Abstract
Bone stress injuries (BSIs) occur in up to 20% of runners and military recruits and those with a history of BSI have a 5-fold higher risk for a subsequent BSI. Yet, little is known about prior training, menstrual status and bone structure in runners who experience multiple BSIs.
PURPOSE:
To determine differences in health and physical activity history, bone density, microarchitecture, and strength among female athletes with a history of multiple BSI, athletes with ≤1 BSI, and non-athletes.
METHODS:
We enrolled 101 women (ages 18–32 years) for this cross-sectional study: non-athlete controls (n=17) and athletes with a history of ≥ 3 BSIs (n=21) or ≤1 BSI (n=63). We collected subjects’ health and training history and measured bone microarchitecture of the distal tibia via high-resolution peripheral quantitative computed tomography (HR-pQCT) and areal bone mineral density (aBMD) of the hip and spine by dual-energy X-ray absorptiometry (DXA).
RESULTS:
Groups did not differ according to age, BMI, age at menarche, aBMD, or tibial bone microarchitecture. Women with multiple BSIs had a higher prevalence of primary and secondary amenorrhea (p<0.01) compared to other groups. Total hours of physical activity in middle school were similar across groups; however, women with multiple BSIs performed more total hours of physical activity in high school (p=0.05), more hours of uniaxial loading in both middle school and high school (p=0.004, p=0.02) and a smaller proportion of multiaxial loading activity compared to other groups.
CONCLUSION:
These observations suggest that participation in sports with multiaxial loading and maintaining normal menstrual status during adolescence and young adulthood may reduce the risk of multiple bone stress injuries.
Keywords: Runners, overuse injury, stress fracture, bone health, women, amenorrhea, BMD
INTRODUCTION
Lower extremity bone stress injuries (BSIs) are a common form of overuse injury in active individuals. Women are particularly at risk for BSI, with incidence rates of up to 20% among runners and military cadets (1, 2). Further, individuals who previously sustained a BSI have a nearly five-fold higher risk of sustaining a future BSI compared to those with no history of BSI (3), BSIs were recurrent in 21.5% and 10.6% of NCAA athletes and military recruits, respectively (4, 5). Multiple BSIs are particularly devastating to an athletic or military career given that rehabilitation timelines generally range from 4–12 weeks for return to activity, depending on injury site and severity (4, 6). Further, it is recommended that individuals sustaining multiple BSIs receive a more extensive clinical workup, including testing of bone mineral density (7).
Risk factors for BSI include those that result from low energy availability (EA). Low EA results from under-fueling relative to exercise energy expenditure, leading to inadequate energy to support physiological functions necessary to maintain optimal health and performance. This can lead to low estrogen and menstrual dysfunction (primary or secondary amenorrhea), which are associated with reduced bone density, deleterious bone microarchitecture and significantly increased risk of BSIs (7, 8). Although the simultaneous prevalence of low EA, menstrual dysfunction and impaired bone health is modest (0 to 16%), 16–60% of all female athletes report having at least one of these three BSI risk factors (9).
Poor bone health is thought to contribute to BSI risk, though prior studies report inconsistent findings regarding the contribution of low aBMD to BSI risk (3, 10–15), perhaps because two-dimensional DXA measurements do not provide information about bone geometry or microarchitecture and can be confounded by body size (16). Factors beyond aBMD, including bone geometry, microarchitecture and volumetric BMD (vBMD) are important in assessing bone strength and can be captured using three-dimensional (3D) bone imaging techniques such as peripheral quantitative computed tomography (pQCT) and high resolution pQCT (HR-pQCT). A recent systematic review concluded that bone geometry and structure are largely similar between athletes/trainees with and without a history of BSI (17). However, many studies in this review did not take into account history of menstrual dysfunction and none specifically evaluated women with a history of multiple BSIs, who may have more pronounced or different risk factors (17).
Physical activity history may also influence the risk for multiple BSI. It is well established that weightbearing physical activity has beneficial effects on bone (18–21). High-impact exercise, such as jumping, increases BMD over time in children and adolescents (22), and has lasting effects on bone mass, bone size and bone strength (23, 24). Multiaxial loading, defined as lower extremity loading that applies forces across multiple planes of motion, may be particularly beneficial and protective against BSI, as military recruits who play ball sports prior to Basic Combat Training have significantly lower incidence of BSI compared to those who do not play ball sports (25). Further, athletes who participate in multidirectional sports have more favorable bone density and geometry compared to runners (26, 27).
Altogether there are limited data on risk factors for multiple BSIs, including whether physical activity patterns, menstrual history during bone acquisition, current menstrual history, and/or skeletal health distinguish individuals with a history of multiple BSIs. Thus, we aimed to identify factors that are associated with a history of multiple BSI in young adult female athletes. We hypothesized that athletes would have better bone properties compared to non-athletes, and that athletes with multiple BSIs would have worse bone properties than other groups. Further, we hypothesized that athletes with multiple BSIs would have a higher prevalence of menstrual dysfunction and less multiaxial physical activity compared to other groups.
METHODS
Participant Selection
We recruited participants from the community using flyers and advertisements placed on institutional research recruitment websites, Facebook, and Craigslist. We enrolled 101 women (ages 18–32 years) for this cross-sectional study: non-athlete controls (n=17) and athletes with a history of ≥ 3 (n=21), 1 (n=23), or 0 (n=40) lower extremity BSIs. Athletes that reported only 2 BSIs (n=22) were not eligible to participate in the study. Participants in the athlete groups were defined as those who report a minimum of 15 miles per week of running or perform an average of ≥4 hours per week of other weightbearing endurance exercise (e.g., lacrosse) over the past six months and report consistent activity in the three months prior to enrollment. This activity could be self-directed exercise or part of organized sport participation. Participants in the BSI groups were required to be at least twelve months out from their most recent BSI diagnosis. Lower extremity BSIs were defined those occurring at or distal to the femoral diaphysis. BSIs in other locations (e.g. sacrum and femoral neck) were not exclusionary if participants in the multiple BSI group had at least 3 additional lower extremity BSIs. All BSI were self-reported to be medically diagnosed using imaging. Non-athlete control participants performed less than two hours per week of any type of physical activity over the 12 months prior to enrollment.
We excluded potential participants with a self-reported history of thyroid dysfunction, cancer, medically diagnosed eating disorder, or use of medications known to affect bone (glucocorticoids, bisphosphonates, chemotherapeutic agents). This study was approved by the Institutional Review Board of Partners Health Care and the Human Research Protection Office at the US Army Medical Research and Development Command, Ft. Detrick, MD. All participants provided written informed consent at the time of enrollment.
