Abstract
Background
Diaphragmatic pacemakers are used to assist respiration in ventilator-dependent patients. Electromagnetic interference with intrinsic cardiac electrical activity is a theoretical risk but has never been reported in the literature. This case highlights a serious complication of cardiac arrest as a result of diaphragmatic pacing.
Case summary
We report a quadriplegic patient with recent diaphragmatic pacemaker implantation who presented with ventricular tachycardia leading to cardiac arrest. Extensive workup was negative for other aetiologies for ventricular arrhythmias. Reduction of the left-sided diaphragmatic pacemaker voltage resulted in cessation of ventricular ectopy.
Discussion
Diaphragmatic pacing at high voltages can cause unwanted transmission of impulses to the cardiac myocytes as a rare complication. This should be noted as a possible complication of intramuscular diaphragmatic pacing, and efforts should be taken to circumvent this risk in the future.
Keywords: Diaphragmatic pacemaker, Ventricular tachycardia, Cardiac arrest, Case report
Learning points
Diaphragmatic pacemakers may be implanted directly to the diaphragm for respiration assistance in ventilator-dependent tetraplegic patients.
At higher voltages, left-sided diaphragmatic electrodes can result in transmission of impulses to the heart, resulting in ventricular ectopy and/or ventricular tachycardia.
Cardiac arrhythmias in these patients can be avoided with reduction of left-sided electrode voltages.
Diaphragmatic pacemaker-induced ventricular arrhythmias is an important entity to consider in the right clinical setting.
Introduction
Direct diaphragmatic pacing is an invasive method of respiration assistance for ventilator-dependent patients. Electrodes are implanted into the diaphragm laparoscopically. The proximity of left-sided diaphragmatic electrodes to the inferior surface of the heart carries a theoretical risk of depolarizing ventricular myocytes and precipitating ventricular electrical conduction, but this phenomenon has not been reported in the literature. Here, we present a novel case of ventricular tachycardia (VT)/ventricular fibrillation (VF) induced by diaphragmatic pacing.
Timeline
| 16 November 2019 | Initial onset of rapidly progressive quadriplegia with intubation |
| 22 November 2019 | Tracheostomy and percutaneous endoscopic gastrostomy tube placed |
| 9 December 2019 | Discharged to long-term care facility (LTAC) |
| 22 December 2019 | Readmitted for diaphragmatic pacemaker implantation, performed without complications |
| 24 January 2020 | Discharged back to LTAC after up-titration of diaphragmatic pacemaker voltage to maximum settings |
| 5 March 2020 | Cardiac arrest at LTAC with return of spontaneous circulation |
| 11 March 2020 | Implantable cardiac defibrillator implantation and reduction of diaphragmatic pacemaker voltage |
| 14 March 2020 | Discharge from hospital |
Case presentation
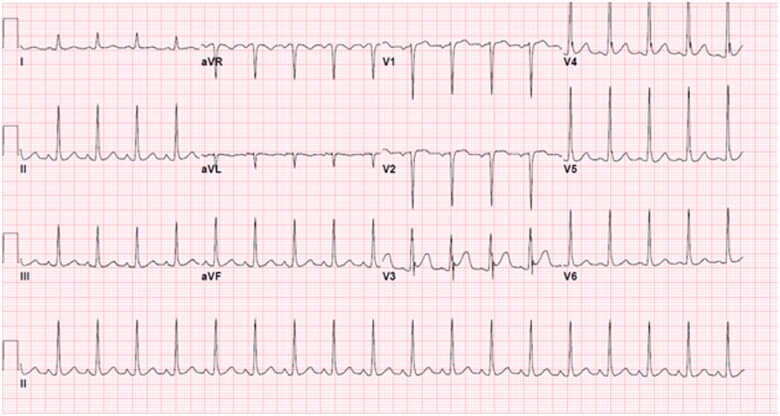
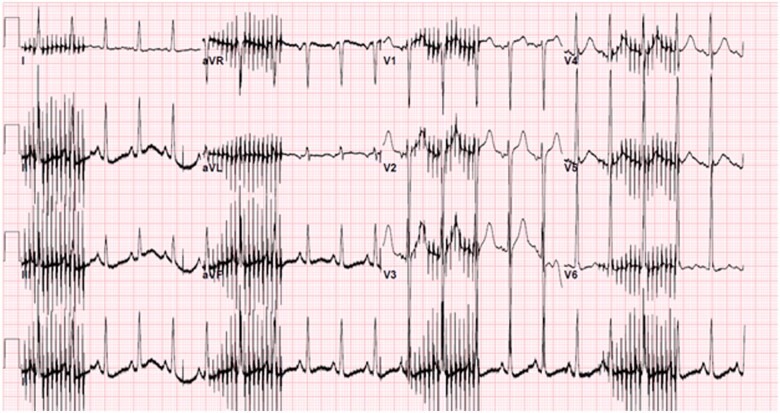
