Abstract
BACKGROUND:
Neurodegeneration of the substantia nigra in Lewy body disease is associated with iron deposition, which increases the magnetic susceptibility of the substantia nigra on MRI. Our objective was to measure iron deposition in the substantia nigra in patients with probable dementia with Lewy bodies (pDLB) and patients who are at risk for pDLB by quantitative susceptibility mapping (QSM).
METHODS:
Participants included pDLB (n=36), mild cognitive impairment with at least one core feature of DLB (MCI-LB; n=15), idiopathic rapid eye movement sleep behavior disorder (iRBD; n=11), and an age-and gender-matched clinically unimpaired control group (CU; n=102). QSM was derived from multi-echo 3D gradient recalled echo MRI at 3T, and groups were compared on mean susceptibility values of the substantia nigra and its relation to parkinsonism severity.
RESULTS:
Patients with pDLB had higher susceptibility in the substantia nigra compared to controls (p<0.001) and MCI-LB (p=0.043). The susceptibility of substantia nigra showed an increasing trend from controls to iRBD and MCI-LB, and to pDLB (p<0.001). Parkinsonism severity was not associated with the mean susceptibility in the substantia nigra in the patient groups.
CONCLUSIONS:
Our data suggested that QSM is sensitive to the increased magnetic susceptibility due to higher iron content in the substantia nigra in pDLB. The trend of increasing susceptibility from controls to iRBD and MCI-LB, and to pDLB suggests that iron deposition in the substantia nigra starts to increase as early as the prodromal stage in DLB and continues to increase as the disease progresses, independent of parkinsonism severity.
Keywords: Quantitative susceptibility mapping, Dementia with Lewy bodies, Mild cognitive impairment, iron
Background
Dementia with Lewy bodies (DLB) is the second most common cause of neurodegenerative dementia after Alzheimer’s disease (AD)1. Mild cognitive impairment with one or more core features of DLB (MCI-LB) is an interim phase that may be present years before the diagnosis of probable DLB and therefore is regarded as the prodromal stage of DLB2. Even earlier than cognitive impairment (MCI-LB), most DLB patients exhibit rapid eye movement (REM) sleep behavior disorder (RBD), which is commonly associated with the synucleinopathies3. Idiopathic RBD (iRBD) is an early feature of evolving DLB, Parkinson’s disease (PD) and multiple system atrophy (MSA), with an onset typically preceding the onset of dementia or parkinsonism by years to decades.
Involvement of the nigrostriatal pathway is observed early in DLB4. The substantia nigra is a distinct region with an extensive network of axonal processes that innervate the basal ganglia and shows increased iron deposition associated with Lewy body pathology5. There appears to be a complex interplay between iron and α-synuclein, promoting disease progression in the synucleinopathies6. Understanding the evolution of iron dysregulation in the substantia nigra is important for potential disease modifying therapies targeting early stages of DLB. Furthermore, imaging biomarkers that can detect the accumulation of iron are needed to track the integrity of substantia nigra during the early stages7.
Quantitative susceptibility mapping (QSM) provides in vivo estimation of regional iron deposition by reconstructing magnetic susceptibility sources from field perturbation8, 9. QSM is sensitive to brain iron content in neurodegenerative diseases including PD10, Parkinson disease with dementia (PDD)11, MSA12 and Alzheimer’s disease13. However, little is known about the QSM findings in patients on the DLB spectrum, especially in those who are at the prodromal stage of the disease and are at risk for developing DLB. The objective of this study was to measure the magnetic susceptibility of the substantia nigra in patients with iRBD, patients with MCI with at least one core feature of DLB (MCI-LB), and probable DLB compared to cognitively unimpaired controls (CU) by QSM.
Methods
Participants
Participants in this study include patients enrolled in the Mayo Clinic Alzheimer’s Disease Research Center (ADRC) between April 2018 and January 2020 and clinically unimpaired controls enrolled in the Mayo Clinic Study of Aging, a population-based study of aging14. A clinical diagnosis was determined for each participant by a consensus committee of study coordinators, neuropsychologists, physicians and neuropsychologists. The diagnosis of dementia was based on Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria. Patient groups included those who diagnosed with probable DLB (pDLB; n=36) according to 4th Consortium Criteria15, MCI with at least one clinical core feature of DLB (MCI-LB, n=15)16, and polysomnography-confirmed idiopathic RBD (iRBD, n=11). The clinically unimpaired adults as controls (CU; n =102) were matched to iRBD, MCI-LB and pDLB patients on age and sex. All healthy controls were characterized as cognitively normal by a consensus of neurologists and neuropsychologists14, 17 and also needed to have a “No” response to acting out dreams during sleep on the Mayo Sleep Questionnaire18 and 0 for the total score on the motor subtest (part III) of the Unified Parkinsonism Disease Rating Scale (UPDRS)19. In the MCSA, 75% of the cohort has a baseline UPDRS-III score of 0 in the same age range (45–89) as the CU control group in current study. We used this criterion in order to exclude CU controls with mild parkinsonism. To avoid circularity, imaging findings were not used to determine group membership.
