Abstract
Bacterial two-component regulatory systems (TCSs) mediate signal transduction by transferring phosphoryl groups between sensor kinase and response regulator proteins, sometimes using intermediary histidine-phosphotransferase (Hpt) domains to form multistep phosphorelays. Because almost all known fungal sensor kinases exhibit a domain architecture characteristic of bacterial TCS phosphorelays, all known fungal Hpts are stand-alone proteins suited to shuttle between cytoplasm and nucleus, and the best-characterized fungal TCS is a canonical phosphorelay, it is widely assumed that most or all fungal TCSs function via phosphorelays. However, fungi generally encode more sensor kinases than Hpts or response regulators, leading to a disparity between putative phosphorelay inputs and outputs. The simplest resolution of this paradox is to hypothesize that most fungal sensor kinases do not participate in phosphorelays. Reimagining how fungal TCSs might function leads to multiple testable predictions.
Keywords: fungi, hybrid histidine kinase, histidine-containing phosphotransfer protein, phosphorelay, response regulator, two-component regulatory systems
Two-Component Regulatory Systems Mediate Signal Transduction
Two-component regulatory systems (TCSs, see Glossary) mediate signal transduction via transient protein phosphorylation in bacteria, archaea, fungi, and plants [1]. TCSs assemble circuits suitable for particular signaling purposes from a set of modular protein domains (reviewed in [2–4]). In a basic bacterial TCS, the unique input and output domains of sensor kinase and response regulator proteins (the two components) are functionally connected via phosphorylation of conserved domains (Figure 1A). The input domain of a sensor kinase detects environmental conditions and regulates the activity of a conserved catalytic and ATP binding (CA) domain to autophosphorylate a His residue in a conserved dimerization/histidine phosphorylation (DHp) domain. The phosphoryl group is then transferred to an Asp residue in the conserved receiver domain of a response regulator. Receiver domains exist in equilibria between inactive and active conformations. Energetically disfavored active conformations are stabilized by phosphorylation. Thus, the phosphorylation state of the receiver controls activity of the output domain, which implements the response. Removal of the phosphoryl group by hydrolysis terminates the response.
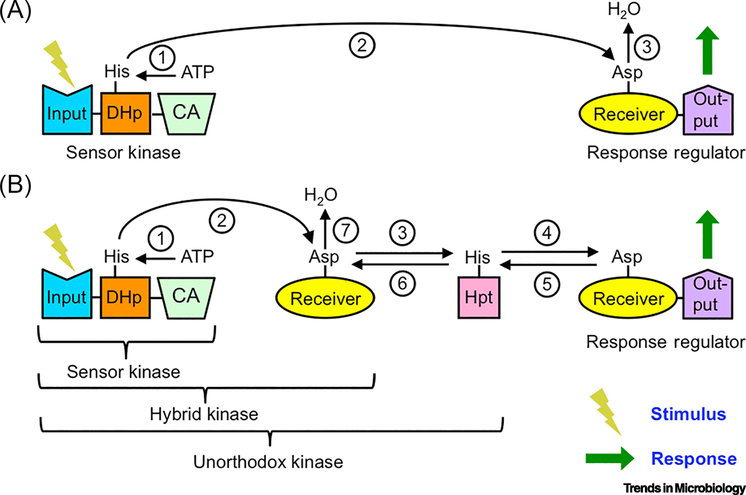
Figure 1. Organization of generic bacterial two-component regulatory systems.
A) Basic two-component system. Reactions are (1) sensor kinase autophosphorylation, (2) His to Asp phosphotransfer, and (3) response regulator dephosphorylation. B) Multistep phosphorelay. Reactions are (1) sensor kinase autophosphorylation, (2) (4) (6) His to Asp phosphotransfer, (3) (5) Asp to His phosphotransfer, and (7) receiver domain dephosphorylation. Incorporation of additional domain(s) into the sensor kinase results in a hybrid kinase or unorthodox kinase. Phosphatase activity is not shown for clarity.
When present, a histidine phosphotransfer domain (Hpt) expands the signaling repertoire of "two-component" systems. Hpts participate in bidirectional phosphotransfer reactions with receiver domains to form multistep phosphorelays (Figure 1B). In bacterial phosphorelays, multiple phosphorylation domains can be incorporated into the same protein. A hybrid histidine kinase (HHK) adds a receiver domain to a basic sensor kinase, whereas an unorthodox kinase further includes an attached Hpt domain.
Phosphorelays offer at least five information-processing benefits compared to basic TCSs: (i) A phosphorelay provides multiple points of regulation [5]. (ii) Signals can be rapidly terminated without phosphatases by reverse phosphotransfer and self-catalyzed receiver domain dephosphorylation (Figure 1B) [6]. (iii) Interaction of one Hpt with three or more receiver domains can form branched networks that integrate (many-to-one) or distribute (one-to-many) information [1]. (iv) A chain of phosphorylation sites can generate nonlinear responses to stimuli [7–10]. (v) In eukaryotes, Hpts can shuttle phosphoryl groups between sensor kinases in the cell membrane or cytoplasm and response regulators in the nucleus [11].
TCSs originated [12] and were first discovered in Bacteria [13], and have been more thoroughly characterized in Bacteria than in Archaea or Eukarya. Organization of TCSs clearly differs between phylogenetic groups [1]. This article points out how biases based on extrapolating extensive knowledge of bacterial TCSs may have crept into mechanistic interpretations of data on fungal TCSs.
Fungal Osmosensing TCSs are Phosphorelays
The osmosensing TCS of Saccharomyces cerevisiae is the first discovered [14, 15] and best characterized [16] fungal TCS. The membrane-bound HHK Sln1 transfers phosphoryl groups to the Hpt protein Ypd1, which serves as phosphodonor for the Skn7 and Ssk1 response regulators. Nuclear Skn7 acts as a transcription factor, whereas cytoplasmic Ssk1 regulates the high osmolarity glycerol (HOG) mitogen-activated protein kinase (MAPK) cascade. Thus, the S. cerevisiae osmosensing TCS functions via a multistep phosphorelay and is organized similarly to a typical bacterial phosphorelay, with the exception of sub-cellularly localized response regulators.
