Abstract
We aimed to investigate the cell density and morphology of the corneal endothelium in ophthalmologically healthy young Japanese, given the lack of normative data in literature. This observational study included eyes without ophthalmologic diseases, besides refractive errors, examined between 1996 and 2015 at Miyata Eye Hospital. Eyes with a history of ophthalmologic diseases or contact lens usage were excluded. Correlation of corneal endothelial cell density (ECD), coefficient of variation (CV), appearance rate of hexagonal cells (6A), and cell area with age were examined. Multivariate linear regression analysis was performed to determine the predictors of corneal parameters. We included 16842 eyes of 8421 individuals (19.6 ± 8.7 years). ECD was 3109.0 ± 303.7 cells/mm2 and significantly reduced with age (p < 0.001). The ECD reduction rate was 0.42%/year in the total population. On multivariate analysis, age and sex were significantly correlated with ECD, CV, 6A, and cell area (all p < 0.001). ECD, 6A, CV, and cell area are significantly associated with age in healthy young Japanese individuals. Monitoring their corneal endothelium is essential to assess the risk of endothelial damage.
Subject terms: Diseases, Eye diseases, Anatomy
Introduction
Corneal endothelial cells constitute a single cell layer and help maintain corneal transparency by the barrier function that could let water and nutrients into the corneal stroma from the anterior chamber and the pumping function performed by Na–K-ATPase, thereby maintaining visual acuity1. Corneal endothelial cells can be damaged by various reasons, such as ophthalmologic surgery2,3, trauma4, uveitis5, contact lens usage6, ultraviolet radiation7, and aging8. Moreover, decreased corneal endothelial cell density (ECD) owing to the reasons mentioned above results in blurred vision or impaired visual acuity, which requires corneal transplantation for function recovery9. Therefore, it is clinically essential to observe the status of corneal endothelial cells.
Although it is necessary to determine its normal range in healthy patients to understand the degree of corneal endothelial cell damage, ECD or corneal endothelial morphology is known to vary across different populations, such as 2610 ± 372 cells/mm2 for Nigerian10, 2648 ± 383 cells/mm2 for Egyptian11, 2732 ± 258 cells/mm2 for Thai12, 2732 ± 305 cells/mm2 for Turkish13, and 2932 ± 363 cells/mm2 for Chinese14 individuals. Although several studies have evaluated the status of ECD or endothelial morphology from various countries10–16, few have reported on healthy data collected from Japanese patients17. Furthermore, previous reports lack information on healthy patients aged < 40 years and none of the reports evaluated the healthy morphology. Because patients who use contact lens for refractive correction tend to be relatively young18, it is essential to determine the value and distribution of corneal endothelial density and morphology of healthy young patients for the accurate assessment of contact lens usage. Therefore, we aimed to analyze corneal ECD and morphology in healthy Japanese individuals without a history of contact lens usage, ophthalmic surgery, or ocular disease.
Results
We included 16842 eyes of 8421 patients in the study (age, 19.6 ± 8.7 years; range, 6–70 years) (Table 1). A total of 6668 eyes of 3334 male patients (age, 19.3 ± 7.8 years) and 10,174 eyes of 5087 female patients (age, 19.8 ± 9.1 years) were included.
Table 1.
Demographic data of ophthalmologically healthy Japanese eyes.
