Abstract
Background:
Replacing damaged anterior cruciate ligaments (ACLs) with tissue-engineered artificial ligaments is challenging because ligament scaffolds must have a multiregional structure that can guide stem cell differentiation. Here, we designed a biphasic scaffold and evaluated its effect on human marrow mesenchymal stem cells (MSCs) under dynamic culture conditions as well as rat ACL reconstruction model in vivo.
Methods:
We designed a novel dual-phase electrospinning strategy wherein the scaffolds comprised randomly arranged phases at the two ends and an aligned phase in the middle. The morphological, mechanical properties and scaffold degradation were investigated. MSCs proliferation, adhesion, morphology and fibroblast markers were evaluated under dynamic culturing. This scaffold were tested if they could induce ligament formation using a rodent model in vivo.
Results:
Compared with other materials, poly(D,L-lactide-co-glycolide)/poly(ε-caprolactone) (PLGA/PCL) with mass ratio of 1:5 showed appropriate mechanical properties and biodegradability that matched ACLs. After 28 days of dynamic culturing, MSCs were fusiform oriented in the aligned phase and randomly arranged in a paving-stone-like morphology in the random phase. The increased expression of fibroblastic markers demonstrated that only the alignment of nanofibers worked with mechanical stimulation to promote effective fibroblast differentiation. This scaffold was a dense collagenous structure, and there was minimal difference in collagen direction in the orientation phase.
Conclusion:
Dual-phase electrospun scaffolds had mechanical properties and degradability similar to those of ACLs. They promoted differences in the morphology of MSCs and induced fibroblast differentiation under dynamic culture conditions. Animal experiments showed that ligamentous tissue regenerated well and supported joint stability.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-021-00376-7.
Keywords: Electrospun, Biphasic, Anterior cruciate ligament, Tissue engineering
Introduction
Anterior cruciate ligament (ACL) injuries account for >30% of knee injuries, and this trend is annually increasing. ACLs have poor regeneration capacity with low cell density, low nutrient and blood supply requirements [1]. More than 300,000 patients undergo surgery to repair injured tendons and ligaments annually in the United States [2, 3]. At present, transplantation reconstruction is the primary treatment method after ACL injury of the knee joint, and tissue replacement is mainly taken from autografts or allografts [4, 5]. However, major problems with autografts include additional surgery and possible lesions and pain at the donor site [6]. Moreover, there are concerns about allografts increasing the risk of disease transmission and the possibility of immunogenic response elicited in the host [7, 8]. Recent studies have indicated that tissue-engineered ligaments can provide sufficient stability, enable rapid recovery, and reduce the possibility of infection in the knee joint, compensating for the insufficiency of autografts and allografts to a certain extent [9–12]. Tissue-engineered ligament required a biocompatible and biodegradable extracellular matrix material, and then cells are planted on the scaffold, which gradually grow into the scaffold and secrete extracellular matrix. Finally a three-dimensional spatial structure that simulates ligaments in both structure and composition is generated. Despite the progress in ligament regeneration techniques, bioengineered tracheal replacement studies are still at the preclinical level.
Natural ACLs are multi-phase structures that include bone, fibrocartilage, and fiber gradient from end to middle in anatomical physiology [13]. As greater attention is paid to bionics, the ideal design of scaffolds for ligament tissue engineering should also be multilayered to mimic the spatial structure of the natural tissues [14]. To fabricate the multi-phase scaffold, nonelectrospinning processes, such as micropattern method, can be used to obtain highly aligned multilayer structures. However, these structures can still be limited by their biodegradation [15, 16]. Subsequently, a technique involving a combination of electrospinning and freeze-drying has emerged, allowing the introduction of spatially distributed biomolecules into the aligned scaffolds [17]. However, this scaffold failed to completely restore tissue function in the treatment. In addition, three-dimensional printing technology can incorporate different components into the scaffold, which holds great promise for customized biomimetic scaffolds [18–20]. However, its application in regenerative medicine is limited by the lack of printable biomaterials. Therefore, it will be more convenient and appealing to use electrospinning by itself to realize the dual-phase characteristic. There are two distinct phases in the structure of natural ligaments: (a) an oriented ligament phase where fibroblasts are highly aligned along the direction of the fibers, (b) a random phase containing osteoblasts. Based on these considerations, we fabricated a dual-phase scaffold that represented the medial oriented region and the two ends of the random region using a specifically designed two-step electrospinning process as shown in Scheme 1.
Scheme 1.
Schematic illustration of the fabrication process of the dual-oriented scaffold
Using tissue-engineered ligaments mandates a slow ligament recovery process. Decline in ACL strength leads to ligament loosening and fracturing during the process of ACL ligamentization, which has a certain impact on the reconstruction effect [21]. Therefore, the artificial ligament must have properties of high strength and low fatigue similar to that of the replaced natural tissue. Previous research found that Ligamys® can support ACL acute repair [22, 23], but it has slower degradation properties [24]. LARS™ (Tille, France) does not provide favorable conditions for cell migration, proliferation, revascularization, or collagen synthesis [25]. Among the various polymers used, poly(e-caprolactone) (PCL) shows excellent stiffness and favorable biocompatibility with inferior degradation [26]. Pektok et al. found that the PCL scaffold had a tensile stress of 4.8 MPa, a mass loss of 1/4 in experimental rats at 24 weeks, and its use significantly increased the cell adhesion rate [27]. Poly(D,L-lactide-co-glycolide) (PLGA) has excellent biodegradability with minimal cytotoxicity, and it has been certified by the US Food and Drug Administration for use in medicine [28, 29]. However, its degradation rate is rapid and it has weak mechanical properties. Recent studies indicated that mixing different polymers can produce ideal materials [24, 30]. Therefore, our hypothesis is that incorporation of PLGA and PCL in varying ratios can form a PLGA/PCL composite with a superior biocompatibility, biodegradation, and mechanical strength.
