Abstract
Paraoxonase 2 (PON2) is an intracellular antioxidant enzyme shown to play an important role in mitigating oxidative stress in the brain. Oxidative stress is a common mechanism of toxicity for neurotoxicants and is increasingly implicated in the etiology of multiple neurological diseases. While PON2 deficiency increases oxidative stress in the brain in-vitro, little is known about its effects on behavior in-vivo and what global transcript changes occur from PON2 deficiency. We sought to characterize the effects of PON2 deficiency on behavior in mice, with an emphasis on locomotion, and evaluate transcriptional changes with RNA-Seq. Behavioral endpoints included home-cage behavior (Noldus PhenoTyper), motor coordination (Rotarod) and various gait metrics (Noldus CatWalk). Home-cage behavior analysis showed PON2 deficient mice had increased activity at night compared to wildtype controls and spent more time in the center of the cage, displaying a possible anxiolytic phenotype. PON2 deficient mice had significantly shorter latency to fall when tested on the rotarod, suggesting impaired motor coordination. Minimal gait alterations were observed, with decreased girdle support posture noted as the only significant change in gait with PON2 deficiency. Beyond one home-cage metric, no significant sex-based behavioral differences were found in this study. Finally, A subset of samples were utilized for RNA-Seq analysis, looking at three discrete brain regions: cerebral cortex, striatum, and cerebellum. Highly regional- and sex-specific changes in RNA expression were found when comparing PON2 deficient and wildtype mice, suggesting PON2 may play distinct regional roles in the brain in a sex-specific manner. Taken together, these findings demonstrates that PON2 deficiency significantly alters the brain on both a biochemical and phenotypic level, with a specific impact on motor function. These data have implications for future gene-environment toxicological studies and warrants further investigation of the role of PON2 in the brain.
Keywords: Paraoxonase 2, PON2, brain, mouse, behavior, RNA-Seq
1. INTRODUCTION
Paraoxonase 2 (PON2) is a member of the paraoxonase gene family, consisting of three closely related genes: PON1, 2 and 3. Phylogenic analysis suggests PON2 to be the oldest of the PONs, from which PON1 and 3 evolved (Draganov & La Du 2004). Although the gene family was named for the esterase activity of PON1 against the organophosphate paraoxon, PON2 does not hydrolyze paraoxon, but may hydrolyze methyl-paraoxon (Bar-Rogovsky et al., 2013). All three PONs possess lactonase activity and have both overlapping and distinct substrates for lactone hydrolysis (Draganov et al., 2005). Of the PONs, PON2 has the highest hydrolytic activity for acyl-homoserine lactones (acyl-HCL), molecules which act as bacterial quorum-sensing signals and function as key modulators of the host inflammatory response to Pseudomonas infection (Stoltz et al., 2007; Teiber et al., 2008; Horke et al., 2010). Outside of quorum quenching, the significance of the lactonase activity of PON2 and the potential native substrates it may target are largely unknown, although recent work has begun to elucidate native targets for PON1 and 3 (Teiber et al., 2018).
While PON1 circulates associated with HDL in plasma and PON3 both circulates and is found intracellularly, PON2 is a ubiquitously expressed intracellular enzyme that does not circulate and is primarily located at the inner mitochondrial membrane (Altenhöfer et al., 2010; Devarajan et al., 2011). It is observed in all tissues examined, with the highest expression found in the lung, liver, intestines, heart, brain, and kidneys respectively (Furlong et al., 2016; Giordano et al., 2011). PON2 has been shown to be developmentally regulated in an organ-specific manner, with different expression patterns over development in the brain and liver (Garrick et al., 2016). At the inner mitochondrial membrane, PON2 associates with co-enzyme Q10, and is thought to play a role maintaining redox balance during oxidative phosphorylation (Devarajan et al., 2011). In addition to the mitochondria, PON2 has also been found at the cell membrane in neurons and astrocytes (Giordano et al., 2011). In-vitro studies in HEK 293T cells have shown that PON2 translocates to the plasma membrane under oxidative stress conditions through increased Ca2+ signaling, and functions to mitigate lipid peroxidation (Hagmann et al., 2014), but it is not known if this mechanism is responsible for the observed membrane expression in neurons and astrocytes.
PON2 has been demonstrated to exert an antioxidant effect in various cell types (Ng et al., 2001; Horke et al., 2007; Levy et al., 2007; Giordano et al., 2011; Giordano et al., 2013) and is necessary for mitochondrial homeostasis, as PON2 deficient mice display mitochondrial dysfunction (Devarajan et al., 2011). Superoxide (O2-) is an incidental by-product of the electron transport chain at the mitochondrial membrane and leads to the production of hydrogen peroxide (H2O2), another reactive oxygen species (ROS), which can further produce hydroxyl radicals (Kehrer & Klotz 2015). This suggests that the mitochondria is an important site for antioxidant action to mitigate cellular damage caused by these radicals. Oxidative stress is a principal mechanism by which endogenous and exogenous neurotoxicants exert toxicity (Sayre et al., 2008; Deavall et al., 2012) and higher levels of ROS are associated with numerous morbidities such as cardiovascular disease (Panth et al., 2016), cancer (Kumari et al., 2018), diabetes (Asmat et al., 2016), and neurodegenerative diseases (Kim et al., 2015), highlighting the clinical importance of proper oxidative balance.
In the brain, PON2 expression has been found highest in dopaminergic regions such as the striatum, nucleus accumbens and substantia nigra (Giordano et al., 2011). Differential expression among cell types has also been observed, with astrocytes expressing significantly more PON2 than neurons (Giordano et al., 2011). PON2 deficiency in both brain cell types impairs their ability to recover from oxidative stress generated by in-vitro exposure to the oxidants hydrogen peroxide (H2O2) and 2,3-dimethoxy-1,4-naphthoquinone (DMNQ) (Giordano et al., 2011). Glutathione, a principal antioxidant in the body, does not appear to be altered by PON2 deficiency, indicating that the observed sensitivity to oxidant challenge in PON2 deficient cells is due to the loss of PON2 and not the loss of additional antioxidant function (Giordano et al., 2011). As such, PON2 appears to be a critical antioxidant enzyme in the brain and warrants additional investigation.
To further characterize the effects of PON2 deficiency in the brain, we sought to determine if PON2 deficient mice display a behavioral phenotype, with particular emphasis on motor behavior, as PON2 is expressed highest in dopaminergic regions and motor deficits are hallmarks of dopaminergic neuron loss (Grosch et al., 2016). Additionally, we conducted brain transcriptomic analyses using RNA-Seq to compare mRNA expression changes in brain tissue from PON2 deficient and wildtype (WT) mice, aimed at further understanding the pathways and processes in which PON2 is involved. Taken together with existing literature, the results of this study begin to provide a more robust understanding of the role of PON2 in the brain and offers compelling evidence for further toxicological study of PON2.
2. MATERIALS AND METHODS
2.1. Mice
Male and female WT and PON2 deficient (PON2-def) mice (Ng et al., 2006) on a C57BL/6J background were used for this study. Seven to ten animals were used per sex per group. Mice were bred from PON2-def heterozygous crosses with resulting WT and PON2-def homozygous littermates used in rolling cohorts. Behavioral testing began at 3 – 4 months of age. Mice were housed in a specific pathogen-free facility on a 14-hour light/ 10-hour dark cycle with ad libitum access to food and water. All procedures were conducted in accordance with the National Institute of Health Guide for the Use and Care of Laboratory Animals and were approved by the University of Washington Institutional Animal Care and Use Committee.
2.2. Tissues
Mice were sacrificed at 3 – 4 months of age upon conclusion of behavioral testing utilizing a carbon dioxide chamber. Cerebral cortex, striatum, and cerebellum were freshly dissected (Spijker 2011, Pacheco et al., 2017) and flash frozen in liquid nitrogen. Tissues were pulverized with a pre-chilled mortar and pestle into a fine powder, stored at −80°C and aliquoted into appropriate buffers for later testing (Yu et al., 2020).
2.3. Noldus PhenoTyper
Home-cage behavior was evaluated as the first behavioral endpoint using the Noldus PhenoTyper system (Noldus Information Technology, Wageningen, The Netherlands). Mice were placed into plexiglass cages measuring 30 × 30 cm with cameras recording from the cage top downward. All cages were recorded simultaneously for 67 hours, beginning at 12PM and ending at 7AM. EthoVision XT software (Noldus Information Technology, Wageningen, The Netherlands) was used to track the movement of the mice for the duration of the experiment. Utilizing this software, the cage is divided into various zones (arena center, arena perimeter, food hopper, water bottle, hidden shelter) and the amount of time spent within each zone, as well as the travel between zones and the speed of their travel is recorded for each mouse. Relative measurements of the arena areas were as follows: arena center 293 cm2, arena perimeter 347 cm2, food hopper 58 cm2, water bottle 40 cm2, and hidden shelter 106 cm2. Standard cob bedding was provided with no nesting material, as any additional nesting material in the cage could interfere with tracking. Mice were able to freely enter a plastic enclosure (termed ‘hidden shelter’) with two entry/exit points.
2.4. Noldus CatWalk
Gait assessment took place 3–5 days after PhenoTyper analysis using the Noldus CatWalk XT system (Noldus Information Technology, Wageningen, The Netherlands). Gait metrics were measured focusing on four domains: temporal parameters (average speed, cadence, swing and swing speed), coordination (sequence regularity index and step pattern), spatial patterns of individual paws (stride length, print intensity, print area, print length and print width) and spatial pattern relationships between paws (base of support and support time). A detailed protocol for this test can be found in Supplemental Protocol 1. One animal (PON2 deficient male) was dropped from this analysis due to non-compliance, as the animal refused to traverse the walkway.
2.5. Rotarod
Mice were moved to the testing room 15 minutes prior to the start of the experiment for acclimation. A five lane Rota-Rod treadmill (Med Associates Inc., Vermont, USA) was utilized with adjacent lanes separated by a disk-wall. Mice were given a training trial at a constant speed of 4 RPM, 1 min on the rod and 1 min of rest for a total of 3 times. The testing consisted of a 5-min trial with rod speed ramping from 4 RPM to 40 RPM over the 5-min period. Time to fall (latency) was recorded when the mouse completely fell off the rod or rode around the rod for a full revolution. Each mouse was given ten 5-min test trials and the five highest latency trials, expected to reflect the maximum ability of the mouse for this test, were averaged to provide their latency score. Mice that repeatedly jumped from the apparatus were removed from the study and not included in further analysis. In total, 9 mice were removed from our study for jumping and the genotype/sex breakdown of those removed were as follows: WT male n = 3, PON2-def male n = 3, WT female n = 2, PON2-def female n = 1.
2.6. RNA-Seq
RNA was extracted from 30 – 50 mg of pulverized brain tissue utilizing a Qiagen RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommended protocol. The quantity of total RNA was determined by measuring the OD260 with a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). RNA purity was assessed by measuring OD260/280 and OD260/230 ratios. RNA integrity was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Only total RNA samples with RNA Integrity Numbers (RIN) > 8, OD260/280 as well as OD260/230 ratios of 1.8 – 2.1 were used for RNA-Seq analysis. Samples were commercially sequenced by Beijing Genomics Inc. (BGI, Shenzen, China) using their proprietary sequencer which generates 150 nt paired-end sequences. The reads were aligned against the Genecode M23 trranscriptome using the Salmon aligner (Patro et al., 2015) to generate estimated counts for each transcript. Transcript counts were summarized to gene counts using the Bioconductor tximport package (Soneson et al., 2015). Gene counts are proportional to the underlying transcript abundance and were utilized as raw data for all analyses. Differential gene expression was assessed using the Bioconductor limma package, using the ‘limma-voom’ pipeline, which fits a weighted analysis of variance (ANOVA) model to the log counts/million counts (Law et al., 2014). Univariate p-values were corrected using false discovery rate (FDR), and genes were selected at an FDR<0.1. The data were further analyzed at the pathway level using iPathwayGuide from Advaita Bioinformatics (https://www.advaitabio.com/ipathwayguide). This analysis tool utilizes the ‘Impact Analysis’ approach which takes into consideration the direction and type of signals on a pathway as well as the role and type of specific genes (Donato et al., 2013). Pathway and predicted upstream regulator analysis p-values were corrected for multiple comparisons utilizing FDR. Biochemical processes and molecular functions data were corrected utilizing a weighted pruning method which assigns weight to each gene annotated to a gene ontology (GO) term based on the scores of neighboring GO terms (Alexa et al., 2006). GO terms consisting of only one gene were excluded from the analysis regardless of p-value.