| Sex | Side of the eye | N (eyes) | Age (years) | ECD (cells/mm2) | CV (%) | 6A (%) | Cell area (μm2) |
|---|---|---|---|---|---|---|---|
| Total | Total | 16842 | 19.6 ± 8.7 | 3109.0 ± 303.7 | 27.6 ± 5.1 | 65.9 ± 11.4 | 324.9 ± 32.5 |
| Right | 8421 | – | 3117.0 ± 303.0 | 27.6 ± 5.1 | 66.0 ± 11.5 | 324.1 ± 32.4 | |
| Left | 8421 | – | 3101.8 ± 304.3 | 27.6 ± 5.1 | 65.9 ± 11.3 | 325.6 ± 32.7 | |
| Male | Total | 6668 | 19.3 ± 7.8 | 3058.2 ± 292.9 | 27.1 ± 4.9 | 67.3 ± 11.2 | 330.2 ± 32.2 |
| Right | 3334 | – | 3067.6 ± 293.4 | 27.1 ± 4.9 | 67.3 ± 11.3 | 329.3 ± 32.1 | |
| Left | 3334 | – | 3048.7 ± 292.2 | 27.1 ± 4.9 | 67.2 ± 11.1 | 331.1 ± 32.3 | |
| Female | Total | 10174 | 19.8 ± 9.1 | 3142.9 ± 306.0 | 27.9 ± 5.1 | 65.1 ± 11.4 | 321.3 ± 32.2 |
| Right | 5087 | – | 3149.3 ± 304.8 | 27.9 ± 5.1 | 65.1 ± 11.3 | 320.7 ± 32.1 | |
| Left | 5087 | – | 3136.6 ± 307.1 | 27.9 ± 5.1 | 65.0 ± 11.4 | 322.0 ± 32.4 |
ECD, corneal endothelial cell density; CV, coefficient of variation in cell area; 6A, appearance rate of hexagonal cells.
In the stratified data based on the age of 10 years, in the 1–10-year group, the mean ECD was the highest, mean the coefficient of variation (CV) was the lowest, mean appearance rate of hexagonal cells (6A) was the highest, and mean cell area was the lowest (Table 2). ECD was significantly negatively correlated with age (p < 0.001, R2 = 0.359, Fig. 1). ECD reduced to − 12.63 cells/mm2 per year, and the reduction rate was 0.42%/year in the total population. In the stratified age category, ECD had the greatest reduction in the 11–20-year group (− 19.3 cells/mm2/year), which was significantly correlated with age (p < 0.001). The CV in cell area was significantly positively correlated with age (p < 0.001, R2 = 0.267, Fig. 2) while the 6A was significantly negatively correlated with age (p < 0.001, R2 = 0.232, Fig. 3). The cell area was also significantly correlated with age (p < 0.001, R2 = 0.387, Fig. 4), gradually increasing with age. Correlations between age and ECD, CV, 6A, and cell area were confirmed with the data of all included eyes (bilaterally) as well as with those of unilateral eyes (all p < 0.001).
Table 2.
Corneal endothelial cell density and morphology of ophthalmologically healthy Japanese eyes according to age category.
| Age category | N (eyes) | Mean age (years) | ECD (cells/mm2) | CV (%) | 6A (%) | Cell area (μm2) |
|---|---|---|---|---|---|---|
| 1–10 | 96 | 9.3 ± 1.4 | 3314.5 ± 334.5 | 26.1 ± 5.3 | 69.8 ± 10.5 | 304.7 ± 33.9 |
| 11–20 | 12540 | 15.8 ± 2.2 | 3160.3 ± 284.5 | 26.9 ± 4.8 | 67.3 ± 11.2 | 319.2 ± 28.8 |
| 21–30 | 2608 | 24.8 ± 2.8 | 3027.6 ± 287.0 | 29.0 ± 4.9 | 62.9 ± 10.8 | 333.9 ± 32.5 |
| 31–40 | 828 | 34.7 ± 2.8 | 2874.2 ± 283.8 | 30.3 ± 5.2 | 60.2 ± 10.7 | 352.6 ± 35.8 |
| 41–50 | 478 | 45.1 ± 2.8 | 2814.4 ± 298.4 | 31.3 ± 5.3 | 59.2 ± 9.8 | 360.3 ± 39.5 |
| 51–60 | 246 | 54.3 ± 2.5 | 2734.5 ± 273.5 | 31.4 ± 5.3 | 58.7 ± 9.5 | 365.5 ± 37.2 |
| 61–70 | 46 | 64.5 ± 2.4 | 2740.0 ± 289.5 | 30.3 ± 4.2 | 61.3 ± 8.9 | 372.6 ± 35.9 |
ECD, corneal endothelial cell density; CV, coefficient of variation in cell area; 6A, appearance rate of hexagonal cells.
Figure 1.
