Abstract
Background:
An in vitro study on rapid culturing method of human gingival fibroblast cells (HGFCs) was established to investigate the potential use of the leukocyte-platelet rich fibrin (L-PRF) in tissue engineering technology, different medical fields, including periodontology and implantology.
Methods:
Eight biopsies were obtained from eight different donors and a modified culturing technique was developed to obtain HGFCs. The modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide MTT assay was used to compare the cell viability when the modified culturing method was used in comparison to the standard method. Blood samples were collected from the same patients and L-PRF was isolated using a standard protocol. The releases of platelet-derived growth factor-AA and transforming growth factor-beta1 at various time intervals were observed using enzyme-linked immunosorbent assay (ELISA) kit. The proliferative effect of L-PRF on HGFCs was assessed by the cell counting kit—8 assay.
Results:
A simple and rapid modified method for in vitro HGFC culture yielded a cellular monolayer within three to nine days after cell culture. L-PRF with three-dimensional polymer fibers released growth factors that peaked during the first three hours and continued to produce up to 10 days. The L-PRF presented a dose-dependent effect on HGFCs proliferation where HGFCs proliferation increased with an increase in L-PRF concentration.
Conclusion:
The modified technique for the culture of HGFCs might be useful for the development of future experimental and clinical studies, besides L-PRF has great therapeutic potential in oral surgery fields.
Keywords: Cell culture, Fibroblast, Growth factors, Platelet-rich fibrin, Tissue engineering
Introduction
Cell cultures of oral human fibroblasts are usually used in tissue engineering research [1] owing to its ability to reduce the gap between the oral mucosa and teeth. Moreover, oral human fibroblasts play a dynamic role in improving the immune defense system besides being recognized for their rapid rate of tissue turnover in the body [2, 3]. Various techniques have been used for the cultivation of human gingival fibroblast cells (HGFCs), including enzymatic and direct explant techniques [4–6]. The enzymatic technique focuses on the isolation of human keratinocytes using enzymes such as trypsin, dispase, or collagenase), whereas the direct explant method is associated with a higher proliferation rate and fewer steps that have been used for nearly 40 years with some modifications [7]. Currently, regenerative medicine, tissue engineering, and therapeutic cloning are research areas of growing interest. Tissue engineering therapies specifically have been applied in different medical fields in the past [8, 9] and have recently gained attention in preventive dentistry, periodontal treatment, preserving the appearance of the gingiva, and associated consequences [10–12].
One of the important key factors in tissue engineering is the kinetics of growth factor release that is highly dependent on plasma concentrate preparation protocols. Platelet concentrates of first-generation include platelet-rich plasma (PRP), which has been utilized in numerous medical fields. However, PRP also contains anti-coagulants that inhibit complete coagulation during the process of tissue wound healing [13–15]. In contrast to PRP, platelet-rich fibrin (PRF) which has been re-termed as leukocyte-PRF or L-PRF contains a higher leukocyte content, devoid of any anti-coagulants and possesses a 3-D fibrin matrix which could be useful for a variety of dental surgical techniques [16, 17]. Recent studies suggest that L-PRF can autogenously produce growth factors that are being used in many areas of modern medicine [18]. Since its introduction in 2001 [19], the primary advantage of using L-PRF was to obtain immune-compatible growth factors without any anti-coagulants at a relatively low cost [20]. Previous studies have shown that L-PRF contains high concentrations of autologous growth factors [e.g., vascular endothelial growth factor (VEGF), platelet-derived growth factors (PDGFs), and transforming growth factor-beta1 (TGF-β1)] [18]. Moreover, L-PRF concentrates displayed a greater total growth factor release over a longer duration compared to the first-generation PRP [18]. However, very few studies have described a rapid culturing method for HGFCs or provided a comparison between the level of PDGF-AA and TGF-β1 release. Depending on the type of released growth factors, L-PRF was reported to accelerate bone formation, induce proliferation of periodontal ligament cells [21], and improve the syndesmosis union rate for total ankle replacement cases [22].
In the present study, a modified method for HGFCs culturing was developed and the effects of L-PRF on HGFCs proliferation were investigated for the first time. Findings from this study are expected to assist in efficient culturing of HGFCs and application of L-PRF to enhance the healing process and tissue regeneration for oral surgery procedures. Therefore, the objectives of this study were to: (1) compare modified and standard method of culturing HGFCs, (2) determine the structure and constituents of L-PRF, (3) analyze the level of growth factor release (e.g., PDGF-AA and TGF-β1) from L-PRF over time, and (4) assess the effect of L-PRF on in vitro HGFCs proliferation. The hypotheses of this study were: (1) L-PRF allows sustained release of growth factors and (2) L-PRF increases the HGFCs proliferation and secretion of neurotrophic factors in a concentration-dependent condition.
Materials and methods
Patients and gingival biopsy details
This experimental study was conducted at the School and Hospital of Stomatology, Jilin University, China, between September 2017 and January 2019. Gingival fragments were collected from eight healthy patients undergoing implant operations (four males and four females) with a mean age of 29 years with clinically normal gingivae (NG). The eight biopsies were obtained from the Department of Implantology, Jilin University, and were conducted following the bio-ethical guidelines outlined by the Jilin University research ethic committee (IRB no. 3). Before the commencement of the study, written informed consents were obtained from the patients participating in this study. Before explanting the gingival tissue, it was wiped with 0.75% of boric acid which is critical to protect the tissue from infection [4, 23]. The explants were collected under local anesthesia (2 × 2 × 1 mm) by a sharp scalpel, then directly minced in a sterile 15 mL centrifuge tube containing Dulbecco’s Modified Eagle Medium (Hyclone DMEM, Logan, UT, USA), 10% fetal bovine serum (Gibco FBS, Grand Island, NY, USA), and 10% penicillin–streptomycin, 10,000 U/mL (Hyclone PS), before being transferred to the laboratory.