A 22-year-old previously healthy man initially presented to an outside hospital with rapidly progressive quadriplegia, found to have an acute spinal cord infarction secondary to fibrocartilaginous emboli from disc material, complicated by respiratory failure requiring chronic mechanical ventilation. The patient underwent diaphragmatic pacemaker system (DPS) implantation to liberate him from the ventilator with no complications. The pacemaker voltage was up-titrated with a stimulus amplitude of 25 mA and pulse width of 200 µs over several weeks and the patient was discharged to a long-term acute care hospital. Two months later, the patient developed frequent premature ventricular contractions (PVCs) on telemetry while afebrile, normotensive and not hypoxic. Three hours afterwards, he developed polymorphic VT followed by VF and cardiac arrest (Figure 1). Return of spontaneous circulation was achieved after prolonged cardiopulmonary resuscitation by advanced cardiac life support algorithm including numerous shocks. The DPS was subsequently turned off and the patient was transferred to the hospital. Potassium and magnesium were within normal limits, and initial lactate was 1.9 mmol/L. Troponin-I down-trended from 5.98 to 5.51 ng/mL. Diphenhydramine was the only potential QT-prolonging medication the patient took, but there was a normal QT interval on the admission electrocardiogram (EKG) taken post-arrest (Figure 2), which excludes drug-induced QT prolongation. Of note, there was J-point elevation in precordial leads suggestive of early repolarization, which has been associated with idiopathic VF.1 An echocardiogram was obtained, which showed a left ventricular ejection fraction (LVEF) of 30% and right ventricular systolic pressure of 33.7 mmHg. Coronary catheterization showed normal coronaries, and computed tomography (CT) of the head and chest showed no acute pathology. A repeat echocardiogram was obtained prior to discharge showing full recovery of LVEF and no structural abnormalities. The initial echocardiogram was thought to represent stunned myocardium in the setting of resuscitated cardiac arrest as evidenced by normalization of LVEF and overall clinical time course. An initial trial of manipulation of the DPS at low voltage was unable to provoke VT (Figure 3). Given the initially unclear aetiology of VT and the patient’s young age and good prognosis, a single chamber implantable cardiac defibrillator (ICD) was implanted. Subsequently, the DPS voltage was increased, resulting in recurrent nonsustained VT on telemetry which resolved when the DPS was turned off. It was restarted at a lower voltage with a stimulus amplitude of 15 mA and pulse width of 130 µs on the left side resulting in no ectopy recurrence. An ICD interrogation 1 month after discharge showed no evidence of further arrhythmias.
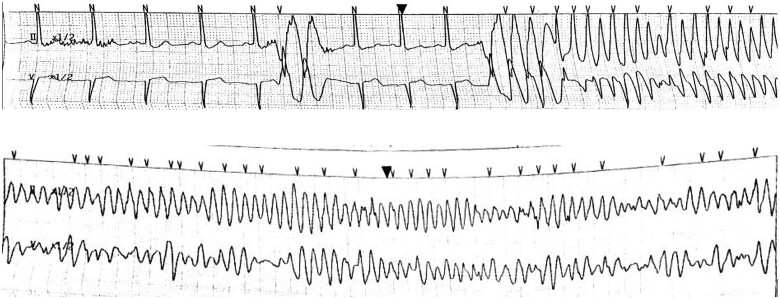
Figure 1.
Onset of ventricular tachycardia (above) which subsequently degenerated to pulseless ventricular fibrillation (below).
Figure 2.
EKG obtained on arrival to University Hospitals Cleveland Medical Center.
Figure 3.
EKG obtained during initial diaphragmatic pacemaker trial.
