Abstract
Focused ultrasound (FUS) in combination with systemically injected microbubbles can be used to non-invasively open the blood-brain barrier (BBB) in targeted regions for a variety of therapeutic applications. Over the past two decades, preclinical research into the safety and efficacy of FUS-induced BBB opening has proven this technique to be transient and efficacious, propelling FUS-induced BBB opening into several clinical trials in recent years. However, as clinical trials further progress, the neuroinflammatory response to FUS-induced BBB opening needs to be better understood. In this study, we provide further insight into the relationship of microbubble cavitation and the resulting innate immune response to FUS-induced BBB opening. By keeping ultrasound parameters fixed (i.e. frequency, pressure, pulse length, etc.), three groups of mice were sonicated using a real-time cavitation controller until a target cavitation dose was reached (1 107 V2•s, 5 107 V2•s, 1 108 V2•s). The change in relative gene expression of the mouse inflammatory cytokines and receptors were evaluated at three different time-points (6 h, 24 h, and 72 h) after FUS. At both 6 and 24 h time-points, significant changes in relative gene expression of inflammatory cytokines and receptors were observed across all cavitation groups. However, the degree of changes in relative expression levels and the number of genes with significant changes in expression varied across the cavitation groups. Groups with a higher cavitation dose exhibited both greater changes in relative expression levels and greater number of significant changes. By 72 h, the gene expression levels returned to baseline in all cavitation dose groups, signifying a transient inflammatory response to FUS-induced BBB opening at the targeted cavitation dose levels. Furthermore, the real-time cavitation controller was able to produce consistent and significantly different BBB permeability enhancement volumes across the three different cavitation dose groups. These results indicate that cavitation monitoring and controlling during FUS-induced BBB opening can be used to potentially modulate or limit the degree of neuroinflammation, further emphasizing the importance of implementing cavitation controllers as FUS-induced BBB opening is translated into the clinic.
Keywords: focused ultrasound, blood-brain barrier, inflammatory response, cavitation controller, brain drug delivery
Introduction
Focused ultrasound (FUS) induced blood-brain barrier (BBB) opening has been established as an effective non-invasive, transient surrogate to deliver therapeutic agents from systemic circulation into the central nervous system (CNS). More recently, aside from a drug delivery adjuvant, FUS-induced BBB opening has been proposed as an immune system stimulator resulting in behavioral improvement [1] and pathological amelioration [2–6]. Extensive pre-clinical research and the corresponding promising findings led to a battery of clinical trials in patients with Glioblastoma (GBM) and Alzheimer’s disease (AD). Initial feasibility and safety has already been established in patients with GBM [7] and AD [8] patients showing safe and reversible BBB openings with and without drug delivery using the MR-guided ExAblate system developed by Insightec (Insightec Inc., Tirat Carmel, Israel). Furthermore, our in-house developed clinical system for neuronavigation-guided FUS-induced BBB opening that does not rely on MR-guidance [9], has started with 6 AD patients and includes assessment of pathological alteration following the procedure (NCT04118764).
Hence, the immense potential of the technique in the clinical setting, greater attention has been focused to the time course of events following the FUS-induced BBB opening. The primary effects unfold immediately following the FUS including the disruption of the tight junctions and the associated protein balance, increased permeability of the cell plasma membrane and elevated transcytotic vesicles [10]. The principal secondary effect that is initiated within hours following the FUS is the inflammatory response [10]. Inflammation is a protective physiologic CNS response to injury and infection, essential in stimuli removal and tissue healing [11]. In acute neuroinflammation, reactive microglia restrain the area of injury through phagocytosing dying cells and releasing pro-inflammatory cytokines [11]. Prolonged neuroinflammation exceeding the bounds of physiological control can result to deleterious effects involving pro-inflammatory signaling pathways, increased oxidative stress, and death of nearby neurons [11].
The resulting bioeffects due to the FUS-induced BBB opening are within the parametric space previously identified to induce safe FUS-induced BBB opening. Previous studies investigated the FUS parameters (frequency, pressure, pulse length) [12–14], the sonication duration [15] as well as the microbubble composition [16–18] and administered concentration [19] have been exploited and optimized to a safety window. The safety profile of this intervention has been evaluated in both wild-type and diseased model animals through a plethora of safety metrics including T2- weighted and susceptibility weighted imaging (SWI) to assess edema and hemorrhage [20], [21], hematoxylin and eosin (H&E) staining to assess histological changes such as red blood cell extravasation[22], [23], immunohistochemistry (IHC) to assess changes in protein expression such as Iba1 and GFAP [2], [24], and behavioral studies to assess potential neurological changes following FUS-induced BBB opening [25–27]. It has been shown that varying the pressure alters the likelihood of red blood cell extravasation in the sonicated locations [26] while varying the combination of FUS pulse lengths and pressures alters the BBB opening probability and closing timeline [12]. In recent years, transcriptomics and proteomics have emerged as another tool to evaluate the safety and efficacy of FUS induced BBB opening [28]–[30]. By studying the innate immune response to the temporary disruption of the BBB, a new metric to further evaluate the safety and efficacy of FUS induced BBB opening is being explored. Consistent with the safety evaluation from previous studies, it has been found that the immune response is dependent on the sonication parameters employed in the FUS-induced BBB opening [29]. With such a large parameter space available, evaluation and understanding of the immune response to FUS-induced BBB opening has become challenging.
One insightful metric that has been often employed in FUS-induced BBB opening is the microbubble cavitation dose, as it can provide real time information on the occurring bioeffects [31], [32]. Previous studies have found that cavitation dose can be correlated with the BBB permeability enhancement volume, BBB opening duration, and potential damage, evaluated using MRI and H&E staining [33]. Using cavitation dose as a unifying metric that is dependent on the sonication parameters can provide meaningful information on the potential bioeffects. Recent studies have begun exploring cavitation-based controllers for safe and effective BBB openings for drug delivery [34], [35], but studies understanding the inflammatory response using a cavitation-based controller system is limited.
Herein, the work in this study looks to exploit the relationship between cavitation level and the inflammatory response following FUS-induced BBB opening. After developing a cavitation-based controller, three different cavitation thresholds were explored at the same, fixed pressure. The inflammatory response at these different cavitation thresholds was evaluated at three different time points to examine both the short term and long-term effects of BBB opening, and whether the inflammation can be controlled using cavitation.
Materials and Methods
A. Animal Use and Study Design
All experimental procedures and protocols involving animals in this study were approved by the Columbia University Institutional Animal Care and Use Committee. Wild-type C57BL/6 mice (sex: male, weight: 25–30 g, age: 6–8 weeks old, Harlan, Indianapolis, IN, USA) were group housed under standard conditions with standard rodent chow and water ad libitum. A total of 36 mice were randomly split into three different cavitation dose groups and three different time points (N = 4/group). The three different cavitation dose groups were controlled using real-time cavitation monitoring, discussed in a later section, and the resulting inflammatory response was assessed at three different time-points of 6 h, 24 h, and 72 h post FUS-mediated BBB opening.
Mice were anesthetized using 1.5% - 2.5% isoflurane with oxygen at 0.8L/min. Anesthetized mice were then placed into a stereotaxic frame (Model 902, David Kopf Instruments, Tujunga, CA, USA), where their heads were shaved and depilatory cream was applied, removing all the hair on the scalp. A 27-guage butterfly needle was placed into the tail vein for intravenous (IV) injections of saline and microbubbles (MBs) during FUS-mediated BBB opening. Throughout the procedure, the anesthesia was maintained at 1.5% - 2% isoflurane with the oxygen flow rate at 0.8L/min.
B. FUS-mediated BBB Opening
FUS-mediated BBB opening was achieved using a combination of FUS ultrasound and systemically injected MBs. A schematic for the experimental setup can be seen in Figure 1A. Throughout this study, a single-element, spherical FUS transducer (center frequency: 1.5 MHz, focal length: 60 mm, diameter: 60 mm, Imasonic, France) was driven by a function generator (Agilent 33220A, Palo Alto, CA) that was amplified using 50-dB power amplifier (324LA, Electronic Navigation Industries, Rochester, NY). The FUS transducer emits an ellipsoidal focal spot with a full width half max (FWHM) lateral length of 1.28 mm and a FWHM axial length of 10.45 mm, as measured in degassed water by a bullet hydrophone (HGL-0200, ±3-dB frequency range of 0.25 – 40 MHz; Onda Corporation, Sunnyvale, CA, USA). A 2D axial cross-sectional view of the focus can be seen in Figure 1B. Confocally aligned with the FUS transducer was another single-element transducer (V320, frequency: 7.5 MHz, focal length 52 mm, diameter: 13mm; Olympus NDT, Waltham, MA) that was used for passive cavitation detection (PCD). The −6 dB bandwidth for the PCD was 59.3%, corresponding to a bandwidth ranging from 5.34 MHz to 9.85 MHz. Real-time cavitation monitoring using this single-element PCD was used for our real-time cavitation controller, which will be explained in the next section.
Figure 1.
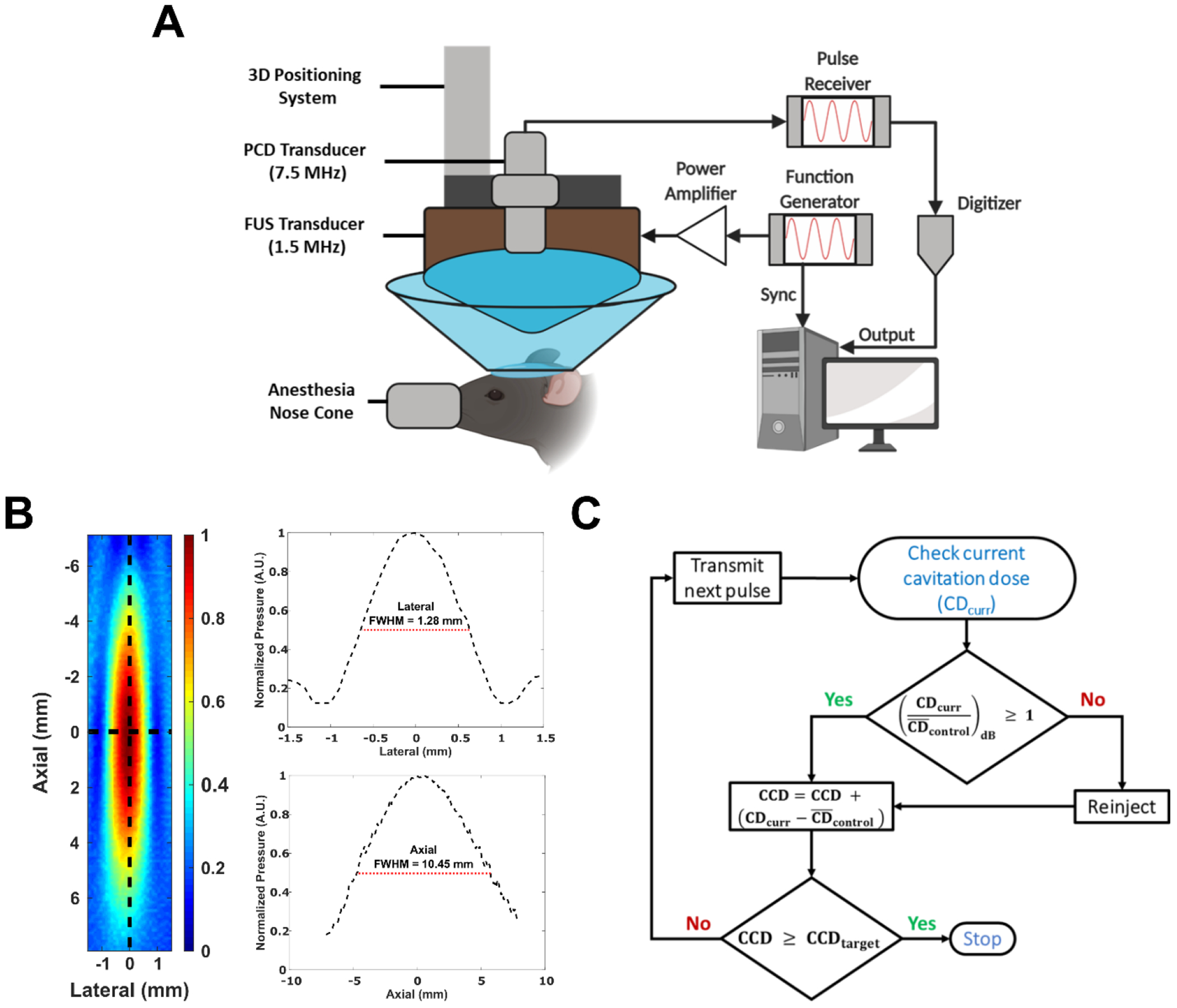
A) Schematic of the experimental setup used throughout this study (Created with Biorender.com). B) 2D axial pressure map of the 1.5 MHz FUS transducer measured with a bullet hydrophone in degassed water. Plots to the right of profile are lateral (top) and axial (bottom) 1-D plots taken along at the center along each respective axis. The full-width half max (FWHM) for each axis is shown. C) Block diagram of cavitation based-controller system used throughout this study.