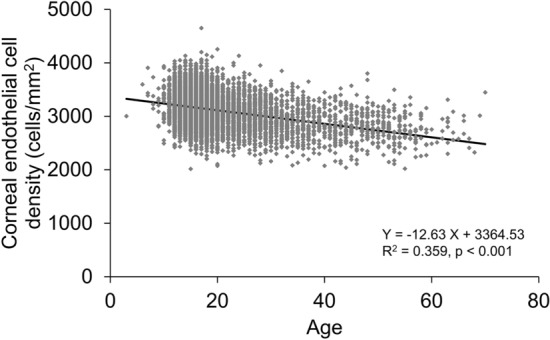
Association between corneal endothelial cell density and age in ophthalmologically healthy Japanese. Based on the linear regression model, a significant mild negative association between corneal endothelial cell density and age was observed (Y = − 12.63 X + 3364.53, R2 = 0.359, p < 0.001).
Figure 2.
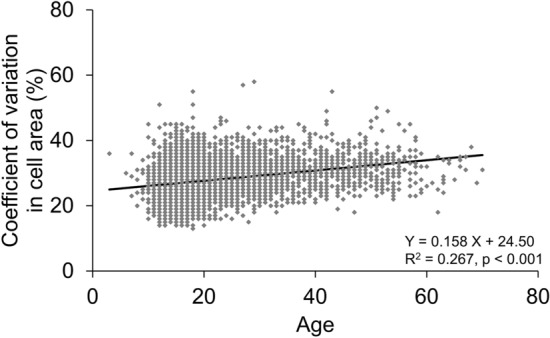
Association between the coefficient of variation in cell area and age in ophthalmologically healthy Japanese. Based on the linear regression model, a significant weak positive association between the coefficient of variation in cell area and age was observed (Y = 0.158 X + 24.50, R2 = 0.267, p < 0.001).
Figure 3.
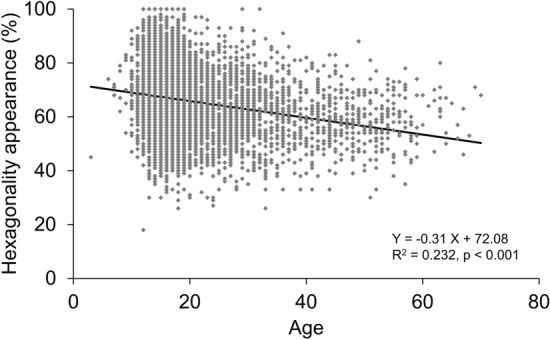
Association between the hexagonal appearance of corneal endothelial cell and age in ophthalmologically healthy Japanese. Based on the linear regression model, a significant weak negative association between the hexagonal appearance of corneal endothelial cell and age was observed (Y = − 0.31 X + 72.08, R2 = 0.232, p < 0.001).
Figure 4.
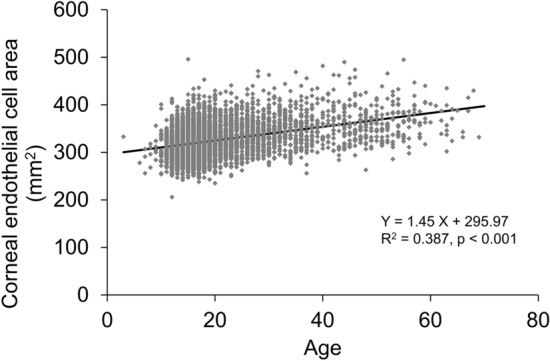
Association between corneal endothelial cell area and age in ophthalmologically healthy Japanese. Based on the linear regression model, a significant mild positive association between corneal endothelial cell area and age was observed (Y = 1.45 X + 295.97, R2 = 0.387, p < 0.001).
Multivariate linear regression analysis revealed that higher age and being male were significantly correlated with lower ECD (p < 0.001, Table 3). Higher age was also significantly related to higher CV, lower 6A, and larger cell area (all p < 0.001, Table 3). These correlations were confirmed with the data of all included eyes (bilaterally) as well as with those of unilateral eyes (all p < 0.001).
