Abstract
For the first time in Europe hundreds of rare disease (RD) experts team up to actively share and jointly analyse existing patient’s data. Solve-RD is a Horizon 2020-supported EU flagship project bringing together >300 clinicians, scientists, and patient representatives of 51 sites from 15 countries. Solve-RD is built upon a core group of four European Reference Networks (ERNs; ERN-ITHACA, ERN-RND, ERN-Euro NMD, ERN-GENTURIS) which annually see more than 270,000 RD patients with respective pathologies. The main ambition is to solve unsolved rare diseases for which a molecular cause is not yet known. This is achieved through an innovative clinical research environment that introduces novel ways to organise expertise and data. Two major approaches are being pursued (i) massive data re-analysis of >19,000 unsolved rare disease patients and (ii) novel combined -omics approaches. The minimum requirement to be eligible for the analysis activities is an inconclusive exome that can be shared with controlled access. The first preliminary data re-analysis has already diagnosed 255 cases form 8393 exomes/genome datasets. This unprecedented degree of collaboration focused on sharing of data and expertise shall identify many new disease genes and enable diagnosis of many so far undiagnosed patients from all over Europe.
Subject terms: Medical genetics, Diseases
Rare Diseases (RD) are individually rare but collectively a common health issue. Around 80% of RD are estimated to have a genetic cause [1]. The time to a genetic diagnosis however often takes several years and initial clinical diagnoses are incorrect in up to 40% of families [2]. Around 50% of patients with a RD remain undiagnosed even in advanced expert clinical settings where whole exome sequencing (WES) is applied routinely as a diagnostic approach. Depending on the exact diagnostic setting, the inclusion criteria and the type of RD, the diagnostic yield from WES ranges between 15 and 51% of cases [3, 4].
At least two scenarios allow boosting the current yield of WES. Firstly, there is a value in re-analysing WES data regularly [5] and on massive scale [6], but not every RD expert has access to tools enabling this systematically. Secondly, it is clear that moving beyond the exome can provide additional benefits [7, 8].
Solve-RD aims to solve a large number of unsolved RD, for which a molecular cause is not yet known, by implementing both strategies mentioned above. To this end, Solve-RD applies innovative ways to effectively organise expertise and data.
Cohorts
To structure its work Solve-RD has defined four types of cohorts. Cohort 1, “Unsolved Cases”, comprises cases with an inconclusive WES or whole genome sequencing (WGS) from any partnering or associated ERN center. These data undergo a comprehensive re-analysis effort. Cohort 2, “Specific ERN Cohorts”, represent disease group specific ERN cohorts that are analysed by newly applied tailored -omics approaches. Cohort 3, “Ultra-Rare Rare Diseases”, includes (groups of) patients with unique phenotypes identified (and matched) by RD experts from all ERN participants. For the diseases included in Cohort 4, “The Unsolvables”, all relevant -omics methodologies will be used to solve highly recognisable, clinically well-defined disease entities for which the disease cause has not been found yet despite considerable previous research investigations including WES and WGS (Table 1).
Table 1.
Examples for the specific ERN cohorts and the unsolvables.
| Cohort | Rationale |
|---|---|
| Cohort 2: Long-read whole genome sequencing (LR-WGS) | |
| X-linked spinal and bulbar muscular atrophy (SBMA) | Suspected expansions of repeat disorder or other hidden structural variants (SV) |
| Hereditary ataxia | Suspected expansions of repeat disorder or other hidden SVs |
| Cohort 2: Genomics and Epigenomics | |
| Unexplained Intellectual Disability (ID): patient-parent trios | De novo mutation prioritisation very powerful filter for de novo methylation changes |
| Diffuse gastric cancer | Hypermethylation of cancer gene promoter known disease mechanism |
| Rare pheochromocytomas and paragangliomas | Hypermethylation of cancer gene promoter known disease mechanism |
| Cohort 4 | |
| Unsolved syndromes available via ERN ITHACA | Aicardi syndrome, Gomez–Lopez Hernandes syndrome, Hallermann–Streiff syndrome are clinically well-defined entities and have been studied by WES and WGS globally and remain unsolved |
In total, Solve-RD is targeting to re-analyse >19,000 datasets for cohort 1, sequence ~3500 short- and long-read WGS for cohorts 2, 3, and 4 and add >3500 additional -omics experiments including RNA sequencing, epigenomics, metabolomics, Deep-WES, and deep molecular phenotyping. Data collected and produced in Solve-RD shall be shared via the European Genome-Phenome Archive (EGA) and the RD-Connect Genome-Phenome Analysis Platform (GPAP) to allow controlled access by other RD initiatives and scientists.