A single location in the left striatum (4.5 mm lateral, 2.6 mm anterior, relative to lambdoid suture) was targeted for BBB opening using an estimated in situ peak negative pressure (PNP) of 490 kPa (mechanical index of 0.4), a pulse repetition frequency (PRF) of 2 Hz, and a pulse length of 10 ms (15,000 cycles). The estimated in-situ PNP pressure was assessed using free-field hydrophone measurements that was derated by 18% to correct for the mouse skull attenuation [36]. The microbubbles used in this study for BBB opening were Definity® (Lantheus Medical Imaging, N. Billerica, MA, USA) and were injected intravenously as a bolus at a concentration of 0.1 µL per gram of body weight (10 times the manufacturer and Food and Drug Administration recommended dosing for clinical imaging). These sonication parameters (PL, PRF, PNP, etc.) and total number of microbubbles administered per bolus injection were chosen based on previous studies showing successful and safe BBB opening at similar parameters [12], [17], [26], [37]. Table 1 summarizes the microbubble dosage used in previous reports that were used to guide this study.
Table 1.
Characterization of microbubble formulations used for clinical imaging with Definity, ten times (10x) the clinical imaging dose of Definity, and previously published reports showing effective FUS-induced BBB opening.
| Reference | Bubble Distribution | Bubble Size | Bubble Concentration | Bubble Dosage | Bubbles per bolus injection (million) |
|---|---|---|---|---|---|
| Clinical Definity | Polydisperse | 1.1 – 3.3 µm | 1.2 × 1010/mL | 0.01 µL/g | 3 |
| 10x Clinical Definity | Polydisperse | 1.1 – 3.3 µm | 1.2 × 109/mL | 1.0 µL/g | 30 |
| Samiotaki et al. 2013 [12] | Polydisperse | 1.1 – 3.3 µm | 6.0 × 108/mL | 1.0 µL/g | 15 |
| Choi et al. 2010 [17] | Size isolated | 4 – 5 µm | 8.5 × 108/mL | 0.9 µL/g | 19 |
| Olumolade et al. 2016 [26] | Polydisperse | ~1.4 µm | 8.0 × 108/mL | 1.0 µL/g | 20 |
| Wu et al. 2015 [37] | Size isolated | 4 – 5 µm | 8.0 × 108/mL | 1.2 – 1.5 µL/g | 24 – 30 |
C. Open-loop Real-time Cavitation Controller
Real-time cavitation monitoring was implemented to monitor the microbubble acoustic emissions during FUS treatment. For each pulse transmitted, microbubble cavitation acquired through the single-element PCD during sonications were transferred to a digitizer (Gage Applied Technologies Inc., Lachine QC, Canada) and then processed in MATLAB (2019a Mathworks, Natick, MA, USA). A fast Fourier transform (FFT) was applied to each PCD pulse acquired and the resulting energy spectral density was filtered with a bandpass filter between the 3rd and 9th harmonics (4.5MHz – 13.5MHz). The total energy within the filtered bandwidth was quantified as the cavitation dose (CD) for the current pulse, seen in the following equation:
where is the raw time-domain signal received by the PCD after each FUS pulse. Prior to microbubble injection, the average CD from 100 acquisitions were taken into account in order to assess the underlying background control signal () and to ensure there were no major reflections or existing bubbles in the FUS path. If the control CD was highly variable, or contained higher harmonic responses, the water bath and ultrasound gel were removed and reapplied until the control CD signal was stable. Furthermore, the CD was broken down into stable and inertial cavitation dose. The stable cavitation dose was quantified either using only the harmonic components (SCDh) or the ultraharmonic components (SCDu) by integrating the energy within the respective harmonic and ultraharmonic bins (bandwidth 200 kHz) between the 3rd and 9th harmonics. The remaining CD energy not contributing to the stable cavitation doses was considered to be the inertial cavitation dose (ICD). Stable (harmonic and ultraharmonic) and inertial cavitation doses were reported relative to their respective cavitation dose during the 100 control sonications acquired prior to microbubble injection.
Throughout this study, an open-loop cavitation controller was implemented to control the BBB permeability enhancement volume, with a cumulative cavitation dose (CCD) as the end-point criteria of the system. The block diagram of the control system is summarized in Figure 1B. Operating at fixed sonication parameters, the CD from the current pulse (CDcurr), minus the average , is added to the CCD, which is the running total of normalized CD accumulated throughout the sonication. Here, the CCD is the metric used to control the sonication duration. Once the CCD reaches the preset target (CCDtarget), the sonication ends. Throughout this study, we used three different CCD targets (1 107 V2•s, 5 107 V2•s, 1 108 V2•s), which herein will be defined as low (lCCD), medium (mCCD), and high (hCCD), respectively. In order to reach the higher CCD target, reinjection of microbubbles was occasionally needed. Bolus reinjections were administered when the current CD during the sonication dropped below 1dB, relative to the average CDcontrol. Subsequent reinjections of microbubbles were at the same initial microbubble dose. These CCD targets and reinjection criteria were determined in preliminary studies performed to emulate different volumes of enhancement and may need to be further refined depending on the application or experimental setup. Previously published controller systems for FUS-induced BBB opening focused on controlling the sonication pressure with fixed treatment durations [38], [39], while the cavitation controller system presented here sonicated with fixed pressure and varied the treatment duration based on the desired CCD targets
D. Magnetic Resonance Imaging (MRI) Data Acquisition and Analysis
Following the FUS induced BBB opening, mice underwent MRI to confirm targeting and BBB opening. Immediately after the sonication, a bolus of 0.2mL of gadodiamide (Omniscan, GE Healthcare, Chicago, IL) was intraperitoneally (IP) administered. The mice were then scanned in a vertical bore 9.4T MRI system (Bruker Biospin, Billercia, MA) and a birdcage coil (diameter: 30mm). Gadodiamide was allowed to circulate for 30 minutes before a T1-weighted 2D FLASH sequence was taken in the axial and coronal planes (TR/TE: 230/3.3ms, flip angle: 70%, NEX: 6, resolution: 100 um x 100 um, slice thickness: 400 um). In addition, a T2-RARE sequence (TR/TE: 2500/30 ms, NEX: 6, resolution: 100 um x 100 um, slice thickness: 400 um) was performed prior to contrast injection to assess for potential hemorrhage or edema. Mice were on isoflurane anesthesia (1–2%, oxygen flow rate of 0.8L/min) throughout the scan.
Contrast-enhanced T1-weighted MR images were processed in MATLAB (2019a, Mathworks, Natick, MA, USA) for quantification of BBB permeability enhancement volume. First, the brain was manually segmented from the skull. Afterwards, a threshold was applied to the brain based on a region of interest (ROI) in the contralateral hemisphere (left striatum). A threshold of two standard deviations above the mean ROI pixel intensity in the contralateral hemisphere was applied to generate a binary mask indicating the area of BBB permeability enhancement. Morphological operations (dilation and erosion) were applied to this mask to clear up small areas of false positive and to connect gaps/holes in the generated mask. This mask generation was applied independently to every axial slice for each mouse and the final enhancement volume was estimated across all axial slices.
E. Histology
Mice used for histology were euthanized and transracially perfused with cold phosphate buffered saline followed by 4% paraformaldehyde. The brain was then extracted from the skull and submerged in 4% paraformaldehyde for at least 3 days. Brains were then embedded in paraffin and sectioned coronally at 5um thickness. Each section level was separated by 150um, with the first 4 consecutive sections collected at the start of each level stained with hematoxylin and eosin (H&E). Each brain was sectioned from the end of the olfactory bulb to the start of the cerebellum, to ensure full coverage of the FUS focus volume within the striatum. Using the contrast enhanced MRI images, representative sections covering the center of the enhancement volume were chosen for histology. Tiled brightfield colored images were acquired for each section at 10X magnification using a Leica Microscope (Leica DM6) and mosaic stitched using the Leica LAS X Navigator software.
F. Quantitative PCR Analysis
Under anesthesia, animals were each rapidly decapitated and the brain was extracted. The brain was washed briefly with 1x USP PBS, in order to remove excess blood and then placed immediately on ice. Using pre-cooled tools, the brain was hemi-sectioned first and while remaining on ice, the striatum was dissected out of each hemisphere. The left and right striatum were kept separate and placed in corresponding pre-labeled eppendorf tubes with 2mL of Invitrogen™ RNAlater™ Stabilization Solution, or enough to submerge the tissue, to stabilize and protect the cellular RNA in each dissected hemisphere. The tissue was kept in the RNAlater™ solution at 4°C until gene expression analysis was performed by the Molecular Pathology Shared Resource (MPSR) core at Columbia University. Frozen tissue sections in RNAlater™ were provided to the MPSR core where RNA was first extracted using the RNeasy Microarry Tissue Mini Kit (Qiagen Inc., Germantown, MD), assessed for quality and concentration using a 2100 Bioanalyzer (Agilent, Santa Clara, CA). An RNA Integrity Number (RIN) greater than 8.0 was used as the cut-off for RNA quality assurance for gene analysis. All samples had RIN values between 9.0 and 10.0. Reverse transcription of the mRNA into cDNA was performed using the RT2 First Strand Kit (Qiagen Inc., Germantown, MD), according to manufacturer’s instructions. Afterwards, real-time quantitative polymerase chain reaction (RT-qPCR) was performed using RT2 SYBR® Green qPCR Mastermix (Qiagen Inc., Germantown, MD) and the Profiler PCR Array (96-Well Format) for Mouse Inflammatory Cytokines & Receptors (Cat. No. 330231 PAMM-011ZA, Qiagen Inc., Germantown, MD) on a Startagene® Mx3005p qPCR system (Agilent, Santa Clara, CA). Gene expression analysis was performed using the method [40], where individual gene cycle thresholds were normalized to the housekeeping gene (Gapdh). Genes with cycle threshold (Ct) values greater than 35 were considered to be insignificant levels of expression and set to a Ct value of 35 for data analysis. Each gene in the treated side (left striatum) was then normalized to the untreated side (right striatum) for each mouse to calculate the log2 fold change in gene expression.
G. Statistical Analysis
Statistical analysis was performed in both Graphpad Prism software (Version 8.4.2, GraphPad Software, La Jolla, CA, USA) and MATLAB (2019a Mathworks, Natick, MA, USA). All error bars presented are expressed in standard error mean (SEM). Inter-group analysis for sonication duration, number of microbubble injections, and BBB permeability enhancement volume was performed using one-way analysis of variance (ANOVA) followed by a post-hoc multiple comparison with Tukey’s multiple comparison correction. Analysis of statistically significant changes in relative gene expression was performed for each gene by taking the delta Ct values for the ipsilateral and contralateral qPCR reactions and performing a paired-sample t-test. Within each group, 84 multiple comparisons were performed for each gene in the qPCR kit, and therefore a false discover rate (FDR) correction was applied using a two-stage step-up method of Benjamini, Krieger and Yekutieli, with an FDR adjusted p-value of 5% (q < 0.05).