Cell culture preparation
The gingival specimen was washed with normal saline or DMEM (with or without antibiotics), then transferred to a culture plate using standard protocols [23, 24]. A new modified method from a previous study was implemented which involved the transfer of the specimen to the laboratory within 15 min after explantation, followed by specimen washing with DMEM containing 10% PS using a pipette. A second wash was then performed with phosphate-buffered saline (PBS) containing 10% PS [23]. The samples were then transferred into another centrifuge tube containing dispase (Sigma-Aldrich, St. Louis, MO, USA) for fixation for 12 h at 4 °C to facilitate the removal of the connective tissue from the epithelial layer for the collection of HGFCs. Sterile scissors and tweezers were used to scrape the connective tissues to ensure the removal of damaged tissues or foreign objects from the wound. Following mincing, the connective tissue was cut into pieces (0.5 mm3) using sharp scissors and placed into a 25 cm2 culture flask. The culture flask was inverted to induce adherence of the small pieces of connective tissue for over 2 h and gently inverted again after the addition of 4 mL of DMEM supplemented with 10% FBS, and cultured at 37 °C in the presence of 5% carbon dioxide. When the cells reached 80% confluency, 0.25% of trypsin/EDTA solution was used for cell passage.
Determination of cellular viability
Cellular viability was determined to compare both standard and modified cell culture technique used in this study. To assess the cell growth, briefly, 25,000 fibroblasts cells in 2 mL of 10% DMEM were allowed to settle in a well of a 24-well culture plate for 2 h. After slight washing with PBS, the medium was replaced with 1% DMEM containing 10% FBS and left for 16 h. Cells were harvested after 3, 5, 7, 9 and 11 days using 0.2% trypsin in PBS, and counted manually with Neubauer Hemocytometer. A modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide MTT assay [25] was also used to determine the level of cell viability for both standard and modified techniques. The cellular suspension in the culture medium was added to 24-well plates at different levels (1000, 2000, 4000, 6000, 8000, and 10,000 cells/mL). After three days, 100 µL of MTT (Sigma-Aldrich) was added to each well at a concentration of 5 mg/mL in PBS, followed by incubation under standard conditions for 4 h. The medium was then removed after the incubation, and solubilization was performed by adding acidified alcohol to each well. The solution was then transferred to a microplate containing 20 µL of sodium dodecyl sulfate. The optical density was determined at 550 nm using an automated microplate reader. Experiments were performed in triplicates for both the standard and modified cell culture techniques.
Cell growth test
In this experiment, only a portion of the gingival specimens was utilised. The same number of 0.5 mm3 pieces of connective tissue were inserted into individual 25 cm3 flasks for culturing purpose. As mentioned in the previous section, each the tissue piece was transferred carefully to a new flask to generate new fibroblast cells to be harvested later. The newly generated fibroblasts were harvested at the predetermined time point, i.e. Day 4, 6, 8, 10, and 12. During the harvesting procedure, the cells would be washed with phosphate buffered saline (PBS) for three repeated times. After that, the cells were detached by applying 0.25% trypsin/EDTA solution and counted with a Counter Cells Neubauer Hemocytometer (Thomas Scientific, Lawrence, KS, USA).
Fabrication and characterization of L-PRF
To prepare the L-PRF, three blood samples were collected from the first three patients from whom the gingival tissues were explanted for the cell cultures. About 9 mL of the blood sample was collected into 10 mL tubes containing EDTA (Vacutainer, BD Biosciences, Allschwil, Switzerland) and was centrifuged instantly at 3000 rpm (1278×g) for 12 min at room temperature (Fixed-angle rotor F-35-30-17, Centrifuge 5702; Eppendorf, Darmstadt, Germany) [26]. Three distinct layers were observed after centrifugation which were: (1) a base (bottom) layer of corpuscle-red cells, (2) an upper layer of serum (the supernatant), and (3) a layer interspersed between the other two layers consisting of the L-PRF clot. The samples were then squeezed between two pieces of gauze to obtain the L-PRF clot which comprised of white cells and platelets enmeshed within a three-dimensional structure of fibrin [27, 28].
Subsequently, morphological features of the L-PRF membrane was evaluated using a scanning electron microscope (SEM, S-3400 N, Hitachi, Tokyo, Japan). To prepare the L-PRF, two blood samples were collected according to the protocol described above. The L-PRF block was squeezed between two sterile gauzes and the resultant L-PRF membrane was divided into three parts. All the specimens were fixed immediately after the preparation in a 2.5% of glutaraldehyde and 0.1% sodium cacodylate buffer for 24 h, then rinsed with sodium cacodylate buffer and distilled water three times for 10 min. Then, the specimens were dried with increasing concentration of ethanol (25–50–75–90–100%) before being sputter-coated with 20 nm gold before SEM evaluation. Photographs were taken at 5 kV using ×2 to ×20 K magnifications to observe and identify the cell bodies trapped in the matrix (leukocytes, platelets, and erythrocytes) and to analyze the overall architecture of the fibrin network.