All patients were evaluated by a behavioral neurologist. Independent evaluations included neurological examination, a clinical interview with patient and informant, and a neuropsychological assessment. Global measures of Clinical Dementia Rating Sum of Boxes (CDR-SOB), and either the MoCA or Short Test of Mental Status20 were used to assess general disease and cognitive severity. A previously established transformation method21 was used to transform the Short Test of Mental Status to MoCA scores which was performed on all 102 MCSA participants and 2 ADRC participants for the further analysis. The presence of REM sleep behavior disorder was obtained clinically by a patient and informant interview and the Mayo Sleep Questionnaire18. Confirmation of the presence of REM sleep without atonia was obtained for the iRBD group. The presence of parkinsonism was based on two of the four cardinal features (tremor, rigidity, bradykinesia, or postural instability) and the severity was quantified using the Unified Parkinson’s Disease Rating Scale19. The presence of fluctuations was based on The Mayo Fluctuations Scale scores of 3 or 422.
The study was approved by the Mayo Clinic Institutional Review Board. Informed consent was obtained from all participants and/or their proxies for participation in this study.
MRI acquisition
All participants underwent MRI on a 3T scanner (Siemens PRISMA, Siemens, Erlangen, Germany) using a 64-channel phased array head coil. For anatomical segmentation and labeling, a 3D high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) was performed using the following parameters: TR/TE /inversion time = 2300/3/900 ms, flip angle 8°, 1 mm isotropic resolution. MR phase measurements used for QSM calculation were acquired using a bipolar multi-echo 3D gradient recalled echo (GRE) sequence with 5 echoes (TR/TE1/ΔTE = 28/6.71/3.91 ms, FOV = 200×200 mm, voxel size = 0.5×0.5×1.8 mm3, NEX=1, flip angle = 15, bandwidth = 280 Hz/px).
MRI analysis
For each participant, the T1 MRI scan was segmented and corrected for intensity inhomogeneity with the unified segmentation algorithm23 implemented in SPM12 using tissue priors and settings from the Mayo Clinic Adult Lifespan Template (MCALT)24 (https://www.nitrc.org/projects/mcalt/). The subject space segmentations were combined to generate a TIV mask, and ANTs25 nonlinear registration was used to register region-of- interest (ROI) masks of bilateral substantia nigra (originally derived from the DISTAL atlas26) from the MCALT space to subject native space. The TIV and ROI masks were re-aligned to the respective magnitude and phase image by applying an affine registration between the T1 and the magnitude image corresponding to the first echo of the multi-echo GRE. Next, Laplacian-based phase unwrapping was used to unwrap the phase from each echo27. The reconstructed phase image was then calculated by summing all unwrapped phase images for all echoes. V-SHARP28 filtering was applied to remove the bias field with the magnitude and TIV mask as additional inputs, and then QSM was calculated by using the iterative least squares (iLSQR) decomposition method29. Finally, ROI masks of bilateral substantia nigra were used to obtain magnetic susceptibility values from the respective QSM images. The mean value of QSM from the left, right and bilateral substantia nigra were measured for each participant.
Statistical Analysis
Participant characteristics were described with means and standard deviations (SD) for continuous variables, and counts and proportions (%) for categorical variables. Continuous variables were compared across the groups using analysis of variance, followed by pair-wise comparisons with contrast statements. Categorical variables were compared using the Chi-square test across the groups, followed by pair-wise comparisons. Pearson correlations were used to assess the associations between magnetic susceptibility values in the substantia nigra and UPDRS-III scores and CDR-SB scores, respectively. A linear regression trend test that sequentially ordered the four groups was performed to evaluate susceptibility values in the substantia nigra from controls to iRBD to MCI-LB and to pDLB patients. The regression assumes that the groups are ordered by severity and that magnetic susceptibility follows a linear trend across the groups, and tests whether the slope of this trend is significantly different from zero. We tested for but did not find evidence of curvature in the trend. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R statistical software version 3.4.2 (Rproject.org).
Results
Characteristics of participants
Demographic and clinical characteristics of the participants are summarized in Table 1. Most participants were men (92% in pDLB, 80% in MCI-LB and 91% in iRBD), with no differences in sex or age between the patient groups. Patients with iRBD had more education than CU (p=0.005). As expected, patients with pDLB had higher CDR-SOB, lower MoCA scores and greater number of core DLB features compared to MCI-LB (p<0.001) and iRBD (p<0.001). Patients with MCI-LB had lower MoCA scores compared to CU (p=0.004). No difference in MoCA scores was observed in iRBD compared to CU (p=0.90). No difference in the frequency of visual hallucinations, fluctuations, parkinsonism and RBD was observed between the MCI-LB and pDLB groups. There was no difference in the number of core DLB features between pDLB and MCI-LB. In terms of number of core DLB features, 73% of patients with iRBD, none of patients with MCI-LB and 8% of patients with pDLB presented with 1 core DLB feature; 18% of patients with iRBD, 60% of patients with MCI-LB and 22% of patients with pDLB presented with 2 core DLB features; 9% of patients with iRBD, 40% of patients with MCI-LB and 69% of patients with pDLB presented with 3 or 4 core DLB features.