Results & Discussion
A. Cavitation Controller Metrics
Throughout this study, a real-time open-loop cavitation-based controller was used. Representative examples of the acquired cavitation dose over time are shown in Figure 2A (top row) for the three different cavitation dose cutoffs (low – left, medium – middle, high – right) used throughout this study, while in the bottom row are examples of the CCD over time, corresponding to the respective cutoffs. Figure 2B breaks down the contribution of power to the cavitation dose from harmonic, ultraharmonic, and broadband emissions. The increase of the cavitation signal relative to the baseline is evident as microbubbles are administered intravenously and begin to circulate, specifically with a much stronger increase in cavitation levels for harmonic emissions than ultraharmonic and broadband emissions. Following this initial increase, microbubble clearance results in the gradual CD decay. To achieve the higher CCD targets, reinjections were necessary in most cases, which are evident from the occasional spikes in CD observed throughout the sonication, indicated by the arrows in the plots in Figure 2A. The average sonication duration was 0.68 ± 0.22 min, 4.0 ± 0.89 min, and 7.2 ± 1.1 min, for the lCCD, mCCD and hCCD groups, respectively (Figure 2C), while the number of injections necessary to reach the target doses were 1.3 ± 0.13 injections, 1.8 ± 0.27 injections, and 3.5 ± 0.45 injections, respectively (Figure 2D). The number of injections and sonication duration was found to be significantly different (p < 0.05) for all groups except for the number of injections required between the lCCD and mCCD groups. The cavitation signal was also further analyzed into stable (harmonics and ultraharmonics) cavitation and inertial (broadband) cavitation (Figure 2E). In all three CCD targets, their average CD (relative to baseline) was similar for harmonic (~12 dB), ultraharmonics (~0.24 dB), and broadband emissions (~0.22 dB). As a result, stable harmonic emissions accounted for the majority of the CD emissions, while ultraharmonics and broadband emissions accounted for a very small percentage in all three CCD targets.
Figure 2.
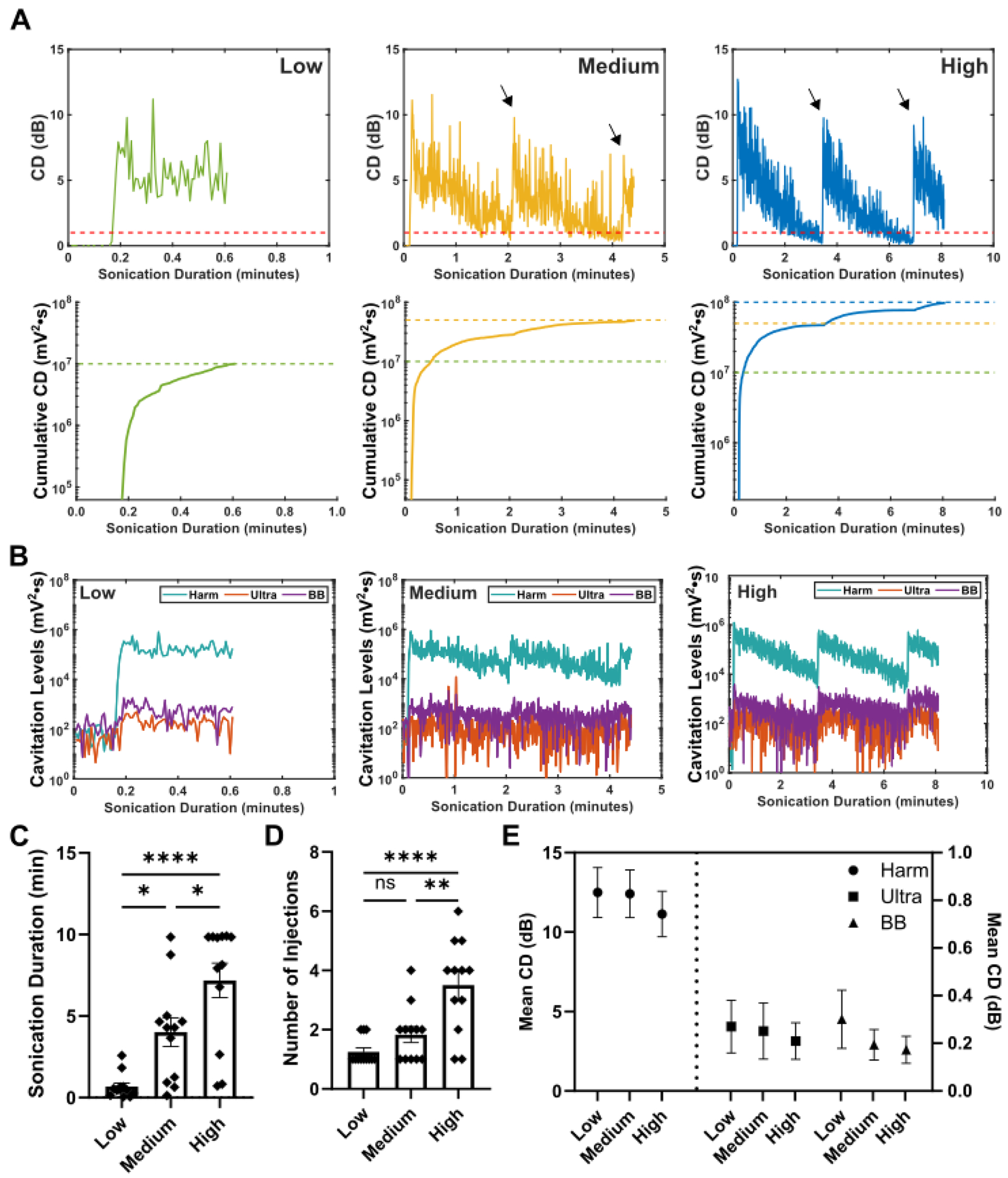
Cavitation Controller Results. A) Representative normalized CD plots (top row) and cumulative CD plots (bottom row) generated in real-time during sonications for CCDtargets of low (green, left column), medium (yellow, middle column), and high (blue, right column). Arrows in the normalized CD plots for the medium and high CCDtargets indicate points of microbubble reinjection. The dashed red lines represent the 1dB CD level for when microbubbles would be reinjected and the dashed colored lines correspond to the respective CCDtargets. Plots are normalized to the average CCD measured during the control (no microbubbles). B) Cavitation levels broken down into harmonic (harm), ultraharmonic (ultra), and broadband (BB) for the three CCDtargets. The spike in harmonic CD at the beginning is when microbubbles are injected. Summary of the C) sonication duration (low: 0.68 ± 0.22 min, medium: 4.0 ± 0.89 min, high: 7.2 ± 1.1 min) and D) number of injections (low: 1.3 ± 0.13 injections, medium: 1.8 ± 0.27 injections, high: 3.5 ± 0.45 injections) for the three different CCDtargets (One-way ANOVA with Tukey’s post-hoc multiple comparisons) E) Breakdown of the CD data into harmonic, ultraharmonic, and broadband (BB) doses. The mean CD over the whole sonication duration are reported for all three CCDtargets. N = 12 mice for each CCDtargets group was used. All error bars represent SEM. * - p < 0.05, ** - p < 0.01, **** - p< 0.0001, ns – not significant (p > 0.05).
The cavitation controller employed three linearly increasing CCD targets, which exhibited a linear increase in sonication times but required a disproportionately different number of microbubble injections for higher CCD targets; there was a drastic increase in the number of injections required for the hCCD group compared to mCCD group. This increase in the number of injections could be due to the microbubble clearance rate, changes in the underlying microvasculature over time, or a combination of both. Our study used oxygen as our anesthesia carrier instead of medical air and previous research has shown that when using oxygen, microbubble clearance rates were found to be 1.5 times faster when compared to medical air as the anesthesia carrier [41]. This faster clearance rate when using oxygen may require increased number of microbubble injections as the duration of sonication increases. We expect a reduction in the number of injections required if medical air was used instead. Additionally, as the sonication duration increases, an exponentially increasing amount of cavitation events are occurring at the focus, changing the underlying microvasculature within, and potentially reducing the amount of bubble perfusing the focal area. This is evident in the lower observed maximum CD peaks following microbubble reinjection than the initial CD peak from the first injection (Figure 1A, arrows indicating reinjections in the middle and right plots). Quantification of the cavitation dose following bolus reinjections of microbubble reveal a significant decrease in peak cavitation dose relative to the peak cavitation dose to the initial microbubble injection (Figure S1). For the 2nd, 3rd, and 4th or more microbubble injection, peak cavitation dose following reinjections were on average 70%, 50% and 56% relative to the initial peak cavitation dose, respectively. Future studies are necessary to properly assess this observed phenomenon.
In this study, the controller system used the total energy emitted from the cavitating microbubbles circulating in the brain to dictate the duration of sonication. By fixing the CCD, predictable and consistent BBB permeability enhancement volume was achievable. Other controller system regimes have been investigated by others as well. O’Reilly et al. developed a controller system that ramped up the pressure until ultraharmonics or subharmonics were detected, and then lowered the pressure by a certain percentage and maintained it for the remaining fixed duration of the sonication [38]. The presence of ultraharmonics and subharmonics during FUS-induced BBB opening have also been investigated and shown to be indicative of certain bioeffects following BBB opening [32], [38]. Another approach reported by Kamimura et al. involved ramping up the pressure to sustain a predefined level of stable cavitation activity, and to decrease the pressure when inertial cavitation activity was detected [39]. Sustained inertial cavitation during FUS-induced BBB opening has been associated with negative and damaging bioeffects such as lesioning and red blood cell extravasation and are often undesirable and avoided during FUS-induced BBB opening experiments [33], [42]. A more recent controller system (Sun et al. 2017) modulated the pressure to sustain certain levels of harmonic cavitation emission, while simultaneously avoiding inertial cavitation emissions [34]. In their study, Sun et al. found a direct relationship between the total harmonic emission and the amount of doxorubicin delivered to the brain. However, their sonications were still dictated by fixed sonications times, and the sonication duration to achieve different delivery doses. The sonications in the present study were guided by the desired CCD, resulting with varied sonication times. Nonetheless, the results presented in this study further bolster that fact that cavitation is one of the most important metrics to monitor as it is highly indicative of the resulting bioeffects, independent of the FUS parameters.
B. FUS-induced BBB opening safety metrics varied dependent on CCD
To assess the bioeffects from using this cavitation-based controller system, MRI and histology was performed. First, contrast enhanced T1-weighted (T1w) MR images were acquired to assess BBB integrity after FUS induced BBB opening. Contrast enhancement in the brain 30 minutes after FUS was used to assess the BBB permeability enhancement volume among the three CCD cutoffs, with representative T1w MR images shown in Figure 3A. Successful opening in the targeted left striatum was found in all mice for all three cutoffs. The average contrast enhancement volume found for the low, medium, and high cutoffs were 10.6 ± 2.88 mm3, 31.3 ± 4.62 mm3, 46.9 ± 4.74 mm3, respectively (Figure 3B). Furthermore, there was significant differences found in contrast enhancement volume among the three cumulative CD cutoffs (lCCD vs mCCD p < 0.01, lCCD vs. hCCD, p < 0.0001, mCCD vs. hCCD, p < 0.05).
Figure 3.
