Abstract
Bone marrow mesenchymal stromal cells are a highly heterogenic cell population containing mesenchymal stem cells as well as other cell types. With the advance of single cell transcriptome analysis, several recent reports identified a prominent subpopulation of mesenchymal stromal cells that specifically express adipocyte markers but do not contain lipid droplets. We name this cell type marrow adipogenic lineage precursor, MALP, and consider it as a major cellular component of marrow adipose tissue. Here, we review the discovery of MALPs and summarize their unique features and regulatory roles in bone. We further discuss how these findings advance our understanding of bone remodeling, mesenchymal niche regulation of hematopoiesis, and marrow vasculature maintenance.
Keywords: marrow adipogenic lineage precursor, single cell RNA-sequencing, mesenchymal stem cells, bone remodeling, hematopoiesis, angiogenesis
Introduction
The primary functions of bone are to provide mechanical support, serve as a reservoir for calcium and phosphorus, and to produce blood cells. To achieve these functions, two lineages of bone-residing cells are essential: mesenchymal and hematopoietic. Mesenchymal cells originate from mesenchymal stem cells (MSCs). First identified by Friedenstein et al. in 1970s 1, bone marrow MSCs have been extensively studied because of their critical roles in skeletal development, maintenance, and injury repair, as well as their clinical applications in regenerative medicine 2. However, their true identity has remained ambiguous or even controversial. Inside the bone, MSCs give rise to marrow stromal cells, marrow adipocytes, and bone forming osteoblasts/osteocytes that synthesize mineralized bone matrix. Meanwhile, osteoclasts are derived from hematopoietic stem and progenitor cells (HSPCs) and are responsible for resorbing existing bone matrix. The dynamic balance between bone formation and resorption, namely bone remodeling, shapes bone structures and maintains mineral metabolism. Shifting this balance toward more bone resorption leads to osteoporosis, a highly prevalent skeletal disorder associated with bone fragility and fracture 3.
Mesenchymal cells in the bone marrow, except for adipocytes, are referred to as marrow stromal cells, a heterogeneous population with unclear cellular composition. To be specific in this review, we reserve the term MSC only for the most primitive form of mesenchymal progenitor cells (MPCs) with self-renewal ability, but not for descendant non-renewing MPCs that have multipotentiality as well but are more abundant. Additionally, we reserve the term adipocyte for cells containing large cytoplasmic lipid droplets. Although prior research has generated an extensive list of markers for MSCs/MPCs, not a single marker or a set of markers has been consistently agreed upon and some of them are even mutually exclusive. This clearly illustrates the heterogeneity of marrow stromal cells and demands further investigation.
The recent emergence of single-cell RNA-sequencing (scRNA-seq) has provided an unprecedented opportunity to investigate cellular heterogeneity in tissues. A seminal study in 2009 reported the gene expression profile of a single mouse blastomere 4. Initial platforms were able to analyze a few hundred cells per sample. Available from 2017, the 10x Genomics platform allows for the analysis of tens to hundreds of thousands of cells at an affordable price 5. Briefly, this technology uses microfluidic partitioning to capture single cells and prepare barcoded, next-generation sequencing cDNA libraries. As a result, all cDNAs from a single cell have the same barcode, allowing the sequencing reads to be mapped back to their cell of origin. Subsequent computational analysis can group cells with similar gene expression profiles together without prior knowledge of any cell type markers. At a large scale, scRNA-seq has the power to interrogate rare cell types, elucidate transitional states, delineate relationships among subpopulations, and predict the course of differentiation or reprogramming 6. By applying this powerful technique to bone, our group discovered a new adipogenic cell population in bone marrow that we named marrow adipogenic lineage precursor (MALP) 7. In this review article, we present the most updated research on MALPs, compare this cell population with previously proposed bone marrow mesenchymal populations and adipoprogenitors in peripheral fat tissues, and explain how this new finding could change the perspectives on marrow adipose tissue (MAT), bone remodeling, and bone marrow environment regulation.
Identification of MALPs as a new bone marrow mesenchymal cell population
We initially sought to determine the mesenchymal cell hierarchy in bone marrow through applying scRNA-seq. To do this, we isolated Tomato (Td)+ cells from enzymatically digested bone marrow of Col2a1-Cre Td mice at 1 (young), 3 (adult), and 16 (aging) months of age. Studies have shown that Col2a1-Cre labels all mesenchymal lineage cells in bone, including all colony forming unit-fibroblast (CFU-F) forming cells, osteoblasts, osteocytes, adipocytes, and all chondrocytes 7-9. Computational analysis identified the following mesenchymal lineage subpopulations among all age group datasets (Fig. 1A): early mesenchymal progenitor (EMP), late mesenchymal progenitor (LMP), lineage committed progenitor (LCP), marrow adipogenic lineage precursor (MALP), osteoblast (OB), osteocyte (Ocy), and chondrocyte (CH, presumably from the growth plate). Pseudotemporal cell trajectory analyses positioned EMPs at one end of a Y shape trajectory, with osteoblasts/osteocytes and MALPs at the opposite, divergent ends. EMP expresses several common stem cell markers (Ly6a/Sca1, Cd34, and Thy1) and thus, resembles previously proposed PDGFRα/Sca1 (PαS) MSCs 10. Sca1 is a common marker for mouse tissue-resident stem and progenitor cells, including hematopoietic stem cells (HSCs) 11. Our datasets show that Pdgfrα expression labels all bone marrow mesenchymal cells and thus the combination of Pdgfrα and Sca1 expression (PαS) identifies MSCs without HSCs. Prior studies show that PDGFRα+Sca1- cells also generate CFU-Fs, an indicator of in vivo MPCs, albeit at a relatively lower frequency than PαS cells 10, 12. Due to their large number compared to PαS cells, PDGFRa+Sca1- cells contain most bone marrow CFU-F forming cells. Since Sca1 expression only labels EMPs, we reason that PDGFRa+Sca1- cells correspond to LMPs and LCPs, which are likely to be abundant, non-renewing progenitors. Genetic lineage tracing and functional studies are required to validate this proposed progenitor cell hierarchy.