Table 1.
Characteristics of participants.
| CU n = 102 |
iRBD n = 11 |
MCI-LB n = 15 |
pDLB n = 36 |
P-value | |
|---|---|---|---|---|---|
| Age, yrs | 69.2 ± 9.7 | 69.5±5.8 | 67.1±9.6 | 69.7±10.2 | 0.85 |
| Males, n (%) | 90 (88%) | 10 (91%) | 12 (80%) | 33 (92%) | 0.69 |
| Education, yrs | 15.7±2.2 | 17.8±2.4 | 16.6±2.8 | 15.5±2.8 | 0.02 |
| CDR sum of boxes | 0.0±0.1 | 0.4±0.5 | 1.7±0.5 | 5.4±3.2 | <0.001 |
| MoCA | 26.3±2.3 | 26.2±2.1 | 23.7±2.6 | 16.3±5.5 | <0.001 |
| UPDRS-III | -- | 3.3±1.6 | 16.4±9.9 | 20.8±13.4 | 0.006 |
| Visual Hallucinations, n (%) | -- | 0 (0%) | 4 (27%) | 19 (53%) | 0.60 |
| Fluctuations, n (%) | -- | 1 (11%) | 6 (40%) | 23 (64%) | 0.009 |
| Parkinsonism, n (%) | -- | 3 (27%) | 13 (87%) | 30 (83%) | <0.001 |
| RBD, n (%) | -- | 11 (100%) | 15 (100%) | 34 (94%) | 0.46 |
| Core DLB features | <0.001 | ||||
| 1 feature, n (%) | -- | 8 (73%) | 0 (0%) | 3 (9%) | |
| 2 features, n (%) | -- | 2 (18%) | 9 (60%) | 8 (23%) | |
| 3 or 4 features, n (%) | -- | 1 (9%) | 6 (40%) | 25 (69%) |
All the data represent mean±standard deviation unless otherwise indicated.
P-values are comparing groups are from an analysis of variance (ANOVA) or chi-squared test.
Abbreviations: CU = cognitive unimpaired adults; iRBD = idiopathic rapid eye movement sleep behavior disorder; MCI = mild cognitive impairment; DLB = dementia with Lewy body; n = number; yrs = years; CDR = clinical dementia rating scale; MoCA = Montreal cognitive assessment(MoCA); UPDRS = unified Parkinson disease rating scale; RBD = rapid eye movement sleep behavior disorder.
Susceptibility in the substantia nigra
The mean susceptibility calculated form QSM in the substantia nigra bilaterally was lowest in CU (0.091 ± 0.025), slightly higher in the iRBD (0.103± 0.024) and MCI-LB (0.096 ± 0.026) groups, and highest in the pDLB group (0.112 ± 0.026). Patients with pDLB had higher susceptibility values in the substantia nigra compared to controls (p<0.001) and MCI-LB (p=0.043, Fig 1). Substantia nigra susceptibility values in MCI-LB (p=0.51) and iRBD (p=0.14) were not different from CU, and no differences were observed between iRBD and MCI-LB (p=0.47) susceptibility values. In addition, the susceptibility of substantia nigra showed an increasing trend from CU to iRBD to MCI-LB and to pDLB (p<0.001, Fig 2). Susceptibility values of the substantia nigra were not associated with the presence or absence of the core clinical features (parkinsonism, RBD, visual hallucinations or fluctuations) in MCI-LB or pDLB.
