Abstract
The recently recognized connection between the gut microbiota and pulmonary disease has been termed the gut-lung axis. However, broader connections link the gut and the lungs and these organ systems are tightly interrelated in both homeostasis and disease. This concept is often ignored in the compartmentalized treatment of pulmonary or gastrointestinal disease. In newborns, the most severe gastrointestinal complication of prematurity, necrotizing enterocolitis, and the most severe pulmonary complication, bronchopulmonary dysplasia, both produce significant systemic morbidity. In this review, we highlight the often neglected pathophysiology of the gut-lung axis contributes to increased risk of bronchopulmonary dysplasia in premature infants with necrotizing enterocolitis.
Introduction
Necrotizing enterocolitis (NEC) continues to be a major cause of mortality and longer-term morbidity in very preterm infants admitted to neonatal ICUs worldwide. In the Vermont Oxford Network, of 473,895 very low birth weight infants (≤1500g or <29w GA) born 2006-2017 and admitted to 820 US centers, 36,130 (7.6%) were diagnosed with NEC, of which 58.3% were medical NEC and 41.7% were surgical NEC. In more recent years, the incidence of medical NEC was 3%, and of surgical NEC was 3.1% (1). In the NICHD Neonatal Research Network consisting of larger academic centers in 2012, 9% of the extremely preterm infants (22-28w GA) developed NEC (2). Many infants with surgical NEC are often ventilator-dependent, and are more likely to be diagnosed with bronchopulmonary dysplasia (BPD) and impairment of growth (3). It is evident that with more extreme immaturity, the risks of both NEC as well as respiratory distress syndrome and subsequent BPD are higher. The term “gut-lung” axis is often used to suggest how alterations in the gut microbiota may predispose to lung disease (e.g asthma and allergic airway disease)(4-7), but this is not the only type of interaction between these two vital systems that share a common embryonic origin. There are physiological interactions between therapeutic respiratory interventions and the gut, and between gastrointestinal interventions and the respiratory system. There are also more complex molecular and cellular interactions between the gut and the lung, as well as between pathophysiology in either the gut (NEC, spontaneous intestinal perforation) and lung (respiratory distress syndrome, BPD). The following is an overview of such potential and proven interactions. First, the effects of respiratory management and of lung disorders on the risk of NEC will be discussed. Next, the effects of NEC on the lung will be described, with a consideration of potential molecular and cellular mechanisms.
Effects of respiratory management on risk of NEC:
Respiratory management in preterm infants generally includes monitoring of gas exchange (e.g. pulse oximetry, blood gas analysis) and support (e.g. oxygen, CPAP, non-invasive or invasive positive pressure mechanical ventilation). The magnitude of gas exchange as well as that of respiratory support provided may impact the risk of NEC.
It is likely that oxygenation needs to be above a certain threshold and prolonged hypoxemia may increase the risk of NEC. In a meta-analysis of individual participant data from five randomized controlled trials enrolling preterm infants <28w GA and evaluating a pulse oximeter (SpO2) target range that was lower (85%-89%) vs higher (91%-95%), it was noted that 484 of 2433 infants (19.9%) died in the lower SpO2 target group versus 418 of 2440 infants (17.1%) in the higher SpO2 target group (risk difference, 2.8% [95% CI, 0.6% to 5.0%]; RR, 1.17 [95% CI, 1.04 to 1.31], P = .01) (8). Importantly, severe NEC occurred in 227 of 2464 infants (9.2%) in the lower SpO2 target group and 170 of 2465 infants (6.9%) in the higher SpO2 target group (risk difference, 2.3% [95% CI, 0.8% to 3.8%]; RR, 1.33 [95% CI, 1.10 to 1.61], P = .003) (8). Hence, it may be advisable to maintain SpO2 in the target range of 91-95% and not lower while the preterm infant needs oxygen supplementation.
However, SpO2 target range may not correlate well with splanchnic tissue oxygenation (StO2) measured with near-infrared spectroscopy. In a prospective study of 92 preterm infants (<32w GA and <1500g birth weight), mean abdominal StO2 during the first postnatal week in those who did not NEC was higher than in those who developed NEC (77.3% ± 14.4% vs 70.7% ± 19.1%, respectively, p = 0.002) (9). StO2 ≤56% identified preterm infants progressing to necrotizing enterocolitis with 86% sensitivity, 64% specificity, 96% negative predictive value, and 30% positive predictive value (9). By logistic regression, StO2 ≤56% was independently associated with increased risk of NEC (OR 14.1; p = 0.01). Infants with NEC also had more variation in StO2 both during and after feeding in the first 2 weeks of life (9). In another study of 52 extremely preterm infants of whom eight developed NEC at a median age of 15 days (range 6-35), infants with a mean StO2<30% had a higher risk for developing NEC compared with those with StO2>30% (crude risk ratio 5.25; 95% CI [1.19-23.01]) (10). Small for gestational age, gestational age, birth weight, postnatal age did not affect the results (10). In another observational study, ten infants who developed NEC at a median age of day 13 (range 4-43d) were compared to 20 matched controls, and Infants with cerebral rSO2 <70% within the first 48 h after birth developed NEC significantly more often than infants with cerebral rSO2 ≥70% (odds ratio 9.00 (95% CI 1.33-61.14) (11). Intestinal fractional tissue oxygen extraction was higher in infants who developed NEC compared to controls during the last near-infrared spectroscopy measurement at median 2 days (range: 1-7) before NEC onset (median 0.65 vs. 0.44) (11). However, other studies have revealed comparable abdominal StO2 and splanchnic-cerebral oxygenation ratio in patients with and without NEC (StO2: 47.3 [20.4] vs. 50.4 [17.8], p = 0.59, splanchnic-cerebral oxygenation ratio: 0.64 [0.26] vs. 0.69 [0.24], p = 0.51) (12). Based on the available data, it may be prudent to maintain abdominal StO2>56% and cerebral rSO2 >70%, with intestinal FTOE <0.50.