Data Collection
Participants completed questionnaires to obtain demographic information, menstrual history, current and previous physical activity, and previous injuries. A positive fracture history did not include fractures to digits or the skull. The current menstrual status of participants was determined to be amenorrhoeic if they reported fewer than 3 menses in the last year or cessation of menstruation for three or more consecutive months; oligo-amenorrhoeic if they reported 4–8 menses in the last year; eumenorrheic if they reported 9 or more menses in the last year; and hormonal contraceptive user if they indicated that they currently used hormonal contraceptives. If participants reported an age of menarche of > 15 years old (primary amenorrhea) or ever having fewer than 3 menses in one year from age 15 or older (secondary amenorrhea), they were recorded as having a positive history of amenorrhea. Self-reported physical activity participation data during ages 11–14 years (middle school) and 15–18 years (high school) was collected via questionnaire. Participants were asked to recall all activities they performed in each time period and the average hours per week, months per year, and number of years they spent on each activity per time period. The total number of hours for each activity was then calculated for middle and high school (28). Each activity was characterized as a multiaxial, uniaxial, or non-weightbearing activity. Multiaxial activities were identified as those that include cutting or lower extremity loading that applies forces across multiple planes of motion. In this cohort, multiaxial activities included baseball/softball, basketball, cheer, dance, field hockey, football, gymnastics, ice hockey, ice skating, lacrosse, martial arts, soccer, tennis, volleyball, weight lifting, and yoga. Uniaxial activities were identified as those with repetitive loading primarily in one direction, and included running and rowing. Non-weightbearing activities included cycling and swimming. The percent multiaxial activity was calculated for each time period, as well as the absolute difference between the percent multiaxial activity in middle and high school as a reflection of changes in activity pattern.
Height was measured using a wall-mounted stadiometer to the nearest centimeter and weight was measured with an electronic scale to the nearest tenth of a pound. Tibia length was measured from the medial tibial plateau to the distal edge of the medial malleolus with an anthropomorphic tape measure to the nearest millimeter. Fasting morning blood was drawn and an immunochemiluminometric assay was used to measure 25(OH) vitamin D (Labcorp; sensitivity 4.0 ng/mL; intra-assay CV 4.8%–7.7%).
Areal Bone Mineral Density
We measured aBMD (g/cm2) at the posterior-anterior (PA) spine, total hip (TH), and femoral neck (FN) via dual-energy X-ray absorptiometry (DXA; QDR4500A; Hologic, Inc., Bedford, MA, USA). Scans were reviewed by a physician-investigator with experience in interpreting DXA results. Quality control scans were performed daily with a phantom. BMD values were converted to Z-scores that standardize BMD to age, sex and ethnicity normative values. Participants with a Z-score of less than −1.0 at any measured skeletal site were categorized as having low BMD (29).
Volumetric Bone Mineral Density and Microarchitecture
We measured trabecular and cortical volumetric BMD (vBMD) and microarchitecture of the ultradistal tibia using HR-pQCT (XtremeCT, Scanco Medical AG, Brutisellen, Switzerland; isotropic voxel size of 82 μm). Starting at 4% of tibial length (distal), the scan region extended proximally for 110 slices (9.02 mm). We scanned the non-dominant tibia unless the participant reported a previous fracture in that leg, in which case the dominant leg was scanned. Leg dominance was determined by asking participants which leg they would use to kick a soccer ball.
The manufacturer’s software (Scanco Medical AG, version 5.11) was used to evaluate total (Tt), cortical (Ct) and trabecular (Tb) area (mm2) and vBMD (mgHA/cm3), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, 1/mm), and trabecular separation (Tb.Sp, mm), and cortical tissue mineral density (Ct.TMD, mgHA/cm3), thickness (Ct.Th, mm), perimeter (Ct.Pm, mm), and porosity (Ct.Po, %). Bone robustness was calculated by dividing total cross-sectional area (mm) by tibia length (mm) (30, 31). Linear micro-finite element analysis (μFEA) was performed using three-dimensional HR-pQCT images to approximate metaphyseal stiffness and failure load of the tibia under axial compression (32, 33). The manufacturer’s phantom was scanned daily for quality control. A technician reviewed all scans for motion artifact and repeated the scan up to two times if the scan was significantly affected by movement. Short-term reproducibility for measurements at the tibia is 0.2 to 1.7% for density parameters, 0.7 to 8.6% for microarchitecture parameters, and 2.1 to 4.8% for μFEA parameters.
Statistical Analysis
A priori, we intended to report comparisons between four groups (athletes with ≥ 3, 1, or 0 BSI history and non-athletes). The 0 (n=40) and 1 BSI (n=23) groups were found to be equivalent for all outcomes (data not shown), thus we combined these groups to form a ≤1 lower extremity BSI group (n=63). Data are reported as mean (SD) or percent (n) unless otherwise noted. We used analysis of variance (ANOVA) to determine differences between groups for continuous outcome variables (age, height, weight, BMI, tibia length, DXA Z-scores, HR-pQCT bone parameters, and historical physical activity parameters). There were no violations of the ANOVA assumptions among these variables. We used analysis of covariance (ANCOVA) to assess differences between groups in bone parameters adjusted for age and BMI. A Tukey-Kramer test was used for pairwise comparisons when results of the ANOVA or ANCOVA reached statistical significance. Fisher’s exact test was used to determine differences between groups for categorical variables (fracture history, age of menarche, current menstrual status, history of amenorrhea, and low BMD). Pairwise comparisons were performed when results of the Fisher’s exact test reached statistical significance. When comparing variables specific to our two athlete groups (serum 25-OH vitamin D, current average hours per week of weightbearing endurance exercise, age of first BSI, and time since most recent BSI), we used paired Student’s t-test . To test for a trend across groups in historical physical activity data, Spearman’s rank-order correlation was used. All analyses were performed in R version 4.0.2, and an alpha of 0.05 was used to determine statistical significance.
RESULTS
Participant Characteristics
Participants were 25.6 ± 4.1 years old and had a mean body mass index (BMI) of 22.0 ± 2.4 kg/m2. Participants with a history of BSI were 4.0 ± 3.5 years post-injury (range 1–18 years) at the time of enrollment. Non-athletes were shorter than participants with ≥ 3 BSIs (p=0.02) and had greater BMI than participants in both BSI groups (p<0.05 for both). There were no differences in lowest ever reported BMI, tibia length, full fracture history, smoking status, or serum 25-hydroxy vitamin D (25-OH Vit D) levels among groups. Current hours per week of weightbearing endurance exercise differed among groups, but this difference was the result of the low number of hours reported by healthy non-athlete group; no significant differences were seen when comparing the athlete groups alone. Women with ≥ 3 BSIs reported an average of 4.2 ± 1.5 BSIs per person (89 total, range 3–7 per subject). Women with ≥ 3 BSI experienced their first BSI significantly earlier than those with ≤1 BSI (16.4 ± 3.7 versus 21.1 ± 4.9 years of age, p=0.0005). The primary site of injury in both BSI groups was the tibia; the second-most common site was the metatarsals in the ≥ 3 BSI group, whereas it was the fibula in the ≤1 BSI group (Table 1).
