Abstract
Background
Optimal management for trauma-induced coagulopathy (TIC) is a clinical conundrum. In conjunction with the transfusion of fresh-frozen plasma (FFP), additional administration of prothrombin complex concentrate (PCC) was proposed to bring about further coagulative benefit. However, investigations evaluating the efficacy as well as corresponding side effects were scarce and inconsistent. The aim of this study was to systematically review current literature and to perform a meta-analysis comparing FFP+PCC with FFP alone.
Methods
Web search followed by manual interrogation was performed to identify relevant literatures fulfilling the following criteria, subjects as TIC patients taking no baseline anticoagulants, without underlying coagulative disorders, and reported clinical consequences. Those comparing FFP alone with PCC alone were excluded. Comprehensive Meta-analysis software was utilized, and statistical results were delineated with odd ratio (OR), mean difference (MD), and 95% confidence interval (CI). I2 was calculated to determine heterogeneity. The primary endpoint was set as all-cause mortality, while the secondary endpoint consisted of international normalized ratio (INR) correction, transfusion of blood product, and thrombosis rate.
Results
One hundred and sixty-four articles were included for preliminary evaluation, 3 of which were qualified for meta-analysis. A total of 840 subjects were pooled for assessment. Minimal heterogeneity was present in the comparisons (I2 < 25%). In the PCC + FFP cohort, reduced mortality rate was observed (OR: 0.631; 95% CI: 0.450–0.884, p = 0.007) after pooling. Meanwhile, INR correction time was shorter under PCC + FFP (MD: –608.300 mins, p < 0.001), whilst the rate showed no difference (p = 0.230). The PCC + FFP group is less likely to mandate transfusion of packed red blood cells (p < 0.001) and plasma (p < 0.001), but not platelet (p = 0.615). The incidence of deep vein thrombosis was comparable in the two groups (p = 0.460).
Conclusions
Compared with FFP only, PCC + FFP demonstrated better survival rate, favorable clinical recovery and no elevation of thromboembolism events after TIC.
Keywords: prothrombin complex concentrate, trauma, coagulopathy, meta-analysis
Introduction
Trauma-induced coagulopathy (TIC) is a well-established yet under-recognized disease entity. Defined as the impairment in coagulation after trauma, TIC could result from the insult itself or iatrogenic reversal against the complication. Previous literature documented longer hospital and intensive care unit stay, escalated transfusion requirement, more reliance on mechanical ventilation support, and greater incidence of multiple organ dysfunction secondary to TIC.1 The presence of TIC not only intertwines with worse prognosis but also independently predicts morbidity and mortality.2
In terms of etiology, TIC was traditionally accounted for the following four aspects: hemodilution due to fluid replenishment, hypothermia, acidosis, and the consumption of coagulative factors with hyperactivation of anti-bleeding cascade and increased fibrino(geno)lysis during acute stage.3-6 Compared with disseminated intravascular coagulation (DIC), although both featured pronounced reduction of fibrinogen and D-Dimer escalation, intravascular fibrin deposition combining the consumption of clotting factors secondary to coagulative system activation was only observed in DIC rather than TIC. TIC was thus described as “DIC with fibrinolytic phenotype,”7 hinting the management of TIC mandated further investigations.
Correspondingly, the concept of goal-directed resuscitation with early factor replacement emerged. Traditionally, fresh-frozen plasma (FFP) played a pivotal role in treating coagulopathy, providing fluid support and the replacement of coagulation factors.8 However, its cumbersome requirements for compatibility or storage and its risk of volume overload, nosocomial infection, as well as other transfusion-related complications prompted the search for improving tactics.9,10 Prothrombin complex concentrate (PCC), composed of pooled plasma with multiple concentrated vitamin K-dependent coagulation factors, thereby became a possible candidate.