In addition to oxygen therapy, many preterm infants also receive either CPAP/nasal positive pressure ventilation or intubation followed by mechanical positive pressure ventilation. It has been shown that infants with lower birth weight and respiratory morbidity (as measured by mean airway pressure x FiO2) are at higher risk not only of death or BPD, but also of NEC (13). It is possible that more extreme immaturity as well as shared genetic predisposition increase the risk of NEC in the infants who also have worse lung disease. One of the common issues noted with use of CPAP is abdominal distension (“CPAP belly”)(14), usually due to increased gas swallowing or ingress via the esophagus secondary to the airway positive pressure. This may be especially noticeable in infants on non-invasive intermittent positive pressure ventilation, as the positive pressure breaths may force more gas down the gastrointestinal tract. As one of the early signs of NEC is abdominal distension, clinicians may investigate infants with such CPAP-induced abdominal distension for NEC. Although CPAP-induced gaseous distension has not been shown to result in NEC (14, 15), radiologic features of NEC such as pneumatosis intestinalis may sometimes be difficult to exclude in the presence of foamy stool in the intestines, and there is the risk of occasional diagnostic error with false positive diagnosis of NEC. This fear of NEC (NEC-phobia) due to CPAP belly may result in prolongation of time to full feeds and a longer duration of parenteral nutrition (16).
A direct effect of positive end expiratory pressure (PEEP) or mean airway pressure (MAP) on cardiovascular variables such as mean arterial pressure, cardiac output, and regional blood flow to the intestines (e.g. superior mesenteric arterial flow or portal venous flow) may also be relevant to NEC, although conclusive data are lacking in the neonatal literature. Mechanical ventilation with high positive end-expiratory pressure has been found to decrease splanchnic perfusion in adults (17). Spontaneous breathing during ventilator support has been shown to improve systemic blood flow and gastrointestinal and splanchnic perfusion(17). At lower intestinal perfusion pressures (e.g. as may occur with hypotension), even moderate levels of PEEP impair local blood flow enough to cause intestinal ischemia in pig models (18). Animal studies have also shown alterations in gut mucosal and muscularis flow with the use of higher peak inspiratory pressures (19). Hence, it is preferable to permit spontaneous breathing (avoiding muscle relaxants) and use sufficient but not excessive PEEP to maintain lung inflation and functional residual capacity (FRC), with limited peak inspiratory pressures, to avoid over-distension and reduction in cardiac output that may impair gut perfusion, potentially increasing the risk of NEC. In the setting of endotoxemia (as may happen with NEC), mechanical ventilation with PEEP increases abdominal edema and inflammation (increased interleukin-6 and tumor necrosis factor-alpha) in the intestine and liver by increasing systemic capillary leakage and impeding abdominal lymph drainage (20). Therefore, mechanical ventilation should be judiciously titrated to achieve adequate gas exchange with the least pressures.
Intestinal microcirculation is also severely compromised by hemodilution (21). Severe anemia is associated with an increased risk of NEC (22), and there is evidence from animal models that intestinal injury worsens with increasing severity and duration of anemia prior to transfusion(23). Although recent data from transfusion thresholds in preterm infants do not suggest any differences in NEC, it may be preferable to maintain sufficient systemic oxygenation (SpO2), intestinal oxygenation as monitored by NIRS (StO2), and adequate gas exchange capacity by avoiding severe anemia.
It is not just management of gas exchange and oxygen transport that impacts the risk for, or the diagnosis of NEC. Pharmacological management of respiratory disorders may also modulate the risk of NEC. Infants with respiratory distress soon after birth are often treated with antimicrobials, as the possibility of pneumonia /sepsis (such as with group B streptococci or E. coli) cannot be excluded. This antimicrobial therapy may alter the neonatal microbiome at multiple sites including the gut and lung, predisposing to subsequent NEC as well as BPD. The prolonged use of empiric antibiotics is associated with increased rates of NEC and death in extremely low birth weight infants (24). Some of these adverse effects of antimicrobials may result from alterations of the microbiome (dysbiosis). Dysbiosis in the gut has been observed in pre-diagnosis samples from infants with established NEC (25, 26). Fecal microbiome from preterm infants with NEC have increased relative abundances of Proteobacteria and decreased relative abundances of Firmicutes prior to NEC onset (26), similar to what has been observed in the airway microbiome of BPD (27). It is known that the lung microbiome plays an important role in the maturation and homeostasis of lung immunity(28). Infectious organisms such as Ureaplasma spp. have been associated not only with increased risk of BPD in infants needing prolonged mechanical ventilation (29-31) but also with a higher risk of NEC(32). NEC was 2.2-fold higher in Ureaplasma-positive (12.3%) than Ureaplasma-negative (5.5%) infants <33 wk (OR 2.43; 95% CI 1.13–5.2; p = 0.02) and 3.3-fold higher in Ureaplasma-positive (14.6%) than Ureaplasma-negative (4.4%) infants ≤28 wk (OR 3.67; 95% CI 1.36–9.93; p = 0.01) (32), though it is not clear if the higher risk of NEC is due directly to inflammation induced by Ureaplasma infection, or because of more severe lung disease. Systemic postnatal corticosteroids are occasionally used to prevent BPD, but there is no evidence to suggest that they affect the rate of NEC (33). It is possible that other medications (e.g. diuretics, bronchodilators, methylxanthines) used for respiratory indications may secondarily have effects on the gut, by modification of intestinal perfusion, motility, or the microbiome. Caffeine (or theophylline) is frequently used to stimulate the respiratory drive and reduce apnea in preterm infants. Retrospective studies have suggested caffeine may either increase NEC(34) or not influence the rate of NEC (35, 36) – the large Caffeine for Apnea of Prematurity (CAP) trial did not show a difference in rate of NEC between caffeine and placebo group (6.3% vs. 6.7%, OR 0.94 (0.65-1.34) adjusted for center and patient characteristics) (37).