Organisation of data
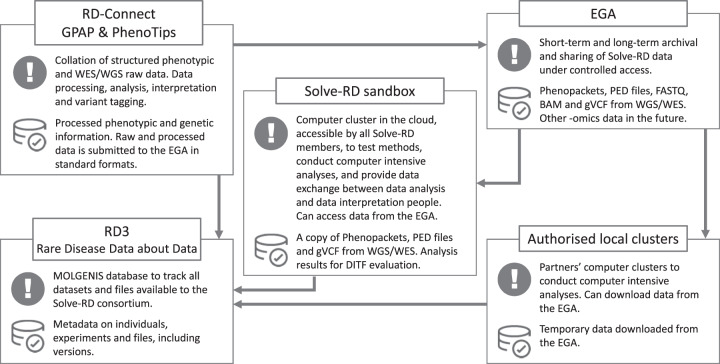
The Solve-RD strategy relies on the availability of large amounts of good quality, standardised genomic and phenotypic data and metadata from undiagnosed RD patients and their relatives. Solve-RD follows a centralised approach, to enable all envisioned analyses. Data sharing in Solve-RD is regulated by policy documents, available on the project’s website. To overcome the technical challenge of centralising large amounts of data, Solve-RD leverages existing infrastructures such as EGA, GPAP, and computing clusters from project partners (Fig. 1). In addition, Solve-RD is developing a cloud-based computing cluster for collaborative analysis and methods testing (the Solve-RD Sandbox) and a central database to control and view all the project’s data and metadata (RD3; rare disease data about data) using the MOLGENIS open source data platform [9]. Clinical data and pedigree structure for all participating individuals is collated through standard terms and ontologies such as HPO, ORDO, and OMIM using GPAP-PhenoStore. To share data within the project and beyond, Solve-RD is an early adopter of the recently GA4GH-approved (Global Alliance for Genomics and Health, https://www.ga4gh.org) PhenoPackets standard to enable exchange of phenotypic and family information [10].
Fig. 1. Solve-RD data infrastructure.
Key components of the Solve-RD infrastructure for multi-omics data analysis, illustrating main use and data available.
For each individual, WES and/or WGS data are submitted to GPAP in FASTQ, BAM, or CRAM format. The sequencing data are processed through a standard pipeline based on GATK (Genomic Analysis Toolkit variant calling software) best practices [11, 12]. After that, PhenoPackets, PED files (for pedigrees), raw data (FASTQ), alignments (BAM) and genetic variants (gVCF) are transferred to the EGA, where they are archived and made available to the consortium (and later on to the broader RD community) for further analysis. Furthermore, Solve-RD data are connected to MatchMaker Exchange via GPAP.
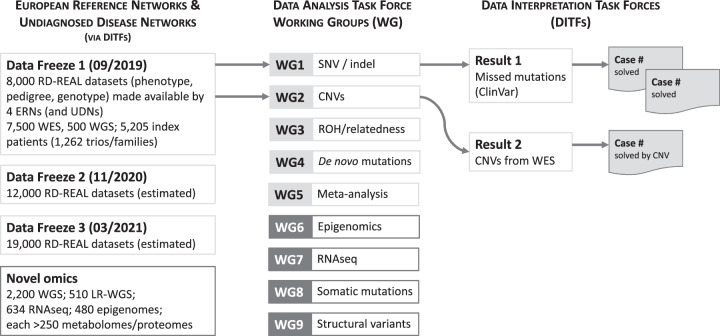
To reach the ambitious goal to collect 19,000 unsolved WES/WGS, Solve-RD has defined several deadlines to submit data to the project. After each deadline, all data are processed and released as a data freeze, which is amenable to corrections via patches. The first data freeze, released in early 2020, includes data from 8,393 individuals.
In parallel to the collection of existing data for cohort 1, new omics data are being generated for cohorts 2, 3, and 4. A common data workflow has been established for all these data types (Fig. 1). The data collated and generated by Solve-RD constitutes a unique collection that will be valuable beyond the project, and the consortium is committed to make it FAIR under controlled access, through the EGA and GPAP.