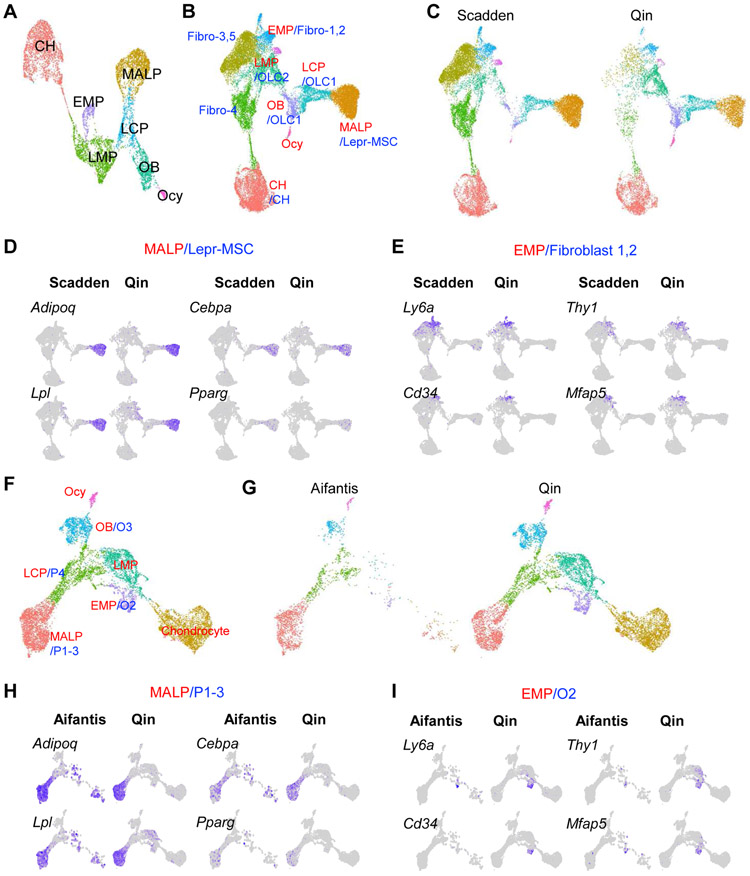
Figure 1. ScRNA-seq datasets of bone marrow mesenchymal lineage cells contain a large MALP subpopulation.
A. UMAP plot of bone marrow mesenchymal lineage cells from Qin group’s 1 month dataset.
B. UMAP plot of integrated dataset containing cells from Qin (GSE145477) and Scadden (GSE128423) scRNA-seq datasets. Newly clustered subpopulations are named by their corresponding cluster names from Qin dataset (red) followed by corresponding cluster names from Scadden dataset (blue). Fibroblast clusters 1,2 (fibro-1,2) from Scadden dataset were previously annotated as MSC-like cells. Fibroblast clusters 3-5 (fibro-3,4,5) from Scadden dataset were previously annotated as tenocyte lineage cells, which are mostly absent in Qin dataset. OLC: osteolineage cell.
C. Split UMAP view of integrated dataset by the original datasets.
D. UMAP plots of MALP/LepR-MSC cluster markers.
E. UMAP plots of EMP/Fibroblast 1 cluster markers.
F. UMAP plot of integrated dataset containing cells from Qin (GSE145477) and Aifantis (GSE108892) scRNA-seq datasets. Newly clustered subpopulations are named by their corresponding cluster names from Qin dataset (red) followed by corresponding cluster names from Aifantis dataset (blue).
G. Split UMAP view of integrated dataset by the original datasets.
H. UMAP plots of MALP/P1-3 cluster markers.
I. UMAP plots of EMP/O2 cluster markers.
Amongst all marrow stromal cells from young animals, MALPs are the only population displaying high enrichment of several bona fide adipocyte markers, including Cebpa, Pparg, Adipoq, and Lpl 7. MALPs also specifically express Zfp423, a marker recently proposed to label a more mature form of pre-adipocyte (CD45-CD31-Sca1-Zfp423+) in the bone marrow 13. On the other hand, MALPs do not express markers associated with lipid metabolism, such as Plin1 (encoding lipid droplet coating protein) and Fabp4 (encoding a fatty acid binding protein), that are necessary for mature adipocyte function. These data suggest that MALPs represent the immediate precursors of marrow adipocytes. Compared to young mice, aged mice had much fewer MPCs and many more MALPs 7, consistent with the general concept that aging is accompanied by a reduced mesenchymal progenitor pool and increased marrow adiposity 14.
In the past two years, five other groups have also analyzed the heterogeneity of mouse bone marrow mesenchymal lineage cells. The Scadden group sorted non-hematopoietic cells from the long bones of young mice for scRNAseq analysis. They identified several clusters, including leptin receptor-expresing MSCs (Lepr-MSCs), osteolineage cells, chondrocytes, fibroblasts (fibro-1-5), endothelial cells, and pericytes 15. Interestingly, the annotated Lepr-MSC cluster also expresses Adipoq, thus resembling our MALPs. Integrated analyses of the dataset from the Scadden group with our 1 month dataset (Fig. 1B) reveals that MALP cluster overlap completely with the Lepr-MSC cluster, while the EMP cluster merges well with fibro-1,2 clusters (Fig. 1C-E). These results suggest that Lepr-MSCs correspond to adipocyte-lineage cells, i.e. MALPs, and may not represent MSCs.
The Aifantis group profiled gene expression in various bone marrow HSC niches, including cells labelled by Lepr-Cre (mesenchymal stromal niche), Cdh5-Cre (vascular niche), and Col2.3-Cre (osteoblast niche) 16. Lepr+ and Col2.3+ cells contained 4 (P1-P4) and 3 (O1-O3) subpopulations, respectively. Strikingly, P1-3 merged very well with MALP, forming a single large cluster (Fig. 1F-H). In addition, O2, O3, and P4 merged with EMP, osteoblasts and LCP, respectively. Thus, similar to the Scadden dataset, the Aifantis dataset also contains a cell population similar to MALP. In addition, all three studies noted that this cell population is enriched with key HSC niche factors SDF1 and SCF, encoded by Cxcl12 and Kitl, respectively.