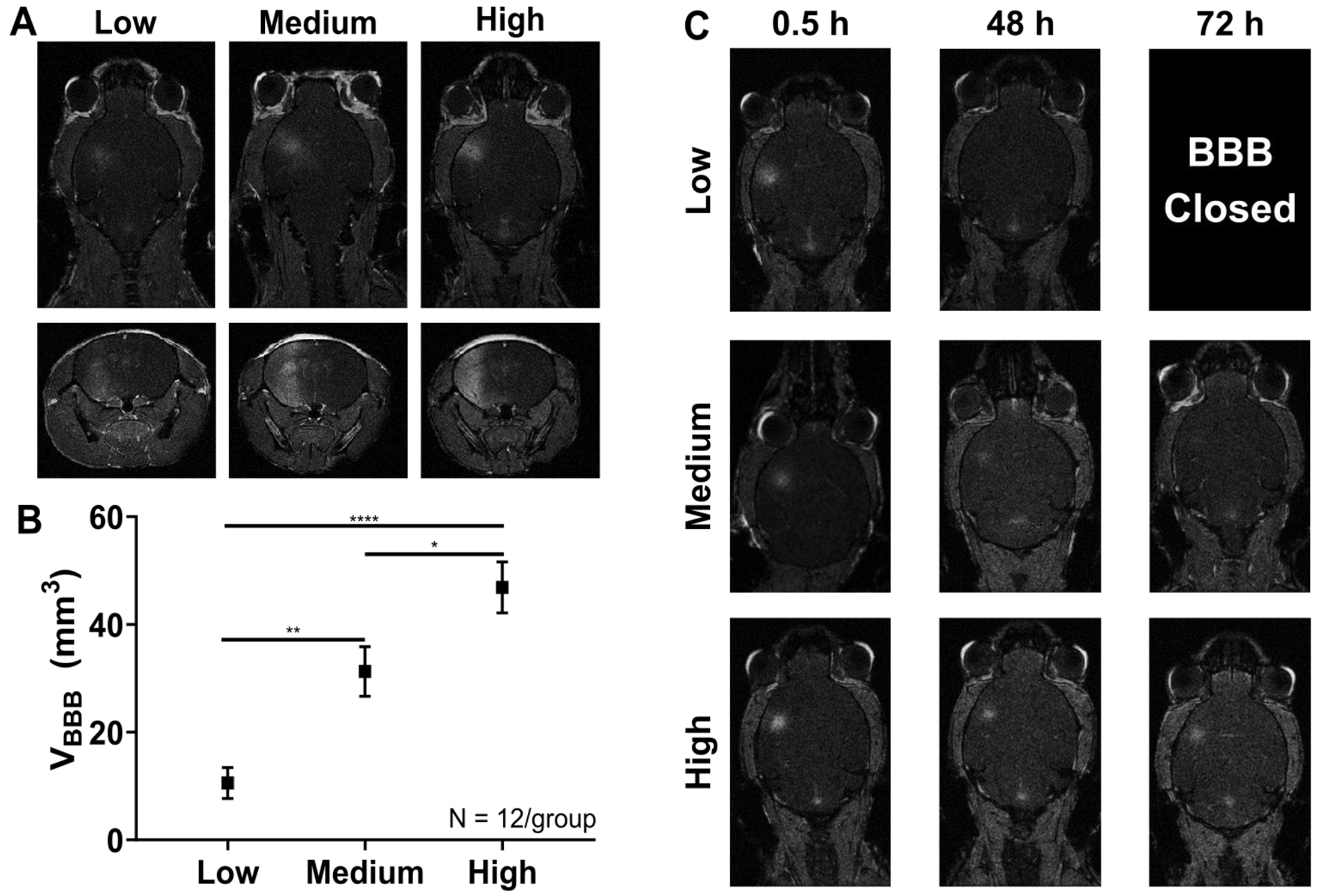
A) Representative axial (top row) and coronal (bottom row) contrast enhanced T1w MRI images after FUS-induced BBB opening using the cavitation-based controller system for the three different CCDtargets. B) Average BBB permeability enhancement volume 30 minutes after gadolinium injection. Statistically significant volumes of enhancement were found in all three CCDtargets groups (One-way ANOVA with Tukey corrected post-hoc comparisons). All error bars represent SEM. * - p < 0.05, ** p < 0.01, ****p < 0.0001. C) Representative axial contrast enhanced T1w MRI images over three time points (0.5 h, 48 h, and 72 h) for all three CCDtargets. The BBB closing timeline varied depending on CCDtargets, ranging from closing within 48 h for lCCD, to being still opened at 72 h for hCCD.
Reporting of cavitation dose as a metric during BBB opening has become common practice in many studies, as it can act as a unifying metric in such a large parameter space for FUS-induced BBB opening. It has been shown that cavitation dose can be predictive of certain bioeffects, such as BBB permeability enhancement volume, duration, and of various safety metrics [14], [33], [43]. However, cavitation-modulated bioeffects has not been extensively explored so far. By employing of a simple cavitation-based controller and standardizing the FUS parameters (pressure, pulse sequence, etc.) and microbubble characteristics (size distribution, shell type, etc.), the present work was able to produce significantly different BBB permeability enhancement volumes using CCD as the only tunable parameter. Furthermore, varying the CCD resulted in different BBB closing timelines observed using contrast enhanced T1w MRI (Figure 3C). For the lCCD group, the barrier closed within 48 h, resulting in no contrast enhancement visible at the 48 h MRI scan. As the CCD increased, the closing timeline increased, with the mCCD group closing by 72 h and the hCCD group still open at 72 h. Though the BBB was still opened for the hCCD group at 72 h, the volume of enhancement is visibly smaller than earlier time points, suggesting that the BBB is being repaired. Other studies have shown similar volumes of enhancement and found closing timelines up to 5 days, which is expected to hold true here as well [16], [44].
Previous studies have indicated that the BBB permeability enhancement volume can be controlled using acoustic pressures [12], [14], [45]. However, in this study, with a fixed acoustic pressure, significantly different BBB permeability enhancement volumes were achievable by controlling the total amount of cavitation activity emitted during the sonication. Furthermore, the FUS parameters used herein relied more heavily on stronger harmonic cavitation levels, rather than weaker ultraharmonic and inertial cavitation levels (Fig. 2E). Sustained inertial cavitation has been long associated with negative bioeffects such as cellular damage during FUS-induced BBB opening [18], [46], [47]. Though the exact CCD cutoffs used here to show a relationship between cavitation dose and BBB opening properties are highly study dependent, the general trend of greater CCD resulting in greater MRI contrast enhancement volume and longer BBB closing timelines can be applied to other studies.
A small safety study was also performed with 12 mice to assess any determinantal bioeffects of the three CCD groups. Two survival time points of 6 h and 72 h after BBB opening (n = 2/group) were chosen and H&E staining was performed to assess any histological changes, such as red blood cell (RBC) extravasation. Additionally, mice survived for 72 h received T2-weighted (T2w) MRI at 48 h and 72 h time points to assess the presence of any edema or hemorrhage. It has been well established from previous reports that using a similar sonication regime (frequency, pressure, pulse length, number of microbubbles per bolus injection, etc.) as this study has produced safe and transient BBB openings [12], [14], [26]. However, the effects of multiple microbubble bolus injections have not been thoroughly investigated within the aforementioned sonication regime. As expected, the lCCD group, which required on average only 1.3 injections and a sonication duration of about 40 seconds, similar to previous reports which used 1 bolus injection and 1 – 2 minutes of sonication, resulted in no histological changes and no detectable edema or hemorrhaging at any of the time points assessed (Figure S2A and S2B). The mCCD group, with on average 1.8 injections and a longer average sonication of 4 minutes, resulted in minimal edema visible at 48 h that mostly subsided by 72 h (Figure 4A). Additionally, histological damage was not found at any time points for the mCCD group (Figure S2C and S2D). Finally, for the hCCD, with on average 3.5 injections and an average sonication duration of 7.2 min, both edema and RBC extravasation were present. The edema presented in the hCCD group was similar to that found in the mCCD, in that the minimal edema present at 48 h had started to reduce drastically by 72 h (Figure 4B). Moreover, minimal RBC extravasation was visible in the hCCD group for both 6 h (Figure 4C) and 72 h (Figure 4D) time points. Overall, through the use of a simple cavitation controller, different bioeffects were elicited without changing the sonication parameters ranging from small BBB openings without any edema or histological changes to larger openings with transient edema effects and minimal RBC extravasation. This allows the potential for the control of different degrees of BBB opening, depending on the application, while simultaneously minimizing inertial cavitation effects. Future studies are still necessary in order to further investigate the effects on specific cell types such as microglia and astrocytes when using this cavitation-based controller system.
Figure 4.
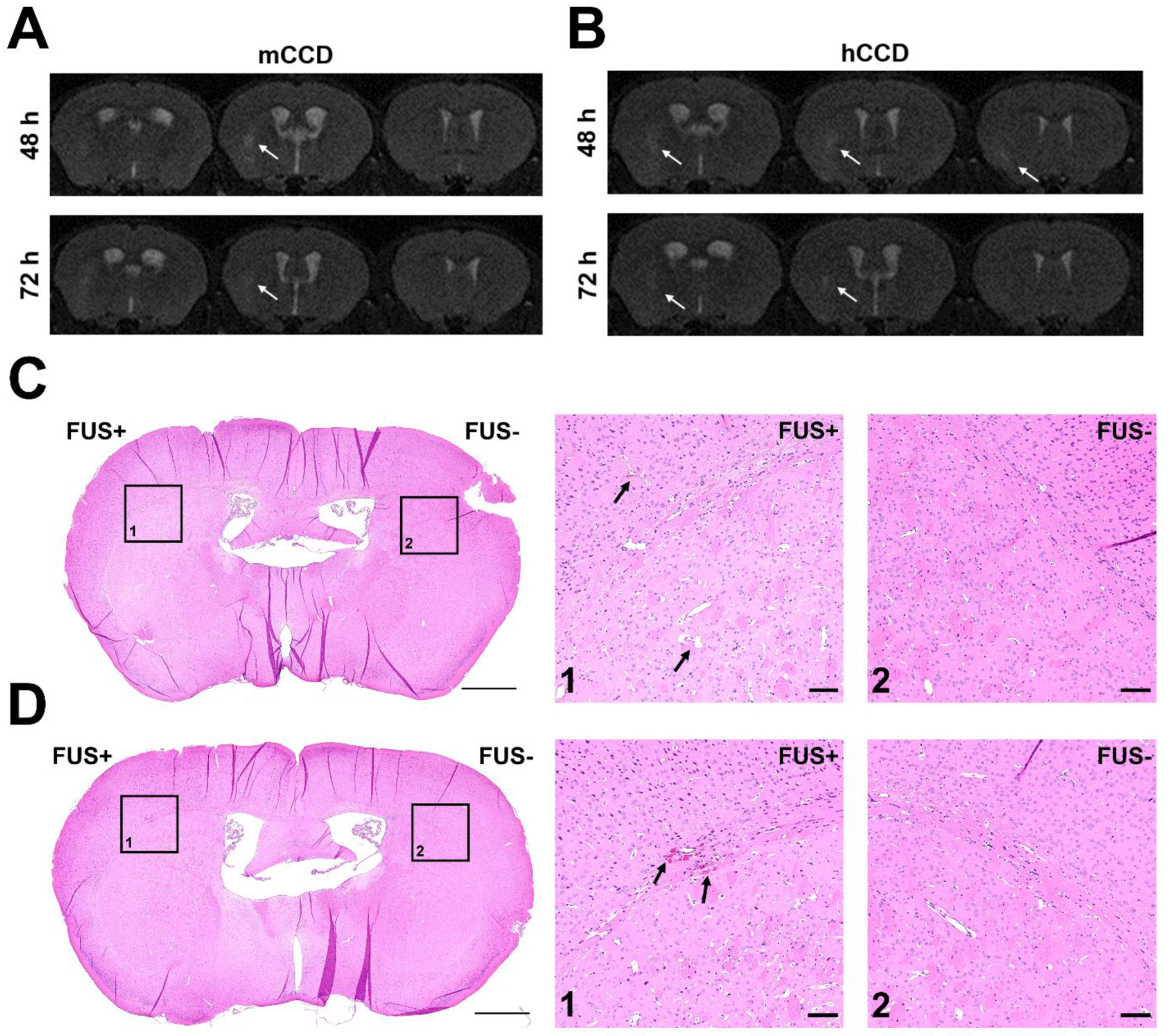
Consecutive coronal T2-weighted MRI images acquired in mice receiving A) medium CCD or B) high CCD taken at 48 hours and 72 hours after FUS-induced BBB opening. Hyperintense areas indicated by the white arrows represent regions of edema, and were only present in the sonicated hemisphere on the left. No hypointense pixels were visible, indicating a lack of hemorrhaging. Histology using H&E staining was performed, and minimal RBC extravasation was found in only the high CCD group at both C) 6 hours and D) 72 hours after FUS-induced BBB opening. The left striatum was sonicated (FUS+) and the right striatum was used as the control (FUS-). RBC extravasation was only found in the boxed region in the left striatum, with the contralateral side shown as a control. The scale bar for full coronal section view is 1 mm and the scale bar for the zoomed in region is 100 um.