The initial indication of PCC administration was for hemophilia B and was later extended to the reversal of vitamin K anticoagulation (VKA)-acquired coagulopathy.11 Studies demonstrated that PCC not only harbors comparable ability to correct bleeding tendency but also carries additional advantages (e.g., rapidity in correction, small-volume administration, and less transfusion-related complications even in TIC patients without preinjury anticoagulant use).12-14 The fifth edition of European guideline on the management of major bleeding and coagulopathy after trauma thus extended the indication of PCC. Aside from FFP-based therapy, PCC served as an alternative regimen regardless of initial coagulative status.15
Although numerous animal studies and real-world investigations with small cohorts have suggested that PCC in conjunction with FFP might be superior to FFP alone in the resuscitation of trauma patients,16-18 there is a paucity of literature considering the safety and efficacy of PCC in combination with FFP to manage patients with TIC. Therefore, the aim of this study is to analyze the clinical role of PCC in combination with FFP against TIC. We hypothesized that adding PCC upon baseline FFP will improve clinical outcomes.
Methods
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement protocol. Public medical databases (e.g., MEDLINE, EMBASE, International Web of Science, Cochrane Central Register of Controlled Trials) were inquired from inception until April 2020. We used Medical Subject Heading Indexation terminology and set the searching strategy as “([prothrombin concentrate complex] OR [PCC]) AND ([fresh frozen plasma] OR [FFP]) AND ([trauma] OR [post trauma hemorrhage]).” Full text of selected manuscripts and references thereof were examined by all authors independently to determine the eligibility for inclusion. Disagreement among the reviewers was resolved by consensus, and inter-observer agreement was determined by averaged Cohen’s kappa value. Manual interrogation of the relevant literature was accomplished to prevent omission.
Studies were included if they fulfill the following criteria. Firstly, the study design needed to be either randomized controlled trial or cohort study. Secondly, the study group focused on those individuals being status post trauma and without baseline anticoagulant or antiplatelet medications. Thirdly, the subjects were human and sustained no underlying coagulopathy, inherited/acquired bone marrow disorder, or bleeding tendency. Lastly, mortality rate, transfusion rate, and the incidence of at least one coagulopathy or transfusion-related complication (e.g., deep vein thrombosis (DVI), pulmonary embolism, mesenteric infarction, etc) were reported. Studies were excluded if the aim was to compare the efficacy between PCC alone and FFP alone. Case report, case series, conference abstracts, editorials, or government publications were ostracized due to insufficient detail for critical appraisal. Author, year, study design, sample size, characteristics of the cohort, intervention strategy, prognostic indexes, and statistical manner were extracted for meta-analysis. The quality of the selected studies was evaluated by the modified New-castle-Ottawa scale.
The primary endpoint for our study was all-cause mortality. Other prognostic indexes, including international normalized ratio (INR) correction, transfusion of blood product, and thrombosis rate, were interrogated as the secondary endpoint. Data for the same outcome measured in all studies were pooled by comprehensive meta-analysis (Cochrane Collaboration, Oxford, UK). Analysis upon dichotomous result was expressed with odds ratio (OR) and their 95% confidence interval (CI), whereas the measurement of continuous data was depicted using mean differences (MD) and 95% CI. Statistical heterogeneity was calculated using the Chi-squared test and I2 test. Values of I2 at 25%, 50%, and 75% were respectively considered low, medium, and high heterogeneity. The random-effect model was exploited if I2 > 50%; otherwise, a fixed-effect model was employed. Statistical significance was established on p < 0.05.
Results
The process of the literature search was summarized (Fig. 1). One hundred and sixty-four manuscripts were selected for preliminary evaluation, 3 of which fulfilled criteria for analysis after excluding redundancy and irrelevancy (Table 1).19-21 Manual search among the references did not yield extra eligible articles. High inter-observer agreement for study selection was reached (Cohen’s Kappa value = 0.829). Pooling together, a total of 840 subjects with clinically-confirmed TIC received either FFP transfusion alone or in combination with PCC. During retrospective reviewing period, OR of 3 selected studies for all-cause mortality comparing PCC + FFP with FFP alone were 0.552 (95% CI: 0.355–0.859), 0.692 (95% CI: 0.294–1.628), and 0.802 (95% CI: 0.414–1.553), respectively. A discrepancy arose as 2 out of the 3 selected studies demonstrated no statistical significance, whilst the one with the largest population favored adjuvant PCC in terms of mortality reduction. After pooling, our meta-analysis suggested a favorable survival rate in the PCC + FFP cohort (OR: 0.631; 95% CI: 0.450–0.884, p = 0.007) (Fig. 2).19-21
Fig. 1. Flowchart of literature review process.