Determination of growth factor in L-PRF
Seven tubes containing 4 mL of DMEM without serum or PS were prepared. The resultant L-PRF was submerged in the first tube for 1 h to estimate the concentration of TGF-β1 and PDGF (subtype AA). The L-PRF was then aspirated and placed into the second tube and the steps were repeated until the seventh tube. The concentration of TGF-β1 and PDGF (subtype AA) at each of the indicated time intervals was estimated (1, 2, 3, 24, 48, and 72 h, and 10 days). The growth factors were measured using a sandwich ELISA kit designed for human use [Cloud-Clone Corp. (CCC), Katy, TX, USA]. The ELISA protocol was performed according to the manufacturer’s instructions and the absorbance of samples were read at a wavelength of 450 nm using a microplate reader (Infinite 200Pro, Switzerland). All results were reported as the total weight of molecules (ng) per 1 mL of supernatant volume. The concentration of growth factors at each interval was determined and the optimum concentration for growth factor release was identified. Besides, the cumulative concentration of growth factors at each interval was compared between the three patients. The analysis was performed in triplicates.
Evaluation of L-PRF treatment on the HGFCs proliferation
As an initial step, L-PRF extract was treated to reduce the differences between the L-PRF membranes. The L-PRF was immersed in fresh DMEM and incubated for 24 h at 37 °C in the presence of 5% of CO2 to obtain adequate cumulative growth factors release as a result of the process discussed above. Then, conditioned mediums were collected, centrifuged at 1000 rpm for 5 min before the extracts were passed through a biological filter (Millex, Millipore Ireland Ltd., Carrigtwohill, Ireland). The extracts were then supplemented with 10% FBS and 1% PS and denoted as 100% L-PRF extract before storage at − 80 °C until further analysis.
A Cell Counting Kit-8 (CCK-8) test was established to determine the cellular proliferation as affected by different concentrations of L-PRF. To examine the impact of L-PRF on cell proliferation, the fourth and fifth generation of HGFCs were seeded into 96-well plates at a cell density of 5000 cells/ well and were kept under a humidified atmosphere (37 °C with 5% CO2) until the next day. After that, the cells were divided into four groups (control, 25%, 50%, and 100%) and a cell viability test was performed on day 1, 2, and 3 using a Cell-Counting Kit-8 (CCK-8) (Dojindo Kagaku Co., Kumamoto, Japan). Upon completion of the different interval of treatment, 10 μL of CCK-8 was added into each well and incubated for an additional of 2 h at 37 °C in the presence of 5% CO2. The absorbance values were recorded at a wavelength of 450 nm using a microplate reader (Infinite 200Pro).
Effect of L-PRF on the proliferation of aged cells
Following the culturing of human gingival fibroblasts, the tenth generation of the HGFCs were incubated at 37 °C for 24 h in 25 × 103 cells/well. The cells were divided into two groups, in which the HGFCs in the control group were inoculated by DMEM medium only as compared to the HGFCs in the tested group that were inoculated by L-PRF extract. After that, the cell growth rate was observed under a microscope for four days.
Results
Cultured fibroblast cells
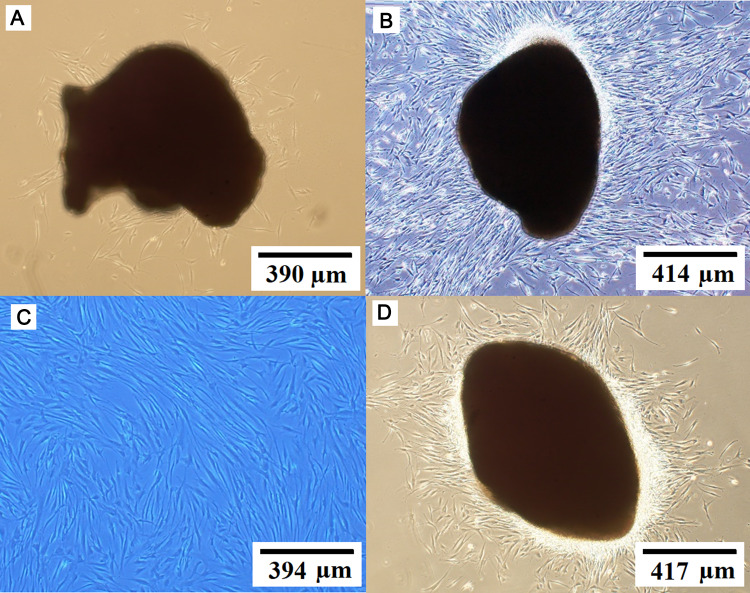
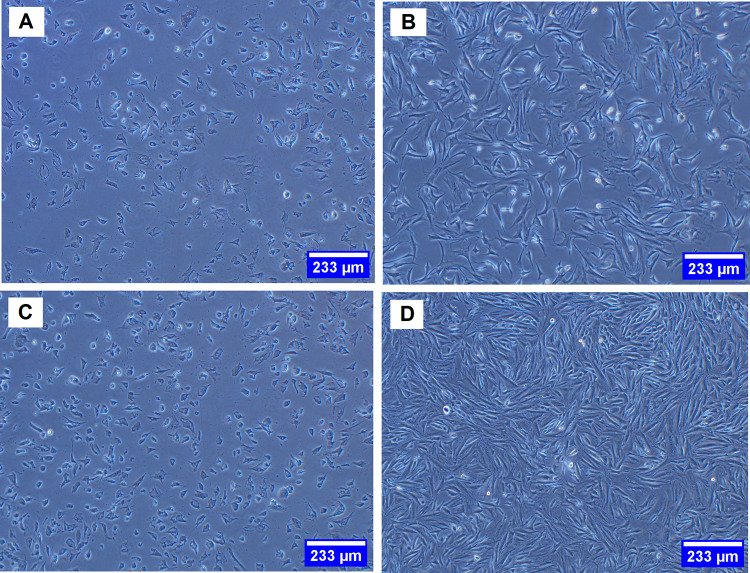
Gingival tissues obtained from biopsies of eight patients were successfully cultured into HGFCs. The first adherent fibroblast cells appeared after four days of cell culturing. Under an optical microscope view, the primary cells displayed a spindle-shaped fibroblast-like morphology (Fig. 1A). For explant technique, fibroblast cell with a smaller size with sharp edges is desirable [6]. After seven to nine days, the cells reached 80% confluence and a full monolayer without overlapping cells was observed (Fig. 1B), indicating the need for sub-culturing. The cells then grew rapidly and appeared radial or whorled after the first generation (Fig. 1C). Therefore, when explant technique is used, gingival tissues are reusable after the first sub-culture and can be used for cell culture for over two months, rapidly (after one day of re-culturing) providing an abundance of HGFCs with typical morphology (Fig. 1D).