Effect of NEC on the lung:
A multicenter retrospective analysis of extremely low birth weight infants in the NICHD Neonatal Research Network indicated that infants with surgical NEC (57%) but not medical NEC (51%) were more likely to have received a diagnosis of BPD, as compared to infants without NEC (43%; p=0.003 for surgical NEC vs. no NEC, and p=0.09 for medical NEC vs. no NEC)(3). A more recent larger multicenter cohort from Spain with 25,821 infants <32w GA also found that infants with surgical NEC had a higher odds ratio (OR) 2.00 (95% CI 1.71-2.33) for BPD after adjustment for gestational age, compared to infants without NEC, and that infants with medical NEC also had increased risk for BPD but to a lesser extent (OR 1.44 (95%CI 1.18-1.77)) (38). It is relatively common for neonatologists to observe that even in infants who do not have severe lung disease to begin with, an episode of necrotizing enterocolitis often leads to multiorgan dysfunction and abdominal distension, both of which cause a significant decline in gas exchange, resulting in the need for a higher oxygen concentration and higher mechanical ventilation settings that often further injures the lungs (ventilation-induced lung injury), contributing to BPD. It is also likely that translocation of gut bacteria through the compromised intestinal wall leads to systemic inflammation and induces a dysbiosis of the lung microbiome.
The mechanisms by which gut necrosis and intestinal inflammation in a preterm infant lead to lung injury, inflammation, or BPD have not been fully defined. There is data from adult models of gut injury that can possibly be extrapolated to the neonatal NEC-lung axis. There is cross-talk and collaboration between the gastrointestinal tract and respiratory tract at multiple levels (microbiome, immunity, metabolites etc.). The intestinal microbiome and its dysbiosis modulates the systemic immune response, by alteration of dendritic cell priming of T-cell subsets, changes in levels of cytokines, and perhaps changes in activation of other immune cells (39). The “intestinal cross-talk” between the intestinal epithelium, immune cells, and microbiome is normally in a state of homeostatic balance (39, 40), but during the early postnatal period in a preterm infant, intestinal epithelial injury, an immature immune system and dysbiosis may combine to a very dysregulated cross-talk in this three-way partnership. It is also possible that the “gut-lymph” theory is relevant to neonatal lung injury during NEC – macrophages and immune cells in the intestine kill the majority of translocating bacteria but surviving bacteria and their fragments/peptides may reach the lungs and activate alveolar macrophages, leading to lung injury (39, 41). Thoracic lymph duct ligation before intestinal ischemia-reperfusion has been shown to reduce pulmonary neutrophil recruitment and plasma extravasation, associated with high levels of tumor necrosis factor in the lymph but not in serum (42). It may be that microbial products rather than circulating microbes are relevant. It has been observed that gut microbiome-derived short-chain fatty acids such as propionate are regulate lung inflammation in mice(43).
There are multiple potential mechanisms by which the gastrointestinal microbiota can regulate lung immunology, and thereby inflammation and pathogenesis of BPD. Toll like receptors (TLRs) are pattern recognition receptors that recognize microbial products, and multiple immune processes, including microbiota-mediated activation of antigen-specific CD4 and CD8 T cells, T-cell priming, dendritic cell (DC) migration, microbe-specific antibodies, and inflammasome regulation are all regulated by TLRs (39). TLR4 signaling has been shown to mediate lung injury and inflammation following intestinal ischemia-reperfusion (44). T cell homing in a tissue-specific manner is induced by direct interaction with mucosal DCs, and T cell homing to the GI tract involves induction of α4β7 and CCR9 by Peyer's patch and mesenteric lymph node (MLN) DCs in a retinoic acid-dependent manner, but it has also been shown that lung DCs also up-regulate the gut-homing integrin α4β7 in vitro and in vivo, and induce T cell migration to the GI tract in vivo (45), indicating that mucosal cross-talk is mediated by DCs. Another mechanism by which lymphocytes may be recruited to multiple mucosal sites is by induction of CCL20 (the ligand for CCR6, which mediates homing of both CD4 T cells and DCs) in either intestinal epithelial or lung epithelial cells by pro-inflammatory signals or TLR agonists (39, 46).
In addition to alterations in adaptive immunity, other pathophysiologic changes may also negatively impact the lung in the setting of ischemic gut injury as seen in NEC. Animal models indicate that neutrophil macroaggregates triggered by dying platelets promote widespread pulmonary thrombosis in the setting of gut ischemia, causing occlusion of pulmonary arteries, veins, and the microvessels (47). Depletion of alveolar macrophages has also been shown to reduce acute lung injury following intestinal ischemia-reperfusion (48).