Table 1.
Demographics and group characteristics in non-athletes, athletes with a history of ≤1 BSI, and athletes with a history of ≥ 3 BSIs. Data are provided in mean (SD) or n (%).
| Non-Athletes (n=17) | ≤ 1 BSI (n=63) | ≥ 3 BSI (n=21) | p-value | |
|---|---|---|---|---|
| Age | 24.2 (1.9) | 26.3 (4.6) | 24.7 (3.5) | 0.10 |
| Height (cm) | 162.1 (6.2) | 164.9 (5.8) | 167.2 (5.5)* | 0.03 |
| Weight (kg) | 61.7 (8.7) | 59.3 (6.4) | 60.2 (6.9) | 0.44 |
| BMI (kg/m2) | 23.4 (2.3) | 21.8 (2.5)* | 21.5 (2.2)* | 0.03 |
| Lowest ever reported BMI | N/A | 19.8 (1.9) | 19.3 (2.5) | 0.35 |
| Tibia Length | 358.5 (28.4) | 363.2 (19.8) | 372.3 (22.1) | 0.13 |
| Fracture History | 4 (23.5%) | 18 (28.6%) | 10 (47.6%) | 0.22 |
| Serum 25-OH Vit D (ng/mL) | N/A | 34.3 (10.8) | 35.2 (11.3) | 0.75 |
| Current average hours/week of exercise | † | |||
| Multiaxial | 0.0 (0.0) | 3.2 (3.1) | 3.3 (5.0) | 0.90† |
| Uniaxial | 0.0 (0.0) | 6.0 (4.0) | 6.8 (4.7) | 0.49† |
| Non-weightbearing | 0.0 (0.0) | 1.1 (2.8) | 1.2 (2.4) | 0.89† |
| Age of first BSI (years) | 21.1 (4.9) | 16.4 (3.7) | < 0.01 | |
| Time since most recent BSI (years) | 4.5 (3.7) | 3.4 (3.2) | 0.09 | |
| Average number of BSI per participant | 0.4 (0.5)‡ | 4.2 (1.5) | ||
| BSI location§ | 23 (100%) | 89 (100%) | ||
| Femur diaphysis | 2 (8.7%) | 3 (3.4%) | ||
| Tibia | 8 (34.8%) | 36 (40.5%) | ||
| Fibula | 6 (26.1%) | 13 (14.2%) | ||
| Metatarsal | 5 (21.7%) | 32 (36.0%) | ||
| Calcaneus | 1 (4.4%) | 1 (1.1%) | ||
| Cuboid | 0 (0.0%) | 1 (1.1%) | ||
| Sesamoid | 1 (4.4%) | 2 (2.3%) | ||
| Sacrum | 0 (0.0%) | 2 (2.3%) |
Group was significantly different from non-athletes in pairwise comparison
Test was performed with athlete groups only
23 out of the 63 participants reported a history of 1 BSI
BSI in the sacrum did not count towards eligibility criteria but were not exclusionary for those with ≥ 3 BSIs
Age of menarche and current menstrual status were similar among groups. Women with ≥ 3 BSIs had a higher prevalence of amenorrhea compared to both those with ≤1 BSI (p=0.04) and non-athletes (p=0.03; Table 2). Interestingly, among women with ≥ 3 BSIs who reported a history of amenorrhea, 40% reported current menstrual dysfunction compared to 25% of women with ≤1 BSI, and 0% of non-athletes.
Table 2.
Menstrual history in non-athletes, athletes with a history of ≤1 BSI, and athletes with a history of ≥ 3 BSIs. Data are provided in n (%).
| Non-Athletes (n=17) | ≤ 1 BSI (n=63) | ≥ 3 BSI (n=21) | p-value | |
|---|---|---|---|---|
| Age of Menarche | 0.13 | |||
| <10 years old | 0 (0.0%) | 0 (0.0%) | 1 (4.8%) | |
| 10–12 years old | 3 (21.4%) | 29 (46.0%) | 6 (28.6%) | |
| 13–15 years old | 10 (71.4%) | 31 (49.2%) | 11 (52.4%) | |
| >15 years old | 1 (7.1%) | 3 (4.8%) | 3 (14.3%) | |
| Not reported | 3 (21.4%) | 0 (0.0%) | 0 (0.0%) | |
| Current Menstrual Status | 0.49 | |||
| Hormonal contraceptive user | 10 (58.8%) | 37 (59.7%) | 9 (42.9%) | |
| Eumenorrheic | 7 (41.2%) | 20 (32.3%) | 8 (38.1%) | |
| Oligo-amenorrhoeic | 0 (0.0%) | 4 (6.5%) | 3 (14.3%) | |
| Amenorrhoeic | 0 (0.0%) | 1 (1.6%) | 1 (4.8%) | |
| History of amenorrhea | 1 (5.9%)* | 12 (19.0%)* | 10 (47.6%) | < 0.01 |
Group was significantly different from ≥3 BSI group in pairwise comparison
All groups had similar average aBMD Z-scores by DXA at the FN, TH, and PA spine (Table 3). However, a greater proportion of women in the ≥ 3 BSI group had low bone density (Z-score < −1.0) at the PA spine compared to women with ≤1 BSI (p=0.04). The groups had similar tibial density, microarchitecture, and strength parameters by HR-pQCT in both an unadjusted model and after adjusting for age and BMI. Bone robustness was also similar across groups.
Table 3.