The HOG pathway and its mechanisms of regulation are broadly conserved in fungi, although with variations [17, 18]. Misregulation of the HOG pathway is lethal, providing a potential drug target that has attracted extensive attention from researchers. In fact, the antifungal agent fludioxonil kills by acting indirectly on Group III HHKs to activate the HOG pathway [19]. Group III HHKs feature multiple HAMP domains, have the widest phylogenetic distribution of any fungal HHK Group, and are involved in morphogenesis in many species [20, 21]. Ypd1 is essential for viability in some species of fungi but not others [22–26], suggesting multiple regulatory controls of the HOG system.
Characteristic Features of Fungal TCSs
Fungal TCSs are typically assumed to function via phosphorelays because of several distinctive features shared by all known fungal TCSs (reviewed in [27, 28]). First, almost all fungal sensor kinases are HHKs, and HHKs are commonly associated with phosphorelays in bacteria. Some fungal sensor kinases take the form of two complete HHKs in the same protein, and there are rare variations on the HHK theme (e.g., no CA domain, no receiver domain, or two receiver domains). Second, all fungal TCSs encode one type of Hpt (Ypd1) and two types of response regulator (Skn7, Ssk1) proteins, as in the S. cerevisiae phosphorelay. A narrow phylogenetic group of fungi (e.g., Candida) also encode a third type of mitochondrial response regulator, Ssr1 [29]. Third, there is good evidence for osmosensing TCS phosphorelays in many fungal species [17, 18]. Finally, all fungal Hpt domains are encoded as distinct proteins and have not been found in unorthodox kinases as in bacteria (Figure 1B), consistent with phosphorelays in which Hpt proteins carry phosphoryl groups between the cytoplasm and the nucleus [11, 30].
The Fungal Hpt Paradox
In striking contrast to the limited repertoire of Hpt and response regulator proteins, fungi often encode many HHKs. Virtually all carry C-terminal DHp, CA, and receiver domains, but differ substantially in their N-terminal regions, which provide the basis for classification into 19 different types (Groups I to XIX) of fungal HHKs [27, 28, 31, 32]. The 52 fungal genomes used to develop HHK classification were all from different genera and encoded a range of 1 to 21 HHKs, with an average of 9 and standard deviation of 5. The well-characterized case of S. cerevisiae, upon which much interpretation of fungal TCSs is based by analogy, is an outlier with only one HHK. What are the implications of the general case of fungi with many HHKs, but one Hpt and few response regulators?
Hpts are promiscuous by design, with an exposed His phosphorylation site. Hpts must interact with at least two different receiver domains to form a phosphorelay (Figure 1B), and S. cerevisiae Ypd1 interacts with three different receiver domains in Sln1, Skn7, and Ssk1 [11]. Purified Hpt and receiver domains from different TCSs (and even different species) can successfully exchange phosphoryl groups in vitro [33–36]. We created an artificial phosphorelay that connected unrelated receiver domains by replacing the entire Hpt protein with the small molecule imidazole (the sidechain of His) [37]. Thus, the need for phosphotransfer reactions between multiple HHKs and a single Ypd1 does not pose a theoretical barrier to the paradigm that fungal TCSs function as phosphorelays.
However, there is a serious objection. Evidence indicates that individual fungal HHKs respond to different stimuli and control distinct physiological processes [38–40]. If fungal TCSs primarily function via phosphorelays, then how can distinct stimuli detected by many different HHKs be funneled through one Hpt to very few response regulators and still implement appropriate responses (Figure 2)? Several clever schemes have been proposed to retain specificity when passing phosphoryl groups through a single Ypd1, including different scaffolds to form specific complexes, different Ypd1 isoforms (splice variants), and frequency modulated transmission [1, 41]. Some of these mechanisms may be utilized [42], but none satisfactorily address the fundamental paradox. Instead, each simply shifts the bottleneck from one Hpt protein to a few response regulators.
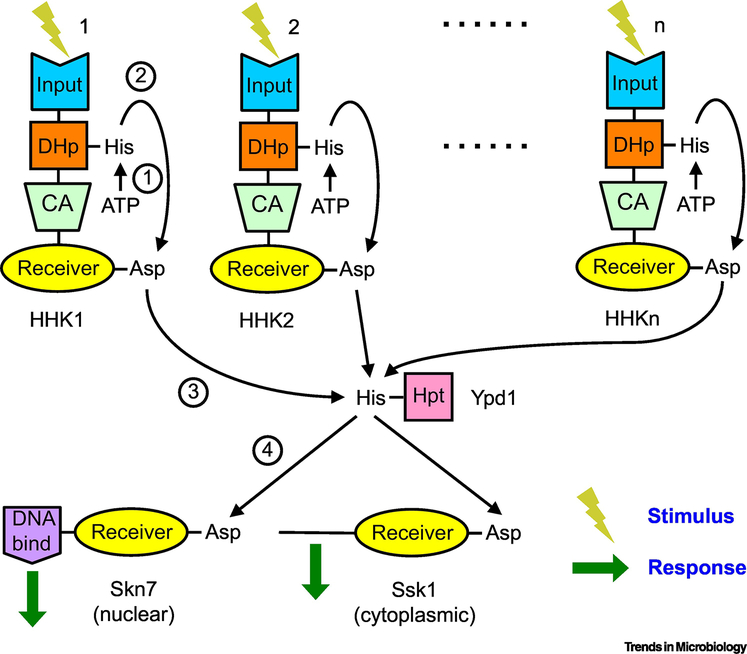
Figure 2. The fungal TCS paradox.