Fig. 1.
Cultured HGFCs prepared from eight gingival biopsy samples. A Spindle-shaped fibroblast cell-like morphology. B Cell line reached about 80% confluence and a full monolayer with non-overlapping cells was observed after seven to nine days of culturing. C Rapidly growing cells appeared radial or whorled after the first generation. D The explant technique offers a reusable gingival tissue after the first sub-culture and can be used for continuous cell culture for over two months, thereby rapidly (after one day of re-culturing) providing an abundance of HGFCs exhibiting a typical morphology. HGFCs: human gingival fibroblast cells
Cellular viability and proliferation rate
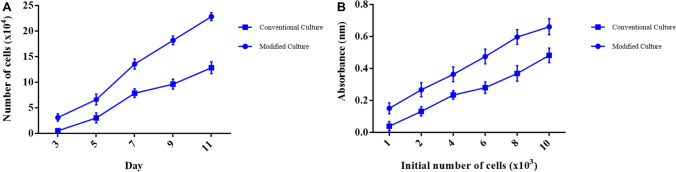
The rate of cellular proliferation and growth of the HGFCs were measured for 11 days for both the standard and modified cell culture technique. An increase in the cell growth rate was noted during the selected periods specifically for cultures prepared using the modified cell culture technique. A significant increase (p < 0.01) in the cellular proliferation rate for the eight gingival biopsy groups during the selected period at each time point was observed as depicted in (Fig. 2A). In previous studies, cellular viability was assessed using colorimetric MTT assay [25] where the reaction occurs only in the living cells by cleaving the tetrazolium salt present in the active mitochondria. After three days of culturing, it was found that the absorbance values increased which indicated a significant increase (p < 0.0001) in the number of viable cells. The means of triplicate wells from five plates are presented in (Fig. 2B).
Fig. 2.
Cellular proliferation and viability. A Cellular proliferation rate from day 3 to day 11 as affected by standard culture and modified culture methods. B Cell viability as affected by standard culture and modified culture methods. Values are represented as mean ± SD (n = 8)
Cellular growth rate
The cellular proliferation and growth rates of the HGFCs between day 4 and 12 were determined. Based on the observation, there was a relative increase in the growth rate during the selected periods. Furthermore, the cellular proliferation rate showed a significant increase between the eight gingival biopsy groups during the selected period at each time point (p < 0.001) (Fig. 3).
Fig. 3.
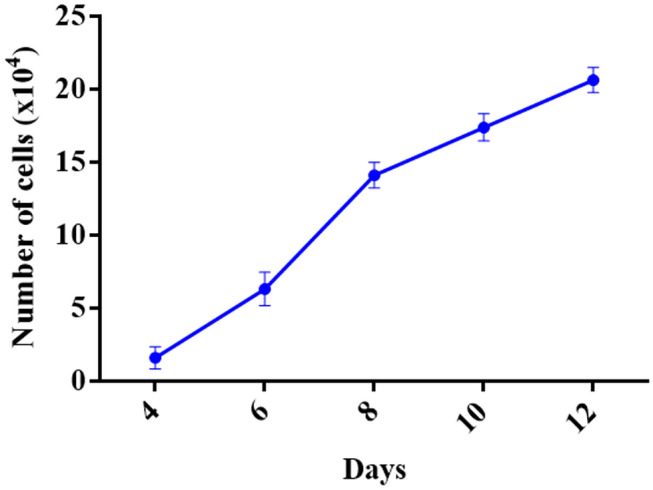
Cell cultures were tested to support the modified methodology. The growth rate of human gingival fibroblast cells (HGFCs) was evaluated between Day 4 to 12
Morphology of cells
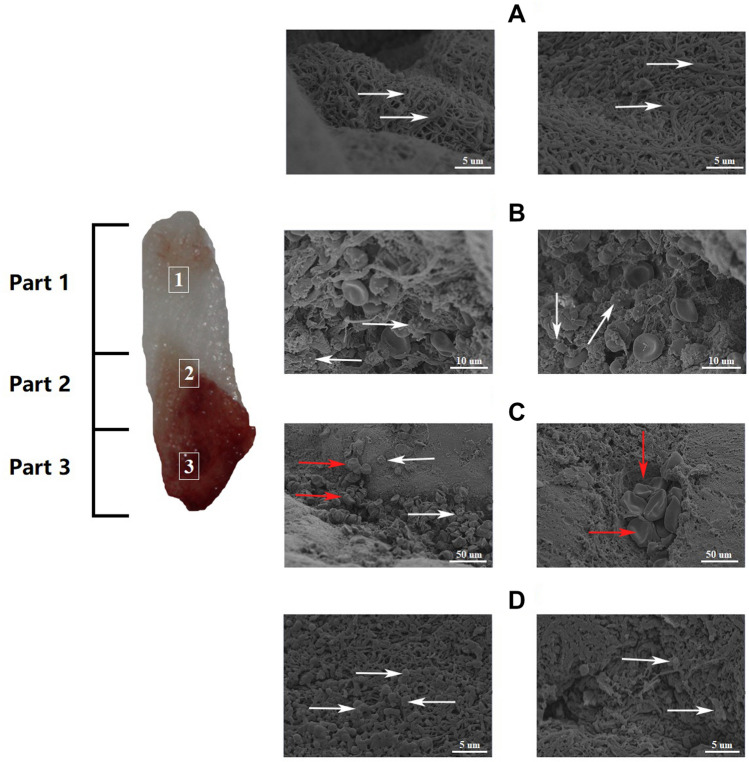
The L-PRF clot was divided into three different parts. To observe the fibrin, one end of the L-PRF membrane was dissected. At high magnification, the fibrin in Part 1 was observed to arrange into thick and dense parallel strips, and the cellular components in the network were difficult to be distinguished. (Fig. 4A). The junction between red and white (light yellow coat area) is Part 2 which represent white blood cells with a distinct spherical structure (Fig. 4B). Most white blood cells are small in size and the observed structure could be lymphocytes. The red part of the L-PRF clot (Part 3) shows the red blood cells that were enmeshed in the fibrin network, the