Discussion
Intramuscular diaphragmatic stimulation initially attained FDA approval for use in ventilator-dependent patients with spinal cord injuries in 2008, based on a prospective study of 50 patients2 with no cases of adverse electrical interference with intrinsic cardiac activity. The DPS subsequently gained FDA approval for amyotrophic lateral sclerosis (ALS) patients in 2011 and central sleep apnoea in 2017.2,3 Experience with the safety and efficacy of DPS have accumulated in the past two decades, with a reported 40–70% of patients weaned from full time ventilator support.4
The initial implantation of DPS electrodes requires careful mapping to locate the area of phrenic nerve insertion into the diaphragm based on intraoperative visualization of diaphragm contraction and change in intraabdominal pressure with stimulation. Two unipolar electrodes are implanted on each side with close monitoring of cardiac rhythm. Electrode voltage is then up-titrated with the goal of reaching a maximum tolerated voltage in order to achieve maximal diaphragm contraction and inspired volume.5,6
Electrical interference due to left-sided pacemaker proximity to the heart have rarely been reported. A review of DPS implantation in 20 patients with concurrent cardiac pacemakers was done in 2010 by Onders et al.7 The investigators noted one case of device-device interference with one diaphragm electrode at the highest amplitude setting. In another case study of a patient who required both diaphragmatic pacing as well as an implantable cardiac pacemaker, impulses from the DPS were falsely detected as native electrical activity in the myocardium due to oversensing, resulting in inappropriate inhibition of the cardiac pacemaker.8
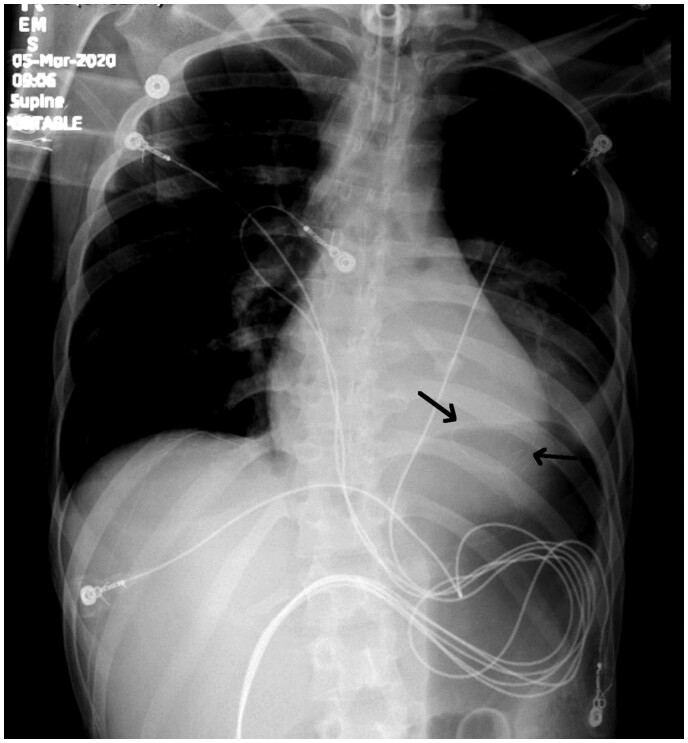
The phenomenon of intramuscular diaphragmatic pacemaker-induced VT has never been described in the literature to our knowledge. In theory, electrical impulses transmitted to the myocardium at a moment of partial myocyte repolarization can result in degeneration into VT. This is analogous to cardiac pacemaker malfunction, such as ventricular undersensing, which can result in poorly timed impulses leading to pacemaker-induced ventricular arrhythmias. PVC’s triggering VF has also been reported in conditions such as cardiac ischaemia, idiopathic VF, and malignant mitral valve prolapse. We performed a search in the Manufacturer and User Facility Device Experience (MAUDE) database between January 2010 and December 2020 of the Neurx diaphragm pacing system and identified six instances of device-induced VT/VF. Of these, two cases were deemed to have insufficient evidence of causation, two were associated with unique off-label uses, and two were real adverse events that report a similar phenomenon. In our case, the left-sided DPS electrodes were the likely culprit of inappropriate ventricular depolarizations due to its proximity to the inferior border of the heart. The close adjacency of the left-sided DPS leads to the heart can be seen in the chest X-ray (Figure 4) obtained on initial presentation as well as in the CT chest slices (Figure 5).
Figure 4.
Chest X-ray obtained post-cardiac arrest demonstrating diaphragmatic pacemaker leads embedded in bilateral diaphragms (black arrows).