If fungal TCSs function exclusively or primarily by phosphorelays, then how do different stimuli detected via multiple HHKs and converted to phosphoryl groups funneled through a single Hpt (Ypd1) to two or three response regulators (Skn7, Ssk1, Srr1) result in specific and appropriate responses? Schemes to maintain signal specificity through Ypd1 (e.g., using scaffolds or splice variants) shift the bottleneck but do not resolve the paradox posed by an excess of HHKs over response regulators. The simplest resolution of either form of the paradox is to hypothesize that only the osmotic stress and closely related pathways utilize a phosphorelay, whereas most fungal TCSs do not. This hypothesis explains why all fungal TCSs encode similar Hpts and response regulators. Reactions are (1) sensor kinase autophosphorylation, (2) (4) His to Asp phosphotransfer, and (3) Asp to His phosphotransfer. Reversible and dephosphorylation reactions are not shown for clarity.
Reimagining Fungal TCSs
The simplest resolution of the paradox is to hypothesize that most fungal TCSs do not form phosphorelays after all. In this view, only the osmotic stress and closely related pathways utilize a phosphorelay, which is sufficient to explain why all fungal TCSs encode similar Hpts and response regulators. Importantly, there appears to be no published evidence to convincingly demonstrate that any fungal TCS other than the osmotic stress response uses a phosphorelay. The hypothesis that most fungal HHKs do not signal via phosphorelays leads to at least four experimentally testable predictions, considered below (Figure 3, Key Figure).
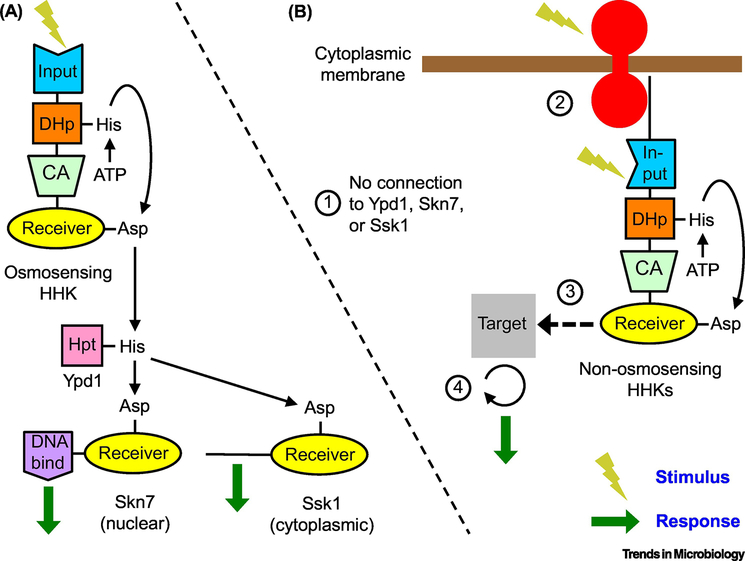
Figure 3. Reimagined fungal TCSs.
A) Canonical: Osmosensing TCS functions as a multi-step phosphorelay from the osmosensing HHK to Ypd1 to Skn7 and Ssk1 essentially as in S. cerevisiae. B) Hypothetical: Non-osmosensing TCSs generally do not function as phosphorelays, which leads to four predictions. (1) Because there is no phosphorelay, the phenotypes resulting from removal of non-osmosensing HHKs will be different than the phenotypes resulting from removal of Ypd1, Skn7, or Ssk1. (2) The HHKs can detect cytoplasmic stimuli via their own input domains, or bind to transmembrane receptors to process external stimuli. Fungal HHKs have substantial N-terminal regions with no identifiable domains, which could serve to bind transmembrane receptors. Furthermore, binding of one type of HHK to a family of receptors could integrate multiple inputs in a manner analogous to bacterial chemoreceptors. (3) The receiver domain of the HHKs binds to downstream targets in a phosphorylation dependent manner. Alternatively, HHKs might interact with downstream targets via non-TCS phosphorylation (e.g., transfer from the HHK receiver domain to a His residue on a non-TCS protein, or phosphorylation mediated by the Ser/Thr kinase domains of Group X and XVI HHKs). (4) Because phosphotransfer within HHKs does not result in signal amplification, some targets of HHKs may have amplification capability (e.g. kinase cascades, generation of second messengers, etc.). Alternatively, activation of multiple HHKs by a single transmembrane receptor as predicted in (2) could result in signal amplification. Reversible and dephosphorylation reactions are not shown for clarity.
Prediction #1 - Most Fungal TCS Pathways Do Not Include Hpts or Response Regulators
The first prediction is that phenotypes arising from deletion or silencing of genes encoding fungal Hpts or response regulators will not mimic phenotypes due to deletion of genes encoding non-osmosensing HHKs, because non-osmosensing HHKs do not signal through Hpts or response regulators (Figure 3B1). This prediction appears to have been rarely tested. In many fungal species, Ypd1 is essential for viability due to regulation of the HOG pathway [18]. Thus, it can be challenging to attribute other phenotypes to loss of Ypd1. However, a Δypd1 mutation is not lethal in a background containing additional mutations that inactivate the HOG pathway. For example, deleting ypd1 in a Δhog1 background in Cryptococcus neoformans affects the same phenotypes observed in Δssk1 or Δskn7 strains [23], consistent with all three genes participating in shared pathways as expected for a phosphorelay. In contrast. Candida albicans Δypd1 strains are initially stressed, but recover wild-type phenotypes upon accumulation of suppressor mutations in the HOG pathway [22], inconsistent with simultaneous involvement of Ypd1 in many distinct signaling pathways.
The most direct test of Prediction #1 appears to have been in Aspergillus fumigatus, which encodes 12 HHKs and two response regulators [38]. Deletion of the gene encoding the Group III HHK results in pleiotropic phenotypes that are not matched by deletion of Ssk1 [43] or Skn7 [44], suggesting that the HHK engages in signaling outside a phosphorelay.