shape is normal, but the fibrin network presents an immature form (Fig. 4C). At low magnification (×10 K), the micrograph showed that important structures were concentrated in one part. The difference between Part 1 and Part 3 was seen when Part 2 was not included. The former consisted of thick fibrin bundles and a small amount of scattered red blood cells, and a more regular and pronounced network structure. When viewed under a low power microscope, the surface of the L-PRF film appeared to be a thread-like footprint. Fibrin is a physiological binder and, therefore, a fibrin clot that is compressed into a film forms a very compact matrix. Platelets/leukocytes accumulated within 1 mm from the formation of the yellow clot, next to the red clot. From the beginning of the yellow clot to the end, the distribution of platelets/leukocytes gradually decreased, and in the latter (upper) half of the yellow clot, there are no visible platelets or white blood cells observed (Fig. 4D).
Fig. 4.
SEM images of three different parts of L-PRF. A Fiber-like appearance and mature structure of fibrin networks as shown by the arrows with the least cellular components observed. B White arrows point white blood cells with a distinct spherical structure. C Red arrows point the platelets while the white arrows point the leukocyte aggregations that are enmeshed within the immature fibrin networks. D The area around the junction between the two parts shows irregular and indistinct mesh structure, arrows pointed on some erythrocytes in standard shape. SEM: scanning electron microscope; L-PRF: Leukocyte-Platelet Rich Fibrin
Growth factors release rate
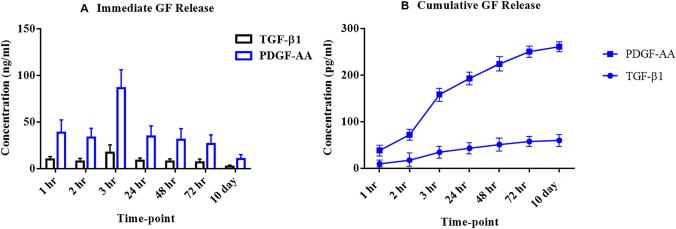
Although in general growth factors release slowly, L-PRF is the most effective in promoting additional growth factor secretion. An analysis of TGF-β1 and PDGF-AA concentrations were quantified at 1 h, 2 h, 3 h, 24 h, 48 h, 72 h, and 10 days after cell culture preparation. The releasing patterns for TGF-β1 and PDGF-AA were similar (Fig. 5). At 1 h and 3 h, TGF-β1 and PDGF-AA concentrations increased and reached a maximum at 3 h. A persistent higher concentration of PDGF-AA compared to the TGF-β1 was noted over time. On day 10, a dramatic decrease of both growth factors concentration was noted (Fig. 5A). As displayed in (Fig. 5B), both growth factors were completely released within the first 24 h.
Fig. 5.
Immediate and cumulative release of growth factors from the L-PRF samples. A Immediate growth factors (PDGF-AA and TGF-β1) release (ng/mL) as a function of time. B Cumulative growth factors (PDGF-AA and TGF-β1) release (pg/mL) as a function of time. L-PRF: leukocyte-platelet rich fibrin; PDGF-AA: platelet-derived growth factors-AA; and TGF-β1: transforming growth factor-beta1
Effect of L-PRF treatment on proliferation of HGFCs
All L-PRF concentrations evaluated displayed excellent cell biocompatibility as demonstrated by the highest number of living cells with very few observable apoptotic cells (Fig. 6). Therefore, the level of L-PRF present in each sample was found to be completely biocompatible when the present in vitro cell culture model was used. The cells treated with 100% L-PRF extract displayed the highest level of cellular proliferation.
Fig. 6.