Because of their similarity to the native extracellular matrix, nanofibers have been used to promote the response and differentiation of mesenchymal stem cells (MSCs). For example, Ma et al. studied the effects of nanofiber alignment on the induction of MSC differentiation in conjunction with the induction medium [31]. Similarly, Grayson et al. showed that a combination of mechanical and chemical stimulations resulted in the upregulation of the differentiation markers of MSCs [32]. Notably, the majority of these studies used chemical factors along with scaffold cues or mechanical stimulation, making it difficult to separate the effects of these distinct stimuli. In addition, scaffold cues were found to direct MSC differentiation without a chemical induction medium. For example, Xue et al. studied nanofibers modulated osteogenic markers expression of MSCs [33]. Similarly, Yao et al. investigated nanofibers that upregulated the expression of neural markers by MSCs [34]. These studies only used scaffold cues, but this study focuses on the relative contributions and interactions of the nanofiber matrix arrangement and mechanical stimulation.
Materials and methods
Scaffold fabrication
Dual-phase scaffolds were fabricated by electrospinning a polymer blend of PCL (Mw = 80,000) and PLGA (Mw = 140,000) (Jinan Daigang Biomaterial Co., Ltd., Jinan, China), with a weight ratio of 1:1, 1:2 and 1:5. The polymers were dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol for 24 h using a magnetic stirrer to form a 16 wt% electrospinning solution. The electrospinning apparatus included a microsyringe pump, a high-voltage power supply, and a rotating stainless-steel drum receiving device (TL-Pro-BM, Shenzhen Litong Micro-Nano Technology Co., Ltd., Shanghai, China) to collect the fibers. The electrospinning process was performed under a positive voltage of 14 ± 1 kV at a tip-to-collector distance of 15 cm using a stainless-steel rotating collector (d = 10 cm) and a constant feeding rate of 1 mL/h with a 23 G stainless-steel needle. PCL and PLGA nanofiber membranes were electrospun and used as controls in several experiments.
Briefly, the dual-oriented scaffold was prepared in two steps as shown in Scheme 1. First, a rotating stainless-steel drum was used to electrospin the nanofiber membranes at a speed of 100 r/min for 2 h. The electrospun membrane was cut into rectangles 1 × 10 cm in size, and two of these cut sheets were curled onto a stainless-steel drum spaced 1.5 cm apart as the random phase. Second, the stainless-steel rotating collector was continuously electrospun at a speed of 1000r/min for 5 h to collect the ligament phase nanofibers. Finally, the membranes were curled into 3.5-cm-long bundles, and the juncture interface was glued using a 5 wt% gelatin aqueous solution. After the electrospinning process was complete, the composite scaffolds were dried under a vacuum for at least 1 week to remove the trace solvent and were then placed in a dryer until use.
Scaffold characterization
Scanning electron microscopy
Morphological investigation of nanofibrous membranes was performed using scanning electron microscopy (SEM). Using sputter coating, the electrospun scaffolds were coated with gold and observed through SEM (Quanta 250 FEG, FEI Company, Hillsboro, OR, USA). SEM images were analyzed using the ImageJ software to determine the average diameter of the nanofibers. Fifty random fibers per image were used to calculate the mean and standard deviation of the fiber diameters. The cells on the scaffolds were fixed overnight in 2.5% glutaraldehyde and dehydrated using a series of ethanol solutions and then dried under vacuum for further SEM characterization (RIGMA þ X-Max20, Zeiss, Oberkochen, Germany).
Mechanical properties
The mechanical characteristics of the PLGA/PCL aligned nanofiber membranes were measured using a universal test device (Inspekt Table Blue, H&P, Nossen, Germany). Samples were punched into dumbbell-shaped specimens over a 75 × 10 mm dumbbell-shaped mold with a gage length of 25 mm using a pneumatic punching machine (GT7016-M1, Goodrechwill, Shanghai, China). These were then tested using a 200-N load cell at a tensile speed of 5 mm/min. The ultimate tensile strength, Young’s modulus, and elongation at break were obtained from the stress–strain curves.
In vitro degradation
In the degradation tests, 10 mg of the vacuum dried nanofiber membranes were precisely weighed as the original weight and sterilized in test tubes; 15.0 mL phosphate-buffered saline (PBS, pH 7.4) was then added to each tube. All tubes were shaken in a horizontal shaker set at 120 rpm and incubated at 37 °C. Samples were collected at 1, 2, 4 and 8 weeks and washed three times with deionized water to remove residual buffer salts and then dried in a vacuum. The residual weight of the membranes after different periods of incubation was calculated. The mass loss was gravimetrically determined by comparing the residual dry weight with the initial weight.