The Welner group used scRNA-seq to analyze non-hematopoietic and non-endothelial cells in bone 17. A total of 7 clusters were identified: pre-adipocyte (P1), adipocyte progenitor (P2), MSC (P3), osteoblast/chondrocyte progenitor (P4), pre-osteoblast/chondrocyte (P5), pro-osteoblast (P6), and pro-chondrocyte (P7). Most cells, P1-4, highly and specifically express LepR, Adipoq, and Cxcl12. The Hass group analyzed total bone marrow cells, followed by progressive depletion of abundant cell types or enrichment of rare populations 18. They identified the following mesenchymal lineage clusters: chondrocyte, osteoblast, Ng2+ MSC, fibroblast-like populations, adipo-lineage CAR (Adipo-CAR) cells, and osteo-lineage CAR (Osteo-CAR) cells. CAR cells refer to Cxcl12-abundant reticular cells that marks bone marrow MPCs 19. The authors noted that among all bone marrow cells, Adipo-CAR and Osteo-CAR cells produce the highest number and levels of cytokines and growth factors, thus naming these cells “professional cytokine producing cells”. Interestingly, the two CAR cell clusters are also enriched for Adipoq expression. Lastly, the Ono group sequenced GFPhigh cells isolated from the bone marrow of Cxcl12-CreER Td Cxcl12-GFP mice at P28 after Tamoxifen induction at P21 20. Three mesenchymal lineage cell clusters were identified and only one of them (cluster 0) is Td+, which is quiescent and enriched for Adipoq, Cxcl12, and Kitl. Most recently, two more scRNA-seq studies, one on Gli1-CreER labeled mouse bone marrow cells (by Dr. Fanxin Long’s group), and the other on human CD271+ bone marrow-derived mononuclear cells (by Dr. Hongwen Deng’s group), identified a large Adipoq+ cell cluster in their datasets (personal communication). Taken together, despite different cell labelling and sorting strategies, all scRNA-seq studies published to date identified one or more mesenchymal cell clusters with a gene signature similar to MALPs,.
Characteristics of MALPs
Recent studies demonstrate that the bone marrow stroma of young mice does contain abundant Adipoq+ cells while having few adipocytes 7, 21, 22. MALPs first appear in marrow perinatally between P0 and P1 21, 2-3 weeks before the emergence of adipocytes. Lineage tracing revealed that MALPs give rise to adipocytes, but not osteoblasts and osteocytes, suggesting that MALPs are fate-determined cells 7, 23. In young mice, MALPs (Adipoq+Plin1−) far exceed adipocytes (Adipoq+Plin1+) in number by 400-1000 folds, depending on location (metaphyseal vs diaphyseal marrow). They constitute 0.2-0.6% (unpublished data, 21) of total bone marrow cells, and thus are a major component of mesenchymal stromal cells. In addition to long bones, MALPs can also be found in calvariae and vertebrates, suggesting a universal distribution in the bone marrow.
Computational cell cycle analysis predicts that MALPs are non-cycling cells 7. Consistently, the Aifantis group predicted that P1 cells, analogous to MALPs, have the lowest proliferative status among all cell clusters 16. In our study, Adipoq-Cre labeled cells did not incorporate EdU in vivo nor form CFU-F colonies 7. By contrast, Zhou et al. show that a subset of Adipoq-CreER labeled cells incorporate BrdU can form CFU-Fs and expand over time after discontinuation of Tam injections 22, suggesting a proliferative capacity. A possible explanation for these apparently discrepancy could be due to tamoxifen treatment, which promotes adipocyte turnover in certain fat depots 24. Tamoxifen may thus induce some level of proliferation and turnover in MPCs and/or MALPs. Using alternative lineage tracing method, such as tetracycline-on transcription system, might resolve this question.
Distinguished from adipocytes, MALPs do not possess lipid content. They exist as pericytes and stromal cells with a central cell body and multiple long dendritic processes. This cell shape is reminiscent of an osteocytic canaliculi network 25. Through their cell processes, MALPs form an extensive 3D network throughout the marrow cavity, making numerous connections among themselves and with other marrow cells, blood vessels, and bone surface. Taken together, MALPs represent committed adipocyte precursor cells in bone marrow. They are evolutionarily beyond multi-potent progenitor stages and positioned as adipogenic precursors in the mesenchymal cell hierarchy.
The relationship between MALPs and LepR+/CAR cells
In addition to adipocyte markers, MALPs highly express some previously defined bone marrow MPC markers, including Lepr and Cxcl12. While this confirms prior observation that LepR+ cells overlap well with CAR cells 12, it also raises two important questions: are LepR+/CAR cells a heterogenic population? And to what extent do MALPs overlap with LepR+/CAR cells?
First reported by the Nagasawa group using Cxcl12-GFP mice, CAR cells were found to be scattered throughout the bone marrow with long processes that create a network 26, 27, a similar morphology we described for MALPs. Subsequent studies indicate that CAR cells are progenitors for osteoblasts and adipocytes, making Cxcl12 a MPC marker 19. In Cxcl12-CreER Td reporter mice, Td labels a majority of LepR+/CAR cells in the diaphyseal marrow and only a small portion of LepR+/CAR cells in the metaphyseal marrow 20. Lineage tracing found that Td gradually labels trabecular osteoblasts from 0% at 9 weeks to a plateau of ~35% at 6 months of age (induction at 8 weeks of age). The adipocyte labeling pattern was not examined in this study.
Lepr was identified by the Morrison group as another MPC marker 12. In Lepr-Cre Td mice, Td labels adipocytes quickly (~70% and 90% at 2 and 6 months of age, respectively) and osteoblasts slowly (~5%, 20%, 40%, and 75% at 2, 6, 10, and 14 months of age, respectively). Accordingly, it was concluded that LepR+ cells are the major source of bone and adipocytes in the adult bone marrow.
Bone remodeling peaks in young mice and declines in adult and aging mice. The rapid bone formation in adolescent mice requires a constant supply of osteoblasts from MPCs. The slow labeling pattern of osteoblasts by LepR+/CAR cells indicates that they do not comprise all MPCs in young mice. Meanwhile, the quick labeling pattern of adipocytes suggests that many of those cells are primed to become adipocytes. Indeed, both CAR cells and LepR+ cells are noticed to express a high level of adipocyte markers, such as Adipoq, Pparg, Cebpb etc, compared to the rest of bone marrow, or more specifically, PαS cells 12, 19. In culture, Lepr and Cxcl12 expression is greatly elevated during the adipogenic differentiation of bone marrow MPCs, qualifying them as adipocyte markers 7.
In our datasets, Cxcl12 and Lepr are also expressed in progenitor clusters, albeit at a much lower level than in MALPs. Thus, we believe that a major portion of LepR+/CAR cells are MALPs with adipo-lineage fate and a small portion of LepR+/CAR cells are MPCs with both adipo- and osteo-lineage fates. Most studies on LepR+ cells are based on a constitutive Cre model. Future studies characterizing inducible mouse lines (Lepr-CreER and Cxcl12-CreER) will provide more detailed insight on the relationship between MALPs and LepR+/CAR cells in mesenchymal cell hierarchy.