Fig 1:
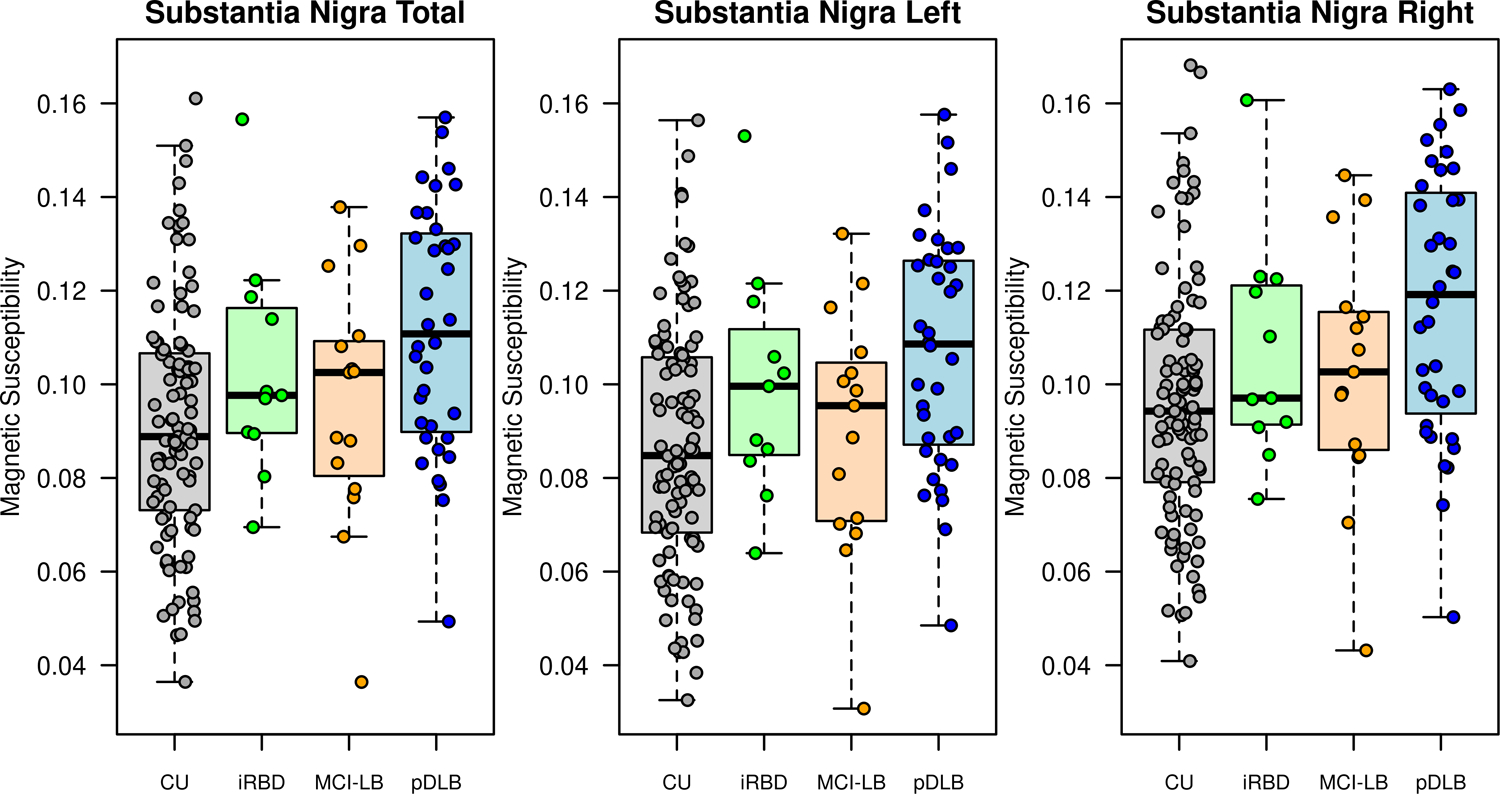
Box-plots of magnetic susceptibility in substantia nigra across 4 groups.
Box-plots show the magnetic susceptibility from left, right and bilateral substantia nigra in cognitive unimpaired controls (CU), patients with idiopathic rapid eye movement sleep behavior disorder (iRBD), mild cognitive impairment with DLB features (MCI-LB) and probable dementia with Lewy bodies (pDLB).
Fig 2:
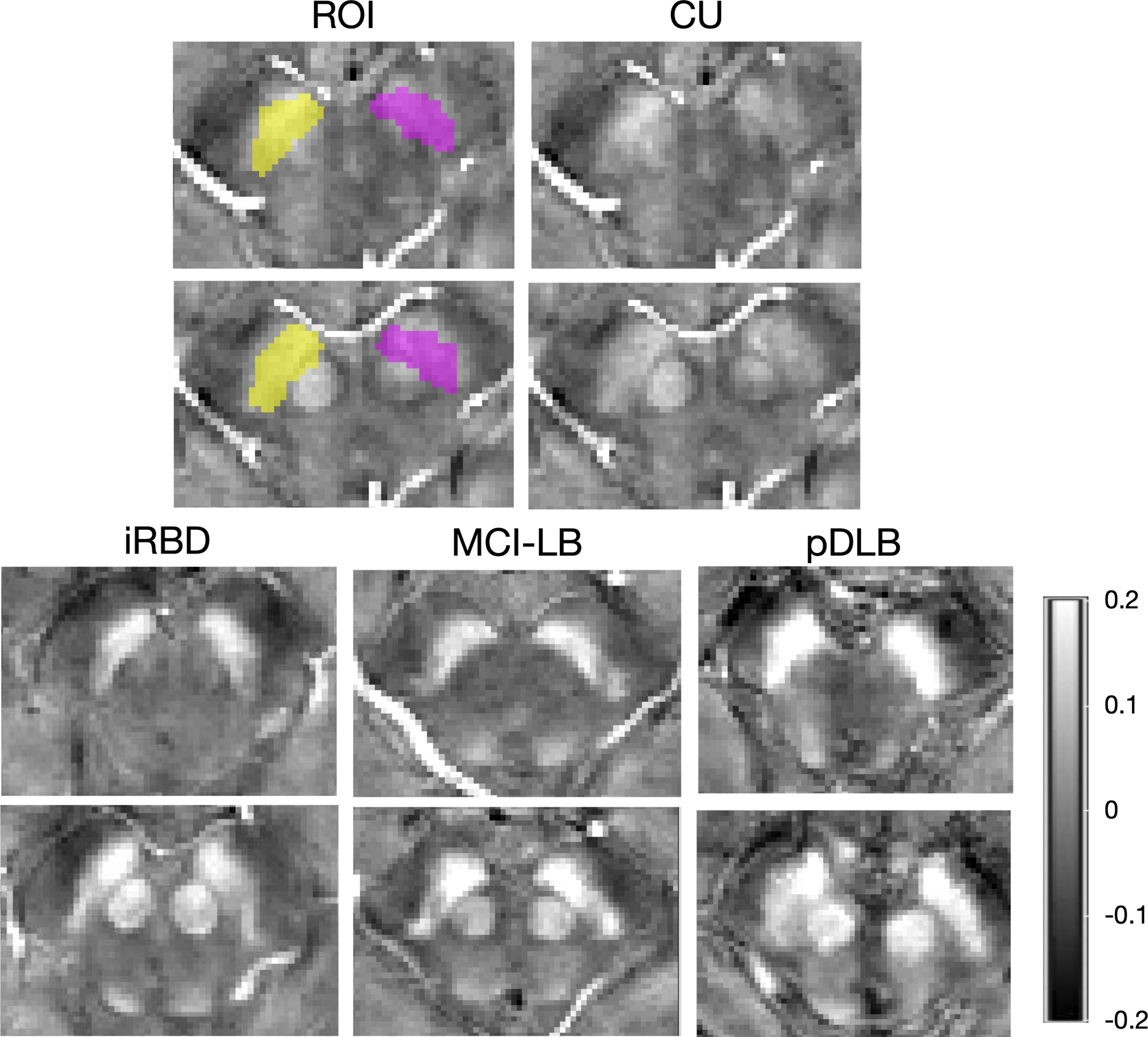
Representative of quantitative susceptibility mapping images and regions of interests of the substantia nigra.
Representative quantitative susceptibility mapping (QSM) images were from a cognitively unimpaired adult (CU) (male, 74 years old), a patient with idiopathic rapid eye movement sleep behavior disorder (iRBD) (male, 69 years old), a patient with mild cognitive impairment and clinical features of dementia with Lewy bodies (MCI-LB) (male, 71 years old) and a patient with probable DLB (pDLB) (male, 72 years old). The upper row shows QSM images in the ventral level of substantia nigra (inferior to the red nucleus) and lower row shows QSM images in the dorsal level of substantia nigra (adjacent to the red nucleus). ROIs are shown overlaid on the native space QSM image of CU from the left (purple) and right (yellow) hemispheres.
Correlation between QSM and Clinical Severity
In the combined group of patients with MCI-LB and pDLB, the mean susceptibility values in the substantia nigra did not correlate with parkinsonism severity derived from the UPDRS-III (r=0.09; p=0.56) or with dementia severity derived from the CDR-SB (r=0.02; p=0.87) or MoCA scores (r= −0.132; p=0.36). When the pDLB group was examined separately, neither UPDRS-III (r=0.02; p=0.93) nor CDR-SOB (r=−0.19; p=0.26) or MoCA scores (r=0.067; p=0.70) were associated with magnetic susceptibility in the substantia nigra.