Organisation of expertise
Solve-RD works on the interphase of many disciplines relevant to solving the unsolved RD. Central to the RD field are clinical geneticists and clinical scientists organised in the respective ERNs. Solve-RD provides expertise in genomics and other -omics data analysis, through data scientists, molecular geneticists, and bioinformaticians.
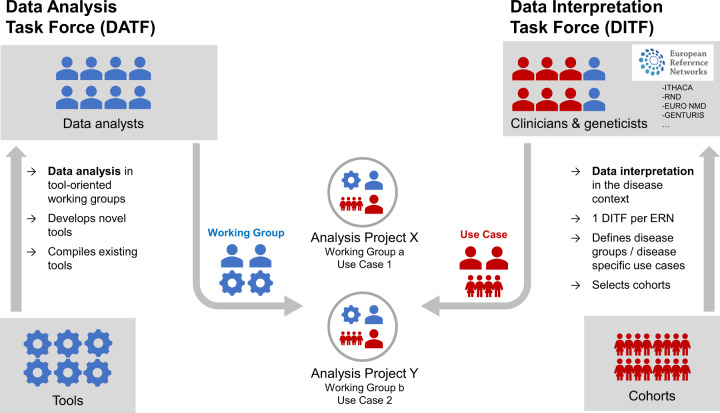
To warrant the best exchange of expertise we have implemented two structures: (i) Data scientists and genomics experts are organised in a Data Analysis Task Force (DATF), (ii) Expert clinicians and geneticists from each ERN are organised in a Data Interpretation Task Force (DITF) (Fig. 2). The tasks for these structures are in brief: ►DITF: define needs of ERN for (a) data re-analysis and (b) novel -omics data; define use cases for re-analysis and novel analysis; discuss/test suitable data output formats for clinical scientists; coordinate collaborative data interpretation; discuss within respective ERN network and feedback to DATF. ►DATF: map expertise in Solve-RD and all (ERN-)partners; create Analysis Projects (Supplementary Table S1) based on ERNs needs; develop state-of-the-art analysis tools; analyse data: (a) data re-analysis and (b) novel omics data; optimise data sharing and output formats for DITF/ERNs.
Fig. 2. The Solve-RD data analysis structure ‘in action’.
Consisting of the Data Analysis Task Force (DATF) and four Data Interpretation Task Forces (DITF)—one per core ERN involved. The DATF established working groups (WGs) for specific analyses. Working groups and DITFs jointly work on analysis projects based on use cases described by the DITF members.
The structure implemented for data re-analysis has proven efficient and versatile [13], and will therefore be applied for novel omics data analysis, with additional working groups for specific -omics technologies (Fig. 3).
Fig. 3. Organisation of new result flow in Solve-RD.
Working groups (WG) 1–5 will re-analyse existing sequencing data. Novel omics data will be analysed by all working groups (as appropriate). RD-REAL refers to Rare Disease - REAnalysis Logistics.
To integrate expertise not available within the Solve-RD consortium, particularly with regards to molecular and functional validation of newly found genes, Solve-RD is implementing an innovative brokerage system (Rare Disease Models and Mechanisms Network—Europe (RDMM-Europe)) that has already been successfully used in Canada [14]. As of 4 December 2020, 14 “brokering” Seeding Grants have been awarded to external model investigators.
Achievements and challenges
The work of the first 3 years of Solve-RD resulted in a practical solution to share and jointly analyse 8393 datasets from all over Europe: Solve-RD organised RD expertise via a DITF and DATF with the respective working group structure described above. The first re-analysis approaches resulted in 255 newly diagnosed cases, mainly by leveraging latest ClinVar entries. As examples we refer to adjacent articles, published jointly in this issue [13, 15–18]. Many more candidate variants and new analysis results are under evaluation.
To achieve its current status Solve-RD has successfully addressed some critical challenges that are (a) European data sharing in accordance with GDPR, (b) heterogeneity in existing WES data (e.g. 26 WES kits so far; multiple sequencing platforms), (c) implementing a centralised analysis approach and (d) addressing the rarity of events.