The presence of many HHKs and few response regulators complicates the interpretation of phenotypes. In Aspergillus nidulans [45] and Magnaporthe oryzae [46], removal of Skn7 and/or Ssk1 has more extreme phenotypes than removal of the Group III HHK. In keeping with the phosphorelay paradigm, the authors interpreted their results as indicating other HHKs also control Skn7 and Ssk1. An alternative interpretation is that the Group III HHKs do not employ a phosphorelay and do not feed signals into Skn7 and Ssk1.
Why Have HHKs Without Phosphorelays?
If most fungal TCSs do not involve phosphorelays, then why do all known fungal sensor kinases take the form of HHKs, an architecture that is generally thought to be synonymous with phosphorelays? Consideration of fundamental signal transduction requirements provides insight. All signal transduction mechanisms (including TCSs) must meet some common functional challenges. Necessary tasks include detection of stimuli, conversion of stimuli into internal signals that regulate output, insulation of parallel pathways with common signaling mechanisms but distinct inputs and outputs, and appropriate kinetics of turning signals on and off to synchronize responses with stimuli.
TCSs are organized differently in bacteria, fungi, archaea, and plants [1]. Because bacterial TCSs are by far the best understood, reviewing their organization (Figure 4) provides a useful perspective before addressing the fungal case. The most common means of signal transduction in bacteria is one-component systems [47], in which input and output domains are directly connected to one another (Figure 4A) (e.g. lac repressor). Because output domains commonly regulate transcription, almost all one-component system proteins are cytoplasmic in order to access DNA. Separation of input and output domains into different proteins in two-component systems (Figure 4B) has important functional consequences. First, about 3/4 of bacterial sensor kinases contain transmembrane segments, which facilitate detection of external stimuli, while the separate response regulators remain free to diffuse to their targets. About 2/3 of bacterial response regulator output domains bind DNA, whereas others bind proteins or RNA, create or destroy second messengers, etc. [48]. Second, separation of input and output functions into different proteins supports signal amplification (one sensor kinase can donate phosphoryl groups to multiple response regulators) and a diversity of circuit designs (branching, nonlinear responses, multiple control points, etc.). Bacterial chemotaxis TCSs are a special case in which further separation of input detection and kinase function into different proteins allows one kinase to integrate inputs from multiple receptors via a scaffolding protein (Figure 4C).
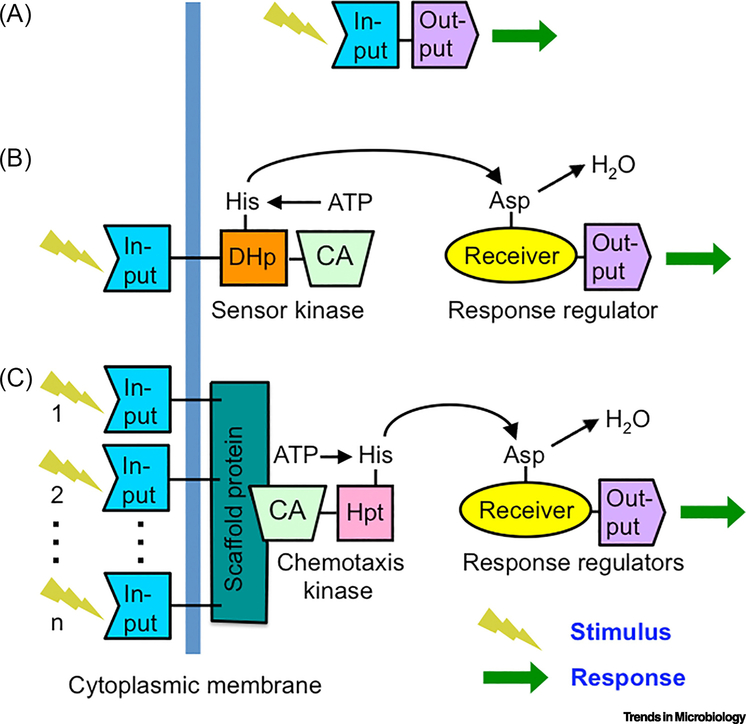
Figure 4. Variants of bacterial two-component systems.
A) One-component systems detect cytoplasmic stimuli. B) Two-component systems with transmembrane sensor kinases detect extracellular stimuli. C) Chemotaxis system with multiple transmembrane chemoreceptors that detect several distinct stimuli. The scaffold protein that connects multiple chemoreceptors with the kinase to integrate information is typically termed CheW. In chemotaxis kinases (typically termed CheA), an Hpt domain replaces the DHp domain of a sensor kinase. The resulting branched pathway (not shown) distributes phosphoryl groups between one kinase and multiple response regulators for excitation and adaptation.
The benefits of separating input and output functions into different proteins in TCSs come with a cost. A single bacterium may have dozens of TCSs operating in parallel and must insulate the pathways from one another to properly match responses with stimuli. Insulation can result from spatial or temporal separation of TCSs, but is primarily achieved through specific binding interfaces between sensor kinases and response regulators [49]. Notably, the DHp and receiver domains of HHKs exhibit reduced interaction specificity that is overcome by high local concentrations due to being part of the same protein [50–52].
Most fungal sensor kinases are cytoplasmic, and those with transmembrane segments tend to have restricted phylogenetic distribution [27, 28]. Thus, fungal TCSs do not take topological advantage of the separation of input and output domains into discrete proteins in the same way that bacterial TCSs do. Fungal TCSs retain input and receiver domains in the same protein. This suggests that the evolutionary rationale for fungal HHKs could be to solve the insulation problem, in a manner similar to bacterial one-component systems. In this view, phosphorylation in fungal TCSs is not primarily used to transfer signals between a HHK and a response regulator, but rather to stably encode information (see Prediction #2) and utilize the binary switch feature of active and inactive receiver domain conformations (see Prediction #3) within a HHK. The receiver domains of fungal HHKs contain the five conserved residues necessary to catalyze phosphorylation and dephosphorylation reactions and thus seem likely to utilize phosphorylation, even if not as part of a phosphorelay.