Bone density, size, morphology, microarchitecture, and strength in non-athletes, athletes with a history of ≤1 BSI, and athletes with a history of ≥ 3 BSIs. Data provided are mean (SD) or n (%).
| Non-Athletes (n=17) | ≤ 1 BSI (n=63) | ≥ 3 BSI (n=21) | p-value | p-value, adjusted† | |
|---|---|---|---|---|---|
| DXA Z-score | |||||
| Femoral Neck | 0.22 (1.69) | 0.09 (0.91) | −0.08 (0.82) | 0.69 | |
| Total Hip | 0.42 (1.17) | 0.23 (0.81) | 0.05 (0.92) | 0.46 | |
| PA Spine | −0.19 (1.06) | −0.29 (0.85) | −0.55 (1.31) | 0.49 | |
| DXA Z-score < −1.0 | |||||
| Femoral Neck | 4 (23.5%) | 7 (11.1%) | 3 (14.3%) | 0.43 | |
| Total Hip | 0 (0.0%) | 2 (3.2%) | 2 (9.5%) | 0.29 | |
| PA Spine | 4 (23.5%) | 10 (15.9%)* | 9 (42.9%) | 0.04 | |
| Any site | 5 (29.4%) | 14 (22.2%)* | 10 (47.6%) | 0.08 | |
| Density | |||||
| Tt.vBMD (mgHA/cm3) | 273 (50) | 274 (42) | 266 (39) | 0.74 | 0.61 |
| Tb.vBMD (mgHA/cm3) | 207 (39) | 210 (33) | 201 (28) | 0.58 | 0.36 |
| Ct.vBMD (mgHA/cm3) | 872 (34) | 866 (44) | 875 (40) | 0.56 | 0.54 |
| Ct.TMD (mgHA/cm3) | 931 (29) | 929 (32) | 927 (32) | 0.93 | 0.92 |
| Size/Morphology | |||||
| Tt.Ar (mm2) | 858 (101) | 839 (111) | 833 (85) | 0.75 | 0.70 |
| Ct.Ar (mm2) | 93.3 (16.8) | 88.6 (13.7) | 86.0 (16.8) | 0.31 | 0.72 |
| Tb.Ar (mm2) | 769 (107) | 754 (112) | 752 (89) | 0.86 | 0.76 |
| Ct.Ar/Tt.Ar (%) | 11.1 (2.8) | 10.7 (2.2) | 10.4 (2.4) | 0.71 | 0.97 |
| Ct.Pm (mm) | 118.7 (8.6) | 117.5 (7.8) | 117.3 (7.1) | 0.83 | 0.75 |
| Robustness (mm) | 2.40 (0.32) | 2.31 (0.31) | 2.24 (0.24) | 0.25 | 0.23 |
| Microarchitecture | |||||
| Ct.Th (mm) | 0.84 (0.19) | 0.79 (0.14) | 0.78 (0.18) | 0.46 | 0.83 |
| Ct.Po (%) | 4.46 (1.59) | 4.78 (1.70) | 3.88 (1.01) | 0.08 | 0.16 |
| Tb.Th (mm) | 0.077 (0.012) | 0.084 (0.013) | 0.081 (0.012) | 0.17 | 0.08 |
| Tb.Sp (mm) | 0.375 (0.048) | 0.398 (0.052) | 0.408 (0.061) | 0.17 | 0.36 |
| Tb.N (1/mm) | 2.23 (0.24) | 2.10 (0.23) | 2.08 (0.27) | 0.10 | 0.23 |
| μFEA | |||||
| Stiffness (kN/mm) | 217.8 (42.7) | 216.7 (35.8) | 208.8 (32.9) | 0.66 | 0.50 |
| Failure Load (kN) | 111.3 (20.0) | 109.8 (16.8) | 105.9 (15.5) | 0.58 | 0.54 |
p-value adjusted for age and BMI
Group was significantly different from ≥3 BSI group in pairwise comparison