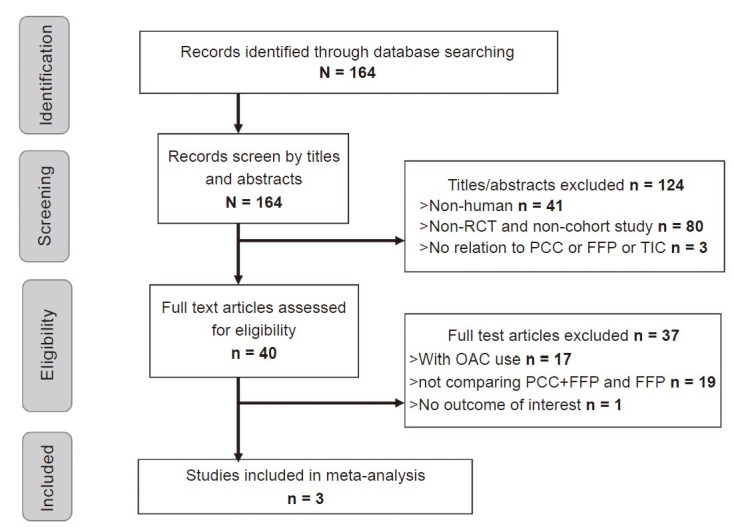
FFP: fresh-frozen plasma; OAC: oral anticoagulant; PCC: prothrombin complex concentrate; RCT: randomized controlled trial; TIC: trauma-induced coagulopathy.
Table 1. Summary of the selected studies for meta-analysis19-21.
|
Author, year |
Study design |
Subject |
Main finding |
Reference |
|
Zeeshan et al., 2019 |
Multicenter, retrospective |
468 patients w/o preinjury anticoagulants. |
Compared to FFP alone, addition of 4-PCC contributed to decreased mortality (17.5% vs. 27.7%, p = 0.01), lower rates of acute respiratory distress syndrome (1.3% vs. 4.7%, p = 0.04), and incidence of acute kidney injury (2.1% vs. 7.3%, p = 0.01). |
19 |
|
Jehan et al., 2018 |
Unicenter, retrospective |
120 patients w/o preinjury anticoagulants |
4-PCC + FFP was associated with an accelerated correction of INR (373 vs. 955 mins; p = 0.001), decreased need of transfusion, and lower mortality. |
20 |
|
Joseph et al., 2014 |
Unicenter, retrospective |
252 patients escorted to trauma center and with initial INR ≥ 1.5 |
Extra PCC with baseline FFP facilitated INR normalization, reduced pRBC or FFP supplementation, and declined mortality. |
21 |
Fig. 2.
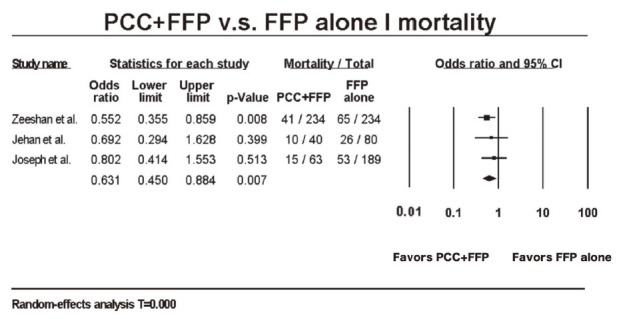
Forest plot for all-cause mortality of patients who received either prothrombin complex concentrate (PCC) + fresh-frozen plasma (FFP) or FFP alone.19-21
CI: confi dence interval.
As for coagulative behavior, 2 out of 3 studies compared INR correction time. Both studies revealed less correction time was needed for patients who undertook FFP + PCC as treatment, and pooled interrogation also demonstrated significantly quicker INR correction (MD: –608.300 mins, p < 0.001) (Fig. 3A).20-21 However, OR of INR correction rate comparing PCC + FFP with FFP alone were 1.541 (95% CI: 0.297–8.001) and 2.440 (95% CI: 0.539–11.045), espectively. Pooled interrogation also showed no significant preference in either group with OR: 1.978 (95% CI: 0.650–6.021, p = 0.230) (Fig. 3B).20,21
Fig. 3.