Discussion
This study demonstrated increased magnetic susceptibility in the substantia nigra in pDLB using quantitative susceptibility mapping, a potential biomarker for increased iron content. There was also evidence of increasing susceptibility from cognitively unimpaired controls to iRBD and MCI-LB to pDLB, suggesting that iron deposition in the substantia nigra starts to increase early in the Lewy body disease process, perhaps years before the onset of dementia with a trending increase with disease severity.
Only a few studies investigated the iron accumulation in DLB with susceptibility-weighted imaging (SWI) or R2*-weighted MRI30, 31. To the best of our knowledge, no study has applied the QSM technique to investigate the iron deposition in patients with pDLB, MCI-LB and iRBD. In the current study, we applied the QSM for accurate quantification of total susceptibility, which is associated with tissue iron content32. SWI is a qualitative estimate of QSM with thresholding limitations from a combination of high-pass filtering phase and a user defined phase multiplicative factor. R2* mapping is confounded by the heterogeneity of the magnetic field, depending on orientation, field strength, and the distribution of susceptibility sources, and is not a quantitative estimate of an inherent tissue property8, 9. Within the context of iron deposition in the substantia nigra in PD, QSM was reported to be more sensitive than R2* mapping33. To date, QSM is considered the MRI technique with highest sensitivity and specificity for brain iron quantification in living patients8, 9, especially in investigations of the pathologic susceptibility changes in the subcortical nuclei34.