Dynamic culture of scaffolds
Cell viability
Before seeding, the scaffolds were sterilized in ozone for 2 h and then under ultraviolet irradiation overnight. Human marrow MSCs (Fenghui, Wuxi, China) were precultured in high-glucose Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% penicillin–streptomycin (Gibco, Grand Island, NY, USA) for 5 days, and then seeded into the biphasic scaffold at a density of 2 × 105 cells/scaffold. The scaffolds were manipulated twice a day in a modular bioreactor system with a tensile strain of 1% and an application frequency of 90 min each time. Cell proliferation was evaluated using the Cell Counting Kit-8, according to the manufacturer's instructions at the set time points (1, 7, 14 and 28 days). The absorbance value at 450 nm was measured using a microplate reader (Epoch, BioTek, Winooski, VT, USA).
Cell adhesion
The cell morphology and spreading as well as cell interaction with the nanofiber membranes were detected using calcein-AM (Sigma-Aldrich, St. Louis, MO, USA) at 28 days. Cell-containing membranes were washed three times with PBS, incubated with a 1 μM calcein-AM for 15 min without light as per the manufacturer's instructions, and washed three times with PBS. A fluorescence imaging microscope (IX73, Olympus, Tokyo, Japan) was used to observe the samples and capture images.
Cell matrix deposition
After 28 days of culturing, all samples were fixed with 4%(w/v)paraformaldehyde at 37 °C for 10 min, and transmembrane treated with 0.1% Triton X-100 solution for 10 min. After washing with PBS, the sample was sealed using 5% donkey serum for 1 h. Then, monoclonal primary antibodies (Invitrogen, Carlsbad, CA, USA) at a dilution of 1:1000 were added and soaked overnight at 4 °C. The unbound primary antibodies were washed off using PBS buffer solution, and the donkey polyclonal secondary antibodies at a dilution of 1:500 were added and incubated for 1 h. After the excess dye was removed, the nuclei were restained with DAPI solution for 10 min, and all samples were imaged using confocal microscopy (Leica TCS SP5, Wetzlar, Germany).
Cell differentiation
To investigate the fibroblastic induction of the composition saffolds, the MSCs were seeded on nanofiber membranes and cultured for 28 days. Gene expression was measured using real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR). Expression of fibroblastic markers type I collagen, type III collagen, fibronectin, tenascin-C, tenomodulin, and scleraxis was determined. Total RNA content of the specimens was extracted using the Trizol reagent (Jiancheng Institute of Biological Engineering, Nanjing, China), and complementary DNA was measured using RT reagent Kit (TaKaRa, Kyoto, Japan). SYBR green master-mix (TaKaRa) was applied for real-time RT-PCR. All primer sequences used in this study are presented in Table 1. The housekeeping gene GAPDH used as a control.
Table 1.
Primer sequences used in this study
| Gene | Sense | Anti-sense |
|---|---|---|
| GAPDH | 5′-GGCGATGCTGGCGCTGAGTA-3′ | 5′-ATCCACAGTCTTCTGGGTGG-3′ |
| Collagen I | 5′-TGGTCCACTTGCTTGAAGAC-3′ | 5′-ACAGATTTGGGAAGGAGTGG-3′ |
| Collagen III | 5′-GGCTACTTCTCGCTCTGCTT-3′ | 5′-CATATTTGGCATGGTTCTGG-3′ |
| Fibronectin | 5′-TTGAACCAACCTACGGATGA-3′ | 5′-AAATGACCACTTCCAAAGCC-3′ |
| Tenascin-C | 5′-TGCCCATTACAGGAGGTACA-3′ | 5′-CACTTTCCTCAAAGCCCTTC-3′ |
| Scleraxis | 5′-CAGCGGCACACGGCGAAC-3′ | 5′-CGTTGCCCAGGTGCGAGATG-3′ |
In vivo culture of scaffolds
ACL reconstruction procedure
The rats were kept in cages with free access to food and water. After administration of anesthesia with a mixture of 80 mg/kg ketamine (Fort Dodge, IA, USA) and 5 mg/kg thiazide (Bayer Corporation, St. Louis, MO, USA) injected intraperitoneally, a longitudinal incision was made at the distal end of the ankle to access the full length of the flexor digitorum longus tendon. The medial parapatellar joint was incised, the ACL excised, and a 1.5-mm bone tunnel was made. The graft replaced the ACL through the bone tunnel, and the proximal and distal ends were secured to the femur and tibia, respectively. The wound was sutured as per the standard method. All animals resumed their normal cage activities on the first day after surgery.
Scaffold matrix deposition
The animals in each group were euthanized via carbon dioxide inhalation at 4 and 8 weeks postoperatively. The harvested tissues were fixed in 10% neutral buffer formalin for 4 days, then decalcified in formalin formic acid and ion-exchange bead (HC-5, Dowex, Sigma-Aldrich) solution for 48 h and dehydrated in increasing concentrations of alcohol (70–100%). The samples were cleared three times in xylene and then embedded in paraffin at 60 °C. A 7-µm-thick section was cut perpendicular to the axis of the ligament. Collagen distribution was observed after Picrosirius red staining. Images were obtained with a polarized light microscope (BX60, Olympus).
Statistics
All data are presented as mean ± standard deviation. Components were compared using one-way analysis of variance (SPSS), and p values < 0.05 were considered statistically significant.