2.7. Statistical Analysis
Data are expressed as the mean ± SEM of at least five independent experiments. For the behavioral analysis, data were first analyzed by two-way ANOVA to detect a significant sex interaction. If no sex interaction was observed, male and female data were combined for increased power. One-way ANOVA followed by the Bonferroni correction for multiple comparisons was utilized for statistical analysis of more than two groups for the CatWalk results, while Student’s t-test was utilized for comparing two groups of the PhenoTyper, Rotarod and CatWalk results.
3. RESULTS
3.1. Behavioral Analysis
3.1.1. PhenoTyper
Home-cage behavior was analyzed for 67 hours using the Noldus PhenoTyper system and EthoVision tracking software (Figure 1A). The arena zones marked in the cage are noted in Figure 1B. Mice were allowed to freely roam the cage and were not disturbed for the duration of the experiment. For analysis purposes, male and female mice were combined to increase power unless a sex interaction was observed. When evaluating movement, PON2-def mice were recorded as having spent more time moving in the cage during the dark cycle than WT mice (Figure 2A), as well as moving longer distances during this time (Figure 2B). Movement during the light cycle was unchanged. When evaluating time spent in various zones of the arena, PON2-def mice spent significantly more time in the center of the arena during the dark cycle than WT mice, while no difference was noted during the light cycle (Figure 2C). Conversely, PON2-def mice spent significantly less time in the hidden shelter during the dark cycle, while no difference was noted during the light cycle (Figure 2D). No differences were observed for time spent in the perimeter of the arena during either light or dark cycle (Figure 2E). A sex interaction was noted for time spent at the food hopper, with female PON2-def mice spending significantly more time at the hopper than WT during the dark cycle, with no difference observed during the light cycle (Figure 2F). Time spent at the food hopper was not significantly different for males during either cycle (Figure 2G). Time spent at the water bottle zone was not significantly different between PON2-def and WT mice at either light or dark cycle (Figure 2H).
Figure 1. PhenoTyper Setup.
A. Schematic demonstrating setup of PhenoTyper cage and camera location. B. Arena zones defined during PhenoTyper experiment.
Figure 2. Home-cage Metrics in Wildtype and PON2 deficient Mice Assessed by PhenoTyper.
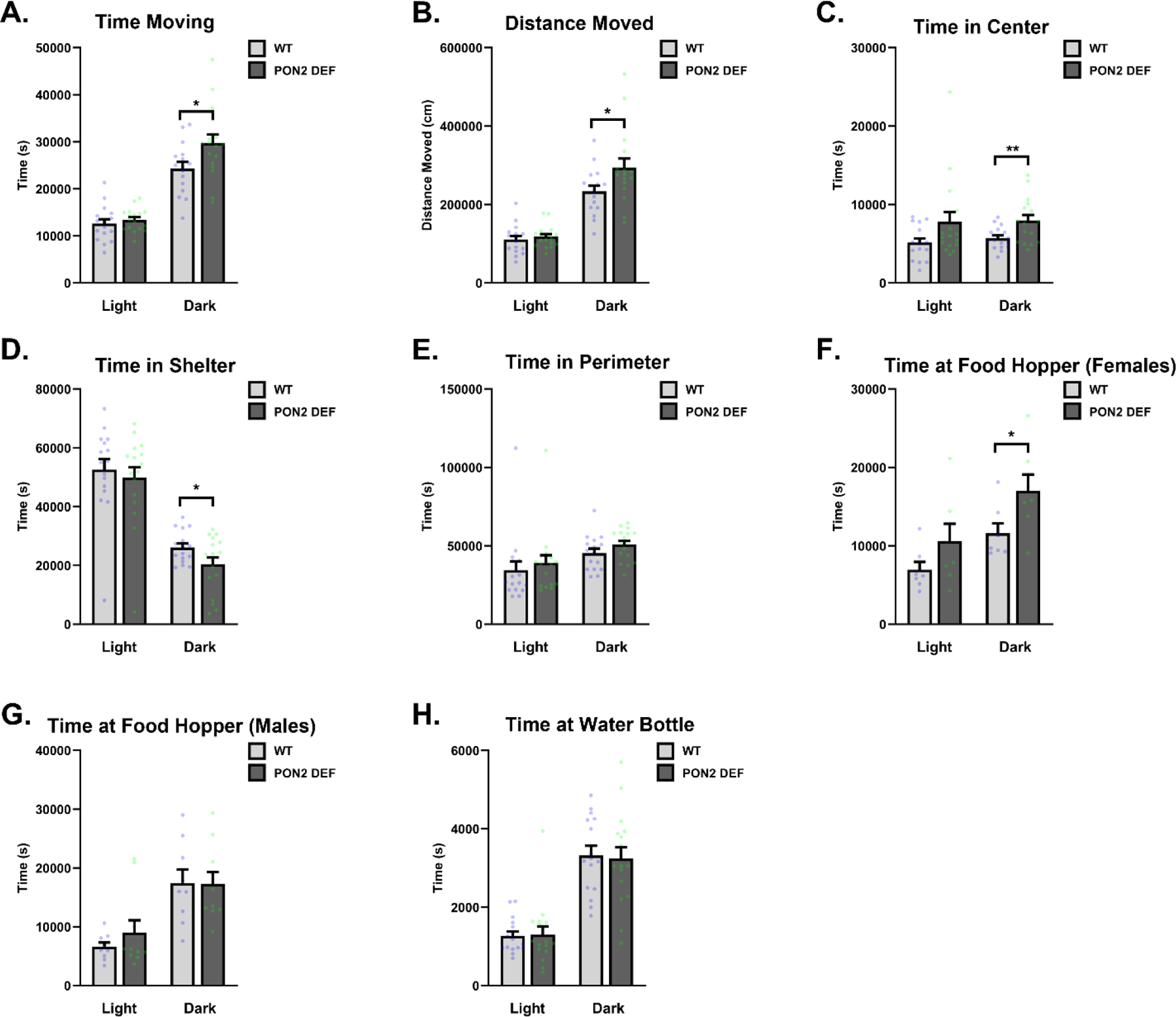
A. Time spent moving in cage during the light and dark cycles (male and female mice combined), measured in seconds (s), n = 16 – 17 per group, ± SEM, * p < 0.05. B. Distance moved in cage during the light and dark cycles (male and female mice combined), measured in centimeters (cm), n = 16 – 17 per group, ± SEM, * p < 0.05. C. Time spent in center of arena during light and dark cycles (male and female mice combined), measured in seconds (s), n = 16 – 17 per group, ± SEM, * p < 0.05. D. Time spent in the hidden shelter during light and dark cycles (male and female mice combined), measured in seconds (s), n = 16 – 17 per group, ± SEM , * p < 0.05. E. Time spent in the perimeter of the arena during light and dark cycles (male and female mice combined), measured in seconds (s), n = 16 – 17 per group, ± SEM. F. Time spent at the food hopper by female mice during the light and dark cycles, measured in seconds (s), n = 7 per group, ± SEM , * p < 0.05. G. Time spent at the food hopper by male mice during the light and dark cycles, measured in seconds (s), n = 9 – 10 per group, ± SEM. H. Time spent at the water bottle zone during the light and dark cycles (male and female mice combined), measured in seconds (s), n = 16 – 17 per group, ± SEM.
3.1.2. Rotarod
Mice were placed on a spinning rod which increased speed at regular intervals, beginning at 4 RPM and ramping up to 40 RPM over a 5-minute span. PON2-def mice had a significantly shorter latency to fall than WT mice (Figure 3), suggesting PON2 deficiency causes motor coordination deficits. No sex interaction was observed in rotarod performance, and male and female data were combined.
Figure 3. Latency to Fall from Rotarod in Wildtype and PON2 deficient Mice.
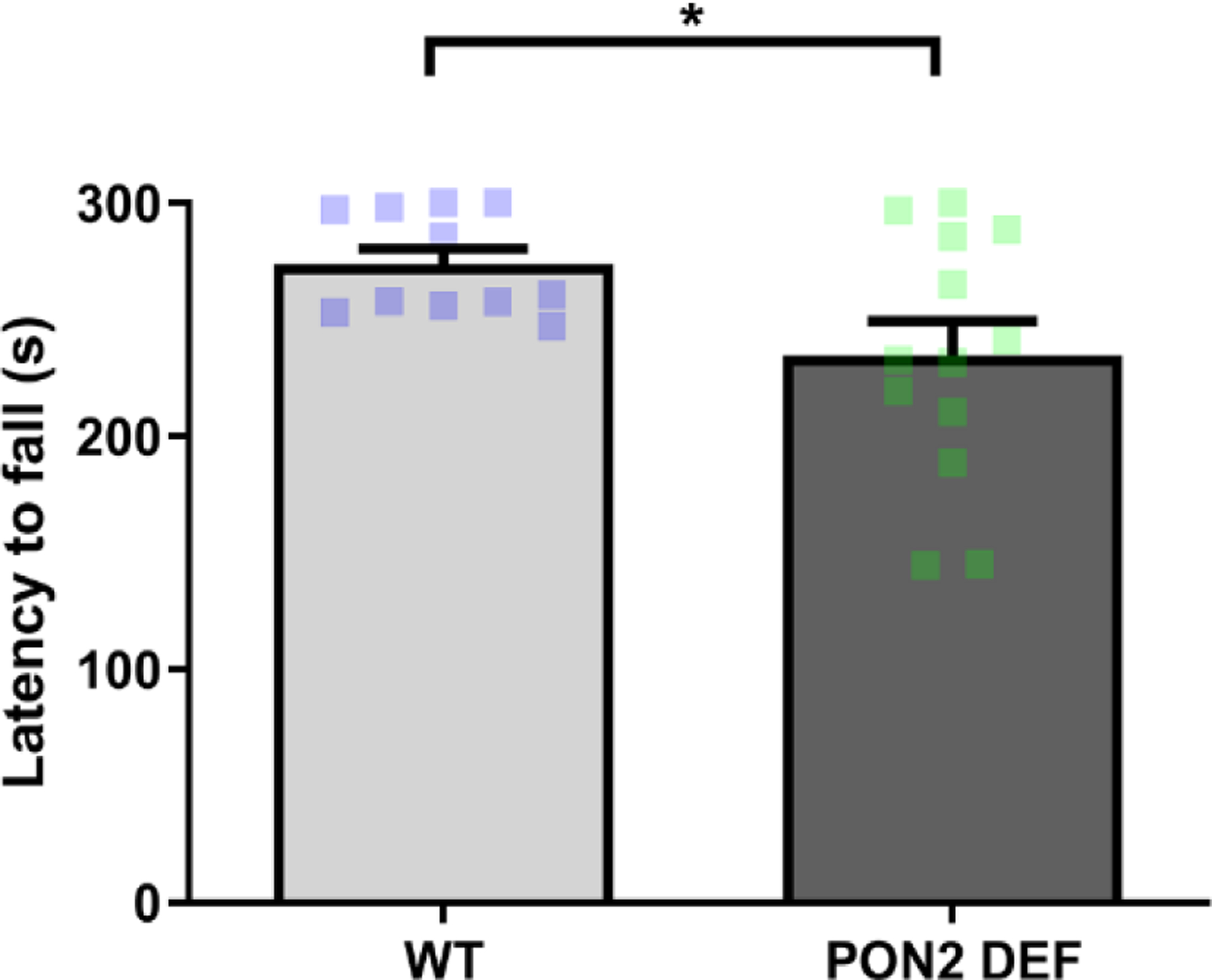
Latency in seconds (s) for mice to fall from rotating rod, starting at 4 RPM and ramping continuously to 40 RPM over a 5-minute period. Male and females combined, N = 11 – 13 per group, ± SEM, * p < 0.05.