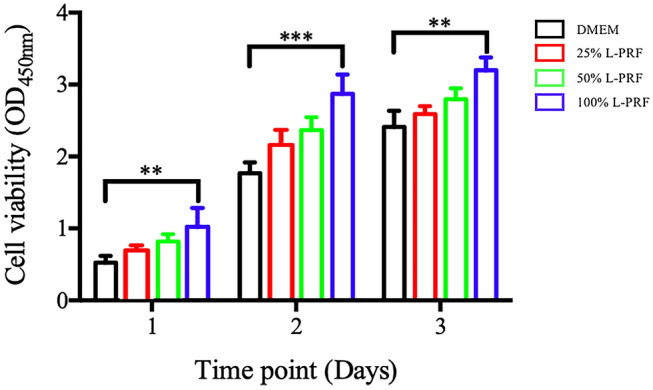
Effect of different concentrations of L-PRF on HGFCs proliferation from day 1 to day 3. Values are presented as mean ± SD (n = 6). Significant difference was indicated by **p < 0.01 and ***p < 0.001. L-PRF: Leukocyte-Platelet Rich Fibrin; HGFCs: Human Gingival Fibroblast Cells
Effect of L-PRF extract on aged cells
Based on the microscopic examination, the aged cells could adhere for two days but the cell morphology was poor. In contrast, HGFCs treated with the L-PRF conditioned medium displayed significant cell proliferation after two days. Furthermore, the cells showed normal long fusiform (Fig. 7A, B). After four days, aging HGFCs continued to proliferate slowly as compared to an obvious proliferation in HGFCs that were treated with L-PRF conditioned medium (Fig. 7C, D).
Fig. 7.
The effect of L-PRF extract on the proliferation of aged gingival fibroblasts. A Aging HGFCs did not proliferate after two days of adherence. B After L-PRF induction, aged HGFCs began to proliferate two days later. C The control group proliferated slowly in the first four days. D Aged HGFCs proliferate rapidly on Day 4 after L-PRF induction
Discussion
Recently, tissue engineering technology and implantology are being applied successfully for versatile applications in the field of dentistry. In the present study, a modified simple and rapid method of HGFC primary tissue culture was evaluated. In particular, HGFCs was isolated from a small biopsy sample (2 × 2 × 1 mm3) and the efficiency of the in vitro culture of these cells was enhanced. In contrast to the method introduced in this study, some studies used traditional explant technique while others used enzymatic treatment for isolation of HGFCs, for instance, the application of dispase, or digestion with collagenase [29]. The fibroblast isolation technique used in the present study has many advantages as follows: (1) HGFCs can be rapidly obtained because a sufficient number of fibroblasts are available within 10 days, (2) fibroblasts can be continuously produced as can be re-used, (3) The companion tissue benefits migrating cells. Cell attachment to the extracellular matrix (ECM) is not dissociated due to the absence of proteolytic enzymes and results in extended isolation time, and (4) Explant culture for HGFCs isolation: tissue piece(s) is placed in the culture dish without any digestion step and cells migrate out of the tissue due to filling of wound condition.
The findings from this study are consistent with the hypothesis where L-PRF has a sustainable release of growth factors. The natural fibrin mesh structure observed in the micrograph of L-PRF suggest a network made up of numerous leukocytes, erythrocytes, and platelets. It is commonly known that the various cells and platelets contents in the L-PRF represent excellent biological properties that can facilitate the release of growth factors. The fibrin matrix is made up of three-dimensional polymer networks with interwoven fibers that hinder platelet degradation and controls the release of growth factors trapped within the network. During the preparation of L-PRF, no damage was incurred on the leukocytes which are clinically impactful as only a small number of leukocytes can be implanted in the membrane. Among the types of leukocytes, small lymphocytes have been associated with the best efficiency in the regulation of inflammatory reactions. Besides, L-PRF requires careful manipulation and handling due to its underlying cell composition such that the viability and stability of the cellular content in this biomaterial can be preserved 26.
There are previous studies that have investigated growth factor concentration and the release kinetics [30]. The present study measured and estimated the growth factor release from L-PRF. The two growth factors PDGF-AA and TGF-β1 were the focus of this study owing to their angiogenesis and anti-inflammatory effects [31]. Naturally, platelet-rich concentrates contain both anabolic growth factors from platelets and catabolic growth factors secreted by leukocytes that facilitate tissue healing. One of the benefits of L-PRF over other platelet concentrates is its ability to be handled as a true solid biomaterial. The most important adjunct molecule in L-PRF is fibrin, which provides a therapeutic advantage over the traditional PRP. Therefore, L-PRF would be an ideal delivery vehicle as a biomaterial [32].
Another important finding derived from this study was the significant influence of L-PRF in increasing the proliferation of HGFCs. L-PRF was able to increase the migration of fibroblasts that could be attributed to the mediating effect by the growth factors released by L-PRF that can promote the proliferation of various cell types. Previous studies presented that TGF-β1 stimulated cancer cell proliferation [33], VEGF facilitated endothelial cell proliferation [34], PDGF enhanced the proliferation of epithelial cells of retinal pigment [35] and the IGF-I promoted the proliferation of various types of peripheral cells [33, 36]. The rapid proliferation of HGFCs provides the necessary bioactive substrates required in epithelial outgrowth to facilitate the healing of surgical sites. In this study, findings from the CCK-8 assay highlighted the role of L-PRF in promoting HGFCs proliferation. There is a dose-dependent effect of L-PRF where with an increase in L-PRF concentration resulted in increased HGFCs proliferation. L-PRF with concentration of 100% can impart maximum beneficial effect on fibroblast cells. The synergistic effect of L-PRF on the proliferation of autogenous HGFCs was also found in this study. Overall, the findings from this study provides a better understanding of the L-PRF clinical effects which can be useful for future studies or applications. Furthermore, this study highlighted the effect of L-PRF on the proliferation of aged HGFCs. The results showed that L-PRF managed to restore the biological characteristics of aged HGFCs and subsequently promote the cell proliferation. This can be attributed to large amounts of growth factors released by L-PRF. These growth factors are likely to restore the metabolic activity of senescent cells. In view of this, the effects of L-PRF on senescent cells and their specific mechanism warrant further evaluation. As shown in this study, fibroblast proliferation plays a vital role in tissue healing and L-PRF is associated with fibroblast proliferation, thus it is highly possible that L-PRF confers certain potential in the promotion of tissue healing [37].