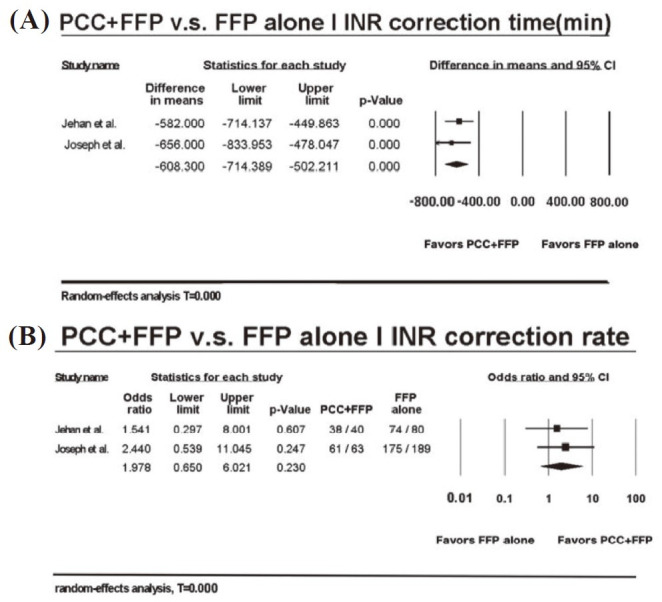
Forest plot for coagulative behavior of patients who received either prothrombin complex concentrate (PCC) + fresh-frozen plasma (FFP) or FFP alone. (A) International normalized ratio (INR) correction time; (B) INR correction rate. 20-21
CI: confidence interval.
Furthermore, the requirement for further blood sample transfusion was assessed. Both packed red blood cell (pRBC) supplementation (p < 0.001) (Fig. 4A)19-21 and plasma replenishment (p < 0.001) (Fig. 4B)19-21 were less indicated in the PCC + FFP group niversally in the 3 studies as well as in the present meta-analysis. Interestingly, none of the studies exhibited statistical differences in platelet transfusion. Pooled interrogation also revealed no preference in either group (p = 0.615) (Fig. 4C).19-21
Fig. 4.

Forest plot for transfusion need of patients who received either prothrombin complex concentrate (PCC) + fresh-frozen plasma (FFP) or FFP alone. (A) Packed red blood cell pRBC) transfusion; (B) plasma transfusion; (C) platelet transfusion. 19-21
CI: confidence interval.
Besides, the occurrence of side effects was inquired. Regarding all thromboembolic events, DVT was the one that all studies have mentioned. OR for the incidence of DVT comparing PCC + FFP with FFP alone were 0.602 (95% CI: 0.245–1.480), .026 (95% CI: 0.123–33.252), and 1.508 (95% CI:0.134–16.918), respectively. After pooling, no significant amplification of DVT incidence in the PCC + FFP groups was detected (OR: 0.738; 95% CI: 0.329–1.654, p = 0.460) (Fig. 5).15-16 Heterogeneity indexes of the above comparisons were low (I2 < 25%).
Fig. 5.
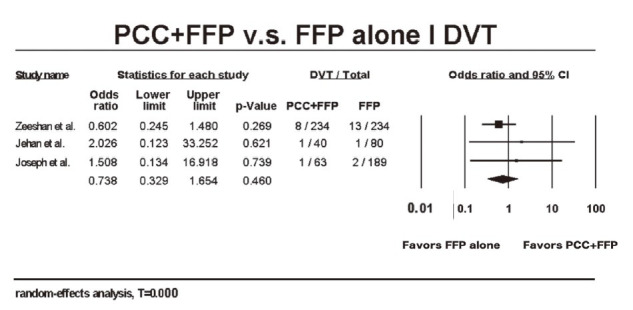
Forest plot for the incidence of deep vein thrombosis (DVT) in patients who received either prothrombin complex concentrate (PCC) + fresh-frozen plasma (FFP) or FFP alone. 19-21
CI: confidence interval.