C. Transient inflammatory response to FUS-induced BBB opening is influenced by the Cumulative Cavitation Dose
Quantitative PCR analysis was used to assess the brain’s immune response to FUS induced BBB opening following the three different CCDs. The gene expression from an array of 84 genes associated with mouse inflammatory cytokines & receptors were assessed.
Volcano plots of relative gene expression (normalized to the non-sonicated contralateral side) for all three CCDs (rows) and all three time points (columns) are shown in Figure 5. At the earliest time point of 6hr, the lCCD group did not have any significant changes in gene expression, which were changes in expression that were both statistically significant (q < 0.05) and biologically relevant (log2(FoldChange) > 1 for upregulated and log2(FoldChange) < −1 for downregulated). However, both mCCD and hCCD groups had multiple genes that were upregulated and biologically relevant, with the hCCD group having a few genes that were statistically significant as well. Additionally, both the mCCD and hCCD group tended to skew towards the right more than the lCCD group, suggesting a trend towards greater upregulation of genes within the inflammatory response pathway examined. These findings indicate that the inflammatory response to FUS-induced BBB opening is consistent with the changes in CCD. The complete list of log2 fold change for all genes assessed in the qPCR array 6 h after BBB opening can be found in Table S1.
Figure 5.
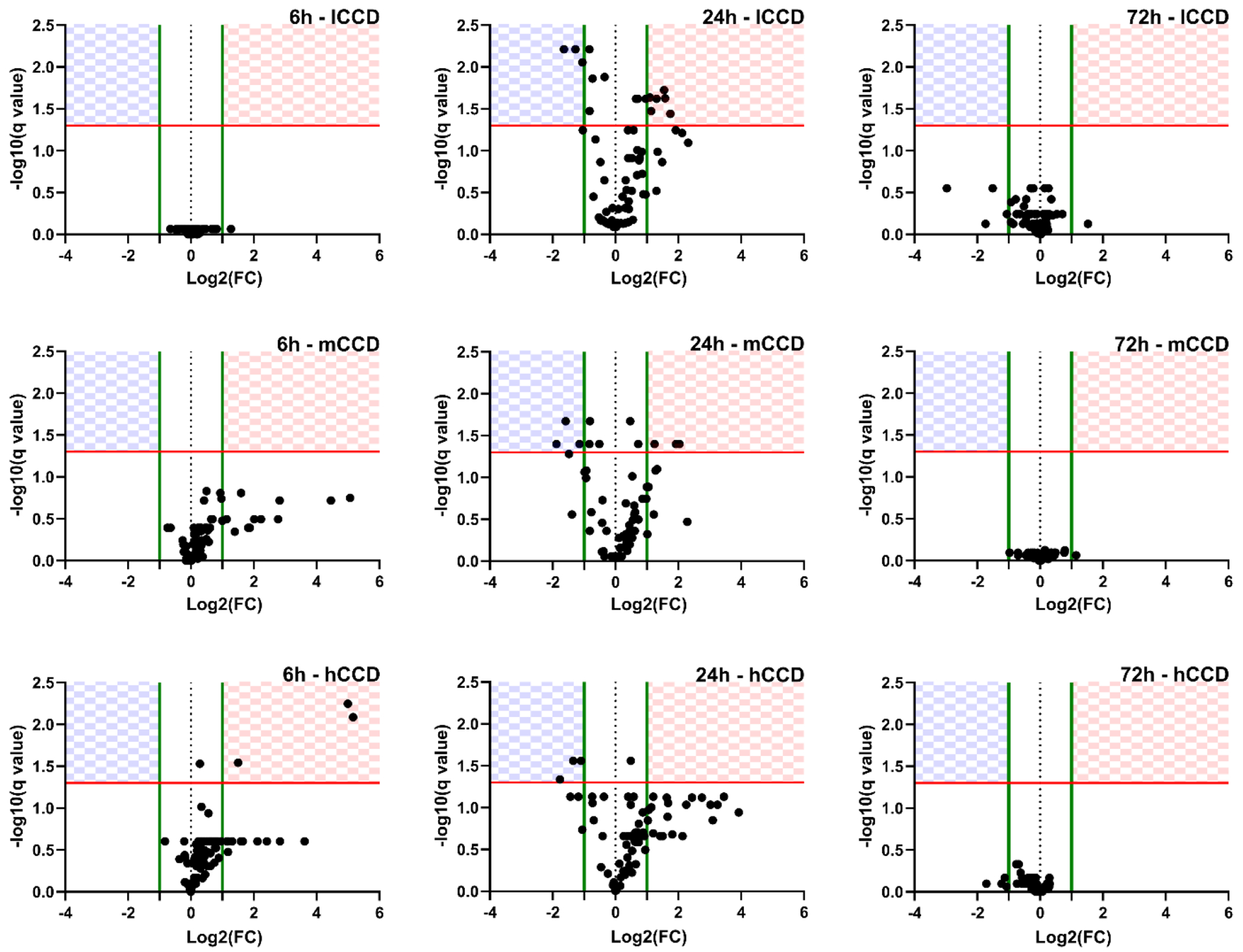
Volcano Plots of Gene Expression Changes. Relative gene expression is expressed as log2 of the fold change relative to the contralateral side. Each row represents data from low, medium, or high CCD (lCCD, mCCD, hCCD) groups while each column is a different time point (6 h, 24 h, 72 h). The checkered regions represent statistically significant changes in gene expression (q < 0.05, red line) and have a log2 fold change greater than 1 for upregulation, and less than −1 for downregulation (green lines). Genes in the top left quadrant (checkered blue) represent genes significantly downregulated and genes in the top right quadrant (checkered red) represent genes significantly upregulated. N = 4 per plot.
At the 24 h time-point, all three CCD groups have a marked change in gene expression compared to their respective 6 h time-points. For the lCCD group, there was an increase in both significantly upregulated and downregulated genes that were not seen at the 6 h time point. The mCCD and hCCD groups continued to have significantly upregulated genes, but also an increase in significantly downregulated genes. Though the hCCD group did not result in any statistically significant gene upregulation, there is still a substantial amount of biologically relevant gene upregulation compared to the lCCD and mCCD groups. Additionally, the hCCD group persistently experienced the right skewedness in data, similar to its 6 h time point. The change in gene expression for all three groups after FUS suggests that the inflammatory response has a temporal component that peaks between 6 h and 72 h. The complete list of log2 fold change for all genes assessed in the qPCR array 24 h after BBB opening can be found in Table S2.
Finally, at the 72 h time point, there was a total shift in gene expression for all three CCD groups, with an absence of both significantly upregulated and significantly downregulated genes. The majority of gene expression in the three CCD groups was not biologically relevant or statistically significant 72 h after FUS-induced BBB opening, indicating a return to baseline gene expression. The complete list of log2 fold change for all genes assessed in the qPCR array 72 h after BBB opening can be found in Table S3. Overall, these results suggest that the inflammatory response to FUS-induced BBB opening is a transient response that peaks around 24 h and is reinstated to baseline within the 72 h time frame. This also fits the BBB closing timeline at the FUS parameters used here [12], [44], indicating that the changes in gene expression may be associated with the BBB opening and repair process. Additionally, the initial response (6 h) and temporal progression (24 h to 72 h) of the measured inflammatory response was consistent with the increase in CCD targets (i.e., the greater the CCD target, the larger the magnitude of the inflammatory response over time). Though these findings indicate a relationship between CCD and the inflammatory response, additional parametric studies employing varying parameters, such as pressure and microbubble composition, are warranted to fully elucidate the relationship between CCD and inflammatory response.
For a more in-depth evaluation of the changes in gene expression, heatmaps showing only the genes with significant changes in expression, grouped by the three CCD groups, are shown in Figure 6. The qPCR kit used in the study focused mainly on the cytokines and receptor genes associated with the inflammation pathway and its associated downstream effects. The cytokine superfamily of proteins is essential in the signaling network between cells and in regulating the immune system [48]. Cytokines and chemotactic cytokines (chemokines) mediate the communication of the immune system [48]. Their biological signals affect the growth and development, hematopoiesis, lymphocyte recruitment, T cell subset differentiation and inflammation [48]. In acute neuroinflammation, the coordinated recruitment and placement of effector cells to the site of inflammation requires the expression of inflammatory cytokines and their respective receptors on target cells [49], [50].
Figure 6.
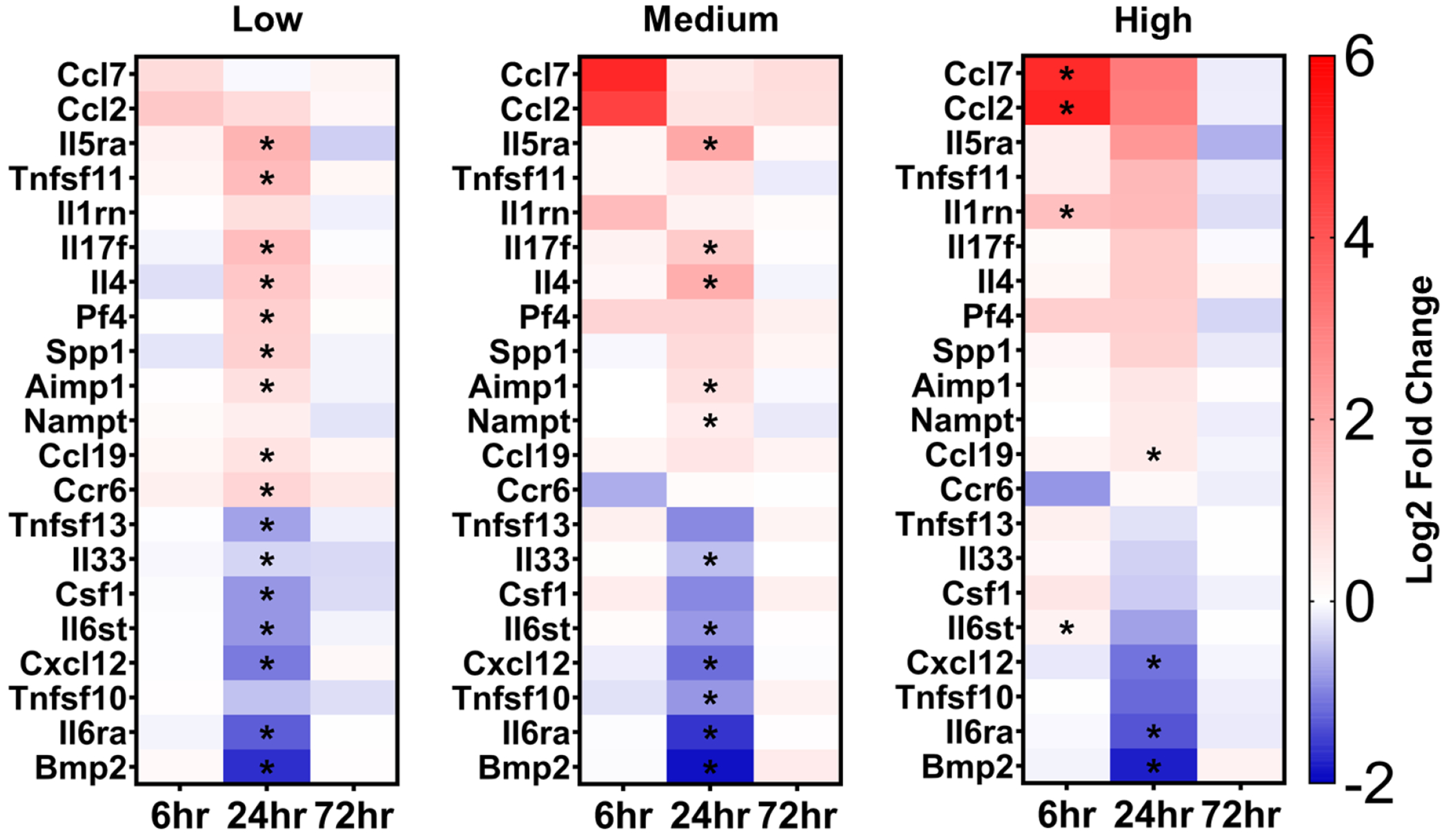
Heat maps of relevant genes with changes in expression levels relative to the non-sonicated contralateral striatum. Each heat map is grouped by the CCD groups (low, medium high) and within each group, the 3 time points (6 h, 24 h, 72 h) are shown. Genes with statistically significant changes and biologically relevant changes (|log2FC| > 1) at any time point or in any group are included. Genes marked with an asterisk signify statistically significant log2 fold change. Genes with positive log2 fold changes were considered upregulated and genes with negative fold2 changes were considered downregulated. Statistically significant changes in gene expression were assessed using paired sampled t-test with FDR multiple comparison correction (q < 0.05). Each point in the heat map is the arithmetic mean log2 fold change of 4 mice.