3.1.3. CatWalk
Gait was assessed using the Noldus CatWalk system, with evaluated metrics falling into four general domains: temporal parameters, coordination, spatial patterns of individual paws and spatial pattern relationships between paws. No sex interaction was found for the reported gait metrics, and male and female data were combined. No differences were observed in PON2-def mice compared to WT for the temporal parameters of average speed of the mouse (Figure 4A), cadence (Figure 4B), swing (Figure 4C) and swing speed (Figure 4D). Likewise, no differences were observed in the coordination metrics of the sequence regularity index (Figure 4E) and percentage measurement of the step sequences (Figure 4F). Metrics of spatial relationships between paws were also largely unaffected, with no observed differences in base of support (Figure 4G) and most postural positions examined (Figure 4H and 4I). However, PON2-def mice spent significantly less time in a girdle support posture compared to WT (Figure 4H), where they place their weight on either their two front or two hind limbs simultaneously. Individual paw metrics of stride length, print intensity, print area, print length, and print width were similarly unaffected by PON2 deficiency (data not shown).
Figure 4. Gait Metrics Measured by CatWalk in Wildtype and PON2 deficient Mice.
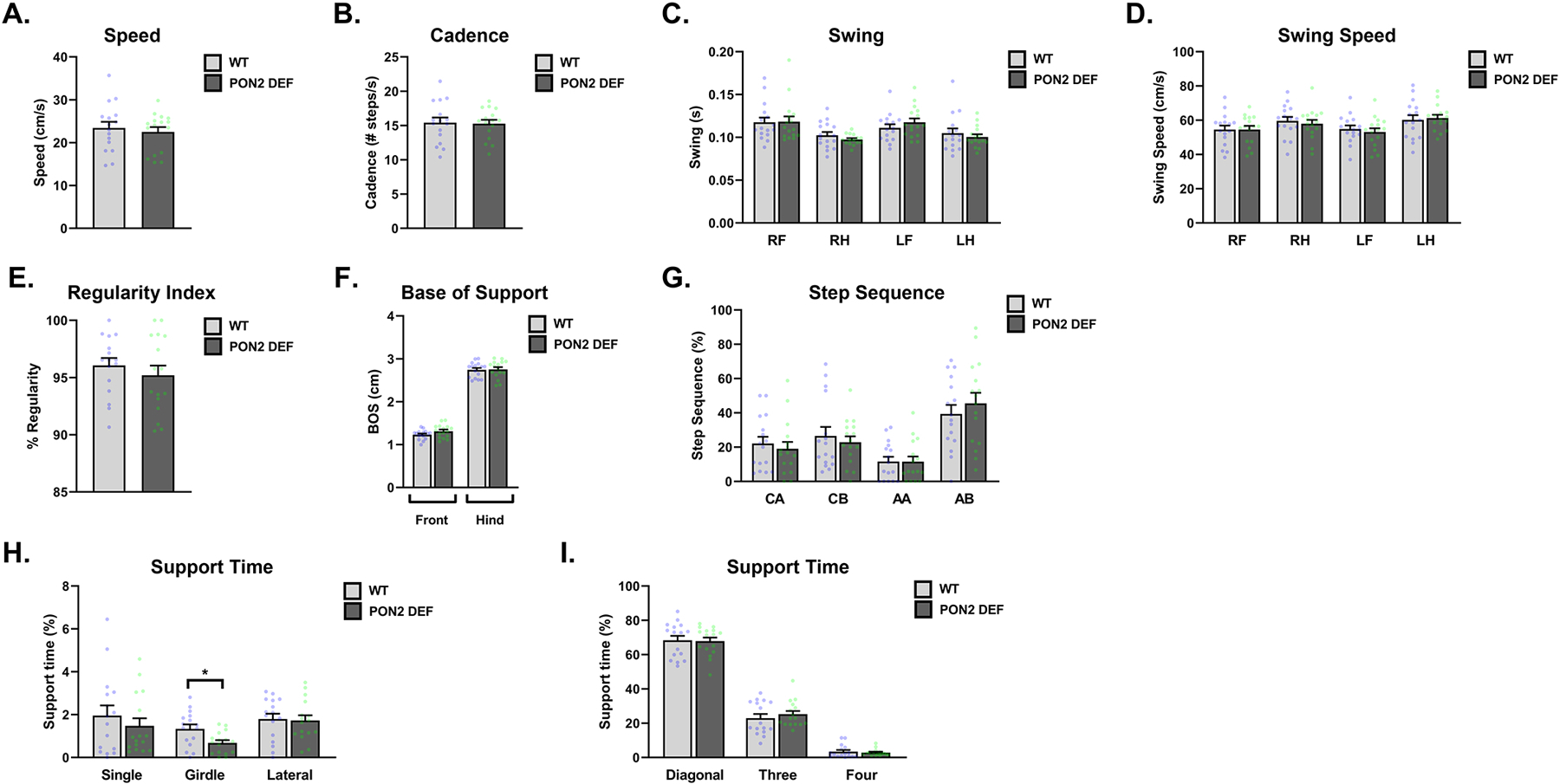
A. Average speed of mice, measured in centimeters/second (cm/s), n = 16 per group, ± SEM. B. Cadence of mice, measured as number of steps/second (steps/s), n = 16 per group, ± SEM. C. Swing of mice, measured in centimeters (cm), n = 16 per group, ± SEM. D. Average swing speed of mice, measured in centimeters/second (cm/s), n = 16 per group, ± SEM. E. Sequence Regularity Index of gait, measured as percent, n = 16 per group, ± SEM. F. Base of support for front and hind limbs, measured in centimeters (cm), n = 16 per group, ± SEM. G. Percentage of steps falling into outlined rodent step sequences, n = 16 per group, ± SEM. H. Percentage of time spent supporting in single, girdle and lateral positions, n = 16 per group, ± SEM , * p < 0.05. I. Percentage of time spent supporting in diagonal, three and four positions, n = 16 per group, ± SEM. All figures combine male and female mice.
3.2. RNA-Seq
3.2.1. Overview
RNA-Seq was conducted using three brain regions (cerebral cortex, striatum, and cerebellum) from WT and PON2-def mice previously tested for behavioral endpoints. PON2-def mice were confirmed to be deficient in all regions examined, with the cerebellum showing the least amount of PON2 expression in both sexes (Figure 5A). When comparing female and male expression profiles, WT mice displayed hundreds of differentially expressed genes (FDR<0.1) between female and males outside of anticipated sex-chromosome specific genes (Figures 5B – E), with gene totals varying depending on region. However, these sex differences appear to be lost in the cerebral cortex and striatum of PON2-def mice, where female and male mice only differ on a handful of sex-chromosome specific genes (Figures 5B – D). In the cerebellum, minor gene differences were observed for both groups, although the population of differentially expressed genes differed (Figures 5B and 5E). This loss of sex-based differential expression does not appear to be mediated by baseline Pon2 levels, as males and females have similar levels of Pon2 in both WT and PON2-def populations (Figure 5A). When comparing PON2-def and WT mice on a regional level, differential expression was highly sex- and region-dependent. While PON2-def female mice exhibited significant differences compared with WT in the striatum, there were no appreciable differences in the cerebral cortex and cerebellum (Figure 6A). Complimentarily, PON2-def male mice exhibited the opposite pattern, with significant differences from WT in the cerebral cortex and cerebellum, but no differences in the striatum (Figure 6A). There was minimal overlap of the differentially expressed genes between regions, supporting discrete regional impacts due to PON2 deficiency (Figure 6B – E).
Figure 5. Overview: PON2 deficiency Removes Sex Differences.

A. Expression of Pon2 by region (cerebral cortex, striatum, cerebellum) and sex (female and male) in WT and PON2 deficient mice, N = 5 per group, ± SEM. B. Differentially expressed genes in WT and PON2 deficient mice per region, comparing female to male expression, filtered by FDR<0.1. C. Venn diagram comparing populations of differentially expressed genes (female vs male expression) between WT (green) and PON2 deficient (purple) mice in cerebral cortex. D. Venn diagram comparing populations of differentially expressed genes (female vs male expression) between WT (green) and PON2 deficient (purple) mice in striatum. E. Venn diagram comparing populations of differentially expressed genes (female vs male expression) between WT (green) and PON2 deficient (purple) mice in cerebellum.
Figure 6. RNA-Seq Overview: Comparing PON2 deficient and WT Brain Regions.
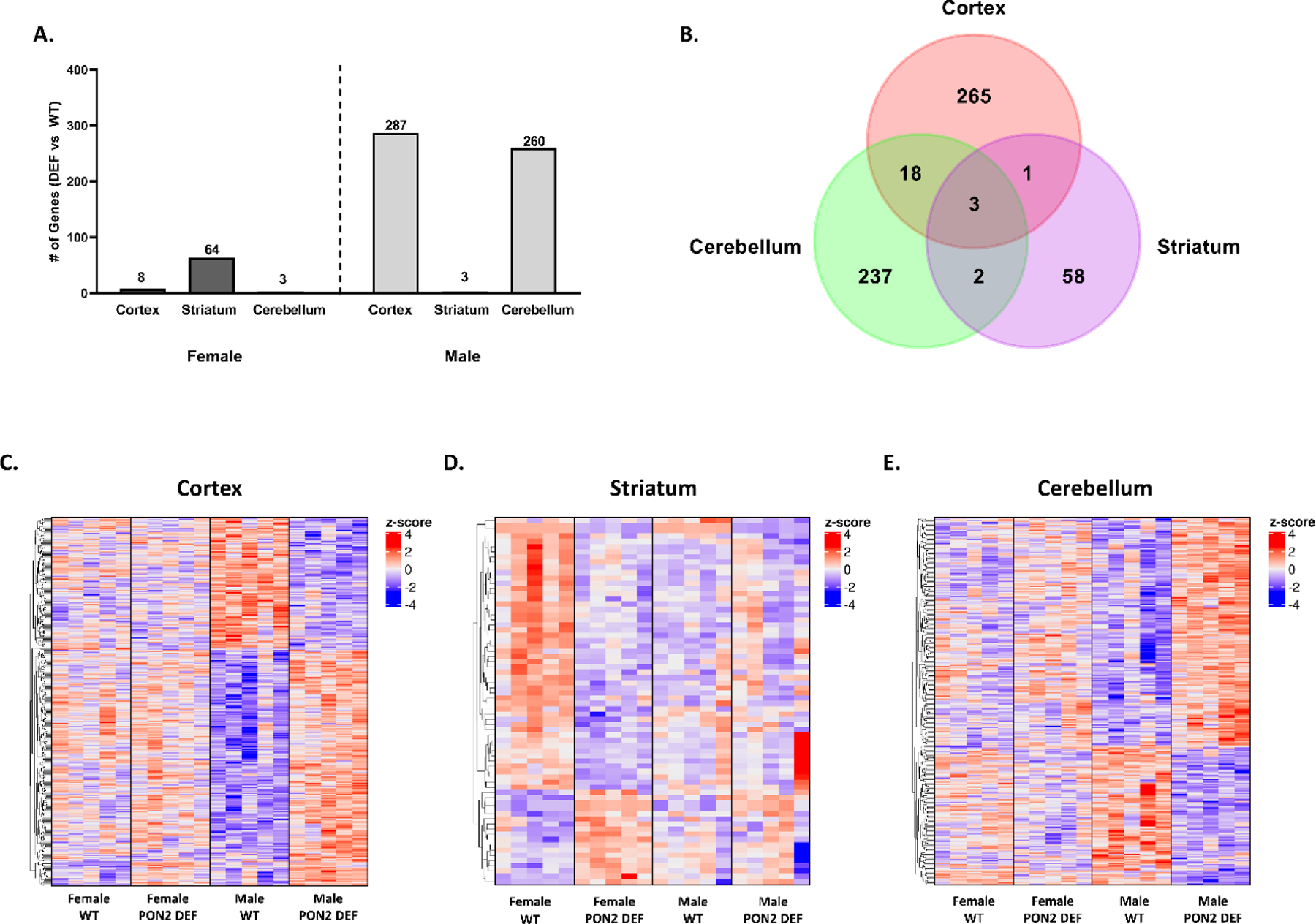
A. Overview of differentially expressed genes by region (cerebral cortex, striatum, cerebellum) and sex (female and male), comparing PON2 deficient to WT, filtered by FDR<0.1. B. Venn diagram of three regions (male cerebral cortex, female striatum, male cerebellum) with significant amount of differentially expressed genes comparing PON2 deficient to WT. C. Heatmap of gene expression in the cerebral cortex for PON2 deficient and WT mice. D. Heatmap of gene expression in the striatum for PON2 deficient and WT mice. E. Heatmap of gene expression in the cerebellum for PON2 deficient and WT mice. Colors in the heatmaps represent row-wise z-scores, e.g., a change of +1 indicates an increase in expression of one standard deviation for a given sample over the average expression of the gene in all samples.