The mechanisms just listed have been mostly demonstrated in adult animal models of gut ischemia. How relevant are they to the preterm infant with NEC, and what differences in mechanisms may be observed? It has been shown that NEC is associated with a systemic pro-inflammatory state with elevated IL-1β, IL-6, IL-8, IL-10, monocyte chemoattractant protein-1/CC-motif ligand-2 (MCP-1), macrophage inflammatory protein-1β/CC-motif ligand-3 (MIP-1β), and C-reactive protein in the blood after the onset of NEC (49). Elevations of these cytokines are also associated with BPD or death in extremely preterm infants (50).
TLR4 expression in the lung gradually increases during postnatal development, and both mice and humans with NEC-associated lung inflammation have more pulmonary TLR4 than age-matched controls (51). NEC in newborn mice results in pulmonary injury that is prevented by TLR4 deletion from the pulmonary epithelium, indicating a role for pulmonary TLR4 in NEC lung injury (51). It was also observed that intestinal epithelial TLR4 activation induced high-mobility group box 1 release from the intestine, which activated pulmonary epithelial TLR4, leading to the induction of the neutrophil recruiting CXCL5 and the influx of neutrophils into lung (51). NEC-induced lung injury in mice and humans is characterized by an influx of Th17 cells and a reduction in T regulatory lymphocytes (52). In mice with NEC, deletion of TLR4 from Sftpc1+ epithelial cells reduced lung injury, while TLR4 activation induced CCL25, the Th17 recruiting chemokine (52). It is therefore evident that TLR4 is a regulator (and potential “druggable” target) of NEC-induced lung injury.
Sensing of commensal bacteria by intestinal mucosal dendritic cells also leads to an influx of IL-22-producing group 3 innate lymphoid cells into the lungs of newborn mice (53). Dysregulation of this process by intestinal luminal pathogens may disturb lung innate immunity. Infants with NEC often receive prolonged courses of antimicrobials, and there is evidence that microbial depletion using broad-spectrum antimicrobials may increase susceptibility to ventilation-induced lung injury (54), which may also contribute to BPD in the setting of NEC. On the other hand, probiotics have been shown to reduce the risk of NEC in preterm infants (risk ratio [RR] 0.52, 95% CI 0.33-0.81, p=0.004), but systematic review of the data from these trials did not demonstrate any effect on BPD at 36w PMA (RR 1.07, 95% CI 0.96-1.20, p=0.203), and meta-regression did not show any significant association between the RR for NEC and that for BPD (55).
In summary, NEC is a multifactorial disorder characterized by intestinal ischemia, dysbiosis, and a state of systemic inflammation that usually results in concomitant lung inflammation and a higher risk for BPD. This effect of NEC on the lung is mediated by many physiological effects as well as molecular and cellular mechanisms related to dysregulated immune processes. Conversely, lung disease and its management in the preterm infant may also impact gut oxygenation, microbiome, and other risk factors for NEC.
Figure 1. Dysbiosis of the lung and gut likely contribute to the development of both BPD and NEC.
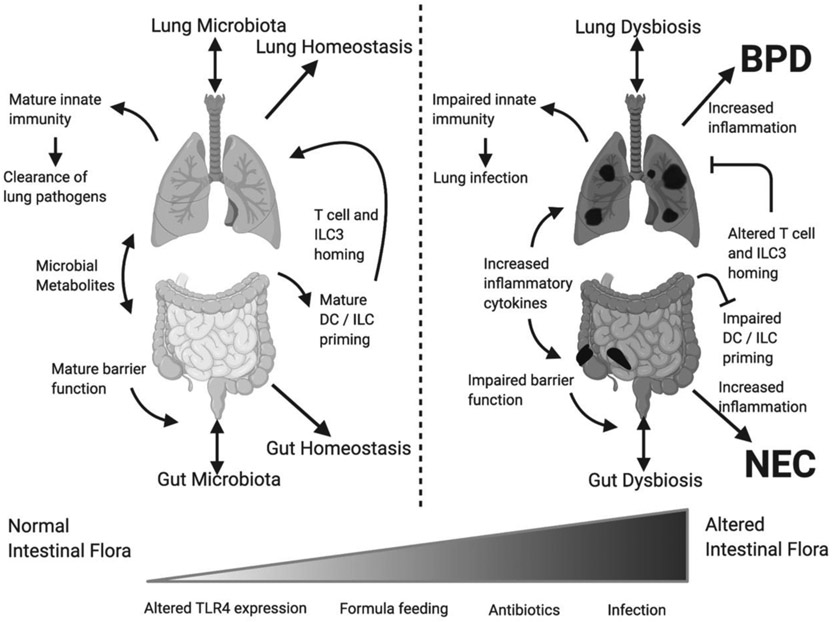
Multiple, likely interrelated, mechanisms may contribute to the development of both diseases. BPD, bronchopulmonary dysplasia; DC, dendritic cell; ILC3, type 3 innate lymphoid cells; NEC, necrotizing enterocolitis.