Cytokines can be broadly grouped based on whether they act on cells of the adaptive immune response, promote or inhibit inflammation [51]. Among the well-characterized pro-inflammatory cytokines is interleukin-1 beta (IL1b) that is secreted by multiple cell types and mediates a wide range of inflammatory signaling responses [51]. IL1b signal transduction results in the activation of mitogen-activated protein kinases (MAPKs) and the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway leading to pro-inflammatory cytokine transduction [51]. Herein, expression levels of Il1b were upregulated only at the 6 h time point in the mCCD and hCCD groups, most likely in response to molecules stimulating pattern recognition receptors (PRR) following BBB opening (Table S1). Along with the upregulation of Il1b expression, upregulation of the interleukin-1 receptor antagonist (Il1rn) was observed, primarily in the mCCD and hCCD groups, which prevents further IL1b binding, inhibits signal transduction, and dampens the inflammatory response [51]. Additionally, upregulation of Il1rn expression was still present for the hCCD group at 24 h, indicating to a potential stronger response due to the larger BBB permeability enhancement volume. Additional key pro-inflammatory cytokines that bind to the same cytokine receptor with IL1b, are tumor necrosis factor (TNF) and interleukin-6 (IL6) [51]. TNF is primarily secreted from activated macrophages and stimulates the proliferation of normal cells, exerts cytolytic or cytostatic activity against tumor cells, and causes inflammatory, antiviral, and immunoregulatory effects [51]. Shortly following the sonication (6 h) at the hCCD group, TNF expression was upregulated but the effect subsided by 24 h, when the expression of tumor necrosis factor ligand superfamily member 10 (Tnfsf10), driving cell apoptosis, was significantly downregulated. Additionally, the expression of tumor necrosis factor ligand superfamily member 11 (Tnfsf11), which augments the ability of dendritic cells to stimulate naive T cell proliferation and regulates the corresponding immune response, was upregulated at the 24h time point. IL6 cytokine is primarily secreted by activated T cells, macrophages and fibroblasts while is involved in hematopoiesis, and is critical in the final maturation of B-cells into antibody-producing plasma cells [51]. Contrary to Il1b and Tnf, expression of IL6 receptor subunit alpha precursor (Il6ra), which in its soluble form extends the half-life of IL6, was significantly downregulated at 24 h in all CCD groups, suggesting resolution of the associated inflammatory signaling.
The cytokines of interest that belong to the adaptive immune system include interleukins 4, 5, and 13 (IL4, IL5, IL13) and macrophage colony stimulating factor (CSF2). IL4 and IL13, that closely maps IL4, are produced by activated T cells, mast cells, basophils and natural killer T cells (NKT) and their biological effect is the inhibition of macrophage activation [48]. The expression levels of these two cytokines were found upregulated 24 h following FUS and could be accounted responsible for the downregulation of inflammatory actions. IL5 is primarily produced by activated T cells and aids in the growth and differentiation of eosinophils and late-developing B cells [48]. The expression levels of IL5 receptor subunit alpha (Il5ra) was found upregulated 24 h following FUS in all three CCD groups.
Finally, interleukin 10 (IL10) that is primarily secreted by microglia and astrocytes in the brain, is an essential anti-inflammatory component mediating immunosuppressive signal, and thus inhibits the synthesis of proinflammatory cytokines [48], [52]. IL10 exerts its anti-inflammatory effects through binding to receptors composed of two subunits, IL10 receptor alpha (IL10ra) and IL10 receptor beta (IL10rb). While most cell types express IL10ra, research has shown that microglia, and potentially astrocytes, express both receptor subunits [52]. Both expression levels of Il10ra and Il10rb were investigated in this study, but changes in expression level were only found in Il10ra. Specifically, biologically relevant downregulation of Il10ra expression was found only at the 24 h after FUS time point in all CCD groups (Table S2). Downregulation of Il10ra expression, particularly if it were to happen in microglia and astrocytes, may result in sustained upregulation of pro-inflammatory cytokine expression observed at the 24 h time point, requiring other cytokines involved in the adaptive immune response to engage in dampening the inflammation.
The chemotactic cytokines, or also known as chemokines, are a large family of small, secreted proteins that signal through chemokine receptors. Chemokines and their receptors control the migration and positioning of immune cells in tissues and are critical for the function of the innate immune system following a protective or destructive immune and inflammatory response [50], [53]. There are two families of chemokines, the CC chemokines that stimulate mainly monocytes, but also basophils, eosinophils, T-lymphocytes, and natural killer (NK) cells and the CXC family that mainly stimulates neutrophil chemotaxis, and is essential for receptor binding [49].
Significant changes in expression levels of a few members of the monocyte chemotactic protein (MCP) subfamily of CC chemokines were observed. The MCP members are primarily regulating the migration and infiltration of monocytes, memory T lymphocytes, and NK cells [54], [55]. The expression of Mcp1/Ccl2 and Mcp3/Ccl7 were found to be the most significant, but a few other MCPs were observed exhibiting biologically relevant changes in expression levels, including Mcp2/Ccl8, Mcp4/Ccl13 and Mcp5/Ccl12. The MCP response to the FUS-induced BBB opening and the corresponding inflammation followed a cavitation-controlled pattern within a well-defined time course. Specifically, expression levels of both Ccl2 and Ccl7 increased linearly with increased CCD levels (i.e., greater CCD resulted in greater upregulation of Ccl2 and Ccl7). CCL2 and CCL7 are rapidly produced by stromal cells and immune cells after pattern-recognition receptor activation or after cytokine stimulation [49]. CCL2 stimulates the chemotaxis and induces a weak cytokine expression in monocytes while CCL7 is structurally similar to CCL2 and is involved in monocyte mobilization [49]. The proportional change in expression levels of Ccl2 and Ccl7 to CCD indicate a potential relationship with CCD and the strength of the immune response and resulting cytokine stimulation.
In the CXC family, chemokines CXCL1–3,5–8 are classified as inflammatory while CXCL4 is plasmatic or platelet related, CXCL12 and CXCL13 as homeostatic and CXCL9, CXCL10, CXCL11 and CXCL16 exhibit a dual function [49]. In acute inflammation, neutrophils are the first cells that infiltrate into the tissue. Neutrophils control the infection by phagocytosing and by releasing other chemotactic mediators, such as CXCL1, CXCL8, CXCL9 and CXCL10 that recruit other leucocytes to the affected tissues [49]. Consistent with these findings is the pattern observed following FUS. Increased neutrophil phagocytosis is believed to cause the relative gene expression upregulation of Cxcl10 with increasing persistence following the CCD. Biologically relevant expression levels of Cxcl10 were not detected for the lCCD group but were relevantly increased at the 6 h time-point of the mCCD group and at both the 6 h and 24 h time points in the hCCD group (Table S1 and Table S2). Additionally, Cxcl12 expression levels were only significantly downregulated at the 24 h time point regardless of the CCD, but around baseline levels at both 6 h and 72 h, suggesting the disruption of normal brain function peaking at around 24 h time point.
Previous research from other groups performing transcriptome analysis on whole brain tissue to investigate the inflammatory response after FUS-induced BBB opening have also found significant changes in gene expression. Kovacs et al. specifically investigated the NF-κB pathway and found alarming levels of gene expression in regions of the brain receiving FUS-induced BBB opening[30]. The type of changes in gene expression associated with the inflammatory response reported by Kovacs et al., however, were drastically greater than what was found in this study. For example, a few of the overlapping chemokine and cytokine genes (e.g. Ccl12, Cxcl1, Il1b, Tnf) reported were severely upregulated with fold changes ranging from 10 to 149 at the 6 h time-point, while in comparison, the same genes in this study were either not significantly upregulated at all in our lCCD group or moderately upregulated (less than 13 fold change) in the mCCD and hCCD groups at the same time point. Furthermore, all the changes in gene expression in this study returned close back to baseline by the 72 h time point. The key difference between our studies is we only sonicated a single region within the striatum while Kovacs et al. required multiple sonications which could increase the potential of overlapping locations, resulting in a potentially stronger inflammatory response. McMahon et al. also investigated the gene expression associated with the NF-κB pathway, but were able to induce much lower levels of gene expression changes [29]. In this study, the types of inflammatory response found follow a similar profile as those reported by McMahon et al. when they employed clinically recommended microbubble dosing and the acoustic controller system developed by O’Reilly et al. [38]. Furthermore, McMahon et al. reported that microbubble dosing was a factor to consider, as they were able to show increased levels of gene expression when using microbubble doses ten times greater than the clinically recommended (10x), albeit, still much lower than that reported by Kovacs et al. The caveat, however, is that in this study, while using a microbubble dosing of 10x, we were still able to induce varying levels of gene expression through controlling the cavitation levels throughout the sonications. Moreover, the requirement of multiple bolus injections for the higher CCD groups may have an influence on the expression levels, as a recent publication has shown that the inflammatory response following FUS induced BBB opening was influenced by the microbubble formulation itself, including the size distribution and total gas volume injected [56]. Further studies investigating microbubble formulations and the changes in cavitation dynamics and inflammatory response are necessary.
More recent reports performing advanced transcriptomics through both bulk and single-cell RNA sequencing have also found compelling variable levels of inflammatory response following FUS-induced BBB opening. By employing bulk RNA sequencing, Mathew et al. found differences in specific enriched and repressed pathways, such as inflammation, metabolism, and repair, dependent on to the specific type of anesthetic used during FUS-induced BBB opening (isoflurane vs. ketamine) [57]. Moreover, Curley et al. performed bulk RNA sequencing and found similar immunomodulatory response after FUS-BBB opening in the BBB and blood-tumor barrier in a tumor mouse model, indicating consistent inflammatory response in a diseased mouse model [58]. Teasing apart the implications of anesthesia and disease on the immunomodulatory effects of FUS-induced BBB opening will be crucial in order to fully characterize the transcriptomic and proteomic changes for clinical applications, as most patients are expected not to be under anesthesia and have some sort of disease (neurodegenerative, cancer, etc.). Additionally, Mathew et al. performed single-cell RNA sequencing of transfected cells after delivery of plasmids with FUS-induced BBB opening, and found transfection efficiency and cell transfection distribution to be FUS pressure dependent [59]. Using a very similar MI in this study, Mathew et al. found greater number of transfected microglia exhibiting stronger changes in differential gene expression when compared to lower FUS pressures. Interestingly, the transfected cells at 48 h after FUS-induced BBB did not exhibit an upregulation of the classical markers associated with sterile inflammation, indicating a potential earlier return to baseline levels than the 72 h time point found here. Finally, Gorick et al. also demonstrated through single-cell RNA sequencing, a pressure dependent change in gene set enrichment and repression in FUS-transfected brain endothelial cells [60]. Overall, active research employing advanced transcriptomics can help further elucidate and understand the immune response to FUS-induced BBB opening, and whether cavitation controller systems are able to modulate the response from specific cell types.