Results
Dual-phase scaffold characterization
SEM
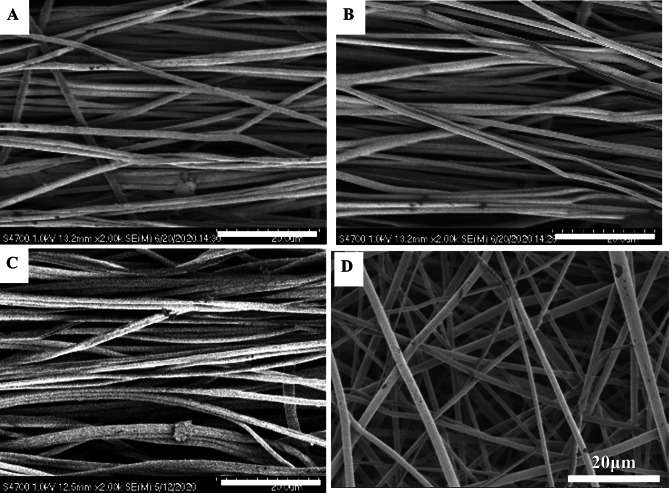
SEM images of the scaffolds indicated that both highly aligned and random nanofibers were prepared successfully (Fig. 1). Importantly, all nanofibers had a highly porous structure and a smooth surface (Fig. 1). The PLGA/PCL nanofibers with quality ratios of 1:1, 1:2 and 1:5 had average diameters of 663 ± 156, 678 ± 109 and 691 ± 181 nm, respectively (Table. 2).
Fig. 1.
Morphology characterization of the dual-oriented scaffold. A–C PLGA/PCL aligned electrospun nanofiber mats with mass ratio of 1:1,1:2 and 1:5; D random electrospun nanofiber mats
Table 2.
Average diameter of nanofibers
| Nanofibers | PLGA | 1:1 PLGA:PCL | 1:2 PLGA:PCL | 1:5 PLGA:PCL | PCL |
|---|---|---|---|---|---|
| Diameter (nm) | 615 ± 152 | 663 ± 156 | 678 ± 109 | 691 ± 181 | 712 ± 146 |
Mechanical properties
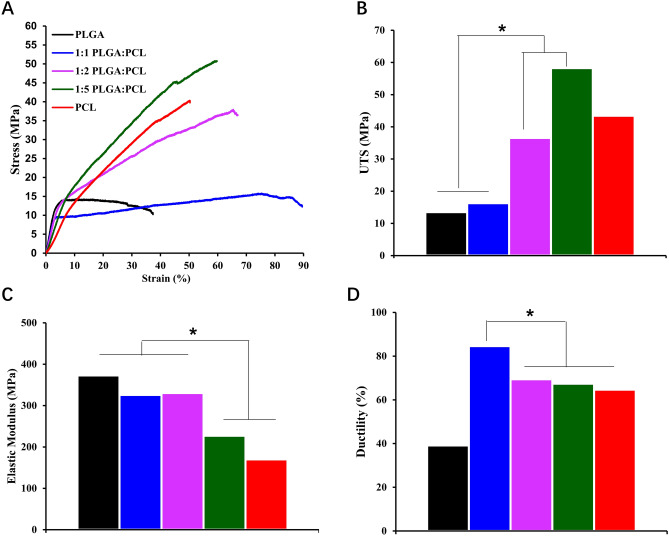
Nanofibers with tunable linear elastic were prepared from the blends of PCL and PLGA. The elastic modulus of the PLGA/PCL (1:1) and (1:2) membranes was 326 ± 31 and 330 ± 37 MPa, respectively. The elastic modulus clearly increased when PLGA was added to the blend. The ultimate tensile stress of PLGA/PCL (1:5) and PLGA/PCL (1:2) was 58.3 ± 7.4 and 43.5 ± 6.7 MPa, respectively, which was higher than that of PLGA/PCL (1:1), indicating that PCL polymer mixing could result in superior mechanical properties. Importantly, the ultimate stress of these complex membranes in the dry state was also consistent with that of the natural ACL ligament. The three types of fibers broke at a strain of approximately 67%–85%, with no significant changes in ductility observed (Fig. 2).
Fig. 2.
Mechanical properties of different PLGA/PCL electrospun nanofiber mats. A Stress–strain curve; B elastic modulus; C ultimate tensile stress; and D elongation break
In vitro degradation
The mass of the nanofiber membrane gradually reduced over 8 weeks. PLGA/PCL membranes with ratios of 1:1, 1:2 and 1:5 retained 85%, 89% and 91% of their original weight, respectively, indicating that the addition of PLGA accelerated the degradation of the entire membrane (Fig. 3).
Fig. 3.