Discussion
TIC is a frequently encountered condition in critically injured patients and occurrence thereof carries prognostic implications.22 However, inconsistency of the theories to appreciate pathophysiology as well as an incomplete understanding of the management gave rise to compromised clinical outcomes. To our knowledge, there is neither universal guideline directing the therapeutic regimen nor meta-analysis determining the efficacy of PCC + FFP for the treatment of TIC. Concerns about thrombus formation by factitious replenishment of coagulative factors in conjunction with ancillary expenditure prevented the integration of PCC usage from being the optimal choice. Our study proposed adding 4-factor concentrate significantly facilitated the abatement of coagulopathy without incrementing adverse events.
Employing PCC was anticipated to reduce death in critical care. To date, four randomized clinical trials were accomplished to appreciate PCC utility for reversing VKA treatment in neurological or cardiac operation. Nevertheless, Cochrane systematic review demonstrated no beneficial effect on the mortality rate between PCC and FFP.23 Six more prospective trials were ongoing for further validation. Surprisingly, all-cause mortality, as the primary endpoint of our analysis, was adequately reduced by PCC with ideal homogeneity. Aside from divergent study design and statistical disparateness, such differences might arguably be attributed to the etiology of TIC as hemodilution and the exhaustion of coagulative factors. Direct replenishment of both plasma volumes in combination with extra clotting factors resolved life-threatening disruption of a hemostatic cascade, which served as the main cause of death after trauma, thereby contributing to the improvement of mortality.
Of notice, in the PCC + FFP group, the need for subsequent transfusion of pRBC and plasma, but not platelet, was effectively reduced. The lethal triad of TIC included coagulopathy but not thrombocytopenia.24 Literature also demonstrated thrombocyte counts were usually maintained at the level that is not enough to cause clinically significant coagulopathy in early TIC.25 Mobilization of platelets from spleen or bone marrow while ongoing bleeding was hypothesized, and the platelet over red blood cell ratio was not related to mortality.26 Together, these explained infrequent platelet transfusion as we reviewed. Studies specifically designed to delineate the behavior of platelets and their corresponding relevance would be mandated.
Besides, orchestrating the clotting profile raised the concern of hypercoagulation. Venous thromboembolism is a well-established adverse event of PCC infusion. Felton et al.27 illustrated administering PCC against Warfarin-associated intracranial hemorrhage in patients with a previous history of DVT and/or pulmonary embolism brought about a 4.5-fold risk of thrombosis within 30 days. Likewise, Maguire et al.28 enrolled 313 subjects who were indicated for urgent Warfarin reversal in emergency rooms. A statistically higher number of venous thromboembolism incidence in 1 month was noted if PCC was given as compared with FFP, marking the necessity to evaluate patient’s baseline characteristics to evaluate the introduction of FFP. However, concomitant administration of PCC and FFP was not discussed in these studies. Our meta-analysis suggested adding PCC to FFP does not further increase DVT incidence. The overall occurrence of thrombosis was also low, arguing the benefit of PCC + FFP in acute traumatic settings exceeded the increment of thrombogenic menace.
There are several limitations regarding our analysis. First, due to scarcity of literature on PCC against TIC, only 3 retrospective studies eventually fulfilled inclusion criteria and no randomized trial was thus far available. Second, studies were all conducted among Caucasians. Future studies with an extended ethnic background are therefore warranted. Third, INR does not depict the overall picture of coagulation. Validation with additional parameters to enhance accuracy is mandatory. Finally, our meta-analysis deduced the prognostic impact from intervention, yet the causal relationship in-between required further elucidation. Together, studies with more comprehensive patient enrollment and longer follow-up would better determine the role of PCC against TIC.
As for future perspectives, refining PCC formula and cultivating practice protocol are urgently needed to better manage TIC. As trauma remained the leading cause of death and permanent disability among individuals under 40 years of age,29 who committed as the main human power in contemporary society, curbing subsequent hypoperfusion and eventual target organ failure is paramount to improve prognosis. Aside from the crystalloid bolus challenge that potentially aggravated coagulopathy, augmentation of clotting factors in an optimized fashion, currently FFP in addition to traditional FFP, brings about survival benefits. Further understanding of the detailed mechanism will assist in the development of the infusion concentrate of new generation.