Our observations complement previously reported findings in the literature, highlighting the importance of control over cavitation levels as a regulatory mechanism of the inflammatory cascade, intensity, and duration. The study presented herein provided new important elements in the role of FUS in eliciting immune responses. Using fixed sonication parameters, the cavitation dose was demonstrated herein as one of the principal regulators of the BBB permeability enhancement volume and the induced inflammatory response. Control of the cavitation level drives consistent volumes of enhancement, resulting in various levels of inflammation consistent with the respective cavitation dose. Previously, Kovacs et al. proposed the release of damage-associated molecular patterns (DAMPs) from injured cells followed by the activation of the NFκB pathway after BBB opening, and concluded a strong correlation between the sonication regime and the immune response [30], [61]. McMahon et al. narrowed down the driving mechanism from sonication regime to microbubble dose as the inflammatory intensity regulator. Among different sonication schemes, their study proved the efficacy of a reduced inflammatory response when a lower dose of microbubbles combined with acoustic feedback control of the FUS pressure level was used [29]. However, the proposed regulatory mechanisms were deemed insufficient predictors of the inflammatory response given the BBB permeability enhancement volume has not yet been studied as a potential regulator [10]. In our study, using a microbubble dosing ten times greater than that approved by the FDA for BBB opening clinical trials, the cavitation controlled sonications allowed for consistent BBB permeability enhancement volumes with not only a distinct, but also a transient inflammatory response; almost all observed genes in the sonicated region returned to expression levels similar to the non-sonicated contralateral region within 72 h after the BBB opening. Instead of relying solely on fixed sonication parameters, our findings highlight the importance of cavitation-based controller systems that adjust and maintain cavitation levels with real-time feedback on the bubble activity and, as shown herein, inferring to the associated inflammatory response. Additional studies are, however, warranted to determine the entire parametric space for a more granular understanding of the relationship between the FUS and microbubble parameters and the resulting types of inflammation. Moreover, employing state-of-the art techniques such as single cell RNA sequencing can help further elucidate the distinct response of different cell types to FUS-induced BBB opening, and allow for the possibility of activation/repression of specific cell populations for therapy.
Limitations
There are several limitations to the present study and the cavitation controller system. The first limitation are the microbubbles dosing and multiple reinjections. Definity microbubbles were injected at a dose 10 times the recommended clinical dose, and in certain cases reinjected multiple times. Though none of the animals in the study had any unanticipated adverse short- or long-term effects prior to their end point, systemic effects of such high volumes of microbubble dose may need to be further evaluated. Additionally, multiple microbubble bolus injections could have been avoided through using microbubble infusion. A recent study has shown that a single microbubble infusion was capable of consistently opening the BBB in multiple locations up to six minutes after the start of infusion [62]. However, even with multiple bolus injections of microbubbles, we were able to achieve consistent and distinct BBB permeability enhancement volumes without tissue damage such as hemorrhage using the cavitation controller system presented herein. As a result, combining an infusion protocol with the cavitation controller may result in lower levels of inflammation, as cavitation levels with infusion rates have been shown to be more predictable and sustained [34], potentially eliminating the need for microbubble reinjections and reducing the probability of broadband responses. Another limitation of this study was that inflammation and safety was only evaluated on the tissue level; evaluation of relative gene expression with qPCR and H&E staining only provide a glimpse into the underlying complex cellular response to FUS-induced BBB opening. Further studies are necessary to assess the different effects CCD may have on specific cell types, such as neurons, microglia or astrocytes, both on a morphological level through immunohistochemistry (IHC) and more importantly, on the transcriptomic level through single-cell RNA sequencing. Currently, there have been very limited studies employing high throughput technologies to investigate changes in transcriptome and biological pathways of specific cell types in response to FUS-induced BBB opening [57–60]. These studies are crucial to be able to elucidate the driving force of the inflammatory response following FUS-induced BBB opening, and whether peripherally circulating immune cells play a role in inflammation and BBB repair. Furthermore, BBB permeability changes evaluated through contrast-enhanced MRI only provide insight in changes of contrast diffusion rate, without providing direct evidence of changes in vascular permeability. Evaluation at a cellular level is necessary in order to elucidate the observed changes in permeability using the cavitation controller. Previous reports have found different types of cellular transport across the BBB (transcytosis, paracellular transport, etc.), which may explain the observed differences in permeability enhancement if different types of transport dominate, depending on the CCD [63], [64]. A third limitation of this study is the clinical translatability of the cumulative cavitation controller system. The FUS transducer was operated at 1.5 MHz and the controller system quantified a large frequency range of cavitation emissions (4.5 MHz – 13.5MHz), all of which have been optimized in murine models. However, this exact system cannot be translated into humans due to the attenuation and aberration of the ultrasound and cavitation signals by the thick human skull. Much lower FUS frequencies (i.e. < 0.5 MHz), and therefore lower cavitation detection bandwidth, are necessary for transcranial FUS-induced BBB opening in humans [65], [66]. Finally, for clinical translatability, it is expected that patients will not be under anesthesia, causing a potential change in cavitation response in awake patients. It is well established that most general anesthetics cause vasodilation in patients [67], which could increase flow rates and microbubble perfusion in sonication regions. Additional research in awake animals is necessary to further understand the potential changes in cavitation dynamics with and without anesthesia. However, the findings in this study further support the importance of both cavitation monitoring and cavitation controlling to be able to produce safe, transient, and consistent BBB openings. Future studies optimizing the cavitation controller with lower frequencies in more relevant animal models are necessary for clinical translation.
Conclusion
FUS-induced BBB opening is widely known as a non-invasive technique for drug delivery to the brain. Histological and behavioral safety studies have shown that within a certain parametric window, FUS-induced BBB opening can be a safe and transient procedure. In recent years, safety studies have shifted toward utilizing transcriptomics and proteomics to evaluate the innate immune response to FUS-induced BBB opening. In this study, the inflammatory response induced by variable cavitation levels and different BBB permeability enhancement volumes were shown to be regulated through the associated cumulative cavitation dose levels. Cytokines responded to the inflammation induced by the opening of the BBB by secreting inflammatory activators as well as inhibitors to resolve the inflammation response by 72 h at the latest. The variations in cavitation level and BBB permeability enhancement volume can dictate the onset of increased cytokine expression and duration. Through comparison of the variable cavitation levels, we can identify possible genes of interest that respond to the smallest disruption and further elucidate the specific cell types and pathways that are affected immediately following sonication. Throughout the study, the parameters used were optimally designed for murine models, this work still highlights the importance and effectiveness of monitoring and controlling cavitation for producing transient and effective BBB openings in pre-clinical applications. Furthermore, depending on the application, controlling the cavitation dose with fixed sonication parameters can be used as to elicit different degrees of BBB openings and inflammatory response. Over the past two decades, many methods of evaluating safety after FUS-induced BBB opening have been proposed including MRI, immunohistochemistry, behavioral studies, and more recently transcriptomics, proteomics. Through all the studies, it has become increasingly clear that safety is an intricate and multifaceted metric that will need to be evaluated on a spectrum, where different outcomes will be assessed, and a cost-benefit analysis is required. As FUS-induced BBB opening transitions more into the clinics, solely relaying on using “one-size-fits-all” sonication parameters for treatment in humans will not be sufficient. The implementation of cavitation-based controller systems, coupled with patient specific pre-planning and targeting, will be paramount for safe and effective treatment of patients in the future, allowing for a precision medicine approach in the treatment of neurological diseases.
Supplementary Material
Acknowledgements
This study was funded by the National Institutes of Health (R01AG038961, R01EB029338, and R01EB009041). The authors wish to acknowledge the Molecular Pathology Shared Resource core at Columbia University, and specifically Dr. Tao Su, for assistance in processing and analyzing the qPCR results. The authors also wish to thank Dr. Antonios Pouliopoulos and Tara Kugelman for providing fruitful discussion in the writing of this manuscript.
Abbreviations
- AD
alzheimer’s disease
- ANOVA
analysis of variance
- BBB
blood-brain barrier
- CCD
cumulative cavitation dose
- CD
cavitation dose
- CNS
central nervous system
- Ct
cycle threshold
- DAMPs
damage-associated molecular patterns
- FFT
fast fourier transform
- FUS
focused ultrasound
- GBM
glioblastoma
- h
hours
- hCCD
high cumulative cavitation dose
- ICD
inertial cavitation dose
- IHC
immunohistochemistry
- IP
intraperitoneally
- lCCD
low cumulative cavitation dose
- MBs
microbubbles
- mCCD
medium cumulative cavitation dose
- MCP
monocyte chemotactic protein
- MPSR
molecular pathology shared resource
- MRI
magnetic resonance imaging
- PCD
passive cavitation detection
- PNP
peak negative pressure
- PRF
pulse repetition frequency
- PRR
pattern recognition receptors
- qPCR
quantitative polymerase chain reaction
- ROI
region of interest
- SCDh
harmonic stable cavitation dose
- SCDu
ultraharmonic stable cavitation dose
- SEM
standard error mean
Footnotes
Competing interests
The authors have declared that no competing interests exist.