It is the vision of Solve-RD that, by the end of the project, the Solve-RD dataset will be the largest well-annotated, standardised, multi-omics RD dataset on the diseases covered by the four core ERNs. In this sense, we hope that the Solve-RD dataset will be as useful to the RD community as the gnomAD consortium is for the genomics community [19], by making -omics data of RD populations available to the community.
Supplementary information
Acknowledgements
The Solve-RD project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 779257. This research is supported (not financially) by four ERNs: (1) The ERN for Intellectual Disability, Telehealth and Congenital Anomalies (ERN-ITHACA)—Project ID No 869189; (2) The ERN on Rare Neurological Diseases (ERN-RND)—Project ID No 739510; (3) The ERN for Neuromuscular Diseases (ERN Euro-NMD)—Project ID No 870177; (4) The ERN on Genetic Tumour Risk Syndromes (ERN GENTURIS)—Project ID No 739547. The ERNs are co-funded by the European Union within the framework of the Third Health Programme.
Solve-RD consortium
Olaf Riess1,11, Tobias B. Haack1, Holm Graessner1,11, Birte Zurek1,11, Kornelia Ellwanger1,11, Stephan Ossowski1, German Demidov1, Marc Sturm1, Julia M. Schulze-Hentrich1, Rebecca Schüle4,5, Christoph Kessler4,5, Melanie Wayand4,5, Matthis Synofzik4,5, Carlo Wilke4,5, Andreas Traschütz4,5, Ludger Schöls4,5, Holger Hengel4,5, Peter Heutink4,5, Han Brunner2,3,12, Hans Scheffer2,12, Nicoline Hoogerbrugge2,7, Alexander Hoischen2,7,20, Peter A.C. ’t Hoen7,9, Lisenka E.L.M. Vissers2,3, Christian Gilissen2,7, Wouter Steyaert2,7, Karolis Sablauskas2, Richarda M. de Voer2,7, Erik-Jan Kamsteeg2, Bart van de Warrenburg3,23, Nienke van Os3,23, Iris te Paske2,7, Erik Janssen2,7, Elke de Boer2,3, Marloes Steehouwer2, Burcu Yaldiz2, Tjitske Kleefstra2,3, Anthony J. Brookes13, Colin Veal13, Spencer Gibson13, Marc Wadsley13, Mehdi Mehtarizadeh13, Umar Riaz13, Greg Warren13, Farid Yavari Dizjikan13, Thomas Shorter13, Ana Töpf6, Volker Straub6, Chiara Marini Bettolo6, Sabine Specht6, Jill Clayton-Smith24, Siddharth Banka24,25, Elizabeth Alexander24, Adam Jackson24, Laurence Faivre10,26,27,28,29, Christel Thauvin10,27,28,29, Antonio Vitobello10, Anne-Sophie Denommé-Pichon10, Yannis Duffourd10,28, Emilie Tisserant10, Ange-Line Bruel10, Christine Peyron30,31, Aurore Pélissier31, Sergi Beltran8,21, Ivo Glynne Gut21, Steven Laurie21, Davide Piscia21, Leslie Matalonga21, Anastasios Papakonstantinou21, Gemma Bullich21, Alberto Corvo21, Carles Garcia21, Marcos Fernandez-Callejo21, Carles Hernández21, Daniel Picó21, Ida Paramonov21, Hanns Lochmüller21, Gulcin Gumus32, Virginie Bros-Facer33, Ana Rath14, Marc Hanauer14, Annie Olry14, David Lagorce14, Svitlana Havrylenko14, Katia Izem14, Fanny Rigour14, Giovanni Stevanin34,35,36,37,38, Alexandra Durr35,36,37,39, Claire-Sophie Davoine35,36,37,38, Léna