MALPs as adipose lineage cells are unique to bone
Research on adipose tissue traditionally focuses on white adipose tissue (WAT) that stores excess energy as triglycerides, and brown adipose tissue (BAT) that regulates thermogenesis by producing energy in the form of heat. Marrow adipose tissue (MAT) has attracted relatively little attention until it recently emerged as a functionally distinct type of adipose tissue. Based on lipid content, the amount of MAT is significant in human, accounting for ~13% of total adipose tissue in lean and heathy individuals 28. Adipocytes in MAT are morphologically similar to adipocytes in WAT with a characteristic unilocular lipid droplet. MAT does not express uncoupling protein-1 (UCP1), a key brown fat-specific component, either during development or after adrenergic stimulation, indicating that MAT is transcriptionally different from BAT 29. The resemblance between MAT and WAT raises an intriguing question as to whether these two types of adipose cells share the same differentiation route from MSCs to adipocytes.
Adipocytes in WAT and BAT are derived from adipocyte progenitor cells (APCs) residing in the stromal vascular faction (SVF). Those APCs are defined by their expression of mesenchymal markers, such as Pdgfra, Cd34, and Sca1, and the lack of expression of hematopoietic lineage markers 30. To date, several scRNA-seq studies profiled gene expression in SVF cells from mouse and human peripheral adipose tissues at a single cell resolution 31-36. While most adipocytes were discarded at the centrifugation step when harvesting SVF, a few of them were still detected in some studies 34, 36. In peripheral fat depots, only adipocytes (Plin1+), but not APCs, express Adipoq. Interestingly, APCs in the newly developing subcutaneous WAT of fetal mice express Plin1 and Adipoq 37, likely representing transitional cells undergoing adipocyte differentiation. However, in peripheral fat depots of adult mice, Adipoq is exclusively expressed in mature Plin1+ adipocytes 38. MALPs thus appear to represent a distinctive adult adipogenic cell type or state that express a subset of mature adipocyte genes and are poised to become mature adipocytes. It remains unclear if mature adipocytes in marrow can lose their lipids and revert or de-differentiate into MALPs. In this regard, it is interesting to note that mature adipocytes in certain fat depots and in the skin have the capacity to de-differentiate into fibroblast cells 39-41.
Analyzing mouse subcutaneous inguinal WAT at P12 identified 3 APC subpopulations (Fig. 2A) 34. Among these, dipeptidyl peptidase 4 (DPP4)+ cells are multipotent early progenitors, capable of giving rise to Icam1+ committed preadipocytes and Cd142+ cells. While DPP4+ cells reside in the reticular interstitium, Icam1+ cells are intercalated among adipocytes and poised to undergo adipocyte differentiation with minimal stimulation. Interestingly, Cd142+ cells resemble adipogenesis-regulatory cells, or Aregs, an APC subpopulation discovered by the Deplancke group 33.
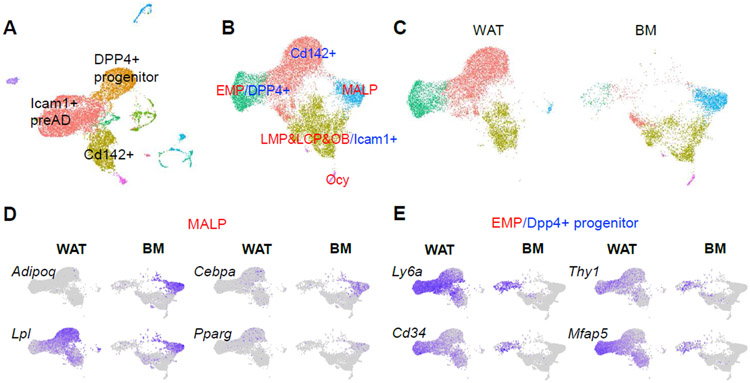
Figure 2. WAT scRNA-seq dataset does not contain a MALP subpopulation.
A. UMAP plot of WAT from the Seale group. PreAD: preadipocytes.
B. UMAP plot of integrated dataset containing cells from WAT (GSE128889) and bone marrow mesenchymal cells (GSE145477). Newly clustered subpopulations are named by their corresponding cluster names from bone marrow dataset (red) followed by corresponding cluster names from WAT dataset (blue).
C. Split UMAP view of integrated dataset by the original datasets.
D. UMAP plots of MALP cluster markers.
E. UMAP plots of EMP/Dpp4+ progenitor cluster markers.
To compare MALPs to APC subpopulations, we integrated this WAT dataset with bone marrow dataset (Fig. 2B). Interestingly, the EMP cluster from bone marrow merged well with the DPP4+ cell cluster from WAT; LMP, LCP, and osteoblast clusters formed a new cluster with Icam1+ preadipocytes but these cells did not overlap very well; MALPs remained as a separate cluster (Fig. 2C, D). Several implications can be drawn from this clustering pattern. First, bone marrow MSCs and WAT MSCs share a high degree of similarity. Second, they adopt different differentiation strategies to produce adipocytes. Compared to WAT MSCs, bone marrow MSCs go through more complicated differentiation steps to become adipocytes, probably reflecting their additional role of generating osteoblasts/osteocytes. Third, MALPs have no counterpart in WAT, and thus, unique to bone. Historically, MAT refers to adipocytes only. The discovery of MALPs greatly expands the content of MAT to include both MALPs and adipocytes.
A master regulator of bone marrow environment
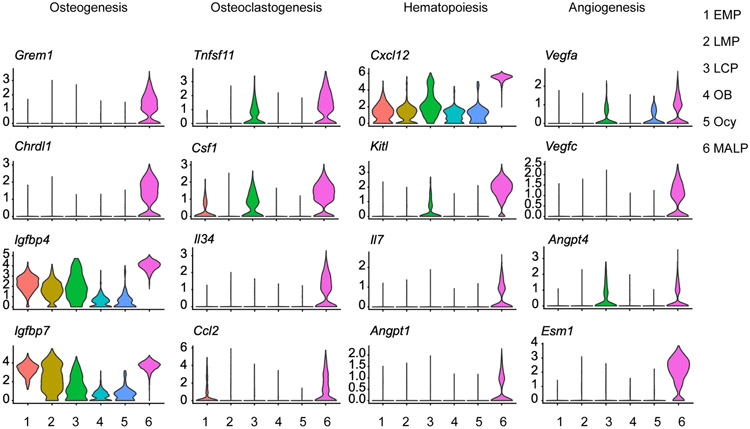
According to the scRNA-seq datasets, the most prominent feature of MALPs is that they express the greatest number of secreted factors at the highest levels among bone mesenchymal lineage cells. These factors are known for their actions in osteogenesis, osteoclastogenesis, hematopoiesis, and angiogenesis (Fig. 3), implying a master regulatory role of MALPs on nearby bone marrow cells.
Figure 3.