Cerebral cortex
Comparing PON2-def and WT gene expression in the cerebral cortex of males, 287 genes were differentially expressed. Utilizing iPathwayGuide analysis, no significant KEGG pathways were identified after controlling for multiple comparisons. The top 5 significant Biological Processes Gene Ontology (GO) categories altered from PON2 deficiency were found to be glutamate reuptake, positive regulation of nuclear-transcribed mRNA poly(A) tail shortening, transcription by RNA polymerase II, meiotic spindle organization, and peptidyl-lysine dimethylation (Figures 7A and 7B). The top 5 significant Molecular Functions GO categories perturbed by PON2 deficiency were cyclo-ligase activity, methylated histone binding, RNA polymerase binding, core promoter sequence-specific DNA binding, and chromatin binding (Figures 7C and 7D). Based on the differentially expressed genes, iPathwayGuide predicts upstream regulators which may be activated or inactivated. In the cerebral cortex, transcription factors MLX interacting protein like (Mlxipl), neuronal PAS domain protein 2 (Npas2), and circadian locomotor output cycles kaput (Clock) were all predicted to be activated in PON2-def mice (Figure 8G) based on the gene expression changes noted in figures 8E and 8F. Chemical, drug and toxicant exposure was also predicted based on gene expression profiles. This prediction tool compared the expression profile of our samples to various compounds in the iPathwayGuide database to identify exposures which PON2 deficiency may mimic on a transcriptional level. PON2 deficiency leads to gene expression changes like those observed after aflatoxin B1 (Figure 8A) and dibutyl phthalate (Figure 8B) exposure, which are both predicted to be present (Figure 8D). Conversely, PON2 deficiency leads to gene expression changes suggesting the absence of troglitazone (Figure 8C and 8D), an anti-diabetic and anti-inflammatory drug. This indicates that PON2-def mice display a transcriptional profile like that of an untreated diabetic and/or an animal experiencing inflammation.
Figure 7. RNA-Seq: PON2 deficient Cerebral Cortex Pathway Analysis.
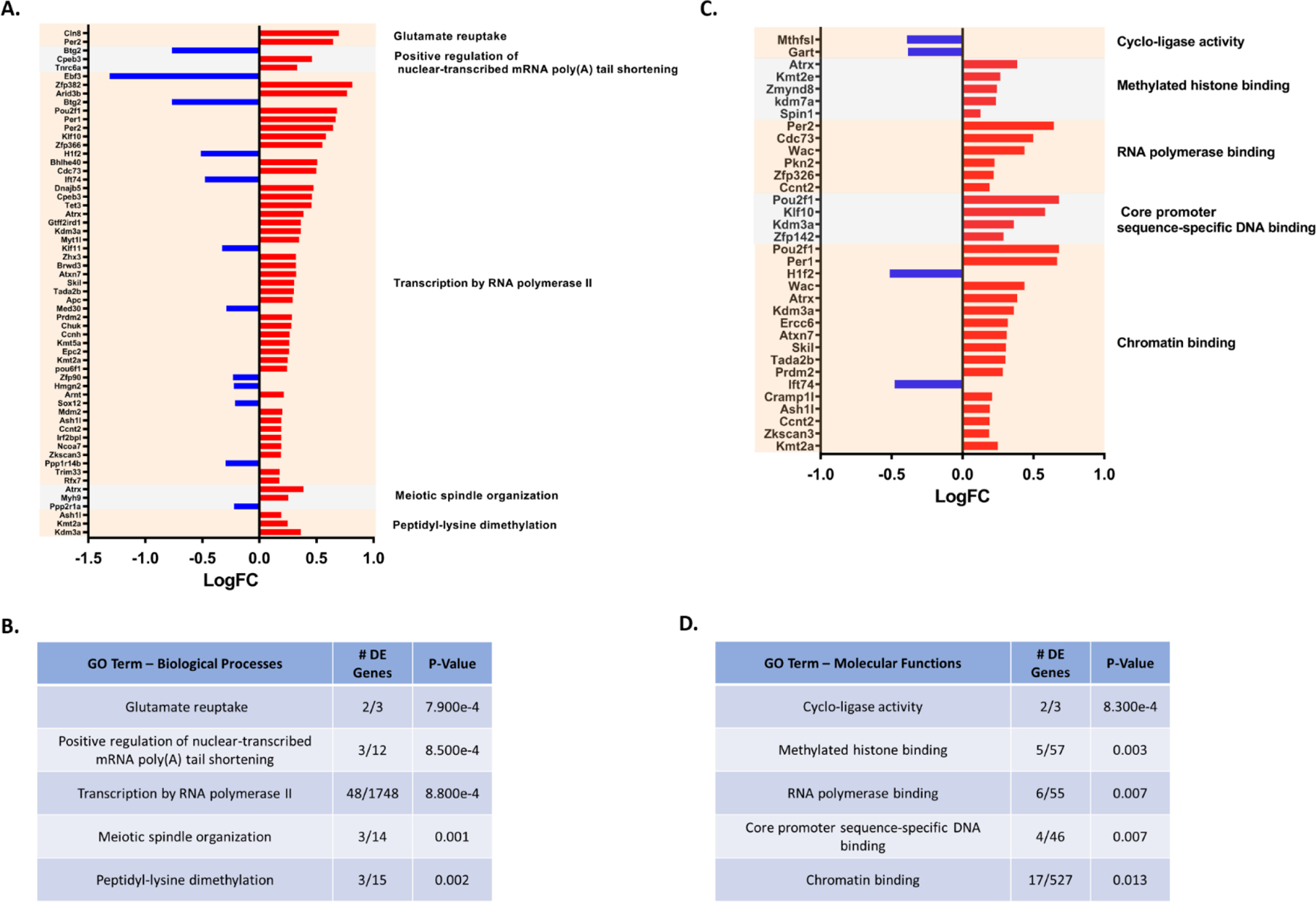
A. Differentially expressed genes (PON2 deficient vs WT) and associated Biological Processes GO categories in the male cerebral cortex, upregulated genes are shown in red and downregulated genes are shown in blue. B. Top 5 Biological Processes GO categories significantly impacted by PON2 deficiency in the male cerebral cortex, corrected for multiple comparisons using false discovery rate (FDR). C. Differentially expressed genes (PON2 deficient vs WT) and associated Molecular Functions GO categories in the male cerebral cortex, upregulated genes are shown in red and downregulated genes are shown in blue. D. Top 5 Molecular Functions GO categories significantly impacted by PON2 deficiency in the male cerebral cortex, corrected for multiple comparisons using false discovery rate (FDR).
Figure 8. RNA-Seq: Predicted Upstream Regulators in the PON2 deficient Cerebral Cortex.
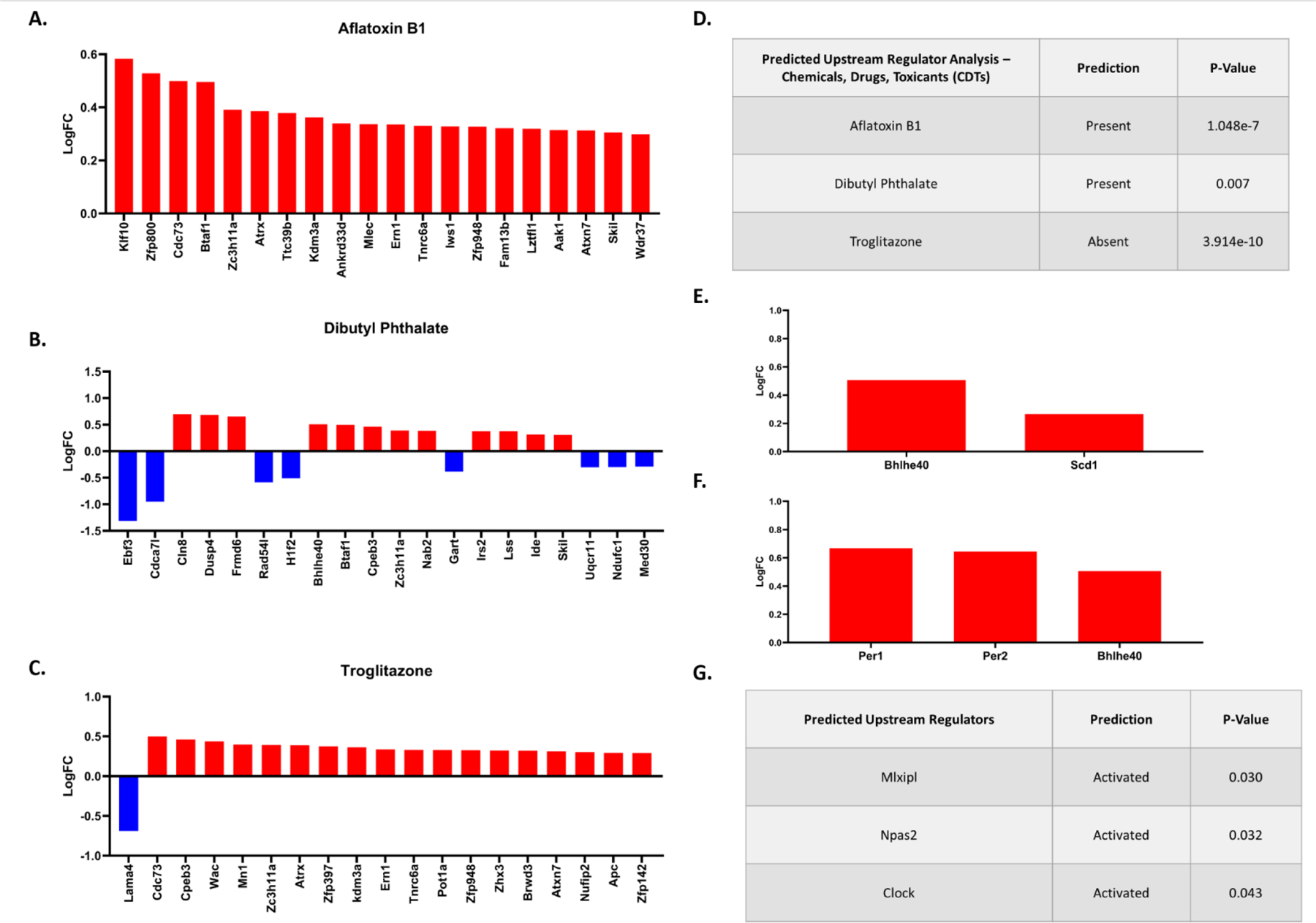
A. Differentially expressed genes in PON2 deficient male cerebral cortex related to aflatoxin B1 exposure. B. Differentially expressed genes in PON2 deficient male cerebral cortex related to dibutyl phthalate exposure. C. Differentially expressed genes in PON2 deficient male cerebral cortex related to troglitazone exposure. D. Predicted Upstream Regulator Analysis of Chemicals, Drugs and Toxicants predicted to be present or absent based on gene expression, corrected for multiple comparisons using false discovery rate (FDR). E. Differentially expressed genes targeted by Mlxipl (MLX interacting protein-like) F. Differentially expressed genes targeted by Npas2 (neuronal PAS domain protein 2) and Clock (circadian locomotor output cycles kaput). G. Predicted Upstream Regulators and their predicted activation status, corrected for multiple comparisons using false discovery rate (FDR).
Striatum
Comparing PON2-def and WT gene expression in the striatum of females, 64 genes were differentially expressed. iPathwayGuide analysis identified the top 5 significantly altered KEGG pathways due to PON2 deficiency as basal cell carcinoma, melanogenesis, hippo signaling pathway, breast cancer and Cushing syndrome (Figure 9A). All pathways were linked to changes in the same gene set (Figure 9B). The top 5 significant Biological Processes GO categories altered from PON2 deficiency were found to be regulation of asymmetric cell division, Wnt signaling pathway involved in midbrain dopaminergic neuron differentiation, forebrain neuroblast division, cerebellar granule cell differentiation, and negative regulation of signaling receptor activity (Figures 9C and 9D). The top 5 significant Molecular Functions GO categories affected by PON2 deficiency were steroid hormone receptor activity, delayed rectifier potassium channel activity, frizzle binding, transcription cofactor binding and GTPase activity (Figures 9E and 9F). No significant upstream regulators were identified in the striatum.