Guillot-Noel35,36,37,38, Anna Heinzmann35,36,37,40, Giulia Coarelli35,36,37,40, Gisèle Bonne15, Teresinha Evangelista15, Valérie Allamand15, Isabelle Nelson15, Rabah Ben Yaou15,41,42, Corinne Metay15,43, Bruno Eymard15,41, Enzo Cohen15, Antonio Atalaia15, Tanya Stojkovic15,41, Milan Macek Jr.44, Marek Turnovec44, Dana Thomasová44, Radka Pourová Kremliková44, Vera Franková44, Markéta Havlovicová44, Vlastimil Kremlik44, Helen Parkinson19, Thomas Keane19, Dylan Spalding19, Alexander Senf19, Peter Robinson45, Daniel Danis45, Glenn Robert46, Alessia Costa46, Christine Patch46,47, Mike Hanna48, Henry Houlden49, Mary Reilly48, Jana Vandrovcova49, Francesco Muntoni50,51, Irina Zaharieva50, Anna Sarkozy50, Vincent Timmerman52,53, Jonathan Baets54,55,56, Liedewei Van de Vondel53,54, Danique Beijer53,54, Peter de Jonghe53,55, Vincenzo Nigro57,58, Sandro Banfi57,58, Annalaura Torella57, Francesco Musacchia57,58, Giulio Piluso57, Alessandra Ferlini59, Rita Selvatici59, Rachele Rossi59, Marcella Neri59, Stefan Aretz60,61, Isabel Spier60,61, Anna Katharina Sommer60, Sophia Peters60, Carla Oliveira62,63,64, Jose Garcia Pelaez62,63, Ana Rita Matos62,63, Celina São José62,63, Marta Ferreira62,63, Irene Gullo62,63,64, Susana Fernandes62,65, Luzia Garrido66, Pedro Ferreira62,63,67, Fátima Carneiro62,63,64, Morris A. Swertz18, Lennart Johansson18, Joeri K. van der Velde18, Gerben van der Vries18, Pieter B. Neerincx18, Dieuwke Roelofs-Prins18, Sebastian Köhler68, Alison Metcalfe46,69, Alain Verloes70,71, Séverine Drunat70,71, Caroline Rooryck72, Aurelien Trimouille73, Raffaele Castello58, Manuela Morleo58, Michele Pinelli58, Alessandra Varavallo58, Manuel Posada De la Paz74, Eva Bermejo Sánchez74, Estrella López Martín74, Beatriz Martínez Delgado74, F. Javier Alonso García de la Rosa74, Andrea Ciolfi75, Bruno Dallapiccola75, Simone Pizzi75, Francesca Clementina Radio75, Marco Tartaglia75, Alessandra Renieri76,77,78, Elisa Benetti76, Peter Balicza79, Maria Judit Molnar79, Ales Maver80, Borut Peterlin80, Alexander Münchau81, Katja Lohmann81, Rebecca Herzog81, Martje Pauly81, Alfons Macaya82, Anna Marcé-Grau82, Andres Nascimiento Osorio83, Daniel Natera de Benito83, Hanns Lochmüller84,85,86, Rachel Thompson85,86, Kiran Polavarapu84, David Beeson87, Judith Cossins87, Pedro M. Rodriguez Cruz87, Peter Hackman88, Mridul Johari88, Marco Savarese88, Bjarne Udd88,89,90, Rita Horvath91, Gabriel Capella92, Laura Valle92, Elke Holinski-Feder93, Andreas Laner93, Verena Steinke-Lange93, Evelin Schröck94, Andreas Rump94,95
Funding
Open Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Members of the Solve-RD consortium are listed below Acknowledgements.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/13/2021
A Correction to this paper has been published: 10.1038/s41431-021-00936-4
Contributor Information
Holm Graessner, Email: holm.graessner@med.uni-tuebingen.de.