Conclusions
Based on our meta-analysis, we demonstrated that administering PCC in addition to FFP effectively improved all-cause mortality, reduced the need for further pRBC as well as plasma transfusion, and accelerated INR correction. No escalation in the risk of thromboembolism was accompanied. Future comprehensive trials would be warranted to further pinpoint the clinical role of PCC in facilitating coagulation.
Conflicts of Interest Statement
The authors declare no conflict of interest.
Acknowledgment
The authors would like to acknowledge Vivianne Yu-Lien Lo, Department of English, National Chengchi University, for grammatical edition and wording refinement.
References
- 1.Cohen MJ, Call M, Nelson M. Critical role of activated protein c in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255:379-385. doi: 10.1097/SLA.0b013e318235d9e6 [DOI] [PMC free article] [PubMed]
- 2.Frith D, Goslings JC, Gaarder C. Definition and drivers of acute traumatic coagulopathy: Clinical and experimental investigations. J Thromb Haemost. 2010;8:1919-1925. doi: 10.1111/j.1538-7836.2010.03945.x [DOI] [PubMed]
- 3.Hayakawa M. Pathophysiology of trauma-induced coagulopathy: Disseminated intravascular coagulation with the fibrinolytic phenotype. J Intensive Care. 2017;5(14). doi: 10.1186/s40560-016-0200-1 [DOI] [PMC free article] [PubMed]
- 4.Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: Initiated by hypoperfusion. doi: 10.1097/01.sla.0000256862.79374.31 [DOI] [PMC free article] [PubMed]
- 5.Hess J, Brohi K, Dutton RP. The coagulopathy of trauma: A review of mechanisms. J Trauma. 2008;65:748-754. doi: 10.1097/TA.0b013e3181877a9c [DOI] [PubMed]
- 6.Esmon CT. Protein c pathway in sepsis. Ann Med. 2002;34:598-605. doi: 10.1080/078538902321117823 [DOI] [PubMed]
- 7.Gando S, Wada H, Thachil J. Scientific and standardization committee on DIC of the international society on thrombosis and haemostasis (ISTH). Differentiating disseminated intravascular coagulation (DIC) with the fibrinolytic phenotype from coagulopathy of trauma and acute coagulopathy of trauma-shock (COT/ACOTS. J Thromb Haemost. 2013;11:826-835. doi: 10.1111/jth.12190 [DOI] [PubMed]
- 8.Gonzalez EA, Moore FA, Holcomb JB. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112-119. doi: 10.1097/01.ta.0000250497.08101.8b [DOI] [PubMed]
- 9.Watson GA, Sperry JL, Rosengart MR. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67:221-227. doi: 10.1097/TA.0b013e3181ad5957 [DOI] [PubMed]
- 10.Inaba K, Branco BC, Rhee P. Impact of the duration of platelet storage in critically ill trauma patients. J Trauma. 2011;71:1766-1773. doi: 10.1097/TA.0b013e31823bdbf9 [DOI] [PubMed]
- 11.Matsushima K, Benjamin E, Demetriades D. Prothrombin complex concentrate in trauma patients. Am J Surg. 2015;209:413-417. doi: 10.1016/j.amjsurg.2014.08.019 [DOI] [PubMed]
- 12.Sarode R, Milling TJ Jr, Refaai MA. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: A randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234-1243. doi: 10.1161/CIRCULATIONAHA.113.002283 [DOI] [PMC free article] [PubMed]
- 13.Nienaber U, Innerhofer P, Westermann I. The impact of fresh frozen plasma vs coagulation factor concentrates on morbidity and mortality in trauma-associated haemorrhage and massive transfusion. Injury. 2011;42:697-701. doi: 10.1016/j.injury.2010.12.015 [DOI] [PubMed]
- 14.Schöchl H, Nienaber U, Hofer G. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. doi: 10.1186/cc8948 [DOI] [PMC free article] [PubMed]