Violin plot of marker genes of osteogenesis, osteoclastogenesis, hemotopoiesis and angiogenesis of bone marrow mesenchymal lineage cells.
Bone formation
The most conspicuous phenotype observed after MALP ablation in young mice is rapid and massive bone formation throughout the marrow cavity, especially in the diaphyseal marrow that is normally devoid of any trabeculae 7, 42, 43. In these studies, MALPs were ablated through toxin gene expression driven by Adipoq-Cre, which also targets peripheral fat depots. However, subcutaneous fat transplantation did not reverse the bone phenotype 7, 42, suggesting that MALPs exert local effects to suppress osteogenesis. Mechanistic studies revealed that the expression of two bone morphogenetic protein (BMP) antagonists, Grem1 and Chrdl1 44, are diminished in the bone marrow after MALP ablation 42. Consistent with this finding, Grem1 and Chrdl1 are highly and specifically expressed in MALPs (Fig. 3). Additionally, MALPs produce a large amount of IGFBPs, which antagonize the IGF signaling pathway. BMPs and IGFs are two of the most robust osteogenic signals for MPCs 45, 46. One more line of evidence supporting the osteogenic inhibitory effect of MALPs comes from a prior study of mice with Pparg deletion using Adipoq-Cre. Since Pparg is a master transcriptional regulator of MSC adipogenesis 47, these mice have no bone marrow adipocytes but they have a remarkable increase of trabecular bone mass 48.
Cultured mouse MPCs are normally obtained from diaphyseal bone marrow. However, the paradox is that those cells do not become osteoblasts nor form bone in vivo. We believe that the ubiquitous distribution of MALPs in the bone marrow blocks nearby MPCs from entering the osteogenic differentiation route and thus prevents unwanted bone formation. Future studies on MALP-specific deletion of osteogenesis inhibitors are required to validate this hypothesis.
Bone resorption
Osteoclast formation relies on two cytokines: macrophage colony-stimulating factor (M-CSF, encoded by Csf1) and receptor activator of NF-κB ligand (RANKL, encoded by Tnfsf11) 49. Previous in vitro studies found that adipocytes derived from bone marrow MPCs support osteoclast formation in culture 50, 51 and that adipogenic differentiation is accompanied by an increase of RANKL 52. Moreover, MPCs from aged mice support osteoclast formation better in the coculture experiment than those from young mice. Strikingly, our scRNA-seq datasets showed that MALPs highly and specifically express not only Rankl and Csf1, but also other osteoclast regulatory factors, such as Il7, Il34, Ccl2, Vcam1, and C3 23. Moreover, MALPs have much more interactions with osteoclast precursors, monocytes/macrophages, than other mesenchymal subpopulations 23. RANKL deletion in MALPs using Adipoq-Cre causes a remarkable gain of trabecular bone mass at a level comparable to or even higher than RANKL depletion in osteocytes 23, a cell type previously demonstrated to be the main stimulator of osteoclastogenesis 53-55. MALP-derived RANKL also plays a critical role in pathological bone loss, such as lipopolysaccharide (LPS)- and ovariectomy-induced bone resorption 23.
Bone remodeling initiates with bone resorption via osteoclasts followed by bone formation via osteoblasts. Many past studies point to osteocytes, particularly apoptotic osteocytes induced by different stimuli, as the initiator of osteoblast activity 56. Considering their characteristic cell shape and marrow location, it is conceivable that MALPs function as a sensor of changed marrow environment and subsequently orchestrate bone remodeling at the specific trabecular bone site. Whether MALPs detect the environmental signal via their cell processes deserves further investigation.
Hematopoiesis
Bone is the primary site for hematopoiesis in adult mammals. Indeed, hematopoietic cells comprise of >98% total bone marrow cells. While at much lower numbers, mesenchymal and endothelial cells provide niches to support HSPC renewal, quiescence, and multi-lineage differentiation 57. It is well-recognized that LepR+/CAR cells are the major producer of SDF1/Cxcl12 and SCF/Kitl in the bone marrow 19. As chemokines, Cxcl12 is required for the retention and maintenance of HSPCs 58 and Kitl is critical in the survival, migration, and differentiation of HSPCs 59. LepR+/CAR cells control many aspects of hematopoiesis, particularly the maintenance of HSPCs under steady state and in regenerative conditions 19, 60-64. As discussed above, LepR+/CAR cells are a heterogenous cell population containing MPCs and MALPs. It would be interesting to tease out the role of each subpopulation in hematopoiesis.
ScRNA-seq datasets indicate that MALPs produce hematopoietic regulatory factors, such as Cxcl12, Kitl, Il7, Angiopoietin 1 (encoded by Angpt1), Adiponectin (encoded by Adipoq) at the highest levels among all mesenchymal subpopulations. Deletion of Kitl using Adipoq-Cre significantly decreases the frequencies and numbers of HSPCs, including Lineage-Sca1+c-Kit+ (LSK) cells, long-term HSCs, common myeloid progenitors (CMPs), megakaryocyte-erythrocyte progenitors (MEPs), and granulocyte-monocyte progenitors (GMPs) in long bones of adult mice 65. These mice also have altered peripheral blood components and develop macrocytic anemia at the steady state. Deletion of Kitl using Adipoq-CreER only affects HSPCs in caudal vertebrates but not in long bones 22, which might reflect the lower recombination efficiency of Adipoq-CreER compared to Adipoq-Cre. However, these mice have severely impaired bone marrow hematopoietic regeneration after irradiation or 5-FU treatment, suggesting a role of MALP-derived Kitl in injury repair.
Il-7 is a cytokine important for B cell development 66. Interestingly, Adipoq-Cre Il7 CKO mice have reduced pro B, pre B, immature B and mature B cells in bone marrow with more severe effects in precursors than mature cells 21, suggesting that B cell development relies on MALP-derived Il7 production. Angpt1 and its receptor Tie2 play dual actions in the maintenance of HSPCs and vasculature 67, 68. Deletion of Angpt1 from LepR+ cells in mouse bone marrow accelerates hematopoietic and vascular recovery after irradiation while increasing vascular leakiness 69, implicating that MALPs could regulate hematopoiesis and angiogenesis through secreting Angpt1. As a major product of MALPs, Adiponectin promotes HSPCs proliferation and retaining them in a functionally immature state 70. Future research on MALP-specific deletion of this adipokine could strengthen the important HSC niche role of MALP.