Solve-RD consortium:
Tobias B. Haack, Birte Zurek, Kornelia Ellwanger, German Demidov, Marc Sturm, Christoph Kessler, Melanie Wayand, Carlo Wilke, Andreas Traschütz, Ludger Schöls, Holger Hengel, Peter Heutink, Han Brunner, Hans Scheffer, Wouter Steyaert, Karolis Sablauskas, Richarda M. de Voer, Erik-Jan Kamsteeg, Bart van de Warrenburg, Nienke van Os, Iris te Paske, Erik Janssen, Elke de Boer, Marloes Steehouwer, Burcu Yaldiz, Tjitske Kleefstra, Colin Veal, Spencer Gibson, Marc Wadsley, Mehdi Mehtarizadeh, Umar Riaz, Greg Warren, Farid Yavari Dizjikan, Thomas Shorter, Volker Straub, Chiara Marini Bettolo, Sabine Specht, Jill Clayton-Smith, Siddharth Banka, Elizabeth Alexander, Adam Jackson, Laurence Faivre, Christel Thauvin, Antonio Vitobello, Anne-Sophie Denommé-Pichon, Yannis Duffourd, Emilie Tisserant, Ange-Line Bruel, Christine Peyron, Aurore Pélissier, Sergi Beltran, Ivo Glynne Gut, Steven Laurie, Davide Piscia, Leslie Matalonga, Anastasios Papakonstantinou, Gemma Bullich, Alberto Corvo, Carles Garcia, Marcos Fernandez-Callejo, Carles Hernández, Daniel Picó, Ida Paramonov, Hanns Lochmüller, Gulcin Gumus, Virginie Bros-Facer, Marc Hanauer, Annie Olry, David Lagorce, Svitlana Havrylenko, Katia Izem, Fanny Rigour, Giovanni Stevanin, Alexandra Durr, Claire-Sophie Davoine, Léna Guillot-Noel, Anna Heinzmann, Giulia Coarelli, Valérie Allamand, Isabelle Nelson, Rabah Ben Yaou, Corinne Metay, Bruno Eymard, Enzo Cohen, Antonio Atalaia, Tanya Stojkovic, Milan Macek, Jr., Marek Turnovec, Dana Thomasová, Radka Pourová Kremliková, Vera Franková, Markéta Havlovicová, Vlastimil Kremlik, Helen Parkinson, Thomas Keane, Alexander Senf, Peter Robinson, Daniel Danis, Glenn Robert, Alessia Costa, Christine Patch, Mike Hanna, Henry Houlden, Mary Reilly, Jana Vandrovcova, Francesco Muntoni, Irina Zaharieva, Anna Sarkozy, Vincent Timmerman, Jonathan Baets, Liedewei Van de Vondel, Danique Beijer, Peter de Jonghe, Vincenzo Nigro, Sandro Banfi, Annalaura Torella, Francesco Musacchia, Giulio Piluso, Alessandra Ferlini, Rita Selvatici, Rachele Rossi, Marcella Neri, Stefan Aretz, Isabel Spier, Anna Katharina Sommer, Sophia Peters, Carla Oliveira, Jose Garcia Pelaez, Ana Rita Matos, Celina São José, Marta Ferreira, Irene Gullo, Susana Fernandes, Luzia Garrido, Pedro Ferreira, Fátima Carneiro, Morris A. Swertz, Lennart Johansson, Joeri K. van der Velde, Gerben van der Vries, Pieter B. Neerincx, Dieuwke Roelofs-Prins, Sebastian Köhler, Alison Metcalfe, Alain Verloes, Séverine Drunat, Caroline Rooryck, Aurelien Trimouille, Raffaele Castello, Manuela Morleo, Michele Pinelli, Alessandra Varavallo, Manuel Posada De la Paz, Eva Bermejo Sánchez, Estrella López Martín, Beatriz Martínez Delgado, F. Javier Alonso García de la Rosa, Andrea Ciolfi, Bruno Dallapiccola, Simone Pizzi, Francesca Clementina Radio, Marco Tartaglia, Alessandra Renieri, Elisa Benetti, Peter Balicza, Maria Judit Molnar, Ales Maver, Borut Peterlin, Alexander Münchau, Katja Lohmann, Rebecca Herzog, Martje Pauly, Alfons Macaya, Anna Marcé-Grau, Andres Nascimiento Osorio, Daniel Natera de Benito, Hanns Lochmüller, Rachel Thompson, Kiran Polavarapu, David Beeson, Judith Cossins, Pedro M. Rodriguez Cruz, Peter Hackman, Mridul Johari, Marco Savarese, Bjarne Udd, Rita Horvath, Gabriel Capella, Laura Valle, Elke Holinski-Feder, Andreas Laner, Verena Steinke-Lange, Evelin Schröck, and Andreas Rump
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-021-00859-0.
References
- 1.Hartley T, Lemire G, Kernohan KD, Howley HE, Adams DR, Boycott KM. New diagnostic approaches for undiagnosed rare genetic diseases. Annu Rev Genomics Hum Genet. 2020;21:351–72. doi: 10.1146/annurev-genom-083118-015345. [DOI] [PubMed] [Google Scholar]
- 2.EURORDIS AKFF. The Voice of 12,000 Patients. Experiences and Expectations of Rare Disease Patients on Diagnosis and Care in Europe. Eurordis; Paris, France; 2009.