Prediction #2 - Some Fungal HHKs Interact with Transmembrane Receptors
Fungal HHKs frequently contain large N-terminal regions that exhibit no annotated domains according to typical protein domain classification schemes [53, 54]. The portions of fungal HHKs without currently identified structure/function may contain intrinsically disordered regions, which often participate in protein/protein interactions and are capable of binding to multiple partners [55]. Thus, one function could be to serve as scaffold-like devices to bind to and receive signals from transmembrane receptors (Figure 3B2). Information about external stimuli could be transmitted across the membrane by a variety of mechanisms including changes in the relative positions of receptor transmembrane segments (piston, rotation, scissor), exposure or occlusion of HHK binding sites, change in receptor multimeric state, etc. The resulting changes in the cytoplasmic portion of the receptor could alter how the HHK interacts with the receptor to modulate HHK activity, via allosterically transmitted conformational changes, changes in relative positions of HHK domains to enhance or inhibit phosphorylation, etc. This scheme would be functionally analogous to the interactions between G-proteins and G-protein coupled receptors [56, 57], but with the receptor affecting intracellular signaling via phosphorylation/dephosphorylation of HHKs rather than the GTP binding/hydrolysis used by G-proteins.
Most fungal HHKs contain CHASE, GAF, HAMP, PAS/PAC or phytochrome domains that could have input function for cytoplasmic stimuli. However, Group II, VII, XIV, and XV HHKs are particularly likely to interact with membrane receptors due to the complete absence of recognizable cytoplasmic input domains combined with lack of transmembrane segments that could position the protein to detect extracellular stimuli.
If one type of fungal HHK bound to different receptors, then it could integrate multiple stimuli in a manner functionally analogous to sensor kinases in bacterial chemotaxis (Figure 4C). Bacterial chemoreceptors of different specificities form mixed arrays that collectively connect to sensor kinases via a common scaffold protein [58]. Alternatively, a population of fungal HHKs might sample various individual receptors to achieve an average activation state that reflects the status of the receptor pool.
Prediction #3 - Fungal HHK Receiver Domains Bind to Output Targets
If most fungal HHKs do not participate in phosphorelays, then their receiver domains likely bind to other distinct targets (Figure 3B3). Binding of the receiver domains of fungal Ssk1 response regulators to Ssk2 in the MAP kinase cascade is an obvious analogy [59]. There are also many examples of bacterial receiver domains in which conformational changes associated with phosphorylation state affect binding affinity to other proteins [4]. The relaxed evolutionary constraints on the receiver domain as a result of being part of the HHK [51] could facilitate the evolution of additional binding specificity for a separate partner. Alvarez et al. suggested that fungal HHKs might migrate to the nucleus and bind transcription factors or histone modifying proteins [1]. Many other targets are possible.
Fungi encode one atypical response regulator (Rim15, reviewed in [60]) bearing a pseudoreceiver domain in which Glu replaces the Asp phosphorylation site [46]. Deletion of the pseudoreceiver domain alters Rim15 function [61], confirming the domain is not cryptic. It is not known if or how Rim15 connects to fungal TCSs, but Rim15 could be a direct target of HHK binding. In bacteria, phosphorylation typically leads to receiver domain dimerization [3]. Furthermore, phosphorylated receiver domains can form heterodimers with unphosphorylated receiver domains that stabilize the latter in an active conformation [62–64]. Introduction of Glu at the phosphorylation site activates some bacterial response regulators but not others [65], perhaps by altering the propensity to adopt an active conformation in the absence of phosphorylation. Taken together, these observations raise the possibility that a phosphorylated HHK receiver domain might bind to and stabilize an active conformation of the Rim15 pseudoreceiver domain, thus altering the activity of Rim15.
Although receiver domain binding seems the most plausible mechanism to pass signals from fungal HHKs to downstream targets without using a canonical TCS phosphorelay, there are other possibilities involving phosphotransfer. First, the phosphoryl group might be transferred from the receiver domain of the HHK to a His residue on a non-TCS protein. His-P residues are well known as enzyme intermediates, but also participate in eukaryotic signal transduction and regulatory processes [66]. Second, fungal Group X and XVI HHKs contain Ser/Thr kinase related domains. The output of these HHKs might be Ser/Thr kinase activity regulated by the receiver domain. Finally, there is bacterial precedent for direct phosphotransfer from the DHp domain of a HHK to a response regulator [67]. However, bypassing an Hpt in this manner would not resolve the paradox of insufficient response regulators compared to fungal HHKs.
Prediction #4 - Some Fungal HHK Targets Amplify Signals
The 1:1 ratio of DHp and receiver domains in HHKs precludes the signal amplification that occurs with separate proteins, where one sensor kinase can donate phosphoryl groups to many response regulators. In the few cases where in vivo stoichiometry has been determined, bacterial TCSs generally contain a molar excess of response regulators over sensor kinases [49, 68]. Therefore, targets of fungal HHKs may have a capability for signal amplification (e.g. kinase cascades, generation of second messengers, etc.) (Figure 3B4). There is precedent for this expectation in the MAP kinase cascade that is the target of fungal osmosensing TCSs. Alternatively, perhaps upstream receptors (Prediction #2) activate a stoichiometric excess of HHKs to achieve amplification. Finally, if HHKs communicate with downstream targets via phosphorylation rather than binding, then one HHK could donate phosphoryl groups to multiple targets.
Concluding Remarks
Pathogenic fungi are a major threat to humans, plants, and animals [69]. Because fungal pathogens and their hosts are eukaryotes, it is challenging to target critical fungal functions without also harming the hosts [70, 71]. TCSs are present in most pathogenic fungi but not humans, so TCSs have long been considered potential targets for new antifungal drugs [72]. However, we have pointed out here that some current perceptions about fungal TCSs may be incorrect, and much remains unknown about how fungal TCSs actually function. Discovering the molecular signaling mechanisms used by fungal TCSs (see Outstanding Questions) could substantially facilitate effective targeting by therapeutic agents.