Our findings of increased magnetic susceptibility in the substantia nigra in patients with pDLB were consistent with the observations in PD10, 35, suggesting that elevated iron content in the substantia nigra preserved a similar pattern in the specific clinical scenarios of synucleinopathy. Abnormal intracellular aggregation of α-synuclein, the main constituent of the Lewy bodies and Lewy neurites, is observed in the substantia nigra early in DLB and PD. The interaction between α-synuclein and iron is intricate. On one hand, iron binds to α-synuclein36, accelerates α-synuclein aggregation37 and causes toxic hydroxyl radical production38. On the other hand, α-synuclein may also accumulate due to alteration of iron uptake and metabolism39. Furthermore, elevated iron concentration in the substantia nigra in DLB might also result from depigmentation of the nigrosome-1 content40 or dopaminergic neuronal loss41. In addition, previous neuromelanin-sensitive MRI and histopathological studies demonstrated an overlap of both massive loss of melanized dopamine neurons and iron accumulation in the substantia nigra, suggesting that elevated iron deposition in the substantia nigra may also be due to the loss of neuromelanin content in DLB7, 42. Although iron accumulation in the substantia nigra as a cause or outcome of cell death still remains in debate, the increased magnetic susceptibility in the substantia nigra due to increased iron content may provide important insights into the pathogenesis of DLB.
In the current study, iron deposition in the substantia nigra demonstrated an increasing trend from controls to iRBD to MCI-LB and to pDLB, suggesting that iron accumulation in the substantia nigra shows a trend of increase as early as the iRBD stage in DLB and continues to increase as the disease progresses. This is consistent with previous findings in PD that the higher susceptibility was found only in the substantia nigra in PD patients compared to controls by QSM35. Furthermore, a recent QSM study demonstrated a disparate ventral to dorsal distribution pattern of iron content in the substantia nigra from early to late stage in PD10. Increased magnetic susceptibility in substantia nigra pars compacta (SNc) was reported in the early stage of PD, and extended to the substantia nigra pars reticulata (SNr) in the late stage43. Taken together, our results expand the previous findings and suggest that increasing burden of iron load in the substantia nigra may be an early biomarker of disease progression starting from the prodromal stages in DLB.
RBD is a strong predictor of synucleinopathy and may occur years or even decades before the motor and/or cognitive symptoms of parkinsonism or dementia3. Our findings concur with the transcranial sonography (TCS) findings in patients with iRBD, with increased iron deposition in the substantia nigra revealed as hyperechogenicity on TCS44. However, the explanation that underlies the presence of iron excess in the substantia nigra in iRBD is not clear. There was no difference in the susceptibility value between iRBD and MCI-LB in groups, and no correlation of susceptibility with CDR-SOB or MoCA. Thus, we would suspect that the abnormal susceptibility in the substantia nigra may be prominent in patients with iRBD independent of cognitive impairment. However, whether the iron deposition patterns differ between iRBD patients who progressed to Parkinson disease, or to pDLB or to MSA is currently unknown. Further studies with a broader representation of patients with Lewy body disease, and a larger sample of iRBD patients in a longitudinal design are needed to investigate the association between the susceptibility in the substantia nigra and clinical phenotypes.
There was no relationship between magnetic susceptibility in the substantia nigra and a measure of parkinsonism severity (UPDRS-III) in patients with MCI-LB and pDLB. It is possible that although iron accumulation may be an early finding in the disease process and associated with synucleinopathy, it may be independent of the extent of nigral loss and of the severity of parkinsonism. Some studies have shown a positive correlation between magnetic susceptibility in the substantia nigra and the Hoehn &Yahr stage in PD45, 46 and between magnetic susceptibility in the substantia nigra and UPDRS-III scores47, while others show a lack of correlation with UPDRS-III scores10, 45, 46. The disparate findings among studies may be due to the variability in disease duration, severity, and symptomatic treatment of parkinsonism in some patients. The overall mild severity of motor features detected by UPDRS-III may have influenced the correlations. It is also possible that increased substantia nigra iron content might be independent of dopaminergic dysfunction as revealed by a 123I-FP-CIT SPECT study48. Future studies are needed to identify the potential mechanisms underlying associations between iron content in the substantia nigra by QSM with clinical outcome measurements.
One limitation of this study is a relatively small sample size; therefore the results may be impacted by influential cases. For example, one of the MCI-LB patients had the lowest magnetic susceptibility in substantia nigra in our cohort. This patient was a 76-year-old woman whose main symptoms were complex visual and auditory hallucinations with mild cognitive impairment but no RBD or parkinsonism. Although Lewy body pathology is commonly observed in the brainstem of DLB in a bottom-up pathological progression as staged by Braak et al.49, there is some evidence that the nigra may be less severely affected in a subgroup of patients50, 51. Second, our data are cross-sectional and cannot provide information on the relationship of QSM values with motor, cognitive and functional progression in patients with DLB and prodromal DLB. Future studies with larger sample sizes in a longitudinal study design assessing the diagnostic utility of QSM in pDLB and prodromal DLB as well as neuropathological validation would increase the utility of the present findings.
In conclusion, our data indicate QSM is sensitive to the increased magnetic susceptibility likely due to higher iron content in the substantia nigra in DLB. The trend of increasing susceptibility from CU to iRBD and MCI-LB and to DLB suggests that iron deposition in the substantia nigra starts to increase early in the Lewy body disease process and continues to increase as the disease progresses independent of the severity of parkinsonism. Our cross-sectional findings support future studies of QSM in the substantia nigra as a potential biomarker for monitoring the disease progression in pDLB and prodromal DLB.