Angiogenesis
Bone is a highly vascularized tissue receiving 10-15% of total cardiac output 71. In addition to serving as a transport conduit system, blood vessels play multifaceted roles in bone development, homeostasis, and regeneration via a spatiotemporally angiogenic-osteogenic coupling mechanism 72. The extensive network of blood vessels in bone marrow include arteries, veins, and capillaries but the vast majority are Emcn+ sinusoidal capillaries 73. Prior work described LepR+/CAR cells as perivascular stromal cells 27, 61. Using whole-mount and 3D confocal imaging technique, we found that MALPs exist as both stromal cells and pericytes 7. Anatomically, pericytes are defined as cells encapsulated under the basal membrane of microvessels where they form a single layer around and in close contact with endothelial cells 74. In a 3D view, MALP stromal cells and pericytes are morphologically similar, possessing a stellate shape with many long dendritic processes extending into the marrow, contacting each other, and surrounding endothelial walls 7. PDGFRβ is a general pericyte marker 75 and basement membrane are made of Laminin and type IV collagen 76. Genes encoding these proteins are highly expressed in MALPs 7. Pericytes regulate the formation, permeability, and stabilization of blood microvessels 75. We found that MALP ablation severely damages marrow vasculature, leading to vessel swelling and a reduction in vessel number 7.
Angiogenesis is mainly controlled by two families of growth factors, VEGF 77 and Angiopoietin 78. Strikingly, MALPs express Vegfa, Vegfc, Angpt1, and Angpt4 at the highest level within all mesenchymal subpopulations. While VEGFc is generally considered as a master lymphangiogenic factor, a recent report found that it is highly expressed in bone marrow LepR+ cells 79. Moreover, LepR+ cells-derived VEGFc contributes to marrow vascular development and vascular and HSPC regeneration after irradiation 79. Interestingly, MALPs also highly express Esm1, encoding Endothelial cell-specific molecule 1 that modulates VEGF signaling in endothelial cells 80. Future studies are needed to delete these factors specifically in MALPs and understand their roles in the development and maintenance of marrow vasculature.
Unanswered questions and perspectives
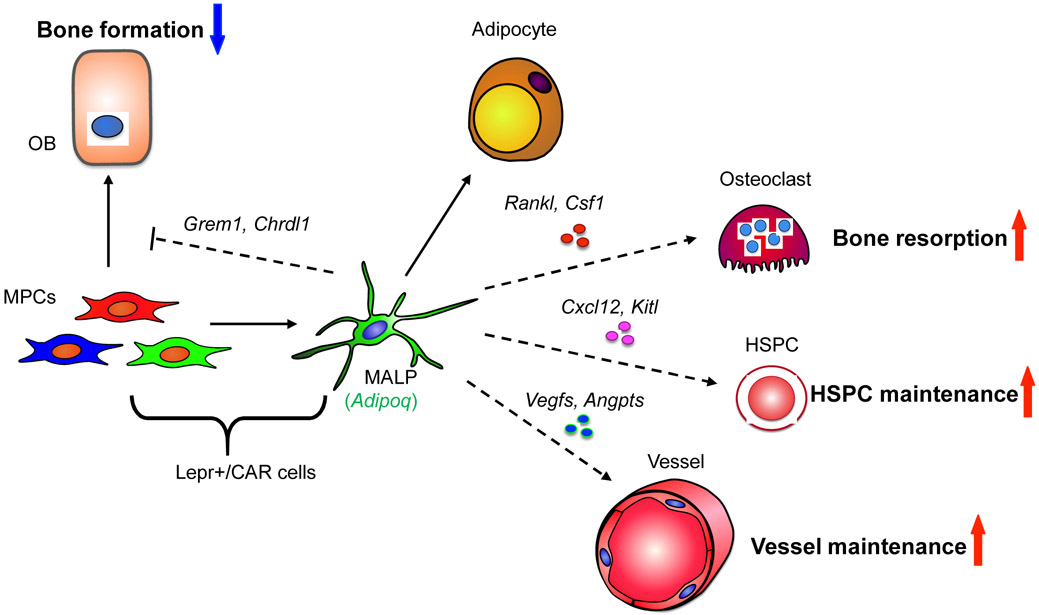
The discovery of MALPs and their profound actions on neighboring cells (Fig. 4) provides new perspectives on some fundamental concepts in the bone biology and has broad impact on stem cell, adipose, and vascular biology. It also invokes many interesting questions that demand future research for clarification.
Figure 4.
A schematic diagram depicts the master regulatory role of MALPs on bone marrow cells.
First, what is the identity of bone marrow MSCs? Theoretically, a single MSC should have the ability to form the entire mesenchymal tissue in bone, just like a single HSC being able to reconstitute the entire hematopoietic system. While scRNA-seq analysis nominate PαS cells as the most likely MSC population in bone, lineage tracing experiments using cluster specific inducible Cre models are required to demonstrate that they descend into non-renewing MPCs, adipocytes, and osteoblasts/osteocytes.
Second, why are MALPs unique to bone? This might reflect distinct tissue organization and functions of different adipose tissues. Peripheral fat pads are primarily made of adipocytes, which are supplied by APCs. On the contrary, MAT is only a small part of bone. Mesenchymal cells, including MALPs, are interspersed among hematopoietic and endothelial cells. Thus, for whatever reasons, MALPs are evolutionally selected to take on additional regulatory roles. For that, its adipogenic differentiation must be de-coupled from lipid accumulation. Comparing the transcriptome profiles of MALPs and marrow adipocyte will provide interesting clues to how adipocyte formation is paused at MALP stage.
Third, what is the function of marrow adipocytes? Are they mere a side product of MALPs or do they have unique functions? Currently available Cre drivers target both MALPs and marrow adipocytes. Past studies on marrow adipocytes cannot exclude the involvement of MALPs and thus should be re-evaluated. Since MALPs outnumber marrow adipocytes, it is imperative to construct a new adipocyte-specific Cre line for studying the function of marrow adipocytes.
Lastly, do MALPs contribute to injury repair? To date most MALP studies are limited to bone development and homeostasis. LepR+/CAR cells play critical roles in marrow regeneration after radiotherapy and chemotherapy. Our current knowledge of MALPs raises a question whether the regenerative ability of LepR+/CAR cells comes from its MALP portion or MPC portion. Moreover, although MALPs do not exist at the periosteum, it will be still interesting to study whether they participate into fracture repair.
No doubt, studying MALPs open new venues of developing therapies for osteoporosis and other bone-related disorders. Discovering new cellular mechanisms to control bone turnover and regeneration will enable fine-tuning of existing therapies or design of novel therapeutics. With the advance of gene-editing technology and novel cell-specific delivery approaches 81, in the future it would be possible to spatiotemporally regulate MALP behavior as a therapy for skeletal and blood diseases. The future of MALP research looks both promising and exciting.