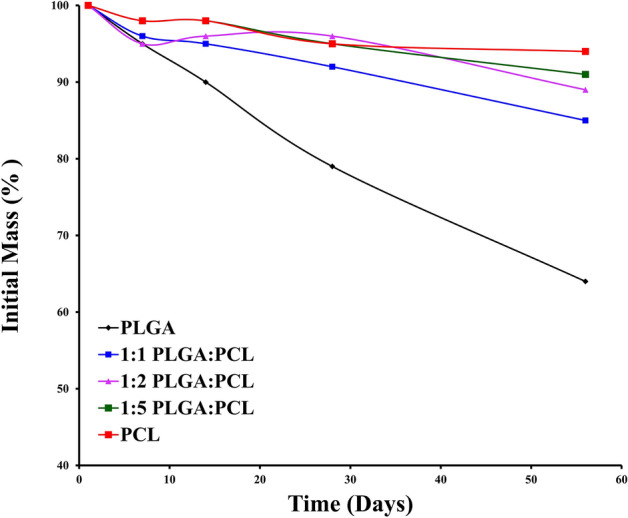
In vitro degradation of PLGA/PCL scaffolds
Dynamic culture
Cell viabilities
There were no significant differences between the groups on days 1, 7 and 14 in the random scaffolds. However, the proliferation rates of MSCs with tension were significantly higher than those of MSCs without tension on day 28 (Fig. 4A); this was mainly due to the effect of tensile force on cell growth. There were no significant differences between the groups on the first day in aligned scaffolds; however, the number of cells significantly increased compared with the previous time point on the seventh day (Fig. 4B). Thus, the oriented scaffold and mechanical stimulation promoted the proliferation of MSCs. The proliferation rates of MSCs on scaffolds with tension were significantly higher than those on scaffolds without tension on days 7 and 14. These results supported the implication that aligned scaffolds allow for greater cell density on nanofibers and that mechanical loading may also promote cell survival over the unloaded groups.
Fig. 4.
Cell viability of MSCs on different phases of the dual-oriented scaffold. Cell proliferative behavior of the A scaffold random phase and B aligned phase
Cell adhesion
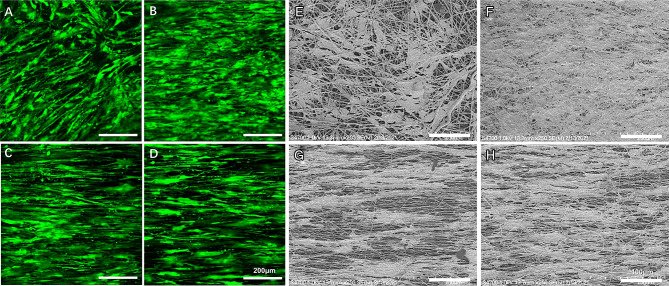
MSCs attached well to both kinds of membranes (Fig. 5). On the random phase, MSCs showed a random arrangement without a clear dominant direction and exhibited paving-stone-like morphologies (Fig. 5A and E), but appeared to be aligned in a distinct direction when a tensile force was applied (Fig. 5B and F). On the aligned phase, MSCs showed distinctly preferential orientations irrespective of whether tension was applied, exhibited a slender spindle-shaped morphology and similar to tendon cells in phenotype (Fig. 5C, G, D and H). In addition, the number of cells in the tension group was higher than that in the nontension group, which was consistent with cell viability results. The results showed that the surface morphology of the scaffold affected the stress of the cells and changed the morphology of the cells.
Fig. 5.
A–H SEM images and calcein-AM staining of MSCs on different phases of the dual-oriented scaffold. Unloaded (A and E) and loaded (B and F) random phase; unloaded (C and G) and loaded (D and H) aligned phase
Cell matrix deposition
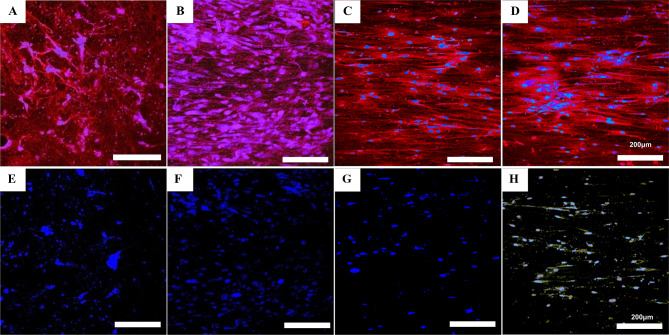
Staining of cells on the random phase for type I collagen revealed that the cells only secreted a small amount of type I collagen in a relatively scattered manner with no clear direction of arrangement (Fig. 6A). Importantly, the secretion of type I collagen increased after the application of tensile force and presented aligned morphologies (Fig. 6B). On the aligned phase, type I collagen was deposited and oriented along the skeleton of the cell (Fig. 6C and D). Interestingly, a matrix of both types I and III collagen was produced by the cells only on nanofibers aligned with the load (Fig. 6E-H). Type I collagen fibers have a very high tensile strength; the directional deposition of new collagen ensures that ligaments can withstand large drafting forces without breaking.
Fig. 6.
A–H Immunofluorescence of type I collagen (red) and type III collagen (yellow) with nuclei counterstain (blue) of MSCs on different phases of the dual-oriented scaffold. Unloaded (A and E) and loaded (B and F) random phase; unloaded (C and G) and loaded (D and H) aligned phase
Cell differentiation
For the random phase, MSCs with tension significantly upregulated the expression levels of gene associated with fibronectin compared with MSCs without tension. Notably, the key fibroblast markers tenosin-C and scleraxis remained at base levels and remained un-upregulated after a period of loading tension on the unaligned nanofibers (Fig. 7A). More importantly, in the aligned phase, nanofibrous membranes with tension showed the highest gene expression levels for type III collagen, fibronectin, tenascin-c and scleraxis compared with those of the other groups, indicating a much more vigorous fibroblast differentiation. Otherwise, there were no significant differences between groups in terms of gene expression of type I collagen (Fig. 7B). The results indicate that costimulation of aligned alignment and mechanical loading was required for the induction of MSC differentiation into fibroblasts.