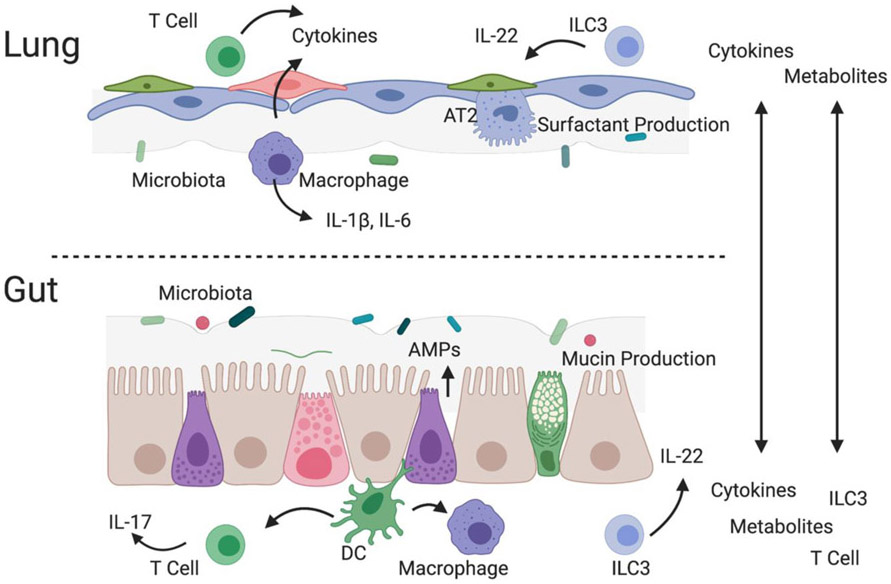
Figure 2. Immune interactions with the intrinsic microbiomes of the gut and lung are critical components of the gut-lung axis.
In addition, to direct transfer of T cells, type 3 innate lymphoid cells (ILC3), metabolites and cytokines, spill over from tissue specific interactions between the microbiota and innate immune cells may also disrupt homeostasis in the opposite organ. AMP, antimicrobial peptides; AT2 alveolar type 2 cell; DC, dendritic cells.
Funding:
NIH, NHLBI: K08HL151907 (KAW), The UAB Microbiome Center (KAW), NIH, NHLBI: U01HL133536 (NA), NIH, NHLBI: R01HL129907 (NA)
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Han SM, Hong CR, Knell J, Edwards EM, Morrow KA, Soll RF, Modi BP, Horbar JD, Jaksic T. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12years: A multicenter cohort analysis. Journal of pediatric surgery. 2020;55(6):998–1001. Epub 2020/03/17. doi: 10.1016/j.jpedsurg.2020.02.046. PubMed PMID: 32173122. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, Sanchez PJ, Van Meurs KP, Wyckoff M, Das A, Hale EC, Ball MB, Newman NS, Schibler K, Poindexter BB, Kennedy KA, Cotten CM, Watterberg KL, D'Angio CT, DeMauro SB, Truog WE, Devaskar U, Higgins RD, Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314(10):1039–51. Epub 2015/09/09. doi: 10.1001/jama.2015.10244. PubMed PMID: 26348753; PMCID: PMC4787615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, Poole WK, Blakely ML, Wright L, Higgins R. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. Epub 2005/03/03. doi: 10.1542/peds.2004-0569. PubMed PMID: 15741374. [DOI] [PubMed] [Google Scholar]
- 4.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T, Investigators CS, Mohn WW, Turvey SE, Finlay BB. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. Epub 2015/10/02. doi: 10.1126/scitranslmed.aab2271. PubMed PMID: 26424567. [DOI] [PubMed] [Google Scholar]
- 5.Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, Gill N, Blanchet MR, Mohn WW, McNagny KM, Finlay BB. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13(5):440–7. Epub 2012/03/17. doi: 10.1038/embor.2012.32. PubMed PMID: 22422004; PMCID: PMC3343350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. Epub 2016/11/01. doi: 10.1038/nrmicro.2016.142. PubMed PMID: 27694885. [DOI] [PubMed] [Google Scholar]
- 7.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, Delhaes L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front Cell Infect Microbiol. 2020;10:9. Epub 2020/03/07. doi: 10.3389/fcimb.2020.00009. PubMed PMID: 32140452; PMCID: PMC7042389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, Davis PG, Carlo WA, Brocklehurst P, Davies LC, Das A, Rich W, Gantz MG, Roberts RS, Whyte RK, Costantini L, Poets C, Asztalos E, Battin M, Halliday HL, Marlow N, Tin W, King A, Juszczak E, Morley CJ, Doyle LW, Gebski V, Hunter KE, Simes RJ, Neonatal Oxygenation Prospective Meta-analysis C. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA. 2018;319(21):2190–201. Epub 2018/06/07. doi: 10.1001/jama.2018.5725. PubMed PMID: 29872859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel AK, Lazar DA, Burrin DG, Smith EO, Magliaro TJ, Stark AR, Brandt ML, Zamora IJ, Sheikh F, Akinkuotu AC, Olutoye OO. Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2014;15(8):735–41. Epub 2014/07/30. doi: 10.1097/PCC.0000000000000211. PubMed PMID: 25068253. [DOI] [PubMed] [Google Scholar]
- 10.Palleri E, Wackernagel D, Wester T, Bartocci M. Low Splanchnic Oxygenation and Risk for Necrotizing Enterocolitis in Extremely Preterm Newborns. Journal of pediatric gastroenterology and nutrition. 2020;71(3):401–6. Epub 2020/05/15. doi: 10.1097/MPG.0000000000002761. PubMed PMID: 32404748. [DOI] [PubMed] [Google Scholar]
- 11.Schat TE, van Zoonen A, van der Laan ME, Mebius MJ, Bos AF, Hulzebos CV, Boezen HM, Hulscher JBF, Kooi EMW. Early cerebral and intestinal oxygenation in the risk assessment of necrotizing enterocolitis in preterm infants. Early human development. 2019;131:75–80. Epub 2019/03/15. doi: 10.1016/j.earlhumdev.2019.03.001. PubMed PMID: 30870625. [DOI] [PubMed] [Google Scholar]
- 12.Le Bouhellec J, Prodhomme O, Mura T, Jacquot A, Combes C, Gamon L, Durand S, Filleron A, Cambonie G. Near-Infrared Spectroscopy: A Tool for Diagnosing Necrotizing Enterocolitis at Onset of Symptoms in Preterm Neonates with Acute Gastrointestinal Symptoms? Am J Perinatol. 2020. Epub 2020/04/24. doi: 10.1055/s-0040-1710033. PubMed PMID: 32325507. [DOI] [PubMed] [Google Scholar]