Cumulative hours of non-weightbearing, uniaxial, and multiaxial physical activity for middle and high school are shown in Figure 1. There were no between-group differences in total hours of physical activity in middle school (p=0.21) However, athletes with ≥ 3 BSI reported more total hours of physical activity in high school compared non-athletes (p=0.05), with and a similar trend of more total hours of physical activity in high school among athletes with ≤ 1 BSI compared to non-athletes (p=0.08). Athletes with ≥ 3 BSIs performed more uniaxial loading activity than non-athletes (p=0.02) and ≤1 BSI athletes (p=0.005) in middle school, and more than the non-athletes (p=0.01) in high school. Though the amount of multiaxial activity was similar across groups in middle and high school (p=0.83 and p=0.30, respectively), the proportion of multiaxial activity relative to total physical activity (Figure 2) differed significantly among groups in high school. Specifically, athletes in the ≥ 3 BSI group performed the lowest proportion of multiaxial activity in high school, whereas non-athletes performed the highest proportion (ρ = −0.25, p=0.01). There was not a statistically significant difference in the proportion of multiaxial activity between groups in middle school.
Figure 1.
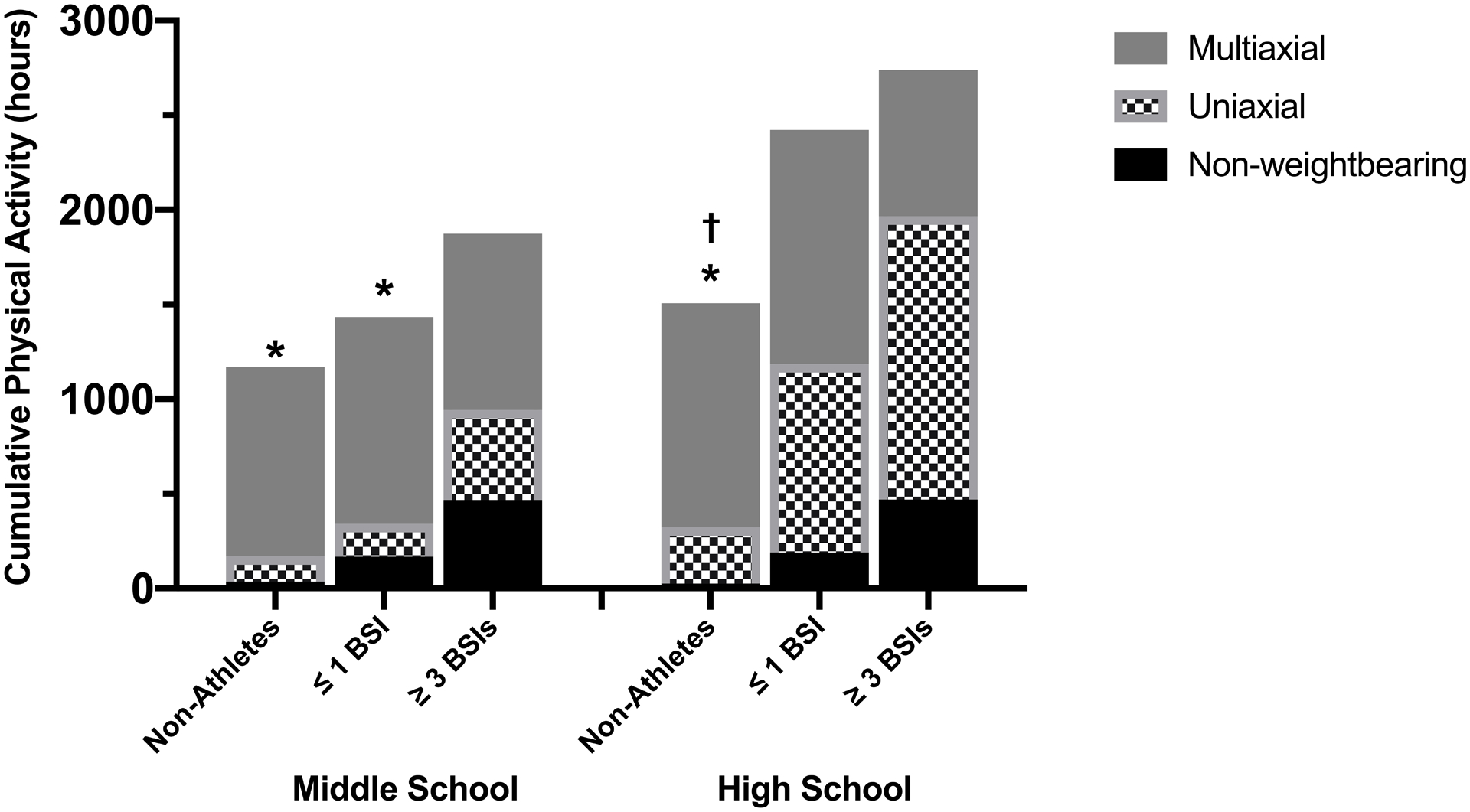
Mean cumulative hours spent participating in multiaxial, uniaxial, and non-weightbearing activity during middle school (left) and high school (right) for non-athletes and runners with a history of ≤ 1 or ≥ 3 BSIs. †p<0.05 compared to ≥ 3 BSI group for total hours physical activity. *p<0.05 compared to ≥ 3 BSI group for uniaxial physical activity.
Figure 2.
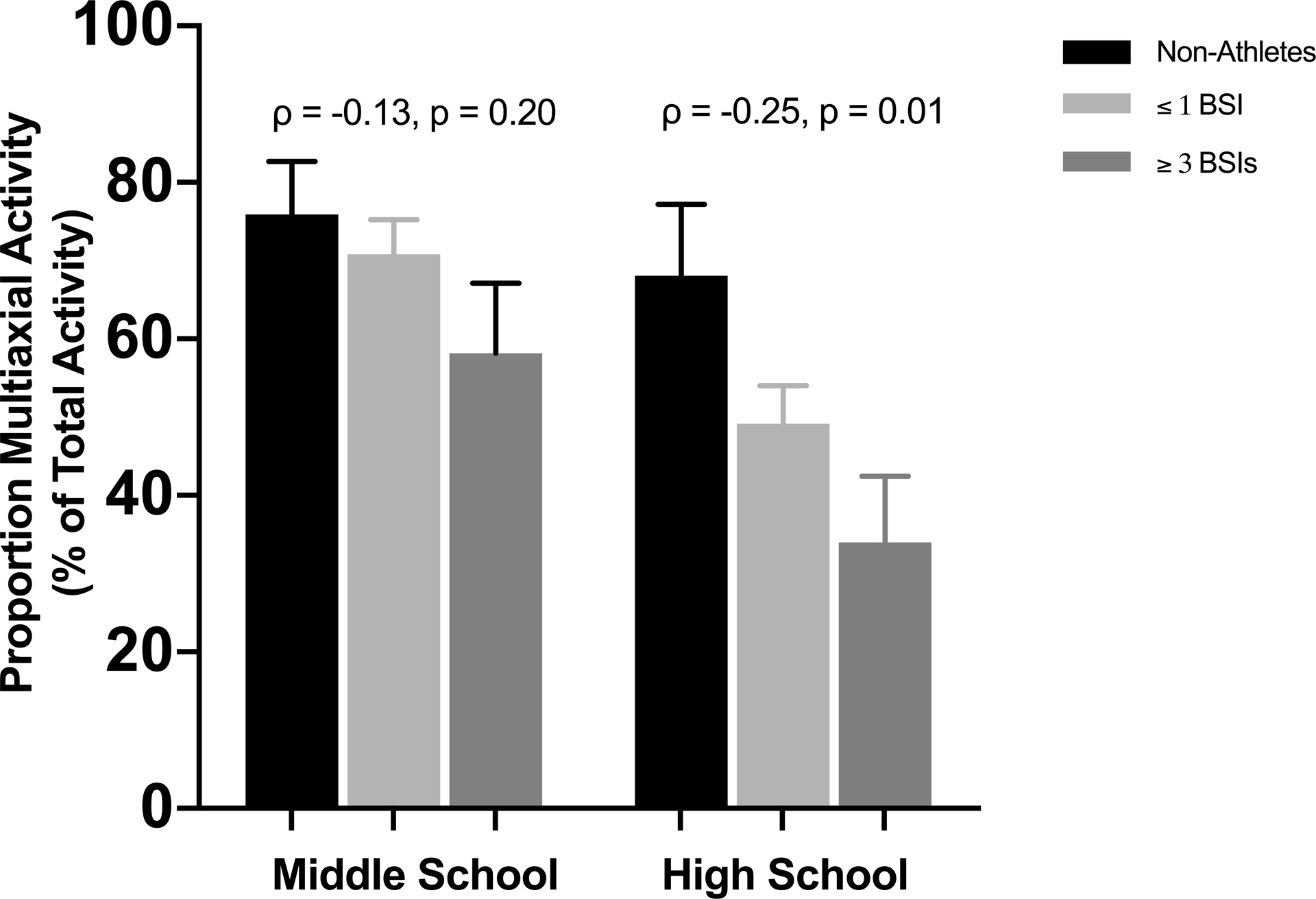
Percent of total activity that is multiaxial for middle school (left) and high school (right) for non-athletes and athletes with a history of ≤ 1 or ≥ 3 BSIs. Spearman correlation coefficients (ρ) and p-values are shown above each time period.
DISCUSSION
To address a lack of information about factors contributing to the occurrence of multiple BSIs, we investigated bone density, microarchitecture and strength as well as medical and physical activity history in female athletes with and without a history of BSIs and in non-athletic controls. We found that bone parameters by HR-pQCT did not differ by group, though a significantly larger proportion of athletes with ≥ 3 BSIs had low aBMD at the spine. Women who had suffered ≥ 3 BSIs were more likely to have experienced primary or secondary amenorrhea. Additionally, women who suffered ≥ 3 BSIs experienced their first BSI at a younger age, namely during high school, whereas women with history of ≤ 1 BSI were more likely to report injury as a young adult. Finally, athletes with ≥ 3 BSIs reported greater historical uniaxial loading activity and the least participation in multiaxial activity during childhood and adolescence.