Figure 9. RNA-Seq: PON2 deficient Striatum Pathway Analysis.
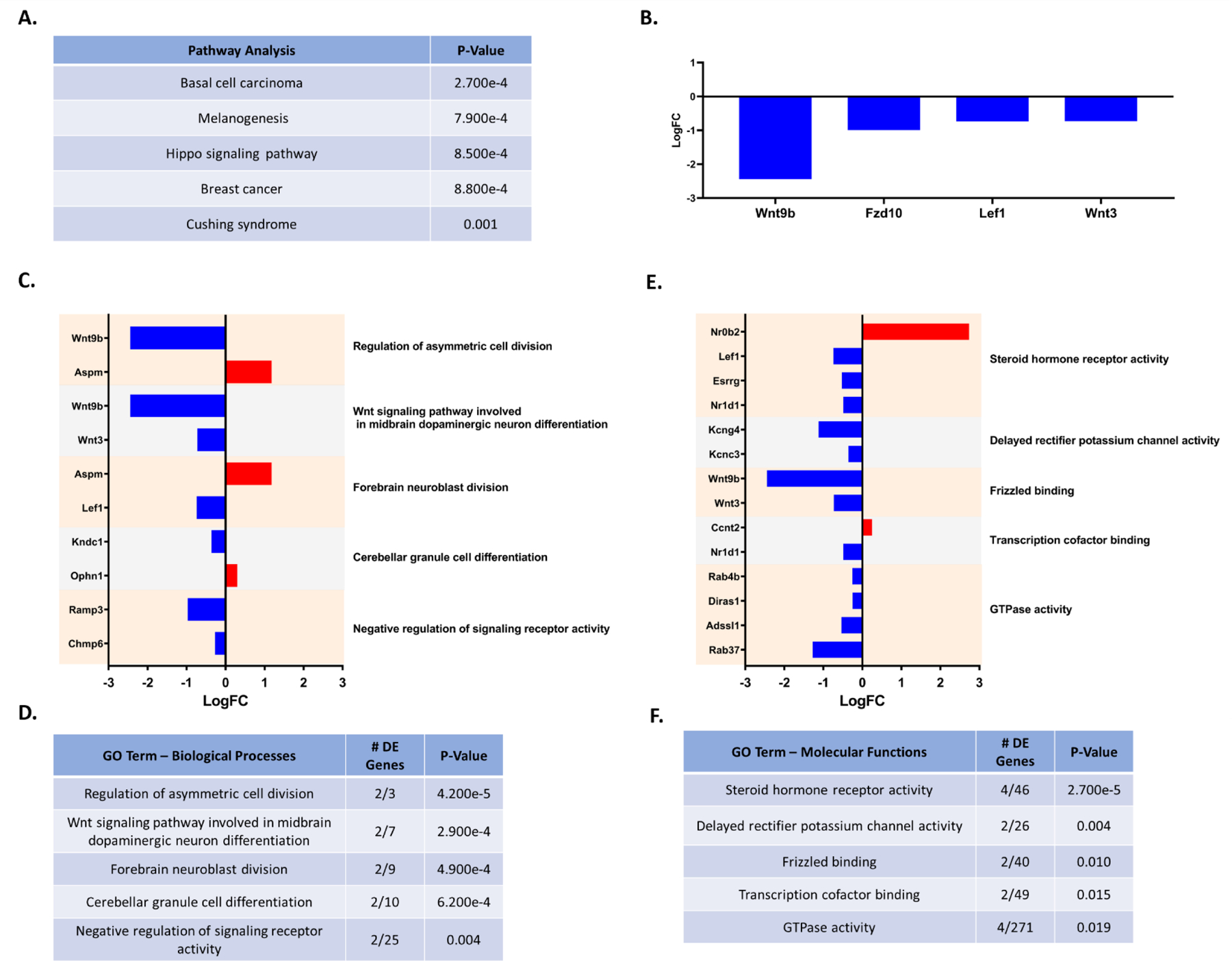
A. Top 5 predicted KEGG pathways affected by PON2 deficiency in the female striatum, corrected for multiple comparisons using false discovery rate (FDR). B. Differentially expressed genes (PON2 deficient vs WT) linked to predicted affected KEGG pathways C. Differentially expressed genes (PON2 deficient vs WT) and associated Biological Processes GO categories in the female striatum, upregulated genes are shown in red and downregulated genes are shown in blue. D. Top 5 Biological Processes GO categories significantly impacted by PON2 deficiency in the female striatum, corrected for multiple comparisons using false discovery rate (FDR). E. Differentially expressed genes (PON2 deficient vs WT) and associated Molecular Functions GO categories in the female striatum, upregulated genes are shown in red and downregulated genes are shown in blue. F. Top 5 Molecular Functions GO categories significantly impacted by PON2 deficiency in the female striatum, corrected for multiple comparisons using false discovery rate (FDR).
3.2.2. Cerebellum
Comparing PON2-def and WT gene expression in the cerebellum of males, 260 genes were differentially expressed. iPathwayGuide analysis identified no significant altered KEGG pathways after controlling for multiple comparisons. The top 5 significant Biological Processes GO categories affected by PON2 deficiency were found to be negative regulation of transcription by RNA polymerase II, inactivation of MAPK activity, ADP biosynthetic process, glutamate reuptake, and histone H3 deacetylation (Figures 10A and 10B). The top 5 Molecular Functions GO categories altered with PON2 deficiency were identified as protein tyrosine/serine/threonine phosphatase activity, transcription cofactor binding, pre-mRNA binding, RNA polymerase II regulatory region sequence-specific DNA binding, and protein kinase B binding (Figures 10C and 10D). Based on the differentially expressed genes noted in Figure 11A, predicted upstream regulators in the cerebellum were transcription neuronal PAS domain protein 2 (Npas2), circadian locomotor output cycles kaput (Clock) and aryl hydrocarbon receptor nuclear translocator like (Arntl) (Figure 11B).
Figure 10. RNA-Seq: PON2 deficient Cerebellum Pathway Analysis.
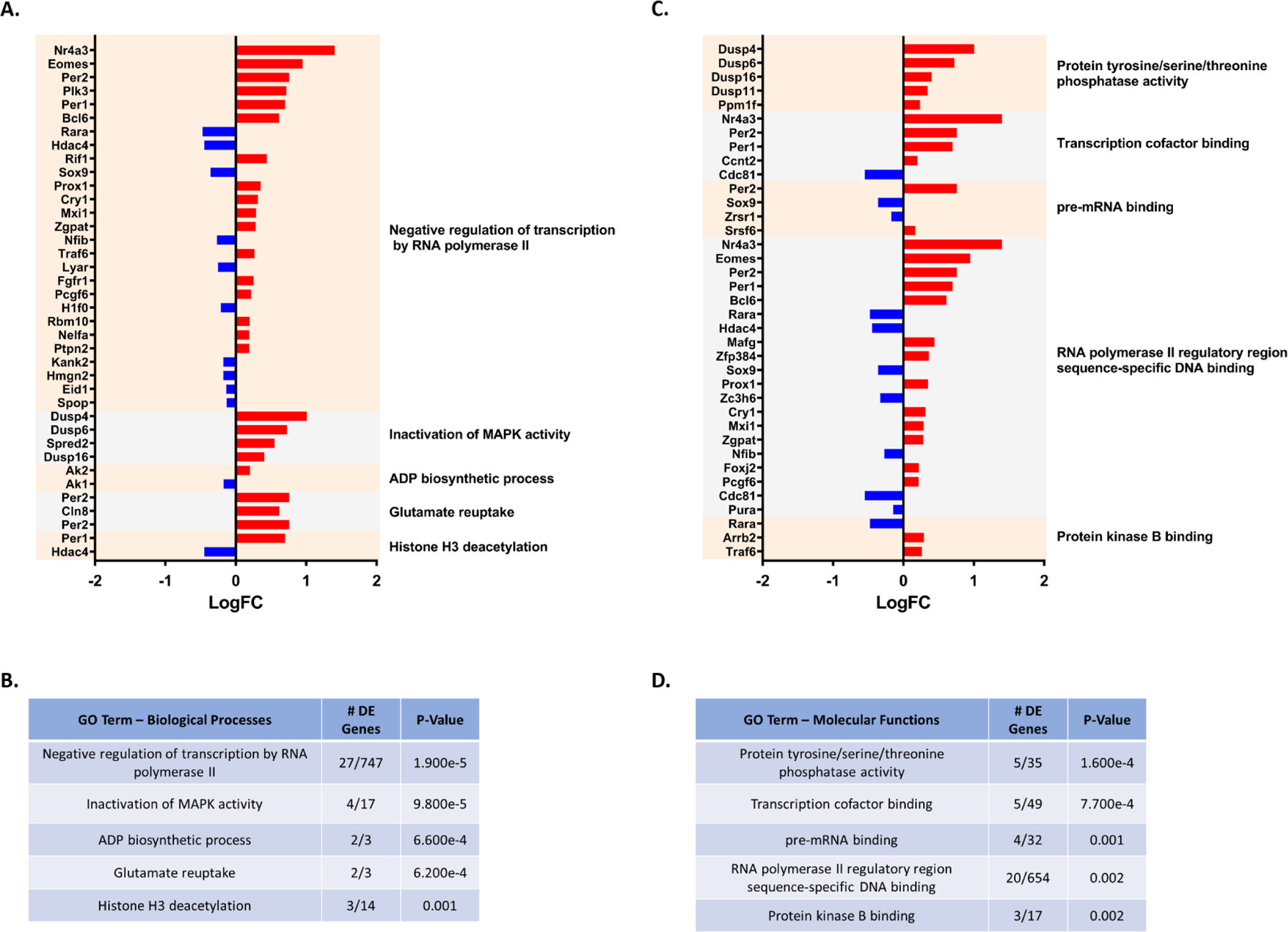
A. Differentially expressed genes (PON2 deficient vs WT) and associated Biological Processes GO categories in the male cerebellum, upregulated genes are shown in red and downregulated genes are shown in blue. B. Top 5 Biological Processes GO categories significantly impacted by PON2 deficiency in the male cerebellum, corrected for multiple comparisons using false discovery rate (FDR). C. Differentially expressed genes (PON2 deficient vs WT) and associated Molecular Functions GO categories in the male cerebellum, upregulated genes are shown in red and downregulated genes are shown in blue. D. Top 5 Molecular Functions GO categories significantly impacted by PON2 deficiency in the male cerebellum, corrected for multiple comparisons using false discovery rate (FDR).
Figure 11. RNA-Seq: Predicted Upstream Regulators in the PON2 deficient Cerebellum.
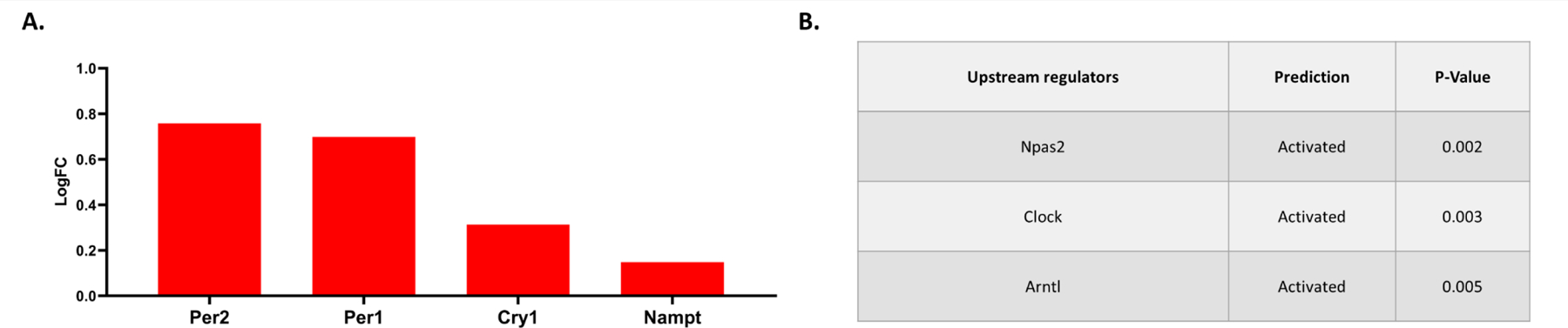
A. Differentially expressed genes targeted by Npas2 (neuronal PAS domain protein 2), Clock (circadian locomotor output cycles kaput) and Arntl (aryl hydrocarbon receptor nuclear translocator like). B. Predicted Upstream Regulators and their predicted activation status, corrected for multiple comparisons using false discovery rate (FDR).