- 13.Thome UH, Dreyhaupt J, Genzel-Boroviczeny O, Bohnhorst B, Schmid M, Fuchs H, Rohde O, Avenarius S, Topf HG, Zimmermann A, Faas D, Timme K, Kleinlein B, Buxmann H, Schenk W, Segerer H, Teig N, Ackermann B, Hentschel R, Heckmann M, Schlosser R, Peters J, Rossi R, Rascher W, Bottger R, Seidenberg J, Hansen G, Bode H, Zernickel M, Muche R, Hummler HD, Group PS. Influence of PCO2 Control on Clinical and Neurodevelopmental Outcomes of Extremely Low Birth Weight Infants. Neonatology. 2018;113(3):221–30. Epub 2018/01/04. doi: 10.1159/000485828. PubMed PMID: 29298438. [DOI] [PubMed] [Google Scholar]
- 14.Jaile JC, Levin T, Wung JT, Abramson SJ, Ruzal-Shapiro C, Berdon WE. Benign gaseous distension of the bowel in premature infants treated with nasal continuous airway pressure: a study of contributing factors. AJR American journal of roentgenology. 1992;158(1):125–7. Epub 1992/01/01. doi: 10.2214/ajr.158.1.1727337. PubMed PMID: 1727337. [DOI] [PubMed] [Google Scholar]
- 15.Aly H, Massaro AN, Hammad TA, Narang S, Essers J. Early nasal continuous positive airway pressure and necrotizing enterocolitis in preterm infants. Pediatrics. 2009;124(1):205–10. Epub 2009/07/01. doi: 10.1542/peds.2008-2588. PubMed PMID: 19564301. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen T, Gronvall J, Petersen S, Andersen GE. "Minitouch" treatment of very low-birth-weight infants. Acta Paediatr. 1993;82(11):934–8. Epub 1993/11/01. doi: 10.1111/j.1651-2227.1993.tb12603.x. PubMed PMID: 8111173. [DOI] [PubMed] [Google Scholar]
- 17.Putensen C, Wrigge H, Hering R. The effects of mechanical ventilation on the gut and abdomen. Current opinion in critical care. 2006;12(2):160–5. Epub 2006/03/18. doi: 10.1097/01.ccx.0000216585.54502.eb. PubMed PMID: 16543794. [DOI] [PubMed] [Google Scholar]
- 18.Lehtipalo S, Biber B, Frojse R, Arnerlov C, Johansson G, Winso O. Effects of positive end-expiratory pressure on intestinal circulation during graded mesenteric artery occlusion. Acta Anaesthesiol Scand. 2001;45(7):875–84. Epub 2001/07/27. doi: 10.1034/j.1399-6576.2001.045007875.x. PubMed PMID: 11472291. [DOI] [PubMed] [Google Scholar]
- 19.Hering R, Kreyer S, Putensen C. Effects of lung protective mechanical ventilation associated with permissive respiratory acidosis on regional extra-pulmonary blood flow in experimental ARDS. BMC Anesthesiol. 2017;17(1):149. Epub 2017/10/29. doi: 10.1186/s12871-017-0439-7. PubMed PMID: 29078756; PMCID: PMC5659005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lattuada M, Bergquist M, Maripuu E, Hedenstierna G. Mechanical ventilation worsens abdominal edema and inflammation in porcine endotoxemia. Critical care. 2013;17(3):R126. Epub 2013/06/27. doi: 10.1186/cc12801. PubMed PMID: 23799965; PMCID: PMC4056092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara G, Kanoore Edul VS, Martins E, Canales HS, Canullan C, Murias G, Pozo MO, Estenssoro E, Ince C, Dubin A. Intestinal and sublingual microcirculation are more severely compromised in hemodilution than in hemorrhage. Journal of applied physiology. 2016;120(10):1132–40. Epub 2016/03/19. doi: 10.1152/japplphysiol.00007.2016. PubMed PMID: 26989219. [DOI] [PubMed] [Google Scholar]
- 22.Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, Easley KA, Josephson CD. Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants. JAMA. 2016;315(9):889–97. Epub 2016/03/05. doi: 10.1001/jama.2016.1204. PubMed PMID: 26934258; PMCID: PMC4805423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MohanKumar K, Namachivayam K, Song T, Jake Cha B, Slate A, Hendrickson JE, Pan H, Wickline SA, Oh JY, Patel RP, He L, Torres BA, Maheshwari A. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun. 2019;10(1):3494. Epub 2019/08/04. doi: 10.1038/s41467-019-11199-5. PubMed PMID: 31375667; PMCID: PMC6677753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, Ambalavanan N, Benjamin DK Jr., Network NNR. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. Epub 2009/01/02. doi: 10.1542/peds.2007-3423. PubMed PMID: 19117861; PMCID: 2760222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sim K, Shaw AG, Randell P, Cox MJ, McClure ZE, Li MS, Haddad M, Langford PR, Cookson WO, Moffatt MF, Kroll JS. Dysbiosis anticipating necrotizing enterocolitis in very premature infants. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(3):389–97. Epub 2014/10/26. doi: 10.1093/cid/ciu822. PubMed PMID: 25344536; PMCID: PMC4415053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, Engstrand L, Lilja HE, Hollister EB, Versalovic J, Neu J. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. Epub 2017/03/10. doi: 10.1186/s40168-017-0248-8. PubMed PMID: 28274256; PMCID: PMC5343300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lal CV, Travers C, Aghai ZH, Eipers P, Jilling T, Halloran B, Carlo WA, Keeley J, Rezonzew G, Kumar R, Morrow C, Bhandari V, Ambalavanan N. The Airway Microbiome at Birth. Sci Rep. 2016;6:31023. Epub 2016/08/05. doi: 10.1038/srep31023. PubMed PMID: 27488092; PMCID: PMC4973241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickson RP, Erb-Downward JR, Falkowski NR, Hunter EM, Ashley SL, Huffnagle GB. The Lung Microbiota of Healthy Mice Are Highly Variable, Cluster by Environment, and Reflect Variation in Baseline Lung Innate Immunity. Am J Respir Crit Care Med. 2018;198(4):497–508. Epub 2018/03/14. doi: 10.1164/rccm.201711-2180OC. PubMed PMID: 29533677; PMCID: PMC6118022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaser K, Gradzka-Luczewska A, Szymankiewicz-Breborowicz M, Kawczynska-Leda N, Henrich B, Waaga-Gasser AM, Speer CP. Perinatal Ureaplasma Exposure Is Associated With Increased Risk of Late Onset Sepsis and Imbalanced Inflammation in Preterm Infants and May Add to Lung Injury. Front Cell Infect Microbiol. 2019;9:68. Epub 2019/04/20. doi: 10.3389/fcimb.2019.00068. PubMed PMID: 31001484; PMCID: PMC6454044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silwedel C, Speer CP, Glaser K. Ureaplasma-associated prenatal, perinatal, and neonatal morbidities. Expert review of clinical immunology. 2017;13(11):1073–87. Epub 2017/09/19. doi: 10.1080/1744666X.2017.1381559. PubMed PMID: 28918659. [DOI] [PubMed] [Google Scholar]
- 31.Viscardi RM, Kallapur SG. Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update. Clin Perinatol. 2015;42(4):719–38. Epub 2015/11/26. doi: 10.1016/j.clp.2015.08.003. PubMed PMID: 26593075; PMCID: PMC4662049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okogbule-Wonodi AC, Gross GW, Sun CC, Agthe AG, Xiao L, Waites KB, Viscardi RM. Necrotizing enterocolitis is associated with ureaplasma colonization in preterm infants. Pediatr Res. 2011;69(5 Pt 1):442–7. Epub 2011/01/25. doi: 10.1203/PDR.0b013e3182111827. PubMed PMID: 21258263; PMCID: PMC3968774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle LW, Cheong JL, Ehrenkranz RA, Halliday HL. Late (> 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017;10:CD001145. Epub 2017/10/25. doi: 10.1002/14651858.CD001145.pub4. PubMed PMID: 29063594; PMCID: PMC6485440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox C, Hashem NG, Tebbs J, Bookstaver PB, Iskersky V. Evaluation of caffeine and the development of necrotizing enterocolitis. J Neonatal Perinatal Med. 2015;8(4):339–47. Epub 2016/01/13. doi: 10.3233/NPM-15814059. PubMed PMID: 26757002. [DOI] [PubMed] [Google Scholar]
- 35.Lampkin SJ, Turner AM, Lakshminrusimha S, Mathew B, Brown J, Fominaya CE, Johnson KK. Association between caffeine citrate exposure and necrotizing enterocolitis in preterm infants. Am J Health Syst Pharm. 2013;70(7):603–8. Epub 2013/03/22. doi: 10.2146/ajhp120457. PubMed PMID: 23515513. [DOI] [PubMed] [Google Scholar]
- 36.Lodha A, Seshia M, McMillan DD, Barrington K, Yang J, Lee SK, Shah PS, Canadian Neonatal N. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA pediatrics. 2015;169(1):33–8. Epub 2014/11/18. doi: 10.1001/jamapediatrics.2014.2223. PubMed PMID: 25402629. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W, Caffeine for Apnea of Prematurity Trial G. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–21. Epub 2006/05/19. doi: 10.1056/NEJMoa054065. PubMed PMID: 16707748. [DOI] [PubMed] [Google Scholar]
- 38.Zozaya C, Garcia Gonzalez I, Avila-Alvarez A, Oikonomopoulou N, Sanchez Tamayo T, Salguero E, Saenz de Pipaon M, Garcia-Munoz Rodrigo F, Couce ML. Incidence, Treatment, and Outcome Trends of Necrotizing Enterocolitis in Preterm Infants: A Multicenter Cohort Study. Frontiers in pediatrics. 2020;8:188. Epub 2020/06/02. doi: 10.3389/fped.2020.00188. PubMed PMID: 32478014; PMCID: PMC7237564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol. 2015;6:1085. Epub 2015/10/27. doi: 10.3389/fmicb.2015.01085. PubMed PMID: 26500629; PMCID: PMC4595839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the "motor" of critical illness. Shock. 2007;28(4):384–93. Epub 2007/06/20. doi: 10.1097/shk.0b013e31805569df PubMed PMID: 17577136; PMCID: PMC2084394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. The Journal of trauma. 2006;60(5):958–65; discussion 65-7. Epub 2006/05/12. doi: 10.1097/01.ta.0000215500.00018.47. PubMed PMID: 16688055. [DOI] [PubMed] [Google Scholar]
- 42.Cavriani G, Domingos HV, Soares AL, Trezena AG, Ligeiro-Oliveira AP, Oliveira-Filho RM, Sudo-Hayashi LS, Tavares de Lima W. Lymphatic system as a path underlying the spread of lung and gut injury after intestinal ischemia/reperfusion in rats. Shock. 2005;23(4):330–6. Epub 2005/04/02. doi: 10.1097/01.shk.0000157303.76749.9b. PubMed PMID: 15803056. [DOI] [PubMed] [Google Scholar]