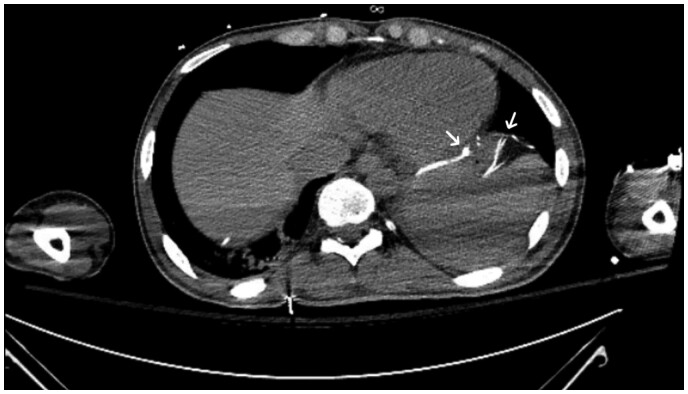
Figure 5.
Computed tomography chest obtained post-cardiac arrest showing diaphragmatic pacemaker leads adjacent to the inferior/posterior border of the heart (white arrows).
The effect was voltage dependent, as reducing the pacemaker voltage on the left side resulted in complete cessation of ventricular ectopy. On close examination of the initial rhythm strip at the onset of VT, the wide complex couplet immediately preceding the pulseless ventricular rhythm correlated with the pacing spikes of the DPS, as the R-R interval was equal to the frequency of the DPS stimulations. Additionally, the PVC’s are of right bundle morphology and superior axis suggestive of origin in inferior LV wall which is adjacent to DPS electrodes. This case report highlights diaphragmatic pacemaker-induced VT as an important diagnosis to consider in patients with recent DPS implantation without other explainable causes of ventricular arrhythmias. In this context, frequent PVC’s may be a precursor to impending VT/VF. Knowledge of this possible complication is critical to identify and potentially eliminate triggers for VT by reducing the pacemaker voltage.
Conclusion
We present a case of a 22-year-old man with diaphragmatic pacemaker-induced VT, leading to cardiac arrest. This rare complication of diaphragmatic pacing has not been published in the literature but is an important entity to consider as a potentially reversible aetiology of VT. Future work should investigate the optimal titration of diaphragmatic pacemaker voltage and ideal maximum electrode voltage, especially on the left-sided diaphragm. Close attention to left-sided electrode placement with regards to proximity to the heart could also be considered to reduce the risk of this life-threatening complication.
Lead author biography

Tony Dong is a second year Internal Medicine resident at University Hospitals Cleveland Medical Center (UHCMC). He completed his undergraduate studies at the University of Pennsylvania and obtained his medical degree at Zucker School of Medicine at Hofstra/Northwell. After completing his residency training, he intends to apply to a Cardiovascular Fellowship.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1.Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L. et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358:2016–2023. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration (FDA). Humanitarian Device Exemption Database. NeuRx DPS RA/4 Respiratory Stimulation System (Synapse Biomedical, Inc., Oberlin, OH). Summary of Safety and Probable Benefit. No. H070003. Rockville, MD: FDA. June 17, 2008. No. H100006. Rockville, MD: FDA. September 28, 2011.
- 3.U.S. Food and Drug Administration (FDA). Center for Devices and Radiological Health (CDRH). Premarket Notification Database. RemedÄ System (Respicardia, Inc., Minnetonka, MN). Summary of Safety and Effectiveness. No. P160039. Rockville, MD: FDA. October 6, 2017.
- 4.Hedges JR.Diaphragmatic stimulation by a pacemaker. JAMA 1978;239:108.doi:10.1001/jama.1978.03280290028009 [PubMed] [Google Scholar]
- 5.DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT.. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 2005;127:671–678. doi:10.1378/chest.127.2.671 [DOI] [PubMed] [Google Scholar]
- 6.Onders RP, Elmo M, Khansarinia S, Bowman B, Yee J, Road J. et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc 2009;23:1433–1440. [DOI] [PubMed] [Google Scholar]
- 7.Onders RP, Khansarinia S, Weiser T, Chin C, Hungness E, Soper N. et al. Multicenter analysis of diaphragm pacing in tetraplegics with cardiac pacemakers: positive implications for ventilator weaning in intensive care units. Surgery 2010;148:893–898. [DOI] [PubMed] [Google Scholar]
- 8.Kolb C, Eicken A, Zrenner B, Schmitt C.. Cardiac pacing in a patient with diaphragm pacing for congenital central hypoventilation syndrome (Ondine’s Curse). J Cardiovasc Electrophysiol 2006;17:789–791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.