We found that a greater proportion of women with a history of ≥ 3 BSIs had low aBMD defined as an aBMD Z-score of <−1 at the spine. While a Z-score of −1 is clinically “normal”, athletes in weight-bearing sports typically have 5–15% higher BMD than nonathletes, suggesting a Z-score < −1 warrants further consideration (29). Results from prior studies assessing the relationship between aBMD and BSI are inconsistent. Part of the difficulty in interpreting results from the existing body of literature stems from the fact that many athletes and military recruits who sustain a BSI tend to have clinically “normal” aBMD (3, 10, 14, 34, 35). Furthermore, even when comparing athletic populations findings are inconsistent, with some reporting lower aBMD among those with a history of BSI compared to those without (11–13, 36) and some showing no difference. (10, 14, 15, 34, 37). Altogether, because of the expected higher BMD among weight-bearing athletes, our findings support the notion that BMD Z-scores <−1 are clinically important, particularly when considering risk for multiple BSIs.
Our findings of no difference in bone microarchitecture, strength or robustness by BSI history are consistent with a recent systematic review reporting that bone geometry outcomes assessed by DXA and pQCT do not differ between those with and without a history of BSI, particularly at the ultradistal tibia (17). The few studies that have assessed bone microarchitecture in female athletes with BSI also suggest that bone microarchitecture and strength are largely similar in athletes with a history of BSI compared to athletic controls (13, 38). In the only study that has assessed differences in bone microarchitecture in female athletes with multiple BSIs, among amenorrhoeic, adolescent athletes, those with a history of two or more BSIs had compromised bone microarchitecture and strength at the tibia compared to those with fewer than two BSIs (38, 39). It is worth noting that the cohort in our study was comprised of adult athletes, most of whom were using hormonal contraceptives or had regular menses at the time of enrollment. The combination of older age, normal menses, and longer average time from most recent BSI in our cohort compared to the adolescent, amenorrhoeic athletes, with a more recent BSI may explain our different findings.
An additional reason that studies utilizing HR-pQCT technology might not reveal differences in bone microarchitecture according to BSI history could be due to the scan locations. Studies utilizing HR-pQCT have performed measurements at the ultradistal tibia or radius. Our prior work using pQCT to measure bone morphology at various sites along the tibia found differences in bone size and geometry between those with and without BSI in the middle third of the tibia, but not in the distal third (40, 41). It is possible that lack of differences according to BSI history in HR-pQCT studies to date could be partially attributed to the skeletal location measured. Future prospective studies assessing BSI risk should measure bone microarchitecture and estimated strength at more proximal tibial sites.
Athletic amenorrhea is often used as a proxy for energy deficiency since it is one of the first signals of low energy availability, leading to increased bone resorption and decreased bone formation through perturbance of multiple hypothalamic-pituitary hormonal axes (42, 43). This maladaptive physiologic state leaves female athletes susceptible to BSI. Evidence also suggests that the length of time an individual spends in an energy-deficient state, and thus, estrogen-deficient state, is proportional to the degree of bone quality impairment (43). Though history of a medically diagnosed eating disorder was an exclusion criteria for the current study and we did not capture data regarding history of energy deficiency or duration of historical menstrual dysfunction, nearly half of our subjects with ≥ 3 BSI reported a history of primary and/or secondary amenorrhea, compared to 19% of women with ≤1 BSI. This finding is consistent with a previous study, where amenorrhoeic athletes had five-fold greater incidence of BSI compared to eumenorrheic athletes (32% versus 5.9%). (38) Moreover, several other studies report a greater history of amenorrhea in women with BSIs, though these studies did not distinguish between women with single versus multiple BSIs (12, 14, 35, 44). Altogether, these findings support the notion that menstrual dysfunction is related to multiple BSI history. Because low EA is one of the primary causes of menstrual dysfunction, both should be clinically addressed as early preventative measures in female athletes to prevent low BMD and BSI. Treatment should generally include increased food intake, but changes in food choices and timing of nutrient consumption may also be important. Early involvement of an appropriately trained expert (e.g., sports dietitian) is recommended. In severe cases, reduced exercise may be necessary(45). Future research is needed to determine the long-term impact of low EA and menstrual dysfunction, including the severity and duration, on adult bone health.
We found that women with ≥ 3 BSIs reported the lowest proportion of multiaxial loading, an observation that is consistent with the concept that exposure to multiaxial, weight-bearing activity, is beneficial for skeletal health (46). Indeed, military recruits who played ball sports for at least two years prior to Basic Combat Training had significantly lower incidence of BSI compared to those who did not (25). Generally, bone strain during walking and running increases with muscle fatigue (47). Yet, individuals who participate in sports with multiaxial loading have lower tibial strain and strain rates during a load carriage compared to runners or healthy military members (48, 49), though it is not clear whether this is due to differences in bone structure and strength or due to other musculoskeletal factors. It is possible that the participation in multiaxial physical activity results in greater soft-tissue strength and endurance, and/or improved neuromotor control, thereby offering protection from BSI independent of bone properties. Future studies should explore whether incorporating multiaxial loading activities such as strength training and plyometrics to uniaxial sports training may mitigate BSI risk.
Finally, our findings of younger age of first BSI in the ≥ 3 BSI group in conjunction with their greater participation in uniaxial activity may reflect behaviors consistent with early sport specialization. Early sport specialization is associated with menstrual dysfunction and low BMD in adolescent female runners (50, 51). Further, uniaxial loading sports are often endurance-based sports and commonly associated with low energy availability(52). Promotion of sports with multi-axial loading, as well as screening for potential tendencies for low EA and disrupted menses may be important to reduce the risk of low BMD and multiple BSI. Moreover, special attention should be paid to early BSI as an indicator of both low EA and future BSI risk.