Table 3.
Multiple linear regression analysis of endothelial cell density and morphology of healthy Japanese eyes.
| Unstandardized coefficient β | Partial correlation coefficient | P-value | ||
|---|---|---|---|---|
| ECD (cells/mm2) | Sex (male to female) | − 87.46 | − 0.15 | < 0.001 |
| Age (year) | − 12.76 | − 0.37 | < 0.001 | |
| CV (%) | Sex (male to female) | − 0.72 | − 0.07 | < 0.001 |
| Age (year) | 0.16 | 0.27 | < 0.001 | |
| 6A (%) | Sex (male to female) | 2.04 | 0.09 | < 0.001 |
| Age (year) | − 0.31 | − 0.23 | < 0.001 | |
| Cell area (μm2) | Sex (male to female) | 9.19 | 0.15 | < 0.001 |
| Age (year) | 1.46 | 0.39 | < 0.001 |
ECD, corneal endothelial cell density; CV, coefficient of variation in cell area; 6A, appearance rate of hexagonal cells.
Regarding difference in sex, ECD and CV were significantly higher in female than male individuals after adjusting for age (p = 0.018 and 0.011, respectively; Table 4). 6A and cell area were significantly higher in male than female individuals (0.046 and < 0.001, respectively; Table 4). These correlations in ECD, CV, 6A, and cell area were confirmed with the data of all included eyes (bilaterally) as well as with those of unilateral eyes (p = 0.0053, < 0.001, < 0.001, and = 0.0237, respectively).
Table 4.
Comparison of endothelial cell density and morphology of healthy Japanese eyes according to sex.
| Sex | Mean age (years) | ECD (cells/mm2) | CV (%) | 6A (%) | Cell area (μm2) |
|---|---|---|---|---|---|
| Male | 19.3 ± 7.8 | 3058.2 ± 292.9 | 27.1 ± 4.9 | 67.3 ± 11.2 | 330.2 ± 32.2 |
| Female | 19.8 ± 9.1 | 3142.9 ± 306.0 | 27.9 ± 5.1 | 65.1 ± 11.4 | 321.3 ± 32.2 |
| P-value | < 0.001 | 0.018 | 0.011 | 0.046 | < 0.001 |
ECD, corneal endothelial cell density; CV, coefficient of variation in cell area; 6A, appearance rate of hexagonal cells.
Discussion
We analyzed corneal ECD and morphology in 16842 eyes of young healthy Japanese patients, which is the largest number of the eyes compared to past reports, and demonstrated that the mean ECD was 3109.0 ± 303.7 cells/mm2 in healthy Japanese patients aged 19.6 ± 8.7 years (range, 6–70 years). Higa et al. previously reported that ECD was 2943 ± 387 cells/mm2 in ophthalmologically healthy Japanese patients aged more than 40 years, and the reduction was associated with age17. Our data were consistent with those of previous reports, including patients aged 40–50 years, from various countries as mentioned above, such as Nigeria (2610 ± 372)10, Egypt (2648 ± 383)11, Thailand (2732 ± 258)12, China (2932 ± 363)14, Turkey (2671 ± 356)16, the Philippines (2798 ± 307)19, and Malaysia (2648 ± 310)20. On the contrary, normal ECD data of young patients were scarce21,22. Thus, our study revealed significant information on corneal parameters in young patients aged less than 20 years and reinforced the previous studies conducted in Japan17. Furthermore, Shen et al. reported that race significantly affected ECD, and Matsuda et al. also reported that the averaged ECD is reportedly higher in Asian eyes than in Caucasian eyes; our results supported those of previous studies23,24.