4. DISCUSSION
Limited attention has been given to the role of PON2 in the central nervous system (CNS). As oxidative stress is increasingly implicated in multiple disease etiologies and known as a common mechanism by which neurotoxicants exert toxicity, further characterization of antioxidant genes, like PON2, in the CNS is warranted to inform gene-environment interaction studies. Single nucleotide polymorphisms (SNPs) affecting PON2 activity, potentially mimicking a ‘deficient’ state, are known to exist in the population (Dasgupta et al., 2011), underscoring a translational benefit to thoroughly understanding the effects of PON2 deficiency.
When evaluating behavior, PON2 deficiency in our study significantly impacted multiple home-cage metrics. Changes were only observed during the dark cycle, when mice are at peak wake activity (Ripperger et al., 2011). PON2-def mice spent more time moving and moved longer distances than WT, suggesting a mild hyperactivity phenotype. PON2-def mice also spent significantly more time in the center of the arena and less time in the hidden shelter. As prey animals, mice possess a defensive instinct and naturally prefer the safety of the hidden shelter, as open areas lend them to predation in the wild. More time spent in the center could support an anxiolytic phenotype, where PON2-def mice are less anxious in the home-cage environment and feel comfortable exploring the center more frequently. Additionally, this behavior could point to a reduction in fear response, where the PON2-def mice are unable to respond to environmental conditions which would usually induce fear behavior. Further exploration of this behavior using targeted tests, such as the elevated plus maze, would be beneficial, as would assessing impacts to their conditioned fear response. The only metric with an observed sex-interaction was time spent at the food hopper, where PON2-def females spent significantly more time at the hopper than WT. PON2 deficiency has recently been investigated in relation to obesity, with PON2-def mice exhibiting diet-prone obesity when fed a high-fat diet (Shih et al., 2019). No differences in body mass were noted between PON2-def and WT mice in our study, nor were there recorded differences in food consumption by hopper kibble weight (data not shown). As such, the additional time spent by female PON2-def mice at the food hopper may be unrelated to food consumption and due to the location of the food hopper within the cage. Alternatively, these mice may be playing with the food for additional enrichment. Mice are singly housed for the duration of this experiment and lack the usual social interaction provided by their cage mates. Feasibly, PON2-def female mice may be more sensitive to disruptions to social interaction and require more enrichment. Further evaluation to measure social and play behavior with PON2 deficiency would be of interest.
PON2-def mice had significantly shorter latency to fall during the rotarod experiment, suggesting impaired motor coordination (Figure 3). When evaluated for gait metrics on the CatWalk system, PON2-def mice also spent significantly less time using girdle postural support (Figure 4H). This posture is when the mice support their weight on either their two front or two hind limbs simultaneously. Given the nature of the rotarod test, this reduction in girdle postural support may influence their success on the rotarod, particularly if their ability to balance on their two front limbs is impaired. A reduction in front limb balancing could reduce their ability to recover from a slip on the rod, as a slip usually begins with the loss of hind limb rod contact and forces the mouse to balance on their two front limbs until they reestablish hind limb contact or fall off the rod completely. Additional targeted testing of cerebellar function, both motor and autonomic, would be of interest as the cerebellum was found to be the tissue most deficient in Pon2.
Transcript changes were noted in a highly region- and sex-specific manner in our study, with the full list of differentially expressed genes available in Supplemental Tables 1 – 3. Although sex differences have been reported in the literature relative to PON2 expression, with females showing higher expression than males (Giordano et al., 2011; Giordano et al., 2013), this was not observed in the present study where Pon2 expression in WT females and males was comparable. However, PON2 deficiency appears to significantly change the differences between female and male expression patterns on a global transcription scale as noted in Figures 5C – E. While WT females had hundreds of differentially expressed genes in the cerebral cortex and striatum compared to males, these differences were not observed in PON2-def females, with only the expected sex chromosome genes notably different. The differential gene expression was less dramatic in the cerebellum, where WT females had a smaller number of differentially expressed genes compared to WT males, and PON2-def females had a similar value. However, the composition of these genes differed between the WT and PON2-def populations, suggesting significant alterations. Taken together, these results support that PON2 deficiency significantly affects sex-specific expression patterns and removes sex differences in the brain at the transcript level, which may have potential consequences on morbidities with sex interactions.
Notably, we did not observe many sex differences when evaluating behavior in our study. Given the transcriptional results, behavioral differences between WT male and female mice might be expected, while PON2 deficiency could presumably reduce or abolish these differences, mimicking the pattern observed with the transcriptional profile. However, of the metrics measured, only time spent at the food hopper in the PhenoTyper home-cage assessment differed in a sex-based manner among PON2-def mice, and no statistically significant differences were found between WT male and female mice for any tests. Sex-based differences are often overlooked in biomedical research, with most historical studies conducted utilizing only male animals (Beery & Zucker 2011). Work to include both sexes and fully understand potential differences has been pushed only recently, thus our understanding of baseline sex differences in control animals is still lacking—one systematic review suggests between 9.9 – 56.6% of experiments exhibit sexual dimorphic results among control animals (Karp et al., 2017). Factors such as age, mouse strain, husbandry, diet and the estrus cycle stage of the females at the time of testing may influence how strong the sex-based differences are within a given cohort (McFadyen et al., 2003; Kovács & Pearce 2013; Chari et al., 2020). Furthermore, these differences may be subtle and could require a higher number of animals than what was utilized in our study to achieve statistical significance. Feasibly, the endpoints measured by the PhenoTyper, Rotarod and CatWalk may not differ by sex at baseline in WT animals and may not be the best endpoints to measure effects on sex differences with PON2 deficiency. In a large-scale study utilizing 4,554 and 5,311 mice, respectively, water maze and open field results were shown to be identical between males and female mice (Fritz et al., 2017). As well, other investigators have found no sex differences in WT animals when evaluating home-cage metrics (Yokota et al., 2017), highlighting that not all behavioral endpoints demonstrate sex-based differences in control animals. However, sex differences in rotarod performance of control animals are mixed in the literature, with some investigators identifying differences (McFadyen et al., 2003; Antzoulatos et al., 2010; Kovács & Pearce 2013) and others finding none (Chen et al., 2019; Chari et al., 2020). Size may influence rotarod performance, with larger animals noted as performing more poorly (McFadyen et al., 2003). As males are generally larger than females, this may be one explanation for the sexual disparity. Cohorts with larger weight disparities between the sexes may exhibit stronger sex-based performance differences on the rotarod and could explain some of the literature discrepancies. Although differences were not observed in the suite of behavioral tests conducted in our study, the observed transcriptional differences could impact other behavioral endpoints which were not addressed, such as memory, social interactions or contextual fear response. Studies have demonstrated memory function and contextual fear response to be sex-mediated (Yagi & Galea 2019) and may be more suitable at detecting sex-based changes with PON2 deficiency. Evaluating additional behavioral endpoints would be of future interest to determine if the transcriptional changes with PON2 deficiency lead to sex-based behavioral alterations in other metrics.
The sex-based differences found with PON2 deficiency have specific implications for toxicological studies, as exposure and response to toxicants is noted to be sexually dimorphic (Gochfeld 2017). Understanding genes which may drastically alter the transcriptome and cause biologically female animals to present transcriptionally as male, or vise-versa, is critical when assessing gene-environment interactions to toxicants. Furthermore, this has impacts for assessing individual toxicant susceptibility based on genotype. Pon2 is known to be polymorphic in the population, with SNPs affecting general PON activity (Dasgupta et al., 2011). This could be of particular importance if individuals with Pon2 polymorphisms which reduce PON2 activity are exposed to a toxicant or prescribed a medication with sexually dimorphic outcomes, as they may have an idiosyncratic response. Although limited work has addressed the functional impact of Pon2 polymorphisms, variability of PON1 levels or activity vary by at least 15-fold among individuals (Furlong 2007), and it is possible that a similar observation may be seen with PON2.
Our study focused on assessing the effect of PON2 deficiency on the baseline transcriptome of specific brain regions. At odds with our hypothesis, oxidative stress pathways were not the predominant pathways perturbed by PON2 deficiency when looking at the iPathwayGuide analysis. These findings were surprising, as PON2 deficiency has been reported to increase oxidative stress in multiple systems by numerous investigators (Devarajan et al., 2011; Giordano et al., 2013; Yang et al., 2015; Sulaiman et al., 2019). Feasibly, impacted oxidative stress pathways may be more apparent if the mice were challenged with an oxidant, an aspect that was not addressed in this study. While no direct pathways were picked up by this analysis, supporting evidence for increased oxidative stress was noted in the male cerebral cortex with the chemical, drug and toxicant exposure prediction tool. This prediction tool aggregates data from multiple exposure studies and identifies genes within a sample set that have been shown to be similarly affected by a specific toxicant exposure. While this tool utilizes data from multiple cell-type studies and is not specific to brain tissue, it is a valuable tool to identify common genes which are both impacted by PON2 deficiency and an exogenous toxicant exposure. When comparing the expression profile of our samples to various compounds in the iPathwayGuide database, PON2 deficiency leads to gene expression changes like those observed after aflatoxin B1 exposure (Figure 8A). While aflatoxin B1 exhibits well-documented hepatotoxicity and is a known carcinogen, it also has neurotoxic effects, largely mediated through increased oxidative stress (Bbosa et al., 2013). Recent work has additionally identified that aflatoxin B1 alters calcium homeostasis and causes mitochondrial dysfunction in human astrocytes in-vitro (Park et al., 2020) and increases ROS in the brain of mice in-vivo (Huang et al., 2020). As such, the predicted presence of aflatoxin B1 exposure by iPathwayGuide may be related to the reported mitochondrial dysfunction and oxidative stress noted with PON2 deficiency. Furthermore, this finding suggests PON2 deficiency may confer increased sensitivity to aflatoxin B1 exposure, if the expression of similar genes targeted by the toxin are already altered. This may point to individuals with lower PON2 levels as a previously unidentified sensitive population to aflatoxin B1 exposure. Considering the wide global distribution of aflatoxin contaminated food commodities, this could pose a significant public health concern and warrants further investigation regarding the relationship of PON2 deficiency and aflatoxin exposure. Similarly, iPathwayGuide predicted PON2 deficiency to mimic dibutyl phthalate exposure (Figure 8B). Dibutyl phthalate is one of the most used phthalate esters in plastics manufacturing and is a ubiquitous environmental contaminant, with demonstrated neurotoxic effects attributed to an increase in ROS (Wójtowicz et al., 2017). In a similar manner to aflatoxin B1, PON2 deficiency may confer sensitivity to dibutyl phthalate exposure and further investigation of this would be of interest. Finally, PON2 deficiency leads to gene expression changes suggesting the absence of troglitazone (Figure 8C), an anti-diabetic and anti-inflammatory drug, supporting an increase in inflammation and/or dysregulation of insulin pathways. Insulin degrading enzyme (Ide) was found significantly upregulated in PON2-def cerebral cortex (Supplementary Table 1), further linking PON2 deficiency and insulin dysregulation.