This study has several limitations. The retrospective design of this study limited our ability to establish causality. On average, the BSI’s occurred four years prior to enrollment in the current study, thus it is possible that changes in activity, menstrual status, and/or diet after the BSI led to different bone density and microarchitecture at study enrollment compared to when the BSI occurred. It is also possible that we are underpowered to detect differences in bone microarchitecture between groups. However, given that very few trends towards significance were observed in our HR-pQCT data, it is unlikely that the lack of differences between groups is due to low statistical power. The self-reported history of image-confirmed BSI is another limitation. The terminology used to characterize a BSI (i.e. stress fracture, stress reaction, or BSI) varies and accordingly the patient interpretation and self-report of these injuries may not be consistent. However, our grouping of ≥3 BSIs versus ≤1 BSI was intended to ensure different populations within groups from a clinical perspective. Thus, although the interpretation of diagnosed BSIs may have differed among our subjects, we do not think it ultimately influenced the group they were assigned to or our overall findings. We also relied on the accuracy of self-reporting for both menstrual status and physical activity data as far back as age eleven, which is subject to recall bias. Finally, although we excluded participants with a medically diagnosed eating disorder, we did not collect data to determine history of disordered eating, a known risk factor for BSI. Despite these limitations, our study adds valuable information to the sparse body of literature on risk factors for multiple BSIs. It is one of the few studies to examine the role that these factors, in addition to physical activity in childhood and adolescence, might play in bone health and BSI risk. Additional studies that collect this information in real time, vis-à-vis a prospective study design, are necessary to fully understand the effects of health history and multiaxial loading on bone health and BSI risk into adulthood.
In summary, our findings suggest that low BMD, a low proportion of multiaxial physical activity performed and history of amenorrhea distinguish women with a history of multiple BSI. This work highlights the importance of physical activity variety as well as establishing and maintaining normal menstrual status during adolescence and young adulthood. From a clinical standpoint, this information reinforces the importance of screening for and treating low energy availability and menstrual dysfunction, even prior to onset of low BMD and/or BSI, as potential early indicators of female athletes at risk for multiple BSIs.
Acknowledgements
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the U.S. Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official U.S. Army, Department of Defense endorsement of approval of the products or services of these organizations. This paper has been approved for public release with unlimited distribution. We acknowledge support from the U.S. Department of Defense, Defense Health Program, and Joint Program Committee (W811XWH-15-C-0024 and W81XWH-16-1-0652) and the National Institutes of Health shared instrumentation grant (S10 RR017868).
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose. The results of this investigation are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of this investigation do not constitute endorsement by ACSM.
REFERENCES
- 1.Bennell KL, Malcolm SA, Thomas SA, Wark JD, Brukner PD. The incidence and distribution of stress fractures in competitive track and field athletes. A twelve-month prospective study. Am J Sports Med. 1996;24(2):211–7. [DOI] [PubMed] [Google Scholar]
- 2.Cosman F, Ruffing J, Zion M, et al. Determinants of stress fracture risk in United States Military Academy cadets. Bone. 2013;55(2):359–66. [DOI] [PubMed] [Google Scholar]
- 3.Wright AA, Taylor JB, Ford KR, Siska L, Smoliga JM. Risk factors associated with lower extremity stress fractures in runners: a systematic review with meta-analysis. Br J Sports Med. 2015;49(23):1517–23. [DOI] [PubMed] [Google Scholar]
- 4.Rizzone KH, Ackerman KE, Roos KG, Dompier TP, Kerr ZY. The Epidemiology of Stress Fractures in Collegiate Student-Athletes, 2004–2005 Through 2013–2014 Academic Years. J Athl Train. 2017;52(10):966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giladi M, Milgrom C, Kashtan H, Stein M, Chisin R, Dizian R. Recurrent stress fractures in military recruits. One-year follow-up of 66 recruits. J Bone Joint Surg Br. 1986;68(3):439–41. [DOI] [PubMed] [Google Scholar]
- 6.Warden SJ, Davis IS, Fredericson M. Management and prevention of bone stress injuries in long-distance runners. J Orthop Sports Phys Ther. 2014;44(10):749–65. [DOI] [PubMed] [Google Scholar]
- 7.De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289. [DOI] [PubMed] [Google Scholar]
- 8.Mountjoy M, Sundgot-Borgen J, Burke L, et al. The IOC consensus statement: beyond the Female Athlete Triad--Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 2014;48(7):491–7. [DOI] [PubMed] [Google Scholar]
- 9.Daily JP, Stumbo JR. Female Athlete Triad. Prim Care. 2018;45(4):615–24. [DOI] [PubMed] [Google Scholar]
- 10.Beck BR, Rudolph K, Matheson GO, Bergman AG, Norling TL. Risk factors for tibial stress injuries: a case-control study. Clin J Sport Med. 2015;25(3):230–6. [DOI] [PubMed] [Google Scholar]
- 11.Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27(3):437–44. [DOI] [PubMed] [Google Scholar]
- 12.Myburgh KH, Hutchins J, Fataar AB, Hough SF, Noakes TD. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113(10):754–9. [DOI] [PubMed] [Google Scholar]
- 13.Schnackenburg KE, Macdonald HM, Ferber R, Wiley JP, Boyd SK. Bone quality and muscle strength in female athletes with lower limb stress fractures. Med Sci Sports Exerc. 2011;43(11):2110–9. [DOI] [PubMed] [Google Scholar]
- 14.Carbon R, Sambrook PN, Deakin V, et al. Bone density of elite female athletes with stress fractures. Med J Aust. 1990;153(7):373–6. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong DW 3rd, Rue JP, Wilckens JH, Frassica FJ. Stress fracture injury in young military men and women. Bone. 2004;35(3):806–16. [DOI] [PubMed] [Google Scholar]
- 16.Petit MA, Beck TJ, Kontulainen SA. Examining the developing bone: What do we measure and how do we do it? J Musculoskelet Neuronal Interact. 2005;5(3):213–24. [PubMed] [Google Scholar]
- 17.Mallinson RJ, Southmayd EA, De Souza MJ. Geometric and “True” Densitometric Characteristics of Bones in Athletes with Stress Fracture and Menstrual Disturbances: A Systematic Review. Sports Med. 2019;49(7):1059–78. [DOI] [PubMed] [Google Scholar]