- 15.Pakulski C, Wudarska B, Surowicz D. Letter: The european guideline on management of major bleeding and coagulopathy following trauma. Neurosurgery. 2019;85:E1123-E1124. doi: 10.1093/neuros/nyz363 [DOI] [PubMed]
- 16.Spahn DR, Bouillon B, Cerny V. Management of bleeding and coagulopathy following major trauma: An updated european guideline. Crit Care. 2013;17:R76. doi: 10.1186/cc12685 [DOI] [PMC free article] [PubMed]
- 17.Zeeshan M, Hamidi M, Kulvatunyou N. 3-factor versus 4-factor PCC in coagulopathy of trauma: Four is better than three. Shock. 2019;52:23-28. doi: 10.1097/SHK.0000000000001240. [DOI] [PubMed]
- 18.Kuckelman J, Barron M, Moe D. Plasma coadministration improves resuscitation with tranexamic acid or prothrombin complex in a porcine hemorrhagic shock model. J Trauma Acute Care Surg. 2018;85:91-100. doi: 10.1097/TA.0000000000001942 [DOI] [PubMed]
- 19.Zeeshan M, Hamidi M, Feinstein AJ. Four-factor prothrombin complex concentrate is associated with improved survival in trauma-related hemorrhage: A nationwide propensity-matched analysis. J Trauma Acute Care Surg. 2019;87:274-281. doi: 10.1097/TA.0000000000002262 [DOI] [PubMed]
- 20.Jehan F, Aziz H, OʼKeeffe T. The role of four-factor prothrombin complex concentrate in coagulopathy of trauma: A propensity matched analysis. J Trauma Acute Care Surg. 2018;85:18-24. doi: 10.1097/TA.0000000000001938 [DOI] [PubMed]
- 21.Joseph B, Aziz H, Pandit V. Prothrombin complex concentrate versus fresh-frozen plasma for reversal of coagulopathy of trauma: Is there a difference? World J Surg. 2014;38:1875-1881. doi: 10.1007/s00268-014-2631-y [DOI] [PubMed]
- 22.Sawamura A, Hayakawa M, Gando S. Disseminated intravascular coagulation with a fibrinolytic phenotype at an early phase of trauma predicts mortality. Thromb Res. 2009;124:608-613. doi: 10.1016/j.thromres.2009.06.034 [DOI] [PubMed]
- 23.Johansen M, Wikkelsø A, Lunde J, et al. Prothrombin complex concentrate for reversal of vitamin K antagonist treatment in bleeding and non-bleeding patients. Cochrane Database Syst Rev. 2015;2015:CD010555:2. doi: 10.1002/14651858.CD010555. [DOI] [PMC free article] [PubMed]
- 24.Vulliamy P, Gillespie S, Gall LS, et al. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J Trauma Acute Care Surg. 2017;83:388-397. doi: 10.1097/TA.0000000000001520 [DOI] [PubMed]
- 25.Frith D, Davenport R, Brohi K. Acute traumatic coagulopathy. Curr Opin Anaesthesiol. 2012;25:229-234. doi: 10.1097/ACO.0b013e3283509675 [DOI] [PubMed]
- 26.Dirks J, Jørgensen H, Jensen CH, et al. Blood product ratio in acute traumatic coagulopathy—effect on mortality in a scandinavian level 1 trauma centre. Scand J Trauma Resusc Emerg Med. 2010;18(65). doi: 10.1186/1757-7241-18-65 [DOI] [PMC free article] [PubMed]
- 27.Felton D, Foley EM, Traub SJ, et al. Risk of venous thromboembolism after receiving prothrombin complex concentrate for warfarin-associated intracranial hemorrhage. J Emerg Med. 2016;50:1-6. doi: 10.1016/j.jemermed.2015.07.001 [DOI] [PubMed]
- 28.Maguire M, Fuh L, Goldstein JN. Thromboembolic risk of 4-factor prothrombin complex concentrate versus fresh frozen plasma for urgent warfarin reversal in the emergency department. West J Emerg Med. 2019;20:619-625. doi: 10.5811/westjem.2019.4.41649 [DOI] [PMC free article] [PubMed]
- 29.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed]