Neurological disorders, ranging from neurodevelopment to aging, are often sex dependent. In early life, males are four times as likely to develop autism spectrum disorder (ASD) than females, with the underlying mechanism for this difference currently unknown but speculated to be a complex combination of genetic and environmental factors (Park et al., 2016). In contrast, females are twice as likely to experience affective disorders such as anxiety, post-traumatic stress disorder, and major depression, with evidence supporting sex hormone interactions as a possible culprit (Bangasser & Valentino 2014). In our study, female PON2-def mice had significantly higher expression of the small heterodimer partner Nr0b2 (nuclear receptor subfamily 0, group B, member 2) in the striatum. Nr0b2 has been shown to interact with estrogen receptors and inhibit their function (Seol et al., 1996), as well as function as a mediator of endocrine homeostasis in male mice (Vega et al., 2015). Indeed, iPathwayGuide analysis showed steroid hormone receptor activity as the highest impacted molecular functions pathway from PON2 deficiency in the striatum (Figure 9F). This alteration of Nr0b2 and steroid hormone receptor activity may inhibit estrogen signaling in the striatum of female mice and contribute to the male-presenting transcriptome observed in this study. Perturbations in estrogen response in-vitro are noted in the literature with PON2 deficiency, where estradiol protects primary WT astrocytes from oxidative damage, but does not protect primary astrocytes from PON2-def mice (Giordano et al., 2013). These results are consistent with those of our study and support the concept that PON2 deficiency may inhibit estrogen signaling, although targeted study of this should be addressed. Behaviorally, PON2-def mice displayed a mild anxiolytic phenotype, as noted by their increased time spent in the center of the arena and decreased time in the hidden shelter during the PhenoTyper assessment. Feasibly, this may also be related to the inhibitory effects on the hypothalamic-pituitary axis (HPA) by Nr0b2 and sex steroid hormone pathways which affect mood and behavior (Bangasser & Valentino 2014).
Neurodegenerative diseases also differ between sexes, with Parkinson’s disease affecting males more than females, and Alzheimer’s disease affecting females at a higher prevalence compared to age-matched males (Ullah et al., 2019). Alzheimer’s disease is marked by aggregation of amyloid beta (Aβ) and progressive neuron loss, with multiple hypotheses as to how these aggregates destroy neurons. One line of evidence suggest Aβ plaques inhibit glutamate uptake, leading to excitotoxic levels of glutamate to bind to postsynaptic glutamate receptors (Danysz &Parsons 2012). In our study, glutamate reuptake related genes Cln8 (ceroid-lipofuscinosis, neuronal 8) and Per2 (period 2) were upregulated in the cerebral cortex and cerebellum of PON2-def male mice. While this would support a potential protective effect against excitotoxicity for neurons, over-clearance of glutamate from the synaptic cleft may have its own deleterious effects, reducing sensitivity to reward and contributing to symptoms of depression. The increase in reuptake may also point to increases in glutamate output, requiring increases in reuptake to prevent excitotoxicity. Additional investigation of reward seeking behavior and depression would be of interest in PON2-def mice, as well as further probing of the glutamatergic system.
In addition to sex differences, regional differences were highly specific, with iPathwayGuide analysis revealing few common GO terms shared among them. In the cerebral cortex and the cerebellum of PON2-def male mice, a variety of RNA processing and transcription-mediating pathway changes were noted (Figures 7 and 10). Changes in general transcription could have downstream implications on countless pathways and would require targeted hypothesis-testing to determine the precise impact of PON2 deficiency in a specific pathway or disease state, which were not addressed in this study. Predicted upstream regulators in these regions were also identical, with the majority of these in the family of circadian regulators, namely Clock and its paralogue Npas2. Although circadian rhythm disruptions were not noted in PON2-def mice during home-cage assessment, the hyperactivity during the dark cycle may be related to activated circadian pathways. Additionally, Per1 and Per2, both upregulated in the cerebral cortex and cerebellum of PON2-def male mice and transcriptionally controlled by the CLOCK complex, have been shown to play an important role in cancer cell biology, where overexpression of PER1 sensitizes human cancer cells to DNA-damage induced apoptosis (Gery et al., 2006). Indeed, multiple cellular division pathways are under circadian control and the involvement of circadian genes in cancer biology is emerging as an important area of study (Masri & Sassone-Corsi 2018). The role of PON2 in cancer biology has also been examined, with PON2 often found upregulated in cancer cells, likely providing apoptotic protection through the management of ROS at the mitochondrial level (Kruger et al., 2015). Whether PER1 expression plays a role in the ability for PON2 to protect cells against apoptosis has not been addressed in the literature but would be of interest for further evaluation. PON2 deficiency in the striatum of female mice in our study had significant decreases in expression of Wnt9b (wingless-type 9b), Fzd10 (frizzled-10), Lef1 (lymphoid enhancer-binding factor 1) and Wnt3 (wingless-type 3) (Figure 9B). These decreases in gene expression are anticipated to impact multiple cancer-related pathways, as noted in Figure 9A. Given the role of WNT signaling in cancer development, decreased expression of WNT may have a protective effect for cancer promotion but have deleterious effects on the brain in normal aspects of WNT signaling, such as cellular differentiation. The protective effects on cancer development and/or progression from PON2 deficiency have not been studied in detail, although targeted PON2 knockdown has been shown to make human cancer cells more susceptible to irradiation damage (Kruger et al., 2015) and the modulation of PON2 in this arena may be of further interest. Our study analyzed whole regions of the cerebral cortex, striatum, and cerebellum, and did not discriminate by subregion. While this method provides a broad overview of PON2 deficiency in the brain, valuable subregion information was not discernable and was a limitation of our study design. Further analysis of subregions would be useful to pinpoint the effects of PON2 deficiency in the brain and determine if the altered RNA expression observed in our study represents a global effect within the region, or if these changes are being driven by a particular subregion.
While PON2 has been identified as an important modulator of oxidative stress in the CNS, limited work has been done to address the effects of PON2 deficiency in the brain on a global level. Our study begins to address key gaps in the literature regarding the impact of PON2 deficiency in the CNS and what global changes, both biochemically and phenotypically, may be caused by the loss of PON2. We have shown that PON2 deficiency leads to behavioral changes, specifically related to locomotion, and significant biochemical alterations at the transcript level impacting a variety of molecular functions that have implications for affective disorders, cellular differentiation, and cancer biology. Highly specific sex and regional changes were observed when looking at RNA expression, indicating that PON2 may play a variety of distinct roles in different regions of the brain in a sex-specific manner. Many of the differentially expressed genes identified in this study were previously unknown to be affected by PON2 deficiency and provide novel directions for future PON2 research. Further investigation looking at temporal elements, additional brain regions and behavioral domains would be of interest to further characterize the role of PON2 in the brain and the consequences of its deficiency.
Supplementary Material
Highlights.
Paraoxonase 2 deficiency causes motor deficits in mice as tested by the Rotarod
Home-cage behavior and gait metrics largely unaffected by paraoxonase 2 deficiency
Paraoxonase 2 deficient mice display regional- and sex-specific changes in transcript expression in the brain compared to wildtype
RNA-Seq analysis demonstrates previously unknown pathways to be affected by paraoxonase 2 deficiency in the brain
ACKNOWLEDGEMENTS
This work was supported in part by the Superfund Research Program (SRP) grant P42ES004696 and the Environmental Pathology/Toxicology training grant T32 ES007032–37 from the National Institute of Environmental Health Sciences. We thank Khoi Dao for her assistance with animal husbandry and Zahra Afsharinejad from the Functional Genomics & Bioinformatics Core of the SRP at the University of Washington for processing of RNA samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
5 REFERENCES
- Alexa A, Rahnenführer J, & Lengauer T (2006). Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics (Oxford, England), 22(13), 1600–1607. 10.1093/bioinformatics/btl140 [DOI] [PubMed] [Google Scholar]
- Altenhöfer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Förstermann U, & Horke S (2010). One Enzyme, Two Functions. The Journal of Biological Chemistry, 285(32), 24398–24403. 10.1074/jbc.M110.118604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzoulatos E, Jakowec MW, Petzinger GM, & Wood RI (2010). Sex differences in motor behavior in the MPTP mouse model of Parkinson’s disease. Pharmacology Biochemistry and Behavior, 95(4), 466–472. 10.1016/j.pbb.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmat U, Abad K, & Ismail K (2016). Diabetes mellitus and oxidative stress—A concise review. Saudi Pharmaceutical Journal, 24(5), 547–553. 10.1016/j.jsps.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, & Valentino RJ (2014). Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Frontiers in Neuroendocrinology, 35(3), 303–319. PubMed. 10.1016/j.yfrne.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Rogovsky H, Hugenmatter A, & Tawfik DS (2013). The Evolutionary Origins of Detoxifying Enzymes: THE MAMMALIAN SERUM PARAOXONASES (PONs) RELATE TO BACTERIAL HOMOSERINE LACTONASES*. Journal of Biological Chemistry, 288(33), 23914–23927. 10.1074/jbc.M112.427922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bbosa GS, Kitya D, Lubega A, Ogwal-Okeng J, Anokbonggo WW, & Kyegombe DB (2013). Review of the Biological and Health Effects of Aflatoxins on Body Organs and Body Systems. Aflatoxins - Recent Advances and Future Prospects 10.5772/51201 [DOI] [Google Scholar]
- Beery AK, & Zucker I (2011). Sex bias in neuroscience and biomedical research. Neuroscience & Biobehavioral Reviews, 35(3), 565–572. 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari T, Griswold S, Andrews NA, & Fagiolini M (2020). The Stage of the Estrus Cycle Is Critical for Interpretation of Female Mouse Social Interaction Behavior. Frontiers in Behavioral Neuroscience, 14. 10.3389/fnbeh.2020.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-H, Wang V, Huang EY-K, Chou Y-C, Kuo T-T, Olson L, & Hoffer BJ (2019). Delayed Dopamine Dysfunction and Motor Deficits in Female Parkinson Model Mice. International Journal of Molecular Sciences, 20(24), 6251. 10.3390/ijms20246251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, & Parsons CG (2012). Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine—Searching for the connections. British Journal of Pharmacology, 167(2), 324–352. PubMed. 10.1111/j.1476-5381.2012.02057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Demirci FY, Dressen AS, Kao AH, Rhew EY, Ramsey-Goldman R, Manzi S, Kammerer CM, & Kamboh MI (2011). Association analysis of PON2 genetic variants with serum paraoxonase activity and systemic lupus erythematosus. BMC Medical Genetics, 12(1), 7. 10.1186/1471-2350-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deavall DG, Martin EA, Horner JM, & Roberts R (2012). Drug-Induced Oxidative Stress and Toxicity. Journal of Toxicology, 2012, 645460. 10.1155/2012/645460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, Clarke CF, Vergnes L, Reue K, Teiber JF, & Reddy ST (2011). Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxidants & Redox Signaling, 14(3), 341–351. PubMed. 10.1089/ars.2010.3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato M, Xu Z, Tomoiaga A, Granneman JG, MacKenzie RG, Bao R, Than NG, Westfall PH, Romero R, & Draghici S (2013). Analysis and correction of crosstalk effects in pathway analysis. Genome Research, 23(11), 1885–1893. 10.1101/gr.153551.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganov DI, & La Du BN (2004). Pharmacogenetics of paraoxonases: A brief review. Naunyn-Schmiedeberg’s Archives of Pharmacology, 369(1), 78–88. 10.1007/s00210-003-0833-1 [DOI] [PubMed] [Google Scholar]