In summary, a simple and rapid novel modified direct explant technique was developed and compared to the standard method of culturing HGFCs. Besides, this technique yielded pure primary cell cultures after the first passage and the gingival tissue is reusable where the HGFCs can be generated for up to two months. A patent application has been submitted for the method presented in this paper and controlled clinical testing is ongoing. Slow-release of PDGF-AA and TGF-β1 and its synergetic effect enhanced the rate of fibroblast proliferation. Moreover, the PDGF-AA concentration was found to be moderately elevated when compared to TGF-β1. Rapid HGFC production and the associated growth factor release over time exhibited by the modified method may facilitate a better understanding of the processes and assist in the development of guidelines for future applications.
Acknowledgements
1This research article was supported by the department of implantology, Stomatology Hospital of Jilin university. 2Jilin Province of Science and Technology for Development (serial number 20160101138JC).
Compliance with ethical standards
Conflict of interest
Authors have no conflict of interests, and the work was not supported or funded by any companies.
Ethical statement
The study protocol was approved by the institutional review board of School and Hospital of Stomatology at Jilin University (IRB no. 3).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mahmoud Mudalal and Zhanqi Wang contributed equally to this work.
Contributor Information
Xiaolin Sun, Email: sxl2673366@126.com.
Yanmin Zhou, Email: zhouym62@126.com.
References
- 1.Reid CB, Cloos J, Snow GB, Braakhuis BJ. A simple and reliable technique for culturing of human oral keratinocytes and fibroblasts. Acta Otolaryngol. 1997;117:628–633. doi: 10.3109/00016489709113450. [DOI] [PubMed] [Google Scholar]
- 2.Mak K, Manji A, Gallant-Behm C, Wiebe C, Hart DA, Larjava H, et al. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci. 2009;56:168–180. doi: 10.1016/j.jdermsci.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Winning TA, Townsend GC. Oral mucosal embryology and histology. Clin Dermatol. 2000;18:499–511. doi: 10.1016/S0738-081X(00)00140-1. [DOI] [PubMed] [Google Scholar]
- 4.Wanichpakorn S, Kedjarune-Laggat U. Primary cell culture from human oral tissue: gingival keratinocytes, gingival fibroblasts and periodontal ligament fibroblasts. Songklanakarin J Sci Technol. 2010;32:327–331. [Google Scholar]
- 5.Kedjarune U, Pongprerachok S, Arpornmaeklong P, Ungkusonmongkhon K. Culturing primary human gingival epithelial cells: comparison of two isolation techniques. J Craniomaxillofac. 2001;29:224–231. doi: 10.1054/jcms.2001.0229. [DOI] [PubMed] [Google Scholar]
- 6.Klingbeil MF, Herson MR, Cristo EB, dos Santos Pinto P, Yoshito D, Mathor MB. Comparison of two cellular harvesting methods for primary human oral culture of keratinocytes. Cell Tissue Bank. 2009;10:197–204. doi: 10.1007/s10561-009-9122-7. [DOI] [PubMed] [Google Scholar]
- 7.Lauer G, Otten JE, von Specht BU, Schilli W. Cultured gingival epithelium: a possible suitable material for pre-prosthetic surgery. J Craniomaxillofac Surg. 1991;19:21–26. doi: 10.1016/S1010-5182(05)80267-7. [DOI] [PubMed] [Google Scholar]
- 8.Malekzadeh R, Hollinger JO, Buck D, Adams DF, McAllister BS. Isolation of human osteoblast-like cells and in vitro amplification for tissue engineering. J Periodontol. 1998;69:1256–1262. doi: 10.1902/jop.1998.69.11.1256. [DOI] [PubMed] [Google Scholar]
- 9.Salkin LM, Freedman AL, Stein MD, Bassiouny MA. A longitudinal study of untreated mucogingival defects. J Periodontol. 1987;58:164–166. doi: 10.1902/jop.1987.58.3.164. [DOI] [PubMed] [Google Scholar]
- 10.Hürzele MB, Weng D. Functional and esthetic outcome enhancement of periodontal surgery by application of plastic surgery principles. Int J Periodontics Restorative Dent. 1999;19:36–43. [PubMed] [Google Scholar]
- 11.Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. St Louis Missouri: Saunders Elsevier; 2006. p. 1023–5.