- 18.Troy KL, Mancuso ME, Butler TA, Johnson JE. Exercise Early and Often: Effects of Physical Activity and Exercise on Women’s Bone Health. Int J Environ Res Public Health. 2018;15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Lombardi G, Jiao W, Banfi G. Effects of Exercise on Bone Status in Female Subjects, from Young Girls to Postmenopausal Women: An Overview of Systematic Reviews and Meta-Analyses. Sports Med. 2016;46(8):1165–82. [DOI] [PubMed] [Google Scholar]
- 20.MacKelvie KJ, Khan KM, McKay HA. Is there a critical period for bone response to weight-bearing exercise in children and adolescents? a systematic review. Br J Sports Med. 2002;36(4):250–7; discussion 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan VP, Macdonald HM, Kim S, et al. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res. 2014;29(10):2161–81. [DOI] [PubMed] [Google Scholar]
- 22.Janz KF, Letuchy EM, Burns TL, Eichenberger Gilmore JM, Torner JC, Levy SM. Objectively measured physical activity trajectories predict adolescent bone strength: Iowa Bone Development Study. Br J Sports Med. 2014;48(13):1032–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunter K, Baxter-Jones AD, Mirwald RL, et al. Jump starting skeletal health: a 4-year longitudinal study assessing the effects of jumping on skeletal development in pre and circum pubertal children. Bone. 2008;42(4):710–8. [DOI] [PubMed] [Google Scholar]
- 24.Warden SJ, Mantila Roosa SM, Kersh ME, et al. Physical activity when young provides lifelong benefits to cortical bone size and strength in men. Proc Natl Acad Sci U S A. 2014;111(14):5337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milgrom C, Simkin A, Eldad A, Nyska M, Finestone A. Using bone’s adaptation ability to lower the incidence of stress fractures. Am J Sports Med. 2000;28(2):245–51. [DOI] [PubMed] [Google Scholar]
- 26.Tenforde AS, Parziale AL, Popp KL, Ackerman KE. Low Bone Mineral Density in Male Athletes Is Associated With Bone Stress Injuries at Anatomic Sites With Greater Trabecular Composition. Am J Sports Med. 2018;46(1):30–6. [DOI] [PubMed] [Google Scholar]
- 27.Tenforde AS, Fredericson M. Influence of sports participation on bone health in the young athlete: a review of the literature. PM R. 2011;3(9):861–7. [DOI] [PubMed] [Google Scholar]
- 28.Popp KL, Turkington V, Hughes JM, et al. Skeletal loading score is associated with bone microarchitecture in young adults. Bone. 2019;127:360–6. [DOI] [PubMed] [Google Scholar]
- 29.Nattiv A, Loucks AB, Manore MM, et al. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–82. [DOI] [PubMed] [Google Scholar]
- 30.Jepsen KJ, Evans R, Negus CH, et al. Variation in tibial functionality and fracture susceptibility among healthy, young adults arises from the acquisition of biologically distinct sets of traits. J Bone Miner Res. 2013;28(6):1290–300. [DOI] [PubMed] [Google Scholar]
- 31.Schlecht SH, Jepsen KJ. Functional integration of skeletal traits: an intraskeletal assessment of bone size, mineralization, and volume covariance. Bone. 2013;56(1):127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pistoia W, van Rietbergen B, Lochmüller EM, Lill CA, Eckstein F, Rüegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842–8. [DOI] [PubMed] [Google Scholar]
- 33.Popp KL, Hughes JM, Martinez-Betancourt A, et al. Bone mass, microarchitecture and strength are influenced by race/ethnicity in young adult men and women. Bone. 2017;103:200–8. [DOI] [PubMed] [Google Scholar]
- 34.Bennell K, Crossley K, Jayarajan J, et al. Ground reaction forces and bone parameters in females with tibial stress fracture. Med Sci Sports Exerc. 2004;36(3):397–404. [DOI] [PubMed] [Google Scholar]
- 35.Bennell KL, Malcolm SA, Thomas SA, et al. Risk factors for stress fractures in female track-and-field athletes: a retrospective analysis. Clin J Sport Med. 1995;5(4):229–35. [DOI] [PubMed] [Google Scholar]
- 36.Duarte Sosa D, Fink Eriksen E. Women with previous stress fractures show reduced bone material strength. Acta Orthop. 2016;87(6):626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weidauer LA, Binkley T, Vukovich M, Specker B. Greater Polar Moment of Inertia at the Tibia in Athletes Who Develop Stress Fractures. Orthop J Sports Med. 2014;2(7):2325967114541411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackerman KE, Cano Sokoloff N, DENM G, Clarke HM, Lee H, Misra M. Fractures in Relation to Menstrual Status and Bone Parameters in Young Athletes. Med Sci Sports Exerc. 2015;47(8):1577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell DM, Tuck P, Ackerman KE, et al. Altered trabecular bone morphology in adolescent and young adult athletes with menstrual dysfunction. Bone. 2015;81:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popp KL, Hughes JM, Smock AJ, et al. Bone geometry, strength, and muscle size in runners with a history of stress fracture. Medicine and Science in Sports and Exercise. 2009;41(12):2145–50. [DOI] [PubMed] [Google Scholar]
- 41.Popp KL, McDermott W, Hughes JM, Baxter SA, Stovitz SD, Petit MA. Bone strength estimates relative to vertical ground reaction force discriminates women runners with stress fracture history. Bone. 2016;94:22–8. [DOI] [PubMed] [Google Scholar]
- 42.Loucks AB, Laughlin GA, Mortola JF, Girton L, Nelson JC, Yen SS. Hypothalamic-pituitary-thyroidal function in eumenorrheic and amenorrheic athletes. J Clin Endocrinol Metab. 1992;75(2):514–8. [DOI] [PubMed] [Google Scholar]
- 43.Schorr M, Miller KK. The endocrine manifestations of anorexia nervosa: mechanisms and management. Nat Rev Endocrinol. 2017;13(3):174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mountjoy M, Sundgot-Borgen JK, Burke LM, et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br J Sports Med. 2018;52(11):687–97. [DOI] [PubMed] [Google Scholar]
- 46.Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc Sport Sci Rev. 2003;31(1):45–50. [DOI] [PubMed] [Google Scholar]
- 47.Yang PF, Brüggemann GP, Rittweger J. What do we currently know from in vivo bone strain measurements in humans? J Musculoskelet Neuronal Interact. 2011;11(1):8–20. [PubMed] [Google Scholar]
- 48.Hughes JM, Dickin DC, Wang H. The relationships between multiaxial loading history and tibial strains during load carriage. J Sci Med Sport. 2019;22(1):48–53. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Kia M, Dickin DC. Influences of load carriage and physical activity history on tibia bone strain. J Sport Health Sci. 2019;8(5):478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rauh MJ, Tenforde AS, Barrack MT, M R, Nichols JF. High School Sports Specialization is Associated with Low Bone Mineral Density in Female High School Distance Runners. J Athl Train. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rauh MJ, Tenforde AS, Barrack MT, Rosenthal MD, Nichols JF. Associations Between Sport Specialization, Running-Related Injury, and Menstrual Dysfunction Among High School Distance Runners. Athletic Training and Sports Health Care. 2018;10(6):260–9. [Google Scholar]
- 52.Loucks AB. Low energy availability in the marathon and other endurance sports. Sports Med. 2007;37(4–5):348–52. [DOI] [PubMed] [Google Scholar]