The negative correlation between ECD and age that was clearly observed in our results was consistent with the results of previous studies10–12,14,16,19,20,25. The ECD reduction rate was 0.42%/year, close to that previously reported (0.3–0.5%)8,14,26. Corneal diameter was hypothesized to be associated with ECD difference among various populations23. However, we did not review the corneal diameter data because these were unavailable in all patients. Moreover, female patients had significantly higher ECD than male patients (Tables 3 and 4), which was consistent with the result of a previous study in Japan17. The reason for the difference between sexes is currently unknown. While some studies reported that females had higher ECD than males, other studies found that there was no difference between the sexes11,20. Regression analysis also revealed age-related changes, such as a decrease in 6A and increases in CV and cell area. These parameters indicate corneal endothelial stability in terms of cell morphology. Because corneal endothelial cells substantially decrease, when the cells die, the remaining cells will change their form and expand to cover the endothelial surface. Polymegathism, which is an increased variation in endothelial cell size, was rarely observed in young patients according to a previous report15. Additionally, our results demonstrated that corneal endothelium in female individuals had significantly higher ECD, higher CV, and lower 6A compared with those in male individuals, although low CV and high 6A are expected in patients with high ECD in general. This observational data was interesting, but the underlying reason is currently unknown based on the available data.
Age-related changes in ECD, CV, 6A, and cell area were suggested to be different among the groups based on the stratified data in Table 2. The result suggested that corneal endothelial cell distribution changed more acutely when patients were young and that the amount of morphological change would decrease with aging. These changes in corneal parameters could be related to the corneal diameter, as mentioned above23, considering that the cornea becomes larger as individuals age. This morphological change would require further analysis by conducting a longitudinal, observational study.
Soft contact lens usage is prevalent among young patients who desire to correct their myopia without glasses. Some users of soft contact lens experience corneal endothelial damage owing to mechanical stress or oxygen pressure reduction6,27. Therefore, it is critical to determine the normal range of ECD in young patients to understand ECD reduction and compare this reduction among individuals. However, surprisingly, the accumulated knowledge about ECD in young patients was insufficient, probably because young ophthalmologically healthy patients rarely visit hospitals. Furthermore, if they visit hospitals, ECD is not always evaluated. Therefore, we focused on ECD in healthy young patients and demonstrated normalized data in young patients without ophthalmologic disease. Because some corneal parameters are different among different populations, a future clinical study on corneal parameters including healthy young patients in various populations is required.
The current study has some limitations. First, the data included in the current study was obtained using three different types of corneal specular microscopes, which could affect the results. However, the specular microscopes used were basically similar with respect to the microscopic examination methodology. Contact specular microscopy is reportedly useful for further accurate evaluation, but a previous report demonstrated no difference when comparing the results of contact and noncontact specular microscopy28. Second, considering the retrospective nature of the study, some examination items were not available. Especially, high myopia was recently reported as correlated with CV and 6A of the corneal endothelium29; the refractive information is also important. To overcome these limitations, further prospective clinical studies with more detailed information of refractive error and history of systemic diseases, such as diabetic mellitus, that could affect the status of corneal endothelial cells should be conducted30.
In conclusion, we demonstrated normalized data of ECD and morphological parameters and changes per year in a large number of young healthy Japanese patients. It is important to monitor corneal endothelial cells in young patients to assess the possible risk of endothelial damage and to avoid future complications.
Methods
This retrospective observational study was approved by the Institutional Review Board of Miyata Eye Hospital (Miyazaki, Japan) and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all the person or guardian by an opt-out procedure.
We included eyes of Japanese patients who did not have ophthalmologic diseases, other than refractive errors, and whose corneal endothelia were evaluated before the initiation of contact lens usage at Miyata Eye Hospital from January 1996 to December 2015. We excluded eyes that had previous history of ophthalmologic diseases or contact lens usage. Additionally, we excluded any patients whose cornea was evaluated only unilaterally. We retrospectively reviewed patients’ age, ECD, and morphological data (e.g., 6A, CV and cell area) obtained from their medical charts.
Data of corneal endothelium were obtained using noncontact specular microscopy (FA-3509, SP-8000, and CA-2308, Konan, Nishinomiya, Japan). An endothelial cell image around the center of the cornea was captured, and all endothelial cells within the captured image of a 0.24 × 0.4-mm area were automatically traced. Moreover, the ECD (cell/ mm2) of the central area of the cornea was automatically calculated. The process was commonly performed in all noncontact specular microscopy techniques. Simultaneously, 6A, CV, and cell area were also evaluated from the image owing to the program set in the noncontact specular microscope. For the statistical analysis regarding the association between age and corneal ECD or morphology, we selected only the right eye from each patient. The included patients were categorized into groups of allocation by stratifying age in a 10-year interval.