Acknowledgements
This study was funded by the NIH (U01 NS100620, R01 AG040042, R01 AG11378, P50 AG16574, U01 AG06786, P30 AG062677 and C06 RR018898), the Foundation Dr. Corinne Schulerand, the Mangurian Foundation for Lewy Body Research, the Elsie and Marvin Dekelboum Family Foundation, and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program. We are grateful to our patients and caregivers for their participation in our detailed annual assessments and for their involvement in current study.
Dr. Boeve has served as an investigator for clinical trials sponsored by Biogen, Alector, and EIP Pharma. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from the NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, the Little Family Foundation, and the Turner Family Foundation.
Dr. Jack consults for Eli Lilly and serves on an independent data monitoring board for Roche but he receives no personal compensation from any commercial entity. He receives research support from the NIH.
KW Kremers receives research funding from AstraZeneca, Biogen, Roche, DOD and NIH.
Dr. Fields receives research support from NIH.
Dr. J. Graff-Radford receives research support from the NIH.
Dr. Knopman serves on the DSMB of the DIAN-TU study, is a site PI for clinical trials sponsored by Biogen, Lilly and the University of Southern California, and is funded by NIH.
Dr. Dickson is an editorial board member for Acta Neuropathologica, Brain, Brain Pathology, Neuropathology and Applied Neurobiology, Annals of Neurology, Neuropathology and editor for the International Journal of Clinical and Experimental Pathology and American Journal of Neurodegenerative Disease. He is supported by the Mangurian Foundation for Lewy body disease research and NIH.
Dr. Ferman receives funding from the Mangurian Foundation for Lewy body research and NIH.
Dr. N. Graff-Radford receives royalties from UpToDate, has participated in multicenter therapy studies by sponsored by Biogen, TauRx, AbbVie, Novartis and Lilly. He receives receives research support from NIH.
Dr. Petersen serves on scientific advisory boards for Elan Pharmaceuticals, Wyeth Pharmaceuticals, and GE Healthcare; receives royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003). He also receives research support from NIH.
Dr. Kantarci serves on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc.; She consults Biogen Inc. She receives research support from the Avid Radiopharmaceuticals, Eli Lilly. She is funded by the Alzheimer’s Drug Discovery Foundation and NIH.
Footnotes
Disclosures
The other authors have nothing to disclose.
References
- 1.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol 2013;70:1396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020: 10.1212/WNL.0000000000009323. [DOI] [PMC free article] [PubMed]
- 3.Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci 2010;1184:15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang SH, Chang CC, Lui CC, et al. Cortical metabolic and nigrostriatal abnormalities associated with clinical stage-specific dementia with Lewy bodies. Clin Nucl Med 2015;40:26–31. [DOI] [PubMed] [Google Scholar]
- 5.Lhermitte J, Kraus WM, McAlpine D. Original Papers: ON THE OCCURRENCE OF ABNORMAL DEPOSITS OF IRON IN THE BRAIN IN PARKINSONISM WITH SPECIAL REFERENCE TO ITS LOCALISATION. J Neurol Psychopathol 1924;5:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndayisaba A, Kaindlstorfer C, Wenning GK. Iron in Neurodegeneration - Cause or Consequence? Front Neurosci 2019;13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langley J, Huddleston DE, Sedlacik J, Boelmans K, Hu XP. Parkinson’s disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Mov Disord 2017;32:441–9. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Liu T. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magn Reson Med 2015;73:82–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haacke EM, Liu S, Buch S, Zheng W, Wu D, Ye Y. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging 2015;33:1–25. [DOI] [PubMed] [Google Scholar]
- 10.Bergsland N, Zivadinov R, Schweser F, Hagemeier J, Lichter D, Guttuso T Jr. Ventral posterior substantia nigra iron increases over 3 years in Parkinson’s disease. Mov Disord 2019;34:1006–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen PH, Cheng SJ, Lin HC, Lee CY, Chou CH. Risk Factors for the Progression of Mild Cognitive Impairment in Different Types of Neurodegenerative Disorders. Behav Neurol 2018;2018:6929732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzucchi S, Frosini D, Costagli M, et al. Quantitative susceptibility mapping in atypical Parkinsonisms. Neuroimage Clin 2019;24:101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiepolt S, Schafer A, Rullmann M, et al. Quantitative Susceptibility Mapping of Amyloid-beta Aggregates in Alzheimer’s Disease with 7T MR. J Alzheimers Dis 2018;64:393–404. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010;75:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med 2011;12:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahn S. Recent developments in Parkinson’s disease. Macmillan health care information 1987;2:293–304. [Google Scholar]
- 20.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol 1991;48:725–8. [DOI] [PubMed] [Google Scholar]