OUTSTANDING QUESTIONS.
How are fungal TCSs insulated from one another, i.e. how do distinct stimuli result in specific responses?
To what extent do fungal TCSs employ multistep phosphorelays?
Do phenotypes attributed to loss of a fungal HHK depend on Ypd1, Ssk1, Skn7 and/or Ssr1?
Do fungal HHK receiver domains have binding partners other than Ypd1? If so, what are the binding partners?
Do fungal HHKs donate phosphoryl groups to proteins other than Ypd1? If so, what are the recipients and reaction mechanisms?
What are the functional role(s) of the large regions in fungal HHKs that contain no recognizable domain structure? Do these uncharacterized regions serve as scaffolds to bind upstream or downstream signaling partners?
Can fungal HHKs directly regulate nuclear targets without using a phosphorelay?
How do signals reach the mitochondrial response regulator Srr1?
Is Rim15 functionally connected to fungal TCSs? If so, how?
HIGHLIGHTS.
Two-component regulatory systems (TCSs) mediate signal transduction in bacteria, archaea, fungi, and plants, but are organized differently in these taxa.
Extrapolation from extensive knowledge of bacterial TCSs, the characteristic features of fungal TCS proteins, and the fact that the best-characterized fungal TCS is a phosphorelay, have led to the dogma that all fungal TCSs function as phosphorelays. Remarkably little support exists in the literature for this position.
The model of fungal TCSs functioning as phosphorelays leads to a paradox of not enough outputs to match inputs. The simplest resolution of the paradox is to propose that the widely conserved osmosensing/HOG pathway is the only fungal TCS that functions as a phosphorelay.
The hypothesis that most fungal TCSs do not use phosphorelays leads to at least four experimentally testable predictions.
ACKNOWLEDGEMENTS
We thank Sarah Barr and two anonymous reviewers for helpful comments on the manuscript. We thank Jeff Tabor for suggesting that fungal HHKs might donate phosphoryl groups to His residues on non-TCS proteins.
This work was funded by National Institutes of Health Grants GM050860 to Robert B. Bourret and AI153523 to William E. Goldman. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
GLOSSARY
- Atypical response regulator
A response regulator containing a pseudoreceiver domain, in which one or more of the five conserved active site residues that catalyze phosphoryl group reactions and bind metal ion has been replaced. Fungal atypical response regulators are typically termed Rim15 after the S. cerevisiae prototype
- CA domain
Catalytic and ATP binding domain (HATPase_c, PF02518)
- DHp domain
Dimerization and histidine phosphorylation domain. Any one of five Pfam domains (HisKA, PF00512; HisKA_3, PF07730; His_Kinase, PF06580; HisKA_2, PF07568; HWE_HK, PF07536). HisKA is the most abundant, followed by HisKA_3
- Groups I to XIX
Types of fungal HHKs defined by the domain architecture of the N-terminal region and phylogenetic relationships
- HHK
Hybrid histidine kinase. Also hybrid sensor kinase. A subset of sensor kinases containing DHp, CA, and receiver domains in that order from N- to C-terminal and not containing an Hpt domain
- HOG pathway
High osmolarity glycerol pathway. Part of the fungal response to osmotic stress. Regulated by a MAP kinase
- Hpt domain
Histidine-containing phosphotransfer domain (Hpt, Pfam01627). Fungal Hpts are typically termed Ypd1 after the S. cerevisiae prototype
- MAP kinase
Mitogen-activated protein kinase. Also MAPK or stress-activated protein kinase (SAPK). Often at the end of a kinase cascade (MAPKKK → MAPKK → MAPK → transcription factor)
- Phosphorelay
Short for "multistep phosphorelay" formed from a sensor kinase, receiver domain, Hpt domain, and response regulator (Figure 1B)
- Receiver domain
(Response_reg, Pfam00072). Also receiver module
- Response regulator
Defined by the presence of a receiver domain and the absence of a CA domain. Often contains an output domain to implement a response. Fungal response regulators are typically termed Skn7, Ssk1, or Srr1 after the S. cerevisiae or Candida albicans prototypes
- Sensor kinase
Also termed a histidine kinase. Defined by the presence of DHp and CA domains in that order from N- to C-terminal. The DHp and CA domains together form a transmitter module. Typically also contains one or more input domains to detect stimuli
- TCS
Two-component regulatory system. Defined by the presence of at least one sensor kinase (with transmitter module) and response regulator (with receiver module)
- Unorthodox kinase
A subset of sensor kinases containing DHp, CA, receiver, and Hpt domains in that order from N- to C-terminal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alvarez AF, et al. (2016) Organization and mode of action of two component system signaling circuits from the various kingdoms of life. Environ. Microbiol 18, 3210–3226 [DOI] [PubMed] [Google Scholar]
- 2.Buschiazzo A and Trajtenberg F (2019) Two-component sensing and regulation: How do histidine kinases talk with response regulators at the molecular level? Annu. Rev. Microbiol 73, 507–528 [DOI] [PubMed] [Google Scholar]
- 3.Gao R, et al. (2019) Structural basis of response regulator function. Annu. Rev. Microbiol 73, 175–197 [DOI] [PubMed] [Google Scholar]
- 4.Zschiedrich CP, et al. (2016) Molecular mechanisms of two-component signal transduction. J. Mol. Biol 428, 3752–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perego M (2001) A new family of aspartyl phosphate phosphatases targeting the sporulation transcription factor Spo0A of Bacillus subtilis. Mol. Microbiol 42, 133–143 [DOI] [PubMed] [Google Scholar]
- 6.Pena-Sandoval GR, et al. (2005) Requirement of the receiver and phosphotransfer domains of ArcB for efficient dephosphorylation of phosphorylated ArcA in vivo. J. Bacteriol 187, 3267–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csikasz-Nagy A, et al. (2011) Response dynamics of phosphorelays suggest their potential utility in cell signalling. J. R. Soc. Interface 8, 480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JR and Cho KH (2006) The multi-step phosphorelay mechanism of unorthodox two-component systems in E. coli realizes ultrasensitivity to stimuli while maintaining robustness to noises. Comput. Biol. Chem 30, 438–444 [DOI] [PubMed] [Google Scholar]