- 43.Tian X, Hellman J, Horswill AR, Crosby HA, Francis KP, Prakash A. Elevated Gut Microbiome-Derived Propionate Levels Are Associated With Reduced Sterile Lung Inflammation and Bacterial Immunity in Mice. Front Microbiol. 2019;10:159. Epub 2019/03/21. doi: 10.3389/fmicb.2019.00159. PubMed PMID: 30891007; PMCID: PMC6413706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ben DF, Yu XY, Ji GY, Zheng DY, Lv KY, Ma B, Xia ZF. TLR4 mediates lung injury and inflammation in intestinal ischemia-reperfusion. The Journal of surgical research. 2012;174(2):326–33. Epub 2011/03/12. doi: 10.1016/j.jss.2010.12.005. PubMed PMID: 21392794. [DOI] [PubMed] [Google Scholar]
- 45.Ruane D, Brane L, Reis BS, Cheong C, Poles J, Do Y, Zhu H, Velinzon K, Choi JH, Studt N, Mayer L, Lavelle EC, Steinman RM, Mucida D, Mehandru S. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. The Journal of experimental medicine. 2013;210(9):1871–88. Epub 2013/08/21. doi: 10.1084/jem.20122762. PubMed PMID: 23960190; PMCID: PMC3754860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito T, Carson WFt, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Experimental cell research. 2011;317(5):613–9. Epub 2011/03/08. doi: 10.1016/j.yexcr.2010.12.018. PubMed PMID: 21376174; PMCID: PMC3063449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan Y, Alwis I, Wu MCL, Kaplan Z, Ashworth K, Bark D Jr., Pham A, McFadyen J, Schoenwaelder SM, Josefsson EC, Kile BT, Jackson SP. Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Sci Transl Med. 2017;9(409). Epub 2017/09/29. doi: 10.1126/scitranslmed.aam5861. PubMed PMID: 28954929. [DOI] [PubMed] [Google Scholar]
- 48.Moraes LB, Murakami AH, Fontes B, Poggetti RS, van Rooijen N, Younes RN, Heimbecker AM, Birolini D. Gut ischemia/reperfusion induced acute lung injury is an alveolar macrophage dependent event. The Journal of trauma. 2008;64(5):1196–200; discussion 200-1. Epub 2008/05/13. doi: 10.1097/TA.0b013e31816c5ca6. PubMed PMID: 18469641. [DOI] [PubMed] [Google Scholar]
- 49.Maheshwari A, Schelonka RL, Dimmitt RA, Carlo WA, Munoz-Hernandez B, Das A, McDonald SA, Thorsen P, Skogstrand K, Hougaard DM, Higgins RD, Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014;76(1):100–8. Epub 2014/04/16. doi: 10.1038/pr.2014.48. PubMed PMID: 24732104; PMCID: 4062583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ambalavanan N, Carlo WA, D'Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD, Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123(4):1132–41. Epub 2009/04/02. doi: 10.1542/peds.2008-0526. PubMed PMID: 19336372; PMCID: PMC2903210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia H, Sodhi CP, Yamaguchi Y, Lu P, Martin LY, Good M, Zhou Q, Sung J, Fulton WB, Nino DF, Prindle T Jr., Ozolek JA, Hackam DJ. Pulmonary Epithelial TLR4 Activation Leads to Lung Injury in Neonatal Necrotizing Enterocolitis. J Immunol. 2016;197(3):859–71. Epub 2016/06/17. doi: 10.4049/jimmunol.1600618. PubMed PMID: 27307558; PMCID: PMC4955761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia H, Sodhi CP, Yamaguchi Y, Lu P, Ladd MR, Werts A, Fulton WB, Wang S, Prindle T Jr., Hackam DJ. Toll Like Receptor 4 Mediated Lymphocyte Imbalance Induces Nec-Induced Lung Injury. Shock. 2018. Epub 2018/08/28. doi: 10.1097/SHK.0000000000001255. PubMed PMID: 30148762; PMCID: PMC6387863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray J, Oehrle K, Worthen G, Alenghat T, Whitsett J, Deshmukh H. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci Transl Med. 2017;9(376). Epub 2017/02/10. doi: 10.1126/scitranslmed.aaf9412. PubMed PMID: 28179507; PMCID: PMC5880204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wienhold SM, Macri M, Nouailles G, Dietert K, Gurtner C, Gruber AD, Heimesaat MM, Lienau J, Schumacher F, Kleuser B, Opitz B, Suttorp N, Witzenrath M, Muller-Redetzky HC. Ventilator-induced lung injury is aggravated by antibiotic mediated microbiota depletion in mice. Critical care. 2018;22(1):282. Epub 2018/10/31. doi: 10.1186/s13054-018-2213-8. PubMed PMID: 30373626; PMCID: PMC6206919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villamor-Martinez E, Pierro M, Cavallaro G, Mosca F, Kramer B, Villamor E. Probiotic Supplementation in Preterm Infants Does Not Affect the Risk of Bronchopulmonary Dysplasia: A Meta-Analysis of Randomized Controlled Trials. Nutrients. 2017;9(11). Epub 2017/11/01. doi: 10.3390/nu9111197. PubMed PMID: 29088103; PMCID: PMC5707669. [DOI] [PMC free article] [PubMed] [Google Scholar]