Fig. 7.
Fibrogenic differentiation of MSCs induced by scaffolds. A random phase; B aligned phase
Additionally, because no specific marker serves to distinguish ligament fibroblasts from other fibroblasts in connective tissues, the type I:III collagen ratio is an important indicator of cell phenotype. The main reason for this is that it significantly differs between ligaments (~ 7:1) and tendons (~ 20:1) and is substantially decreased in scar tissue (~ 1:1). In this study, cells on aligned nanofibers had a type I:III collagen expression ratio of 8.35 ± 2.12 after 28 days of loading, thereby suggesting that cells assumed a ligament fibroblast-like phenotype in response to mechanical stimulation only on the aligned nanofibers (Table. 3).
Table 3.
The type I:III collagen ratio of MSCs on different phases of the dual-oriented scaffold
| MSCs | Random phase without load | Random phase with load | Aligned phase without load | Aligned phase with load |
|---|---|---|---|---|
| Type I:III collagen ratio | 0.51 ± 1.26 | 1.03 ± 0.99 | 8.35 ± 2.12 | 2.07 ± 1.11 |
In vivo scaffold matrix deposition
Collagen matrix visualized after Picrosirius red staining showed similar collagen distribution in all groups at 4 and 8 weeks. Evaluation of collagen orientation indicated minimal differences in collagen direction in the control ligament phase and the scaffold orientation groups at 4 and 8 weeks. Collagen fibers appeared green under polarized light, indicating that smaller-diameter fibers were present in the control bone phase and the scaffold random groups after 8 weeks. Green fibers were also seen around the grafts in each group, corresponding to the contours of the ligaments (Fig. 8).
Fig. 8.
A-H Picrosirius red staining of collagen on different phases of the dual-oriented scaffold. Control group’s ligament phase (A) and bone phase (B) at 4 weeks; scaffold’s aligned phase (C) and random phase (D) at 4 weeks; control group’s ligament phase (E) and bone phase (F) at 8 weeks; scaffold’s aligned phase (G) and random phase (H) at 8 weeks
Discussion
As previously reported, the tensile strength of autologous ligaments is approximately13.7–36.4 MPa, and the elongation at break of scaffold should be 19–36% [35]. In this study, PLGA/PCL (1:5) fiber scaffolds had a high ultimate tensile strength (58.3 ± 7.4 MPa) and elongation at break (67 ± 1.8%), which were both higher than the reported criteria. The scaffold showed the characteristics of high strength and low fatigue, which provided sufficient mechanical properties in the early stage of recovery. Interestingly, the PLGA:PCL (1:1) nanofibers had the greatest ductility in this study, which suggested interactive effects between PLGA and PCL, resulting in unique mechanical properties when the two polymers are blended together. In addition, the PLGA/PCL membrane with a ratio of 1:5 retained 91% of its original weight as measured, indicating that the addition of PLGA indeed accelerated the degradation of the entire scaffold. The slow degradation of the scaffolds at the later stage gives space for cells to grow and provides more durable mechanical properties, depending on the structure of the newly generated tissue.
Compared with other ligament saffolds, the dual-orientation ligament saffold prepared using the two-step spinning method in this study has the following advantages: First, the PLGA:PCL (1:5) had mechanical properties and degradability similar to those of ACLs. Secondly, in terms of cell arrangement and morphology, nanofiber tissue arrangement is a major determinant of cell arrangement, and contact guidance drives long-term cell arrangement. Cell alignment along the strain direction in the random nanofibers suggested that this alignment can also be regulated by exogenous factors and directed in a more physiologically relevant alignment. Thirdly, the production of type III collagen is essential for the fibroblastic differentiation of cells, and it provides tendons and ligaments with strength and elasticity by forming heterotypic fibers incorporating type I collagen. The production of type III collagen by MSCs in response to tensile strain only occurs on aligned nanofibers. These observations corroborate previous reports of elevated MSC biosynthesis, such as the study by Baker et al., who applied both tensile and chemical stimulation to MSCs cultured on PCL nanofibers as well as to human fibroblasts cultured on polyurethane nanofibers and subjected to tensile strain [36]. However, in contrast to these reports, the results of this study showed that mechanical stimulation alone can enhance MSC matrix deposition in the absence of growth factors. In addition, the qRT-PCR results showed that MSCs in the random areas of scaffolds could adjust their cell morphology to the direction of loading under tensile force. MSCs could differentiate into fibroblasts only when they were cultured on oriented nanofibers and stimulated by mechanical load. An interaction between matrix arrangement and mechanical loading was observed, and it was determined that costimulation is necessary to induce the differentiation of MSCs into fibroblasts. At last, histological analysis demonstrated that the graft was intact as a dense collagenous structure and surrounded by abundant collagenous tissue, as observed after Picrosirius red staining. This indicated that collagen molecules directionally deposited on nanofibers and assembled into collagen fibers with increased culture time and that ligaments can withstand large forces without breaking, providing long-term stability for joint activity.