- Draganov Dragomir I., Teiber JF, Speelman A, Osawa Y, Sunahara R, & La Du BN (2005). Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. Journal of Lipid Research, 46(6), 1239–1247. 10.1194/jlr.M400511-JLR200 [DOI] [PubMed] [Google Scholar]
- Fritz A-K, Amrein I, & Wolfer DP (2017). Similar reliability and equivalent performance of female and male mice in the open field and water-maze place navigation task. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 175(3), 380–391. 10.1002/ajmg.c.31565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong CE (2007). Genetic variability in the cytochrome P450-paraoxonase 1 (PON1) pathway for detoxication of organophosphorus compounds. Journal of Biochemical and Molecular Toxicology, 21(4), 197–205. 10.1002/jbt.20181 [DOI] [PubMed] [Google Scholar]
- Furlong CE, Marsillach J, Jarvik GP, & Costa LG (2016). Paraoxonases-1, −2 and −3: What are their functions? Chemico-Biological Interactions, 259(Pt B), 51–62. PubMed. 10.1016/j.cbi.2016.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick JM, Dao K, de Laat R, Elsworth J, Cole TB, Marsillach J, Furlong CE, & Costa LG (2016). Developmental expression of paraoxonase 2. Chemico-Biological Interactions, 259(Pt B), 168–174. PubMed. 10.1016/j.cbi.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, & Koeffler HP (2006). The Circadian Gene Per1 Plays an Important Role in Cell Growth and DNA Damage Control in Human Cancer Cells. Molecular Cell, 22(3), 375–382. 10.1016/j.molcel.2006.03.038 [DOI] [PubMed] [Google Scholar]
- Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, & Costa LG (2013). Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radical Biology & Medicine, 58, 98–108. PubMed. 10.1016/j.freeradbiomed.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, & Costa LG (2013). Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radical Biology & Medicine, 58, 98–108. 10.1016/j.freeradbiomed.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano Gennaro, Cole TB, Furlong CE, & Costa LG (2011). Paraoxonase 2 (PON2) in the mouse central nervous system: A neuroprotective role? Toxicology and Applied Pharmacology, 256(3), 369–378. 10.1016/j.taap.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochfeld M (2017). Sex Differences in Human and Animal Toxicology: Toxicokinetics. Toxicologic Pathology, 45(1), 172–189. 10.1177/0192623316677327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosch J, Winkler J, & Kohl Z (2016). Early Degeneration of Both Dopaminergic and Serotonergic Axons – A Common Mechanism in Parkinson’s Disease. Frontiers in Cellular Neuroscience, 10. 10.3389/fncel.2016.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann H, Kuczkowski A, Ruehl M, Lamkemeyer T, Brodesser S, Horke S, Dryer S, Schermer B, Benzing T, & Brinkkoetter PT (2014). Breaking the chain at the membrane: Paraoxonase 2 counteracts lipid peroxidation at the plasma membrane. The FASEB Journal, 28(4), 1769–1779. 10.1096/fj.13-240309 [DOI] [PubMed] [Google Scholar]
- Horke S, Witte I, Altenhöfer S, Wilgenbus P, Goldeck M, Förstermann U, Xiao J, Kramer GL, Haines DC, Chowdhary PK, Haley RW, & Teiber JF (2010). Paraoxonase 2 is down-regulated by the Pseudomonas aeruginosa quorumsensing signal N-(3-oxododecanoyl)-L-homoserine lactone and attenuates oxidative stress induced by pyocyanin. The Biochemical Journal, 426(1), 73–83. 10.1042/BJ20091414 [DOI] [PubMed] [Google Scholar]
- Horke S, Witte I, Wilgenbus P, Krüger M, Strand D, & Förstermann U (2007). Paraoxonase-2 reduces oxidative stress in vascular cells and decreases endoplasmic reticulum stress-induced caspase activation. Circulation, 115(15), 2055–2064. 10.1161/CIRCULATIONAHA.106.681700 [DOI] [PubMed] [Google Scholar]
- Huang B, Chen Q, Wang L, Gao X, Zhu W, Mu P, & Deng Y (2020). Aflatoxin B1 Induces Neurotoxicity through Reactive Oxygen Species Generation, DNA Damage, Apoptosis, and S-Phase Cell Cycle Arrest. International Journal of Molecular Sciences, 21(18). 10.3390/ijms21186517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp NA, Mason J, Beaudet AL, Benjamini Y, Bower L, Braun RE, Brown SDM, Chesler EJ, Dickinson ME, Flenniken AM, Fuchs H, Angelis M. H. de, Gao X, Guo S, Greenaway S, Heller R, Herault Y, Justice MJ, Kurbatova N, … White JK (2017). Prevalence of sexual dimorphism in mammalian phenotypic traits. Nature Communications, 8(1), 15475. 10.1038/ncomms15475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer JP, & Klotz L-O (2015). Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Critical Reviews in Toxicology, 45(9), 765–798. 10.3109/10408444.2015.1074159 [DOI] [PubMed] [Google Scholar]
- Kim GH, Kim JE, Rhie SJ, & Yoon S (2015). The Role of Oxidative Stress in Neurodegenerative Diseases. Experimental Neurobiology, 24(4), 325–340. PubMed. 10.5607/en.2015.24.4.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács AD, & Pearce DA (2013). Location- and sex-specific differences in weight and motor coordination in two commonly used mouse strains. Scientific Reports, 3. 10.1038/srep02116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Badana AK, G, M. M., G, S., & Malla R (2018). Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomarker Insights, 13, 1177271918755391–1177271918755391. PubMed. 10.1177/1177271918755391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Trudel K, Bendayan M, Seidman E, Delvin E, Elchebly M, Lavoie J-C, Precourt L-P, Amre D, & Sinnett D (2007). Biological role, protein expression, subcellular localization, and oxidative stress response of paraoxonase 2 in the intestine of humans and rats. American Journal of Physiology. Gastrointestinal and Liver Physiology, 293(6), G1252–1261. 10.1152/ajpgi.00369.2007 [DOI] [PubMed] [Google Scholar]
- Masri S, & Sassone-Corsi P (2018). The emerging link between cancer, metabolism, and circadian rhythms. Nature Medicine, 24(12), 1795–1803. 10.1038/s41591-018-0271-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen MP, Kusek G, Bolivar VJ, & Flaherty L (2003). Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes, Brain and Behavior, 2(4), 214–219. 10.1034/j.1601-183X.2003.00028.x [DOI] [PubMed] [Google Scholar]
- Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, & Reddy ST (2001). Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. The Journal of Biological Chemistry, 276(48), 44444–44449. 10.1074/jbc.M105660200 [DOI] [PubMed] [Google Scholar]
- Ng Carey J., Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, Fogelman AM, Lusis AJ, Young S, & Reddy ST (2006). Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: Anti-atherogenic role for paraoxonase-2. The Journal of Biological Chemistry, 281(40), 29491–29500. 10.1074/jbc.M605379200 [DOI] [PubMed] [Google Scholar]
- Pacheco NL, Heaven MR, Holt LM, Crossman DK, Boggio KJ, Shaffer SA, Flint DL, & Olsen ML (2017). RNA sequencing and proteomics approaches reveal novel deficits in the cortex of Mecp2-deficient mice, a model for Rett syndrome. Molecular Autism, 8(1), 56. 10.1186/s13229-017-0174-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panth N, Paudel KR, & Parajuli K (2016). Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Advances in Medicine, 2016, 9152732–9152732. PubMed. 10.1155/2016/9152732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Lee JM, Moon HE, Lee DS, Kim B-N, Kim J, Kim DG, & Paek SH (2016). A Short Review on the Current Understanding of Autism Spectrum Disorders. Experimental Neurobiology, 25(1), 1–13. PubMed. 10.5607/en.2016.25.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee J-Y, You S, Song G, & Lim W (2020). Neurotoxic effects of aflatoxin B1 on human astrocytes in vitro and on glial cell development in zebrafish in vivo. Journal of Hazardous Materials, 386, 121639. 10.1016/j.jhazmat.2019.121639 [DOI] [PubMed] [Google Scholar]
- Patro R, Duggal G, & Kingsford C (2015). Salmon: Accurate, Versatile and Ultrafast Quantification from RNA-seq Data using Lightweight-Alignment. BioRxiv, 021592. 10.1101/021592 [DOI] [Google Scholar]
- Ripperger JA, Jud C, & Albrecht U (2011). The daily rhythm of mice. Circadian Rhythms, 585(10), 1384–1392. 10.1016/j.febslet.2011.02.027 [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, & Smith MA (2008). Oxidative Stress and Neurotoxicity. Chemical Research in Toxicology, 21(1), 172–188. 10.1021/tx700210j [DOI] [PubMed] [Google Scholar]
- Seol W, Choi H-S, & Moore DD (1996). An Orphan Nuclear Hormone Receptor That Lacks a DNA Binding Domain and Heterodimerizes with Other Receptors. Science, 272(5266), 1336. 10.1126/science.272.5266.1336 [DOI] [PubMed] [Google Scholar]
- Shih DM, Meng Y, Sallam T, Vergnes L, Shu ML, Reue K, Tontonoz P, Fogelman AM, Lusis AJ, & Reddy ST (2019). PON2 Deficiency Leads to Increased Susceptibility to Diet-Induced Obesity. Antioxidants (Basel, Switzerland), 8(1), 19. PubMed. 10.3390/antiox8010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneson C, Love MI, & Robinson MD (2015). Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research, 4, 1521. 10.12688/f1000research.7563.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker S (2011). Dissection of Rodent Brain Regions (Vol. 57, pp. 13–26). 10.1007/978-1-61779-111-6_2 [DOI] [Google Scholar]
- Stoltz DA, Ozer EA, Ng CJ, Yu JM, Reddy ST, Lusis AJ, Bourquard N, Parsek MR, Zabner J, & Shih DM (2007). Paraoxonase-2 deficiency enhances Pseudomonas aeruginosa quorum sensing in murine tracheal epithelia. American Journal of Physiology. Lung Cellular and Molecular Physiology, 292(4), L852–860. 10.1152/ajplung.00370.2006 [DOI] [PubMed] [Google Scholar]
- Sulaiman D, Li J, Devarajan A, Cunningham CM, Li M, Fishbein GA, Fogelman AM, Eghbali M, & Reddy ST (2019). Paraoxonase 2 protects against acute myocardial ischemia-reperfusion injury by modulating mitochondrial function and oxidative stress via the PI3K/Akt/GSK-3β RISK pathway. Journal of Molecular and Cellular Cardiology, 129, 154–164. 10.1016/j.yjmcc.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Teiber JF, Horke S, Haines DC, Chowdhary PK, Xiao J, Kramer GL, Haley RW, & Draganov DI (2008). Dominant role of paraoxonases in inactivation of the Pseudomonas aeruginosa quorum-sensing signal N-(3-oxododecanoyl)-L-homoserine lactone. Infection and Immunity, 76(6), 2512–2519. 10.1128/IAI.01606-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teiber JF, Xiao J, Kramer GL, Ogawa S, Ebner C, Wolleb H, Carreira EM, Shih DM, & Haley RW (2018). Identification of biologically active δ-lactone eicosanoids as paraoxonase substrates. Biochemical and Biophysical Research Communications, 505(1), 87–92. 10.1016/j.bbrc.2018.09.083 [DOI] [PubMed] [Google Scholar]
- Ullah MF, Ahmad A, Bhat SH, Abu-Duhier FM, Barreto GE, & Ashraf GM (2019). Impact of sex differences and gender specificity on behavioral characteristics and pathophysiology of neurodegenerative disorders. Neuroscience & Biobehavioral Reviews, 102, 95–105. 10.1016/j.neubiorev.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Vega A, Martinot E, Baptissart M, De Haze A, Saru J-P, Baron S, Caira F, Schoonjans K, Lobaccaro J-MA, & Volle DH (2015). Identification of the Link Between the Hypothalamo-Pituitary Axis and the Testicular Orphan Nuclear Receptor NR0B2 in Adult Male Mice. Endocrinology, 156(2), 660–669. 10.1210/en.2014-1418 [DOI] [PubMed] [Google Scholar]
- Wójtowicz AK, Szychowski KA, Wnuk A, & Kajta M (2017). Dibutyl Phthalate (DBP)-Induced Apoptosis and Neurotoxicity are Mediated via the Aryl Hydrocarbon Receptor (AhR) but not by Estrogen Receptor Alpha (ERα), Estrogen Receptor Beta (ERβ), or Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) in Mouse Cortical Neurons. Neurotoxicity Research, 31(1), 77–89. 10.1007/s12640-016-9665-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yang Y, Yu P, Yang J, Jiang X, Villar V. a. M., Sibley DR, Jose PA, & Zeng C (2015). Dopamine D1 and D5 receptors differentially regulate oxidative stress through paraoxonase 2 in kidney cells. Free Radical Research, 49(4), 397–410. 10.3109/10715762.2015.1006215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S, & Galea LAM (2019). Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology, 44(1), 200–213. 10.1038/s41386-018-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Suzuki Y, Hamami K, Harada A, & Komai S (2017). Sex differences in avoidance behavior after perceiving potential risk in mice. Behavioral and Brain Functions, 13(1), 9. 10.1186/s12993-017-0126-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang Y, Ma H, Zeng R, Liu R, Wang P, Jin X, & Zhao Y (2020). Epitranscriptomic profiling of N6-methyladenosine-related RNA methylation in rat cerebral cortex following traumatic brain injury. Molecular Brain, 13(1), 11. 10.1186/s13041-020-0554-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