- 9.Knudsen M, et al. (2012) Exact analysis of intrinsic qualitative features of phosphorelays using mathematical models. J. Theor. Biol 300, 7–18 [DOI] [PubMed] [Google Scholar]
- 10.Kothamachu VB, et al. (2013) Phosphorelays provide tunable signal processing capabilities for the cell. PLoS Comput. Biol 9, e1003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fassler JS and West AH (2013) Histidine phosphotransfer proteins in fungal two-component signal transduction pathways. Eukaryot. Cell 12, 1052–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koretke KK, et al. (2000) Evolution of two-component signal transduction. Mol. Biol. Evol 17, 1956–1970 [DOI] [PubMed] [Google Scholar]
- 13.Nixon BT, et al. (1986) Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation genes ntrB and ntrC. Proc. Natl. Acad. Sci. U.S.A 83, 7850–7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ota IM and Varshavsky A (1993) A yeast protein similar to bacterial two-component regulators. Science 262, 566–569 [DOI] [PubMed] [Google Scholar]
- 15.Posas F, et al. (1996) Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 "two-component" osmosensor. Cell 86, 865–875 [DOI] [PubMed] [Google Scholar]
- 16.Brewster JL and Gustin MC (2014) Hog1: 20 years of discovery and impact. Sci Signal 7, re7. [DOI] [PubMed] [Google Scholar]
- 17.Day AM and Quinn J (2019) Stress-activated protein kinases in human fungal pathogens. Front. Cell Infect. Microbiol 9, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahn YS (2008) Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot. Cell 7, 2017–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandhorst TT, et al. (2019) Phenylpyrrole fungicides act on triosephosphate isomerase to induce methylglyoxal stress and alter hybrid histidine kinase activity. Sci. Rep 9, 5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Defosse TA, et al. (2015) Hybrid histidine kinases in pathogenic fungi. Mol Microbiol 95, 914–924 [DOI] [PubMed] [Google Scholar]
- 21.Herivaux A, et al. (2016) Major sensing proteins in pathogenic fungi: The hybrid histidine kinase family. PLoS Pathog 12, e1005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day AM, et al. (2017) Blocking two-component signalling enhances Candida albicans virulence and reveals adaptive mechanisms that counteract sustained SAPK activation. PLoS Pathog 13, e1006131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J-W, et al. (2011) Multiple role of Ypd1 phosphotransfer protein in viability, stress response, and virulence factor regulation in Cryptococcus neoformas. Eukaryot. Cell 10, 998–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen AN, et al. (2000) Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol. Biol. Cell 11, 1169–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Gonzalez M, et al. (2017) Role of the Sln1-phosphorelay pathway in the response to hyperosmotic stress in the yeast Kluyveromyces lactis. Mol. Microbiol 104, 822–836 [DOI] [PubMed] [Google Scholar]
- 26.Jacob S, et al. (2015) High osmolarity glycerol (HOG) signalling in Magnaporthe oryzae: Identification of MoYPD1 and its role in osmoregulation, fungicide action, and pathogenicity. Fungal Biol 119, 580–594 [DOI] [PubMed] [Google Scholar]
- 27.Defosse TA, et al. (2015) Hybrid histidine kinases in pathogenic fungi. Mol. Microbiol 95, 914–924 [DOI] [PubMed] [Google Scholar]
- 28.Kabbara S, et al. (2019) Diversity and evolution of sensor histidine kinases in eukaryotes. Genome Biol. Evol 11, 86–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herivaux A, et al. (2018) Progressive loss of hybrid histidine kinase genes during the evolution of budding yeasts (Saccharomycotina). Curr. Genet 64, 841–851 [DOI] [PubMed] [Google Scholar]
- 30.Lu JM-Y, et al. (2003) Saccharomyces cerevisiae histidine phosphotransferase Ypd1p shuttles between the nucleus and cytoplasm for SLN1-dependent phosphorylation of Ssk1p and Skn7p. Eukaryot. Cell 2, 1304–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catlett NL, et al. (2003) Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot. Cell 2, 1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavin JL, et al. (2010) Genomic analysis of two-component signal transduction proteins in basidiomycetes. J. Mol. Microbiol. Biotechnol 18, 63–73 [DOI] [PubMed] [Google Scholar]
- 33.Janiak-Spens F, et al. (1999) Differential stabilities of phosphorylated response regulator domains reflect functional roles of the yeast osmoregulatory SLN1 and SSK1 proteins. J. Bacteriol 181, 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaku H, et al. (1997) Interaction between the CheY response regulator and the histidine-containing phosphotransfer (HPt) domain of the ArcB sensory kinase in Escerichia coli. FEBS Lett 408, 337–340 [DOI] [PubMed] [Google Scholar]
- 35.Kennedy EN, et al. (2016) Extended N-terminal region of the essential phosphorelay signaling protein Ypd1 from Cryptococcus neoformans contributes to structural stability, phosphostability, and binding of calcium ions. FEMS Yeast Res 16, fow068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawry SM, et al. (2017) Fludioxonil induces Drk1, a fungal Group III hybrid histidine kinase, to dephosphorylate its downstream target, Ypd1. Antimicrob. Agents. Chemother 61, e01414–01416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page SC, et al. (2015) Imidazole as a small molecule analogue in two-component signal transduction. Biochemistry 54, 7248–7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapeland-Leclerc F, et al. (2015) Systematic gene deletion and functional characterization of histidine kinase phosphorelay receptors (HKRs) in the human pathogenic fungus Aspergillus fumigatus. Fungal Genet. Biol 84, 1–11 [DOI] [PubMed] [Google Scholar]