- 12.Anfossi G, Trovati M, Mularoni E, Massucco P, Calcamuggi G, Emanuelli G. Influence of propranolol on platelet aggregation and thromboxane B2 production from platelet-rich plasma and whole blood. Prostaglandins Leukot Essent Fatty Acids. 1989;36:1–7. doi: 10.1016/0952-3278(89)90154-3. [DOI] [PubMed] [Google Scholar]
- 13.Fijnheer R, Pietersz RN, De Korte D, Gouwerok CW, Dekker WJ, Reesink HW. Platelet activation during preparation of platelet concentrates: a comparison of the platelet-rich plasma and the buffy coat methods. Transfusion. 1990;30:634–638. doi: 10.1046/j.1537-2995.1990.30790385523.x. [DOI] [PubMed] [Google Scholar]
- 14.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Lekovic V, Milinkovic I, Aleksic Z, Jankovic S, Stankovic P, Kenney EB, et al. Platelet-rich fibrin and bovine porous bone mineral versus platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodontal Res. 2012;47:409–417. doi: 10.1111/j.1600-0765.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- 16.Shivashankar VY, Johns DA, Vidyanath S, Sam G. Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesion. J Conserv Dent. 2013;16:261–264. doi: 10.4103/0972-0707.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina-Porqueres I, Alvarez-Juarez P. The efficacy of platelet-rich plasma injection in the management of hip osteoarthritis: a systematic review protocol. Musculoskelet Care. 2016;14(2):121–125. doi: 10.1002/msc.1115. [DOI] [PubMed] [Google Scholar]
- 18.Mudalal M, Zhou Y. Biological additives and platelet concentrates for tissue engineering on regenerative dentistry basic Science and concise review. Asian J Pharm. 2017;11:255–263. [Google Scholar]
- 19.Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implantodontie. 2001;42:e62. [Google Scholar]
- 20.Salamanna F, Veronesi F, Maglio M, Della Bella E, Sartori M, Fini M. New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: general overview on still open questions and outlook. Biomed Res Int. 2015;2015:846045. doi: 10.1155/2015/846045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YC, Zhao JH. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dent J. 2011;56:365–371. doi: 10.1111/j.1834-7819.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 22.Qin J, Wang L, Sun Y, Sun X, Wen C, Shahmoradi M, et al. Concentrated growth factor increases Schwann cell proliferation and neurotrophic factor secretion and promotes functional nerve recovery in vivo. Int J Mol Med. 2016;37:493–500. [DOI] [PubMed]
- 23.Saczko J, Dominiak M, Kulbacka J, Chwiłkowska A, Krawczykowska H. A simple and established method of tissue culture of human gingival fibroblasts for gingival augmentation. Folia Histochem Cytobiol. 2008;46(1):117–119. doi: 10.2478/v10042-008-0017-4. [DOI] [PubMed] [Google Scholar]
- 24.Hassell T, Stanek EJ., III Evidence that healthy human gingiva contains functionally heterogeneous fibroblast subpopulations. Arch Oral Biol. 1983;28:617–625. doi: 10.1016/0003-9969(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Miron R, Choukroun J, Ghanaati S. Controversies related to scientific report describing g-forces from studies on platelet-rich fibrin: necessity for standardization of relative centrifugal force values. Int J Growth Factors Stem Cells Dent. 2018;1:80–6. doi: 10.4103/GFSC.GFSC_23_18. [DOI] [Google Scholar]
- 27.Kobayashi M, Kawase T, Horimizu M, Okuda K, Wolff LF, Yoshie H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals. 2012;40:323–329. doi: 10.1016/j.biologicals.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Dohan Ehrenfest DM, Doglioli P, de Peppo GM, Del Corso M, Charrier JB. Choukroun’s platelet-rich fibrin (PRF) stimulates in vitro proliferation and differentiation of human oral bone mesenchymal stem cell in a dose-dependent way. Arch Oral Biol. 2010;55:185–194. doi: 10.1016/j.archoralbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Pini Prato GP, Rotundo R, Mognoni C, Soranzo C. Tissue engineering technology for gingival augmentation procedures: a case report. Int J Periodontics Restorative Dent. 2000;20:552–559. [PubMed] [Google Scholar]
- 30.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sprots Med. 2011;39:266–271. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 31.Mudalal M, Sun X, Li X, Zhou Y. The evaluation of leukocyte-platelet rich fibrin as an antiinflammatory autologous biological additive: a novel in vitro study. Saudi Med J. 2019;40:657–668. doi: 10.15537/smj.2019.7.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boora P, Rathee M, Bhoria M. Effect of platelet rich fibrin (PRF) on peri-implant soft tissue and crestal bone in one-stage implant placement: a randomized controlled trial. J Clin Diagn Res. 2015;9:ZC18-21. doi: 10.7860/JCDR/2015/12636.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Mudalal M, Sun Y, Liu Y, Wang J, Wang Y, et al. The effects of leukocyte-platelet rich fibrin (L-PRF) on suppression of the expressions of the pro-inflammatory cytokines, and proliferation of Schwann cell, and neurotrophic factors. Sci Rep. 2020;10:2421. doi: 10.1038/s41598-020-59319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, et al. Vascular endothelial growth factor–stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86:892–896. doi: 10.1161/01.RES.86.8.892. [DOI] [PubMed] [Google Scholar]
- 35.Chan CM, Chang HH, Wang VC, Huang CL, Hung CF. Inhibitory effects of resveratrol on PDGF-BB-induced retinal pigment epithelial cell migration via PDGFRβ, PI3K/Akt and MAPK pathways. PLoS One. 2013;8:e56819. doi: 10.1371/journal.pone.0056819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maucksch C, McGregor A, Yang M, Gordon RJ, Connor B. IGF-I redirects doublecortin-positive cell migration in the normal adult rat brain. Neuroscience. 2013;241(106):115. doi: 10.1016/j.neuroscience.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Tohidnezhad M, Bayer A, Rasuo B, Hock JVP, Kweider N, Fragoulis A, et al. Platelet-released growth factors modulate the secretion of cytokines in synoviocytes under inflammatory joint disease. Mediators Inflamm. 2017;2017:1046438. doi: 10.1155/2017/1046438. [DOI] [PMC free article] [PubMed] [Google Scholar]