Practice Points.
Bone marrow adipose tissue consists of MALPs and mature adipocytes.
MALPs represent a unique adipocyte-lineage cell or state in bone that are not found in peripheral fat depots.
MALPs are a major constituent of the marrow stroma and play critical roles in regulating bone turnover, hematopoiesis and angiogenesis by elaborating cytokines and growth factors that target adjacent cells.
MALP represents a new cellular target for treating bone disorders, such as osteoporosis.
Research Agenda.
Genetic lineage tracing experiments are needed to ascertain the existence and functional properties of multipotent MSCs in bone marrow.
Transcriptome analysis of MALPs and marrow adipocytes will provide insights into the pathways that regulate the persistence of MALPs in bone.
Generating a mature adipocyte-specific Cre will tease out the distinctive functions of MALPs and marrow adipocytes.
Understanding the role of MALPs in bone injury is imperative for targeting these cells for novel therapies.
Acknowledgments
We thank Dr. Ernestina Schipani from the University of Pennsylvania and Dr. Noriaki Ono from the University of Michigan for critics on this review. We also thank NIH grants NIH/NIAMS R01AR066098 and R21AR074570 (to L.Q.) for supporting our research related to this review.
Abbreviation:
- Adipo-CAR:
adipo-lineage CXCL12-abundant reticular
- Adipoq:
Adiponectin
- Angpt1:
Angiopoietin 1
- APC:
adipocyte progenitor cell
- BAT:
brown adipose tissue
- BMP:
bone morphogenetic protein
- CFU-F:
colony forming unit-fibroblast
- CH:
chondrocyte
- CMP:
common myeloid progenitor
- Dpp4:
dipeptidyl peptidase 4
- EMP:
early mesenchymal progenitor
- GMP:
granulocyte-monocyte progenitor
- HSC:
hematopoietic stem cell
- HSPC:
hematopoietic stem and progenitor cell
- LCP:
lineage committed progenitor
- LMP:
late mesenchymal progenitor
- LPS:
lipopolysaccharide
- LSK:
Lineage-Sca1+c-Kit+
- MALP:
marrow adipogenic lineage precursor
- MAT:
marrow adipose tissue
- M-CSF:
macrophage colony-stimulating factor
- MEP:
megakaryocyte-erythrocyte progenitor
- MPC:
mesenchymal progenitor cell
- MSC:
mesenchymal stem cell
- OB:
osteoblast
- Ocy:
osteocyte
- Osteo-CAR:
osteo-lineage CXCL12-abundant reticular
- PαS:
PDGFRα/Sca1
- RANKL:
receptor activator of NF-κB ligand
- scRNA-seq:
single-cell RNA-sequencing
- SVF:
stromal vascular faction
- Td:
Tomato
- UCP1:
uncoupling protein 1
- WAT:
white adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology. 1976; 4: 267–274. [PubMed] [Google Scholar]
- 2.Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regenerative Medicine. 2019; 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019; 393: 364–376. [DOI] [PubMed] [Google Scholar]
- 4.Tang F, Barbacioru C, Wang Y, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nature Methods. 2009; 6: 377–382. [DOI] [PubMed] [Google Scholar]
- 5.Zheng GX, Terry JM, Belgrader P, et al. Massively parallel digital transcriptional profiling of single cells. Nature Communications. 2017; 8: 14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu AR, Wang J, Streets AM, Huang Y. Single-Cell transcriptional analysis. Annual Review of Analytical Chemistry. 2017; 10: 439–462. [DOI] [PubMed] [Google Scholar]
- 7.Zhong L, Yao L, Tower RJ, et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife. 2020; 9: e54695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra A, Lin T, Young T, et al. Suppression of Sclerostin Alleviates Radiation-Induced Bone Loss by Protecting Bone-Forming Cells and Their Progenitors Through Distinct Mechanisms. Journal of Bone and Mineral Research. 2017; 32: 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nature Cell Biology. 2014; 16: 1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. Journal of Experimental Medicine. 2009; 206: 2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007; 25: 1339–1347. [DOI] [PubMed] [Google Scholar]
- 12.Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014; 15: 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrosi TH, Scialdone A, Graja A, et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell. 2017; 20: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bethel M, Chitteti BR, Srour EF, Kacena MA. The changing balance between osteoblastogenesis and adipogenesis in aging and its impact on hematopoiesis. Current Osteoporosis Reports. 2013; 11: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baryawno N, Przybylski D, Kowalczyk MS, et al. A Cellular Taxonomy of the Bone Marrow Stroma in Homeostasis and Leukemia. Cell. 2019; 177: 1915–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikhonova AN, Dolgalev I, Hu H, et al. The bone marrow microenvironment at single-cell resolution. Nature. 2019; 569: 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolock SL, Krishnan I, Tenen DE, et al. Mapping Distinct Bone Marrow Niche Populations and Their Differentiation Paths. Cell Reports. 2019; 28: 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baccin C, Al-Sabah J, Velten L, et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nature Cell Biology. 2020; 22: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omatsu Y, Sugiyama T, Kohara H, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010; 33: 387–399. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita Y, Nagata M, Kozloff KM, et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nature Communications. 2020; 11: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukohira H, Hara T, Abe S, et al. Mesenchymal stromal cells in bone marrow express adiponectin and are efficiently targeted by an adiponectin promoter-driven Cre transgene. International Immunology. 2019; 31: 729–742. [DOI] [PubMed] [Google Scholar]
- 22.Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nature Cell Biology. 2017; 19: 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu W, Zhong L, Yao L, et al. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. Journal of Clinical Investigation 2021; 131: e140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye R, Wang QA, Tao C, et al. Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Molecular Metabolism 2015; 4: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011; 26: 229–238. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokoyoda K, Egawa T, Sugiyama T, et al. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004; 20: 707–718. [DOI] [PubMed] [Google Scholar]
- 27.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006; 25: 977–988. [DOI] [PubMed] [Google Scholar]
- 28.Cawthorn WP, Scheller EL, Learman BS, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metabolism. 2014; 20: 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craft CS, Robles H, Lorenz MR, et al. Bone marrow adipose tissue does not express UCP1 during development or adrenergic-induced remodeling. Scientific Reports. 2019; 9: 17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hepler C, Vishvanath L, Gupta RK. Sorting out adipocyte precursors and their role in physiology and disease. Genes & Development. 2017; 31: 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hepler C, Shan B, Zhang Q, et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. Elife. 2018; 7: e39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijay J, Gauthier MF, Biswell RL, et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nature Metabolism. 2020; 2: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwalie PC, Dong H, Zachara M, et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. 2018; 559: 103–108. [DOI] [PubMed] [Google Scholar]