- 39.Jacob S, et al. (2014) Histidine kinases mediate differentiation, stress response, and pathogenecity in Magnaporthe oryzae. Microbiol. Open 3, 668–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herivaux A, et al. (2016) Major sensing proteins in pathogenic fungi: The hybrid histidine kinase family. PLoS Pathog 12, e1005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob S and Thines E (2017) Multistep phosphorelay in fungi: the enigma of multiple signals and a limited number of signaling pathways. Mycol. Progress 16, 1007–1013 [Google Scholar]
- 42.Monahan VC, et al. (2017) Fungal histidine phosphotransferase plays a crucial role in photomorphogenesis and pathogenesis in Magnaporthe oryzae. Front. Chem 5, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagiwara D, et al. (2013) NikA/TcsC histidine kinase is involved in conidiation, hyphal morphology, and responses to osmotic stress and antifungal chemicals in Aspergillus fumigatus. PLoS One 8, e80881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamarre C, et al. (2007) Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet. Biol 44, 682–690 [DOI] [PubMed] [Google Scholar]
- 45.Vargas-Perez I, et al. (2007) Response regulators SsrA and SskA are central components of a phosphorealy system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot. Cell 6, 1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motoyama T, et al. (2008) Involvement of putative response regulator genes of the rice blast fungus Magnaporthe oryzae in osmotic stress response, fungicide action, and pathogenicity. Curr. Genet 54, 185–195 [DOI] [PubMed] [Google Scholar]
- 47.Ulrich LE, et al. (2005) One-component systems dominate signal transduction in prokaryotes. Trends Microbiol 13, 52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galperin MY (2010) Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol 13, 150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podgornaia AI and Laub MT (2013) Determinants of specificity in two-component signal transduction. Curr. Opin. Microbiol 16, 156–162 [DOI] [PubMed] [Google Scholar]
- 50.Wegener-Feldbrugge S and Sogaard-Andersen L (2009) The atypical hybrid histidine protein kinase RodK in Myxococcus xanthus: Spatial proximity supersedes kinetic preference in phosphotransfer reactions. J. Bacteriol 191, 1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capra EJ, et al. (2012) Spatial tethering of kinases to their substrates relaxes evolutionary constraints on specificity. Mol. Microbiol 86, 1393–1403 [DOI] [PubMed] [Google Scholar]
- 52.Townsend GEI, et al. (2013) Intromolecular arrangement of sensor and regulator overcomes relaxed specificity in hybrid two-component systems. Proc. Natl. Acad. Sci. U.S.A 110, E161–E169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Gebali S, et al. (2019) The Pfam protein families database in 2019. Nucleic Acids Res 47, D427–D432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell AL, et al. (2019) InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res 47, D3351–D3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oldfield CJ and Dunker AK (2014) Intrinsically disordered proteins and intrinsically disordered protein regions. Annu. Rev. Biochem 83, 553–584 [DOI] [PubMed] [Google Scholar]
- 56.Park H-S, et al. (2020) Heterotrimeric G-protein signalers and RGSs in Aspergillus fumigatus. Pathogens 9, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, et al. (2007) Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol 61, 423–452 [DOI] [PubMed] [Google Scholar]
- 58.Bi S and Sourjik V (2018) Stimulus sensing and signal processing in bacterial chemotaxis. Curr. Opin. Microbiol 45, 22–29 [DOI] [PubMed] [Google Scholar]
- 59.Posas F and Saito H (1998) Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J 17, 1385–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swinnen E, et al. (2006) Rim15 and the crossroads of nutrient signalling pathways in Saccharomyces cerevisiae. Cell Div 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watanabe D, et al. (2012) A loss-of-function mutation in the PAS kinase Rim15p is related to defective quiescence entry and high fermentation rates of Saccharomyces cerevisiae sake yeast strains. Appl. Environ. Microbiol 78, 4008–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Creager-Allen RL, et al. (2013) A link between dimerization and autophosphorylation of the response regulator PhoB. J. Biol. Chem 288, 21755–21769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trajtenberg F, et al. (2014) Allosteric activation of bacterial response regulators: the role of the cognate histidine kinase beyond phosphorylation. mBio 5, e02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernadez I, et al. (2105) Snapshots of conformational changes shed light into the NtrX receiver domain signal transduction mechanism. J. Mol. Biol 427, 3258–3272 [DOI] [PubMed] [Google Scholar]
- 65.Smith JG, et al. (2004) A search for amino acid substitutions that universally activate response regulators. Mol. Microbiol 51, 887–901 [DOI] [PubMed] [Google Scholar]
- 66.Fuhs SR and Hunter T (2017) pHisphorylation: the emergence of histidine phosphorylation as a reversible regulatory modulation. Curr. Opin. Cell Biol 45, 8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mukhopadhyay A, et al. (2004) Integrating input from multiple signals: the VirA/VirG two-component system of Agrobacterium tumefaciens. Chembiochem 5, 1535–1542 [DOI] [PubMed] [Google Scholar]
- 68.Dutta A, et al. (2020) Evidence of robustness in a two-component system synthetic circuit. J. Bacteriol 202, e00672–00619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher MC, et al. (2020) Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 11, e00449–00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li D, et al. (2020) The antifungal pipeline: the need is established. Are there new compounds? FEMS Yeast Res 20, foaa023. [DOI] [PubMed] [Google Scholar]
- 71.Perfect JR (2017) The antifungal pipeline: a reality check. Nat. Rev. Drug. Discov 16, 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shor E and Chauhan N (2015) A case for two-component signaling systems as antifungal drug targets. PLoS Pathog 11, e1004632. [DOI] [PMC free article] [PubMed] [Google Scholar]