References
- [1].Downs ME et al. , “Correction: Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task,” PLoS One, vol. 10, no. 6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jordão JF et al. , “Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound,” Exp. Neurol., vol. 248, pp. 16–29, October. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burgess A et al. , “Alzheimer Disease in a Mouse Model: MR Imaging–guided Focused Ultrasound Targeted to the Hippocampus Opens the Blood-Brain Barrier and Improves Pathologic Abnormalities and Behavior,” Radiology, vol. 273, no. 3, pp. 736–745, December. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Leinenga G and Gotz J, “Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer’s disease mouse model,” Sci Transl Med, vol. 7, no. 278, p. 278ra33, 2015. [DOI] [PubMed] [Google Scholar]
- [5].Pandit R, Leinenga G, and Götz J, “Repeated ultrasound treatment of tau transgenic mice clears neuronal tau by autophagy and improves behavioral functions,” Theranostics, vol. 9, no. 13, pp. 1–33, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karakatsani ME et al. , “Unilateral Focused Ultrasound-Induced Blood-Brain Barrier Opening Reduces Phosphorylated Tau from The rTg4510 Mouse Model,” Theranostics, vol. 9, no. 18, pp. 5396–5411, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Idbaih A et al. , “Safety and Feasibility of Repeated and Transient Blood–Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma,” Clin. Cancer Res, vol. 25, no. 13, pp. 3793–3801, July. 2019. [DOI] [PubMed] [Google Scholar]
- [8].Lipsman N et al. , “Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound,” Nat. Commun, vol. 9, no. 1, p. 2336, December. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pouliopoulos AN, Wu S-Y, Burgess MT, Karakatsani ME, Kamimura HAS, and Konofagou EE, “A Clinical System for Non-invasive Blood–Brain Barrier Opening Using a Neuronavigation-Guided Single-Element Focused Ultrasound Transducer,” Ultrasound Med. Biol, vol. 46, no. 1, pp. 73–89, January. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Todd N, Angolano C, Ferran C, Devor A, Borsook D, and McDannold N, “Secondary effects on brain physiology caused by focused ultrasound-mediated disruption of the blood–brain barrier,” J. Control. Release, vol. 324, no. 61, pp. 450–459, August. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dong H, Zhang X, and Qian Y, “Mast cells and neuroinflammation,” Med. Sci. Monit. Basic Res, vol. 20, pp. 200–206, December. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Samiotaki G and Konofagou EE, “Dependence of the reversibility of focused-ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control, vol. 60, no. 11, pp. 2257–2265, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tung Y-S, Vlachos F, Feshitan J. a., Borden M. a., and Konofagou EE, “The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice,” J. Acoust. Soc. Am, vol. 130, no. 5, p. 3059, November. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen H and Konofagou EE, “The Size of Blood–Brain Barrier Opening Induced by Focused Ultrasound is Dictated by the Acoustic Pressure,” J. Cereb. Blood Flow Metab, vol. 34, no. 7, pp. 1197–1204, July. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shin J et al. , “Focused ultrasound–mediated noninvasive blood-brain barrier modulation: preclinical examination of efficacy and safety in various sonication parameters,” Neurosurg. Focus, vol. 44, no. 2, p. E15, February. 2018. [DOI] [PubMed] [Google Scholar]
- [16].Samiotaki G, Vlachos F, Tung Y-S, and Konofagou EE, “A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI,” Magn. Reson. Med, vol. 67, no. 3, pp. 769–777, March. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Choi JJ et al. , “Microbubble-Size Dependence of Focused Ultrasound-Induced Blood–Brain Barrier Opening in Mice In Vivo,” IEEE Trans. Biomed. Eng, vol. 57, no. 1, pp. 145–154, January. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang S, Samiotaki G, Olumolade O, Feshitan JA, and Konofagou EE, “Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening,” Ultrasound Med. Biol, vol. 40, no. 1, pp. 130–137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McDannold N, Vykhodtseva N, and Hynynen K, “Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption,” Ultrasound Med. Biol, vol. 34, no. 6, pp. 930–937, June. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vlachos F, Tung Y-S, and Konofagou EE, “Permeability assessment of the focused ultrasound-induced blood–brain barrier opening using dynamic contrast-enhanced MRI,” Phys. Med. Biol, vol. 55, no. 18, pp. 5451–5466, September. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].O’Reilly MA, Jones RM, Barrett E, Schwab A, Head E, and Hynynen K, “Investigation of the safety of focused ultrasound-induced blood-brain barrier opening in a natural canine model of aging,” Theranostics, vol. 7, no. 14, pp. 3573–3584, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Baseri B et al. , “Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood–brain barrier using focused ultrasound and microbubbles,” Phys. Med. Biol, vol. 57, no. 7, pp. N65–N81, March. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Karakatsani ME et al. , “Amelioration of the nigrostriatal pathway facilitated by ultrasound-mediated neurotrophic delivery in early Parkinson’s disease,” J. Control. Release, vol. 303, pp. 289–301, June. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McMahon D, Oakden W, and Hynynen K, “Investigating the effects of dexamethasone on blood-brain barrier permeability and inflammatory response following focused ultrasound and microbubble exposure,” Theranostics, vol. 10, no. 4, pp. 1604–1618, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Downs ME et al. , “Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task,” PLoS One, vol. 10, no. 5, p. e0125911, May2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Olumolade OO, Wang S, Samiotaki G, and Konofagou EE, “Longitudinal Motor and Behavioral Assessment of Blood–Brain Barrier Opening with Transcranial Focused Ultrasound,” Ultrasound Med. Biol, vol. 42, no. 9, pp. 2270–2282, September. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ji R et al. , “Focused ultrasound enhanced intranasal delivery of brain derived neurotrophic factor produces neurorestorative effects in a Parkinson’s disease mouse model,” Sci. Rep, vol. 9, no. 1, p. 19402, December. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McMahon D, Bendayan R, and Hynynen K, “Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome,” Sci. Rep, vol. 7, p. 45657, April. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McMahon D and Hynynen K, “Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound is Dependent on Microbubble Dose,” Theranostics, vol. 7, no. 16, pp. 3989–4000, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kovacs ZI et al. , “Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation,” Proc. Natl. Acad. Sci, vol. 114, no. 1, pp. E75–E84, January. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marquet F et al. , “Real-time, transcranial monitoring of safe blood-brain barrier opening in non-human primates,” PLoS One, vol. 9, no. 2, p. e84310, February. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsai CH, Zhang JW, Liao YY, and Liu HL, “Real-time monitoring of focused ultrasound blood-brain barrier opening via subharmonic acoustic emission detection: Implementation of confocal dual-frequency piezoelectric transducers,” Phys. Med. Biol, vol. 61, no. 7, pp. 2926–2946, 2016. [DOI] [PubMed] [Google Scholar]
- [33].Sun T, Samiotaki G, Wang S, Acosta C, Chen CC, and Konofagou EE, “Acoustic cavitation-based monitoring of the reversibility and permeability of ultrasound-induced blood-brain barrier opening,” Phys. Med. Biol, vol. 60, no. 23, pp. 9079–9094, December. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sun T et al. , “Closed-loop control of targeted ultrasound drug delivery across the blood–brain/tumor barriers in a rat glioma model,” Proc. Natl. Acad. Sci, vol. 114, no. 48, pp. E10281–E10290, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McDannold N et al. , “Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model,” Theranostics, vol. 9, no. 21, pp. 6284–6299, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Choi JJ, Pernot M, Small SA, and Konofagou EE, “Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice,” Ultrasound Med. Biol, vol. 33, no. 1, pp. 95–104, January. 2007. [DOI] [PubMed] [Google Scholar]
- [37].Wu S-Y, Chen CC, Tung Y-S, Olumolade OO, and Konofagou EE, “Effects of the microbubble shell physicochemical properties on ultrasound-mediated drug delivery to the brain,” J. Control. Release, vol. 212, pp. 30–40, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].O’Reilly MA and Hynynen K, “Blood-brain barrier: Real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller,” Radiology, vol. 263, no. 1, pp. 96–106, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kamimura HA et al. , “Feedback control of microbubble cavitation for ultrasound-mediated blood–brain barrier disruption in non-human primates under magnetic resonance guidance,” J. Cereb. Blood Flow Metab, p. 0271678X1775351, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Livak KJ and Schmittgen TD, “Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method,” Methods, vol. 25, no. 4, pp. 402–408, December. 2001. [DOI] [PubMed] [Google Scholar]
- [41].McDannold N, Zhang Y, and Vykhodtseva N, “The Effects of Oxygen on Ultrasound-Induced Blood–Brain Barrier Disruption in Mice,” Ultrasound Med. Biol, vol. 43, no. 2, pp. 469–475, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McDannold N, Vykhodtseva N, and Hynynen K, “Targeted disruption of the blood-brain barrier with focused ultrasound: Association with inertial cavitation,” Proc. - IEEE Ultrason. Symp, vol. 2, pp. 1249–1252, 2005. [DOI] [PubMed] [Google Scholar]
- [43].Chu PC, Chai WY, Tsai CH, Kang ST, Yeh CK, and Liu HL, “Focused Ultrasound-Induced Blood-Brain Barrier Opening: Association with Mechanical Index and Cavitation Index Analyzed by Dynamic Contrast-Enhanced Magnetic-Resonance Imaging,” Sci. Rep, vol. 6, no. May, pp. 1–13, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Samiotaki G, Acosta C, Wang S, and Konofagou EE, “Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood-brain barrier opening in vivo.,” J. Cereb. Blood Flow Metab, vol. 35, no. October 2014, pp. 611–22, January. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McDannold N, Vykhodtseva N, and Hynynen K, “Targeted disruption of the blood-brain barrier with focused ultrasound: Association with inertial cavitation,” Proc. - IEEE Ultrason. Symp, vol. 2, no. 4, pp. 1249–1252, February. 2005. [DOI] [PubMed] [Google Scholar]
- [46].Tung YS, Vlachos F, Choi JJ, Deffieux T, Selert K, and Konofagou EE, “In vivo transcranial cavitation detection during ultrasound-inducedblood- brain barrier opening,” Proc. - IEEE Ultrason. Symp, vol. 55, no. 20, pp. 1518–1521, October. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hwang JH, Tu J, Brayman AA, Matula TJ, and Crum LA, “Correlation between inertial cavitation dose and endothelial cell damage in vivo,” Ultrasound Med. Biol, vol. 32, no. 10, pp. 1611–1619, October. 2006. [DOI] [PubMed] [Google Scholar]
- [48].Cameron MJ and Kelvin DJ, “Cytokines, Chemokines and Their Receptors,” in Landes Bioscience, Austin. [Google Scholar]
- [49].Palomino DCT and Marti LC, “Chemokines and immunity.,” Einstein (Sao Paulo), vol. 13, no. 3, pp. 469–473, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sokol CL and Luster AD, “The chemokine system in innate immunity.,” Cold Spring Harb. Perspect. Biol, vol. 7, no. 5, January. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Turner MD, Nedjai B, Hurst T, and Pennington DJ, “Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease,” Biochim. Biophys. Acta - Mol. Cell Res, vol. 1843, no. 11, pp. 2563–2582, 2014. [DOI] [PubMed] [Google Scholar]
- [52].Burmeister AR and Marriott I, “The Interleukin-10 Family of Cytokines and Their Role in the CNS,” Front. Cell. Neurosci, vol. 12, no. November, pp. 1–13, November. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hughes CE and Nibbs RJB, “A guide to chemokines and their receptors.,” FEBS J, vol. 285, no. 16, pp. 2944–2971, August. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Maddaluno M et al. , “Monocyte chemotactic protein-3 induces human coronary smooth muscle cell proliferation,” Atherosclerosis, vol. 217, no. 1, pp. 113–119, 2011. [DOI] [PubMed] [Google Scholar]
- [55].Deshmane SL, Kremlev S, Amini S, and Sawaya BE, “Monocyte chemoattractant protein-1 (MCP-1): An overview,” J. Interf. Cytokine Res, vol. 29, no. 6, pp. 313–325, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].McMahon D, Lassus A, Gaud E, Jeannot V, and Hynynen K, “Microbubble formulation influences inflammatory response to focused ultrasound exposure in the brain,” Sci. Rep, vol. 10, no. 1, p. 21534, December. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mathew AS et al. , “Transcriptomic response of brain tissue to focused ultrasound-mediated blood–brain barrier disruption depends strongly on anesthesia,” Bioeng. Transl. Med, no. October, pp. 1–14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Curley CT et al. , “Immunomodulation of intracranial melanoma in response to blood-tumor barrier opening with focused ultrasound,” Theranostics, vol. 10, no. 19, pp. 8821–8833, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mathew AS, Gorick CM, and Price RJ, “Single-cell mapping of focused ultrasound-transfected brain,” Gene Ther, February. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gorick CM et al. , “Sonoselective transfection of cerebral vasculature without blood–brain barrier disruption,” Proc. Natl. Acad. Sci, vol. 117, no. 11, pp. 5644–5654, March. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kovacs ZI, Burks SR, and Frank JA, “Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions,” Theranostics, vol. 8, no. 8, pp. 2245–2248, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lapin NA, Gill K, Shah BR, and Chopra R, “Consistent opening of the blood brain barrier using focused ultrasound with constant intravenous infusion of microbubble agent,” Sci. Rep, vol. 10, no. 1, p. 16546, December. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, and Hynynen K, “Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles,” Ultrasound Med. Biol, vol. 30, no. 7, pp. 979–989, July. 2004. [DOI] [PubMed] [Google Scholar]
- [64].Sheikov N, McDannold N, Sharma S, and Hynynen K, “Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium,” Ultrasound Med. Biol, vol. 34, no. 7, pp. 1093–1104, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wu S-Y et al. , “Transcranial cavitation detection in primates during blood-brain barrier opening-a performance assessment study,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control, vol. 61, no. 6, pp. 966–978, June. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jones RM, O’Reilly MA, and Hynynen K, “Experimental demonstration of passive acoustic imaging in the human skull cavity using CT-based aberration corrections,” Med. Phys, vol. 42, no. 7, pp. 4385–4400, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Akata T and Warltier DC, “General Anesthetics and Vascular Smooth Muscle,” Anesthesiology, vol. 106, no. 2, pp. 365–391, February. 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