- 34.Merrick D, Sakers A, Irgebay Z, et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019; 364: eaav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oguri Y, Shinoda K, Kim H, et al. CD81 Controls Beige Fat Progenitor Cell Growth and Energy Balance via FAK Signaling. Cell. 2020; 182: 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burl RB, Ramseyer VD, Rondini EA, et al. Deconstructing Adipogenesis Induced by β3-Adrenergic Receptor Activation with Single-Cell Expression Profiling. Cell Metabolism. 2018; 28: 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong KY, Bae H, Park I, et al. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development. 2015; 142: 2623–2632. [DOI] [PubMed] [Google Scholar]
- 38.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine 2013; 19: 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shook BA, Wasko RR, Mano O, et al. Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell. 2020; 26: 880–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Shao M, Hepler C, et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. Journal of Clinical Investigation 2019; 129: 5327–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang QA, Song A, Chen W, et al. Reversible De-differentiation of Mature White Adipocytes into Preadipocyte-like Precursors during Lactation. Cell Metabolism 2018; 28: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou W, Rohatgi N, Brestoff JR, et al. Ablation of Fat Cells in Adult Mice Induces Massive Bone Gain. Cell Metabolism. 2020; 32: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou W, Rohatgi N, Brestoff JR, et al. Congenital lipodystrophy induces severe osteosclerosis. PLoS Genetics. 2019; 15: e1008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brazil DP, Church RH, Surae S, et al. BMP signalling: agony and antagony in the family. Trends in Cell Biology. 2015; 25: 249–264. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Bikle DD, Chang W. Autocrine and Paracrine Actions of IGF-I Signaling in Skeletal Development. Bone Research. 2013; 1: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sánchez-Duffhues G, Hiepen C, Knaus P, Ten Dijke P. Bone morphogenetic protein signaling in bone homeostasis. Bone. 2015; 80: 43–59. [DOI] [PubMed] [Google Scholar]
- 47.Lefterova MI, Zhang Y, Steger DJ, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes & Development. 2008; 22: 2941–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Mullican SE, DiSpirito JR, et al. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proceedings of the National Academy of Sciences of the United States of America. 2013; 110: 18656–18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ono T, Nakashima T. Recent advances in osteoclast biology. Histochem Cell Biol. 2018; 149: 325–341. [DOI] [PubMed] [Google Scholar]
- 50.Goto H, Osaki M, Fukushima T, et al. Human bone marrow adipocytes support dexamethasone-induced osteoclast differentiation and function through RANKL expression. Biomed Res. 2011; 32: 37–44. [DOI] [PubMed] [Google Scholar]
- 51.Goto H, Hozumi A, Osaki M, et al. Primary human bone marrow adipocytes support TNF-alpha-induced osteoclast differentiation and function through RANKL expression. Cytokine. 2011; 56: 662–668. [DOI] [PubMed] [Google Scholar]
- 52.Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014; 289: 16699–16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011; 17: 1231–1234. [DOI] [PubMed] [Google Scholar]
- 54.Xiong J, Onal M, Jilka RL, et al. Matrix-embedded cells control osteoclast formation. Nat Med. 2011; 17: 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong J, Piemontese M, Onal M, et al. Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS One. 2015; 10: e0138189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellido T Osteocyte-driven bone remodeling. Calcified Tissue International. 2014; 94: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzeng YS, Li H, Kang YL, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011; 117: 429–439. [DOI] [PubMed] [Google Scholar]
- 59.Lennartsson J, Rönnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiological Reviews. 2012; 92: 1619–1649. [DOI] [PubMed] [Google Scholar]
- 60.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013; 495: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012; 481: 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seike M, Omatsu Y, Watanabe H, et al. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes & Development. 2018; 32: 359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comazzetto S, Murphy MM, Berto S, et al. Restricted Hematopoietic Progenitors and Erythropoiesis Require SCF from Leptin Receptor+ Niche Cells in the Bone Marrow. Cell Stem Cell. 2019; 24: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Omatsu Y, Seike M, Sugiyama T, et al. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature. 2014; 508: 536–540. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Huang Z, Ong B, et al. Bone marrow adipose tissue-derived stem cell factor mediates metabolic regulation of hematopoiesis. Haematologica. 2019; 104: 1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barata JT, Durum SK, Seddon B. Flip the coin: IL-7 and IL-7R in health and disease. Nature Immunology. 2019; 20: 1584–1593. [DOI] [PubMed] [Google Scholar]
- 67.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004; 118: 149–161. [DOI] [PubMed] [Google Scholar]
- 68.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circulation Research. 2006; 98: 1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou BO, Ding L, Morrison SJ. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. Elife. 2015; 4: e05521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiMascio L, Voermans C, Uqoezwa M, et al. Identification of adiponectin as a novel hemopoietic stem cell growth factor. Journal of Immunology. 2007; 178: 3511–3520. [DOI] [PubMed] [Google Scholar]
- 71.Tomlinson RE, Silva MJ. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Research. 2013; 1: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. Journal of Bone and Mineral Research. 2006; 21: 183–192. [DOI] [PubMed] [Google Scholar]
- 73.Sivaraj KK, Adams RH. Blood vessel formation and function in bone. Development. 2016; 143: 2706–2715. [DOI] [PubMed] [Google Scholar]
- 74.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs 2001; 169: 1–11. [DOI] [PubMed] [Google Scholar]
- 75.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011; 21: 193–215. . [DOI] [PubMed] [Google Scholar]
- 76.Glentis A, Gurchenkov V, Matic Vignjevic D. Assembly, heterogeneity, and breaching of the basement membranes. Cell Adhesion & Migration. 2014; 8: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shibuya M Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. Journal of Biochemistry. 2013; 153: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eklund L, Kangas J, Saharinen P. Angiopoietin-Tie signalling in the cardiovascular and lymphatic systems. Clinical Science. 2017; 131: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fang S, Chen S, Nurmi H, et al. VEGF-C protects the integrity of the bone marrow perivascular niche in mice. Blood. 2020; 136: 1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rocha SF, Schiller M, Jing D, et al. Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circulation Research 2014; 115: 581–590. [DOI] [PubMed] [Google Scholar]
- 81.Broeders M, Herrero-Hernandez P, Ernst MPT, et al. Sharpening the Molecular Scissors: Advances in Gene-Editing Technology. iScience. 2020; 23: 100789. [DOI] [PMC free article] [PubMed] [Google Scholar]