For statistical analysis, linear regression model was applied to assess the association between age and ECD, 6A, CV, or cell area. Stepwise multivariate linear regression analyses were performed to determine the correlations among ECD, sex, and age. Analysis of covariance was performed to compare ECD, 6A, CV, or cell area to adjust for the covariates of age. Statistical analyses were performed using the BellCurve for Excel (Social Survey Research Information, Tokyo, Japan) and GraphPad Prism 9 (GraphPad Software Inc., CA, USA). All data are expressed as mean ± standard deviation unless otherwise mentioned. Two-tailed P-value < 0.05 was considered statistically significant.
Author contributions
The authors who contributed to the design and conduct of the study were T.O., Y.M., and T.I.; to the collection, management, analysis, and interpretation of data were T.O., Y.M., and R.N.; and to the preparation, review, and approval of the manuscript were T.M. and K.M.
Data availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available because they contain information that could compromise the privacy of the research participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geroski DH, Edelhauser HF. Quantitation of Na/K ATPase pump sites in the rabbit corneal endothelium. Invest. Ophthalmol. Vis. Sci. 1984;25:1056–1060. [PubMed] [Google Scholar]
- 2.Rosado-Adames N, Afshari NA. The changing fate of the corneal endothelium in cataract surgery. Curr. Opin. Ophthalmol. 2012;23:3–6. doi: 10.1097/ICU.0b013e32834e4b5f. [DOI] [PubMed] [Google Scholar]
- 3.Hau S, Barton K. Corneal complications of glaucoma surgery. Curr. Opin. Ophthalmol. 2009;20:131–136. doi: 10.1097/ICU.0b013e328325a54b. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa A. Risk factors for reduced corneal endothelial cell density before cataract surgery. J. Cataract Refract. Surg. 2002;28:1982–1992. doi: 10.1016/s0886-3350(02)01502-x. [DOI] [PubMed] [Google Scholar]
- 5.Guclu H, Gurlu V. Comparison of corneal endothelial cell analysis in patients with uveitis and healthy subjects. Int. Ophthalmol. 2019;39:287–294. doi: 10.1007/s10792-017-0809-7. [DOI] [PubMed] [Google Scholar]
- 6.Liesegang TJ. Physiologic changes of the cornea with contact lens wear. CLAO J. 2002;28:12–27. [PubMed] [Google Scholar]
- 7.Karai I, Matsumura S, Takise S, Horiguchi S, Matsuda M. Morphological change in the corneal endothelium due to ultraviolet radiation in welders. Br. J. Ophthalmol. 1984;68:544–548. doi: 10.1136/bjo.68.8.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: A laser scanning in vivo confocal microscopy study. Br. J. Ophthalmol. 2007;91:1165–1169. doi: 10.1136/bjo.2006.112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishima S. Clinical investigations on the corneal endothelium-XXXVIII. Am. J. Ophthalmol. 1982;93:1–29. doi: 10.1016/0002-9394(82)90693-6. [DOI] [PubMed] [Google Scholar]
- 10.Ewete T, Ani EU, Alabi AS. Normal corneal endothelial cell density in Nigerians. Clin. Ophthalmol. 2016;10:497–501. doi: 10.2147/OPTH.S97070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdellah MM, et al. Corneal endothelial cell density and morphology in healthy Egyptian eyes. J. Ophthalmol. 2019;2019:6370241. doi: 10.1155/2019/6370241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tananuvat N, Khumchoo N. Corneal thickness and endothelial morphology in Normal Thai eyes. BMC Ophthalmol. 2020;20:167. doi: 10.1186/s12886-020-01385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman R, Tok Çevik M, Görkem Çevik S, Duman R, Perente İ. Corneal endothelial cell density in healthy Caucasian population. Saudi J. Ophthalmol. 2016;30:236–239. doi: 10.1016/j.sjopt.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yunliang S, et al. Corneal endothelial cell density and morphology in healthy Chinese eyes. Cornea. 2007;26:130–132. doi: 10.1097/ICO.0b013e31802be63e. [DOI] [PubMed] [Google Scholar]