- 3.Smith HS, Swint JM, Lalani SR, Yamal JM, de Oliveira Otto MC, Castellanos S, et al. Clinical application of genome and exome sequencing as a diagnostic tool for pediatric patients: a scoping review of the literature. Genet Med. 2019;21:3–16. doi: 10.1038/s41436-018-0024-6. [DOI] [PubMed] [Google Scholar]
- 4.Wise AL, Manolio TA, Mensah GA, Peterson JF, Roden DM, Tamburro C, et al. Genomic medicine for undiagnosed diseases. Lancet. 2019;394:533–40. doi: 10.1016/S0140-6736(19)31274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P, Meng L, Normand EA, Xia F, Song X, Ghazi A, et al. Reanalysis of clinical exome sequencing data. N. Engl J Med. 2019;380:2478–80. doi: 10.1056/NEJMc1812033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplanis J, Samocha KE, Wiel L, Zhang Z, Arvai KJ, Eberhardt RY, et al. Integrating healthcare and research genetic data empowers the discovery of 28 novel developmental disorders. bioRxiv. 2020. 10.1101/797787.
- 7.Short PJ, McRae JF, Gallone G, Sifrim A, Won H, Geschwind DH, et al. De novo mutations in regulatory elements in neurodevelopmental disorders. Nature. 2018;555:611–6. doi: 10.1038/nature25983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kremer LS, Bader DM, Mertes C, Kopajtich R, Pichler G, Iuso A, et al. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat Commun. 2017;8:15824. doi: 10.1038/ncomms15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Velde KJ, Imhann F, Charbon B, Pang C, van Enckevort D, Slofstra M, et al. MOLGENIS research: advanced bioinformatics data software for non-bioinformaticians. Bioinformatics. 2019;35:1076–8. doi: 10.1093/bioinformatics/bty742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M, Havrilla JM, Fang L, Chen Y, Peng J, Liu C, et al. Phen2Gene: rapid phenotype-driven gene prioritization for rare diseases. NAR Genom Bioinform. 2020;2:lqaa032. doi: 10.1093/nargab/lqaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurie S, Fernandez-Callejo M, Marco-Sola S, Trotta JR, Camps J, Chacón A, et al. From wet-lab to variations: concordance and speed of bioinformatics pipelines for whole genome and whole exome sequencing. Hum Mutat. 2016;37:1263–71. doi: 10.1002/humu.23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matalonga L, Hernández-Ferrer C, Piscia D, Solve-RD SNV-indel working group, Vissers LELM, Schüle R, et al. Diagnosis of rare disease patients through programmatic reanalysis of genome-phenome data. Manuscript submitted to EJHG (703-20-EJHG).
- 14.Boycott KM, Campeau PM, Howley HE, Pavlidis P, Rogic S, Oriel C, et al. The Canadian Rare Diseases Models and Mechanisms (RDMM) Network: Connecting Understudied Genes to Model Organisms. Am J Hum Genet. 2020;106:143–52. doi: 10.1016/j.ajhg.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer E, Ockeloen CW, Matalonga L, Horvath R, Solve-RD SNV-indel working group, Rodenburg RJ, et al. A pathogenic MT-TL1 variant identified by whole exome sequencing in an individual with unexplained intellectual disability, epilepsy and spastic tetraparesis. Manuscript submitted to EJHG (699-20-EJHG). [DOI] [PMC free article] [PubMed]
- 16.Schüle R, Timmann D, Erasmus CE, Reichbauer J, Wayand M, van de Warrenburg BPC, et al. Common pitfalls in genetic diagnosis of rare neurological diseases. Manuscript submitted to EJHG (705-20-EJHG).
- 17.Töpf A, Pyle A, Griffin H, Matalonga L, Schon K, Solve RD SNV indel working group, et al. Exome reanalysis and proteomic profiling identified TRIP4 as a novel cause of cerebellar hypoplasia and spinal muscular atrophy (PCH1). Manuscript submitted to EJHG (700-20-EJHG). [DOI] [PMC free article] [PubMed]
- 18.te Paske I, Garcia-Pelaez J, Sommer AK, Matalonga L, Starzynska T, Jakubowska A, et al. A Mosaic PIK3CA Variant in a Young Adult with Diffuse Gastric Cancer: Case Report. Manuscript submitted to EJHG (704-20-EJHG). [DOI] [PMC free article] [PubMed]
- 19.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–43. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.