- 21.Townley RA, Syrjanen JA, Botha H, et al. Comparison of the Short Test of Mental Status and the Montreal Cognitive Assessment Across the Cognitive Spectrum. Mayo Clin Proc 2019;94:1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004;62:181–7. [DOI] [PubMed] [Google Scholar]
- 23.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–51. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz CG, Gunter JL, Ward CP, et al. The Mayo Clinic Adult Lifespan Template: better quantification across the lifespan. Alzheimers Dement 2017;13:P93–P4. [Google Scholar]
- 25.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 2018;170:271–82. [DOI] [PubMed] [Google Scholar]
- 27.Schofield MA, Zhu Y. Fast phase unwrapping algorithm for interferometric applications. Opt Lett 2003;28:1194–6. [DOI] [PubMed] [Google Scholar]
- 28.Wu B, Li W, Guidon A, Liu C. Whole brain susceptibility mapping using compressed sensing. Magn Reson Med 2012;67:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. Neuroimage 2011;55:1645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shams S, Fallmar D, Schwarz S, et al. MRI of the Swallow Tail Sign: A Useful Marker in the Diagnosis of Lewy Body Dementia? AJNR Am J Neuroradiol 2017;38:1737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamagata K, Nakatsuka T, Sakakibara R, et al. Diagnostic imaging of dementia with Lewy bodies by susceptibility-weighted imaging of nigrosomes versus striatal dopamine transporter single-photon emission computed tomography: a retrospective observational study. Neuroradiology 2017;59:89–98. [DOI] [PubMed] [Google Scholar]
- 32.Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage 2012;62:1593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis MM, Du G, Baccon J, et al. Susceptibility MRI captures nigral pathology in patients with parkinsonian syndromes. Mov Disord 2018;33:1432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbosa JH, Santos AC, Tumas V, et al. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2. Magn Reson Imaging 2015;33:559–65. [DOI] [PubMed] [Google Scholar]
- 35.Ghassaban K, He N, Sethi SK, et al. Regional High Iron in the Substantia Nigra Differentiates Parkinson’s Disease Patients From Healthy Controls. Front Aging Neurosci 2019;11:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharathi Rao KS. Molecular understanding of copper and iron interaction with alpha-synuclein by fluorescence analysis. J Mol Neurosci 2008;35:273–81. [DOI] [PubMed] [Google Scholar]
- 37.Kostka M, Hogen T, Danzer KM, et al. Single particle characterization of iron-induced pore-forming alpha-synuclein oligomers. J Biol Chem 2008;283:10992–1003. [DOI] [PubMed] [Google Scholar]
- 38.Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D. alpha-Synuclein implicated in Parkinson’s disease catalyses the formation of hydrogen peroxide in vitro. Free Radic Biol Med 2001;30:1163–70. [DOI] [PubMed] [Google Scholar]
- 39.Patel D, Xu C, Nagarajan S, et al. Alpha-synuclein inhibits Snx3-retromer-mediated retrograde recycling of iron transporters in S. cerevisiae and C. elegans models of Parkinson’s disease. Hum Mol Genet 2018;27:1514–32. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz ST, Mougin O, Xing Y, et al. Parkinson’s disease related signal change in the nigrosomes 1–5 and the substantia nigra using T2* weighted 7T MRI. Neuroimage Clin 2018;19:683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin-Bastida A, Lao-Kaim NP, Loane C, et al. Motor associations of iron accumulation in deep grey matter nuclei in Parkinson’s disease: a cross-sectional study of iron-related magnetic resonance imaging susceptibility. Eur J Neurol 2017;24:357–65. [DOI] [PubMed] [Google Scholar]
- 42.Castellanos G, Fernandez-Seara MA, Lorenzo-Betancor O, et al. Automated neuromelanin imaging as a diagnostic biomarker for Parkinson’s disease. Mov Disord 2015;30:945–52. [DOI] [PubMed] [Google Scholar]
- 43.Chen Q, Chen Y, Zhang Y, et al. Iron deposition in Parkinson’s disease by quantitative susceptibility mapping. BMC Neurosci 2019;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iranzo A, Lomena F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected]. Lancet Neurol 2010;9:1070–7. [DOI] [PubMed] [Google Scholar]
- 45.An H, Zeng X, Niu T, et al. Quantifying iron deposition within the substantia nigra of Parkinson’s disease by quantitative susceptibility mapping. J Neurol Sci 2018;386:46–52. [DOI] [PubMed] [Google Scholar]
- 46.Langkammer C, Pirpamer L, Seiler S, et al. Quantitative Susceptibility Mapping in Parkinson’s Disease. PLoS One 2016;11:e0162460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pesch B, Casjens S, Woitalla D, et al. Impairment of Motor Function Correlates with Neurometabolite and Brain Iron Alterations in Parkinson’s Disease. Cells 2019;8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartova P, Kraft O, Bernatek J, et al. Transcranial sonography and (123)I-FP-CIT single photon emission computed tomography in movement disorders. Ultrasound Med Biol 2014;40:2365–71. [DOI] [PubMed] [Google Scholar]
- 49.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 50.Nedelska Z, Ferman TJ, Boeve BF, et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol Aging 2015;36:452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heitz C, Noblet V, Cretin B, et al. Neural correlates of visual hallucinations in dementia with Lewy bodies. Alzheimers Res Ther 2015;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]