- 15.Doughty MJ. A prospective analysis of corneal endothelial polymegethism and cell density in young adult Asians. Clin. Exp. Optom. 2014;97:256–263. doi: 10.1111/cxo.12127. [DOI] [PubMed] [Google Scholar]
- 16.Arıcı C, Arslan OS, Dikkaya F. Corneal endothelial cell density and morphology in healthy Turkish eyes. J. Ophthalmol. 2014;2014:852624. doi: 10.1155/2014/852624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higa A, et al. Corneal endothelial cell density and associated factors in a population-based study in Japan: The Kumejima study. Am. J. Ophthalmol. 2010;149:794–799. doi: 10.1016/j.ajo.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Supiyaphun C, Jongkhajornpong P. Contact lens use patterns, behavior and knowledge Among university students in Thailand. Clin. Ophthalmol. 2021;15:1249–1258. doi: 10.2147/OPTH.S304735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padilla MD, Sibayan SA, Gonzales CS. Corneal endothelial cell density and morphology in normal Filipino eyes. Cornea. 2004;23:129–135. doi: 10.1097/00003226-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Mohammad-Salih PA. Corneal endothelial cell density and morphology in normal Malay eyes. Med. J. Malaysia. 2011;66:300–303. [PubMed] [Google Scholar]
- 21.Anbar M, Ammar H, Mahmoud RA. Corneal endothelial morphology in children with Type 1 diabetes. J. Diabetes Res. 2016;2016:7319047. doi: 10.1155/2016/7319047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiles DA, Biglan AW, Fetherolf EC. Central corneal endothelial cell counts in children. J. Am. Intraocul Implant Soc. 1979;5:292–300. doi: 10.1016/s0146-2776(79)80078-6. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, Yee RW, Edelhauser HF. Comparison of the corneal endothelium in an American and a Japanese population. Arch. Ophthalmol. 1985;103:68–70. doi: 10.1001/archopht.1985.01050010072023. [DOI] [PubMed] [Google Scholar]
- 24.Sheng H, Bullimore MA. Factors affecting corneal endothelial morphology. Cornea. 2007;26:520–525. doi: 10.1097/ICO.0b013e318033a6da. [DOI] [PubMed] [Google Scholar]
- 25.Hashemian MN, Moghimi S, Fard MA, Fallah MR, Mansouri MR. Corneal endothelial cell density and morphology in normal Iranian eyes. BMC Ophthalmol. 2006;6:9. doi: 10.1186/1471-2415-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SK, et al. Corneal endothelial cell density and morphology in normal Indian eyes. Cornea. 2000;19:820–823. doi: 10.1097/00003226-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Odenthal MT, Gan IM, Oosting J, Kijlstra A, Beekhuis WH. Long-term changes in corneal endothelial morphology after discontinuation of low gas-permeable contact lens wear. Cornea. 2005;24:32–38. doi: 10.1097/01.ico.0000138860.97302.5a. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Mori Y, Ogata M, Minami K, Miyata K. Central and peripheral corneal endothelial cell analysis with slit-scanning wide-field contact specular microscopy: Agreement with noncontact specular microscopy. Cornea. 2019;38:1137–1141. doi: 10.1097/ICO.0000000000001976. [DOI] [PubMed] [Google Scholar]
- 29.Aketa N, et al. Myopia, corneal endothelial cell density and morphology in a Japanese population-based cross-sectional study: the JPHC-NEXT Eye Study. Sci. Rep. 2021;11:6366. doi: 10.1038/s41598-021-85617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein AS, Janson BJ, Skeie JM, Ling JJ, Greiner MA. The effects of diabetes mellitus on the corneal endothelium: A review. Surv. Ophthalmol. 2020;65:438–450. doi: 10.1016/j.survophthal.2019.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available because they contain information that could compromise the privacy of the research participants.


