et al. Impact of a ketogenic diet intervention during radiotherapy on body composition: III–final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res 2020; 22:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khodabakhshi A, Seyfried TN, Kalamian M, Beheshti M, Davoodi SH. Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: a randomized controlled clinical trial. Nutr J 2020; 19:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shingler E, Perry R, Mitchell A, England C, Perks C, Herbert G, et al. Dietary restriction during the treatment of cancer: results of a systematic scoping review. BMC Cancer 2019; 19:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simons AL, Fath MA, Mattson DM, Smith BJ, Walsh SA, Graham MM, et al. Enhanced response of human head and neck cancer xenograft tumors to cisplatin combined with 2-deoxy-D-glucose correlates with increased (18)F-FDG uptake as determined by PET imaging. Int J Radiat Oncol Biol Phys 2007; 69:1222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 1985; 113:548–55. [DOI] [PubMed] [Google Scholar]
- 31.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 1980; 106:207–12. [DOI] [PubMed] [Google Scholar]
- 32.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951; 193:265–75. [PubMed] [Google Scholar]
- 33.Koyfman SA, Adelstein DJ. Enteral feeding tubes in patients undergoing definitive chemoradiation therapy for head-and-neck cancer: a critical review. Int J Radiat Oncol Biol Phys 2012; 84:581–9. [DOI] [PubMed] [Google Scholar]
- 34.Ok JH, Lee H, Chung HY, Lee SH, Choi EJ, Kang CM, et al. The potential use of a ketogenic diet in pancreatobiliary cancer patients after pancreatectomy. AntiCancer Res 2018; 38:6519–27. [DOI] [PubMed] [Google Scholar]
- 35.Klement RJ, Schafer G, Sweeney RA. A ketogenic diet exerts beneficial effects on body composition of cancer patients during radiotherapy: An interim analysis of the KETOCOMP study. J Tradit Complement Med 2020; 10:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Healey LA, Wheaton EA, Cutler RE, Demartini FE. Uric acid excretion in diabetic ketoacidosis. Diabetes 1966; 15:357–9. [DOI] [PubMed] [Google Scholar]
- 37.Goldfinger S, Klinenberg E Jr., Seegmiller JE. Renal retention of uric acid induced by infusion of beta-hydroxybutyrate and acetoacetate. N Engl J Med 1965; 272:351–5. [DOI] [PubMed] [Google Scholar]
- 38.Djiogue S, Nwabo Kamdje AH, Vecchio L, Kipanyula MJ, Farahna M, Aldebasi Y, et al. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer 2013; 20:R1–R17. [DOI] [PubMed] [Google Scholar]
- 39.Klement RJ. Restricting carbohydrates to fight head and neck cancer–is this realistic? Cancer Biol Med 2014; 11:145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stafford P, Abdelwahab MG, Kim do Y, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab 2010; 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camargo CQ, Brunetta HS, Nunes EA. Effects of cotreatment with omega-3 polyunsaturated fatty acids and anticancer agents on oxidative stress parameters: a systematic review of in vitro, animal, and human studies. Nutr Rev 2018; 76:65–777. [DOI] [PubMed] [Google Scholar]
- 42.Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 2010; 299:E1096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner BA, Buettner GR, Burns CP. Free radical-mediated lipid peroxidation in cells: oxidizability is a function of cell lipid bisallylic hydrogen content. Biochemistry 1994; 33:4449–53. [DOI] [PubMed] [Google Scholar]
- 44.Klement RJ, Sweeney RA, Gross EC, Champ CE. Problems associated with a highly artificial ketogenic diet: Letter to the Editor Re: van der Louw EJTM, Olieman JF, van den Bemt PMLA, et al. ‘Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: a feasibility and safety study’. Ther Adv Med Oncol 2019; 11:1758835919879268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson CM, Lee CM, Saunders DP, Curtis A, Dunlap N, Nangia C, et al. Phase IIb, randomized, double-blind trial of GC4419 versus placebo to reduce severe oral mucositis due to concurrent radiotherapy and cisplatin for head and neck cancer. J Clin Oncol 2019; 37:3256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye F, Li XJ, Jiang WL, Sun HB, Liu J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis. J Clin Neurol 2015; 11:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung SJ, Lee SH, Lee YJ, Park HS, Bunger R, Kang YH. Pyruvate protection against endothelial cytotoxicity induced by blockade of glucose uptake. J Biochem Mol Biol 2004; 37:239–45. [DOI] [PubMed] [Google Scholar]
- 48.Ogryzlo MA. Hyperuricemia induced by high fat diets and starvation. Arthritis Rheum 1965; 8:799–822. [DOI] [PubMed] [Google Scholar]