This manufacturing strategy solved the key problem of how to construct random fibers distributed only at the ends of the ligaments and showed that the scaffold had similar mechanical properties and degradability to ACL. The results demonstrated that MSCs adapted their morphology to align in the direction of loading on random nanofibers, only MSCs cultured on oriented nanofibers and stimulated by mechanical load can differentiate into fibroblasts and deposit a ligament-like matrix. Animal experiments showed that ligamentous tissue regenerated well and supported joint stability. Our fabrication strategy gives us spatial control over fiber composition, allowing us to tailor individual construction for the regeneration of specific tissues. Therefore, these scaffolds are potentially suited for any soft tissues, including tendons, ligaments, osteochondral zones, or the periodontium.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by grants from the Doctor Foundation of Guizhou Provincial People’s Hospital (GZSYBS [2017] 11), the National Nature Science Foundation of China (Grant No. 51663004).
Author contribution
YT Carried out the experimental part, take a part in planning the experiment, writing the manuscript and data analysis. JLT Carried out the histological assessment, contributed in study design, planning the experiment writing, supervision and analysis of data. LL Supervised the practical work, helped in explaining the results, and contributed in revising the manuscript writing and organization LH Helped in explaining the results, and contributed in revising the manuscript writing and organization QS, YJ and SZG Carried out the Stem cells culture, contributed in study design,writing, supervision and analysis of data.
Compliance with ethical standards
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/4/2021
A Correction to this paper has been published: 10.1007/s13770-021-00386-5
References
- 1.Nau T, Lavoie P, Duval N. A new generation of artificial ligaments in reconstruction of the anterior cruciate ligament. Two-year follow-up of a randomised trial. J Bone Joint Surg Br. 2002;84:356–60. doi: 10.1302/0301-620X.84B3.0840356. [DOI] [PubMed] [Google Scholar]
- 2.Hamido F, Misfer AK, Al Harran H, Khadrawe TA, Soliman A, Talaat A, et al. The use of the LARS artificial ligament to augment a short or undersized ACL hamstrings tendon graft. Knee. 2011;18:373–378. doi: 10.1016/j.knee.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Lim WL, Liau LL, Ng MH, Chowdhury SR, Law JX. Current progress in tendon and ligament tissue engineering. Tissue Eng Regen Med. 2019;16:549–571. doi: 10.1007/s13770-019-00196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:20. doi: 10.1186/1758-2555-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bistolfi A, Capella M, Guidotti C, Sabatini L, Artiaco S, Massè A, et al. Functional results of allograft vs. Autograft tendons in anterior cruciate ligament (ACL) reconstruction at 10-year follow-up. Eur J Orthop Surg Traumatol. 2021;31:729–35. doi: 10.1007/s00590-020-02823-y. [DOI] [PubMed] [Google Scholar]
- 6.Carey JL, Dunn WR, Dahm DL, Zeger SL, Spindler KP. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J Bone Joint Surg Am. 2009;91:2242–2250. doi: 10.2106/JBJS.I.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nau T, Teuschl A. Regeneration of the anterior cruciate ligament: current strategies in tissue engineering. World J Orthop. 2015;6:127–136. doi: 10.5312/wjo.v6.i1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leong NL, Petrigliano FA, McAllister DR. Current tissue engineering strategies in anterior cruciate ligament reconstruction. J Biomed Mater Res A. 2014;102:1614–1624. doi: 10.1002/jbm.a.34820. [DOI] [PubMed] [Google Scholar]
- 9.Padilla S, Sánchez M, Orive G, Anitua E. Human-based biological and biomimetic autologous therapies for musculoskeletal tissue regeneration. Trends Biotechnol. 2017;35:192–202. doi: 10.1016/j.tibtech.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Sankar S, Sharma CS, Rath SN, Ramakrishna S. Electrospun nanofibres to mimic natural hierarchical structure of tissues: application in musculoskeletal regeneration. J Tissue Eng Regen Med. 2018;12:e604–e619. doi: 10.1002/term.2335. [DOI] [PubMed] [Google Scholar]
- 11.Fisher MB, Henning EA, Söegaard N, Bostrom M, Esterhai JL, Mauck RL. Engineering meniscus structure and function via multi-layered mesenchymal stem cell-seeded nanofibrous scaffolds. J Biomech. 2015;48:1412–1419. doi: 10.1016/j.jbiomech.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elangomannan S, Louis K, Dharmaraj BM, Kandasamy VS, Soundarapandian K, Gopi D. Carbon nanofiber/polycaprolactone/mineralized hydroxyapatite nanofibrous scaffolds for potential orthopedic applications. ACS Appl Mater Interfaces. 2017;9:6342–6355. doi: 10.1021/acsami.6b13058. [DOI] [PubMed] [Google Scholar]
- 13.Samavedi S, Guelcher SA, Goldstein AS, Whittington AR. Response of bone marrow stromal cells to graded co-electrospun scaffolds and its implications for engineering the ligament-bone interface. Biomaterials. 2012;33:7727–7735. doi: 10.1016/j.biomaterials.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Woo SL, Abramowitch SD, Kilger R, Liang R. Biomechanics of knee ligaments: injury, healing and repair. J Biomech. 2006;39:1–20. doi: 10.1016/j.jbiomech.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Xu H, Li H, Ke Q, Chang J. An anisotropically and heterogeneously aligned patterned electrospun scaffold with tailored mechanical property and improved bioactivity for vascular tissue engineering. ACS Appl Mater Interfaces. 2015;7:8706–8718. doi: 10.1021/acsami.5b00996. [DOI] [PubMed] [Google Scholar]
- 16.Uttayarat P, Perets A, Li M, Pimton P, Stachelek SJ, Alferiev I, et al. Micropatterning of three-dimensional electrospun polyurethane vascular grafts. Acta Biomater. 2010;6:4229–4237. doi: 10.1016/j.actbio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Chen Z, Zhang H, Zhuang Y, Shen H, Chen Y, et al. Aligned scaffolds with biomolecular gradients for regenerative medicine. Polymers (Basel) 2019;11:341. doi: 10.3390/polym11020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Zhang YS, Heinrich MA, De Ferrari F, Jang HL, Bakht SM, et al. Rapid continuous multimaterial extrusion bioprinting. Adv Mater. 2017;29:1604630. doi: 10.1002/adma.201604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ying GL, Jiang N, Maharjan S, Yin YX, Chai RR, Cao X, et al. Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv Mater. 2018;30:e1805460. doi: 10.1002/adma.201805460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miri AK, Nieto D, Iglesias L, Goodarzi Hosseinabadi H, Maharjan S, Ruiz-Esparza GU, et al. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv Mater. 2018;30:e1800242. doi: 10.1002/adma.201800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shybut TB, Pahk B, Hall G, Meislin RJ, Rokito AS, Rosen J, et al. Functional outcomes of anterior cruciate ligament reconstruction with tibialis anterior allograft. Bull Hosp Jt Dis. 2013;71:138–143. [PubMed] [Google Scholar]
- 22.Ahmad SS, Schürholz K, Liechti EF, Hirschmann MT, Kohl S, Klenke FM. Seventy percent long-term survival of the repaired ACL after dynamic intraligamentary stabilization. Knee Surg Sports Traumatol Arthrosc. 2020;28:594–598. doi: 10.1007/s00167-019-05749-z. [DOI] [PubMed] [Google Scholar]
- 23.Malahias MA, Chytas D, Nakamura K, Raoulis V, Yokota M, Nikolaou VS. A narrative review of four different new techniques in primary anterior cruciate ligament repair: "back to the future" or another trend. Sports Med Open. 2018;4:37. doi: 10.1186/s40798-018-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker BM, Nerurkar NL, Burdick JA, Elliott DM, Mauck RL. Fabrication and modeling of dynamic multipolymer nanofibrous scaffolds. J Biomech Eng. 2009;131:101012. doi: 10.1115/1.3192140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu SB, Yang RH, Zuo ZN, Dong QR. Histological characteristics and ultrastructure of polyethylene terephthalate LARS ligament after the reconstruction of anterior cruciate ligament in rabbits. Int J Clin Exp Med. 2014;7:2511–2518. [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikari U, An X, Rijal N, Hopkins T, Khanal S, Chavez T, et al. Embedding magnesium metallic particles in polycaprolactone nanofiber mesh improves applicability for biomedical applications. Acta Biomater. 2019;98:215–234. doi: 10.1016/j.actbio.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 27.Pektok E, Nottelet B, Tille JC, Gurny R, Kalangos A, Moeller M, et al. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 2008;118:2563–2570. doi: 10.1161/CIRCULATIONAHA.108.795732. [DOI] [PubMed] [Google Scholar]
- 28.Hua N, Ti VL, Xu Y. Biodegradable effect of PLGA membrane in alveolar bone regeneration on beagle dog. Cell Biochem Biophys. 2014;70:1051–1055. doi: 10.1007/s12013-014-0022-5. [DOI] [PubMed] [Google Scholar]
- 29.Terriza A, Vilches-Pérez JI, González-Caballero JL, Orden E, Yubero F, Barranco A, et al. Osteoblasts interaction with PLGA membranes functionalized with titanium film nanolayer by PECVD. In vitro assessment of surface influence on cell adhesion during initial cell to material interaction. Materials (Basel) 2014;7:1687–708. doi: 10.3390/ma7031687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang H, Husch JFA, Zhang Y, Jansen JA, Yang F, Beucken JJJP. Coculture with monocytes/macrophages modulates osteogenic differentiation of adipose-derived mesenchymal stromal cells on poly(lactic-co-glycolic) acid/polycaprolactone scaffolds. J Tissue Eng Regen Med. 2019;13:785–798. doi: 10.1002/term.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, He X, Jabbari E. Osteogenic differentiation of marrow stromal cells on random and aligned electrospun poly(L-lactide) nanofibers. Ann Biomed Eng. 2011;39:14–25. doi: 10.1007/s10439-010-0106-3. [DOI] [PubMed] [Google Scholar]
- 32.Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan E, Cannizzaro C, et al. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;107:3299–304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue R, Qian Y, Li L, Yao G, Yang L, Sun Y. Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells. Stem Cell Res Ther. 2017;8:148. doi: 10.1186/s13287-017-0588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao Q, Cosme JG, Xu T, Miszuk JM, Picciani PH, Fong H, et al. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–127. doi: 10.1016/j.biomaterials.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luetkemeyer CM, Rosario RA, Estrada JB, Arruda EM. Fiber splay precludes the direct identification of ligament material properties: implications for ACL graft selection. J Biomech. 2020;113:110104. doi: 10.1016/j.jbiomech.2020.110104. [DOI] [PubMed] [Google Scholar]
- 36.Baker BM, Shah RP, Huang AH, Mauck RL. Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Eng Part A. 2011;17:1445–1455. doi: 10.1089/ten.tea.2010.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.