Abstract
Background
Patients with myeloproliferative neoplasms (MPNs), such as polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF), are at an increased risk of recurrent thromboembolic events (TEs) and hemorrhagic complications. Anticoagulation with vitamin K antagonists (VKAs) had been the standard of care until the recent US Food and Drug Administration approval of direct oral anticoagulants (DOACs) for treatment of cancer‐associated thrombosis. However, since patients with MPNs were underrepresented in large studies, the use of DOACs in patients with MPN‐associated thrombosis remains understudied.
Objectives
The primary objective of this study was to establish the incidence of recurrent TEs and hemorrhagic complications in patients with MPN‐associated thrombosis treated with DOACs versus VKAs as first‐line therapy.
Methods
Data from 30 patients ≥18 years old with established diagnoses of PV or ET who were treated with either DOACs or VKAs as the first‐line anticoagulant for arterial and/or venous thrombosis were reviewed to determine the incidence of recurrent TEs as well as hemorrhagic complications.
Results
Nineteen patients were treated with DOACs, and 11 were treated with VKAs. Of those on DOACs, 1 had a recurrent thrombosis, and 4 had bleeding events. Of the 11 patients treated with VKAs, 1 had a recurrent thrombotic event, and 1 had a bleeding event.
Conclusion
Our data did not demonstrate a significant difference in recurrent TEs or bleeding events in patients with MPN‐associated thrombosis anticoagulated with either DOACs or VKAs.
Keywords: anticoagulants, myeloproliferative disorders, neoplasms, thrombosis, treatment outcome
Essentials.
The ideal choice of anticoagulant for thrombosis in myeloproliferative neoplasms is unclear.
We assessed the use of direct oral anticoagulants (DOACs) and warfarin by chart review.
Frequency of recurrent thrombosis was comparable between DOAC and warfarin users.
Frequency of bleeding complications was comparable between DOAC and warfarin users.
1. INTRODUCTION
Myeloproliferative neoplasms (MPNs) are clonal hematologic malignancies, which include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).1, 2 MPNs are rare, with a crude incidence rate of 2.58 cases per 100 000 patients per year across the globe.3 When compared with the general population, patients with MPNs have a significantly higher risk of both thrombotic and hemorrhagic complications. The average annual incidence of thrombotic events (TEs) for patients with MPNs ranges between 0.74 and 10.9 per 100 patient‐years.4 Nearly 10% of patients have their first thrombotic event within 30 days of diagnosis, and thrombotic events appear to be more common in PV than in ET and PMF. Overall, venous thrombotic events (VTEs) are significantly more common than arterial thrombotic events (ATEs), with hazard ratios of 9.7 and 2.5, respectively.5 The risk of recurrent events is such a major factor in overall morbidity and mortality that the major risk stratification models for ET and PV, including the International Prognostic Score for ET (IPSET), the IPSET‐Thrombosis, and the Conventional Score for Prediction of Vascular Complications, heavily weight prior history of thrombosis.1
Historically, the treatment for cancer‐associated thrombosis has been with vitamin K antagonists (VKAs) or low‐molecular‐weight heparins (LMWHs). Both patients and health care providers may find these therapies burdensome, as they require blood tests, dose adjustments, dietary restrictions, and self‐injections. DOACs, on the other hand, may provide higher treatment satisfaction.6, 7 Widespread use of DOACs for the treatment of patients with cancer‐associated thrombosis increased after several investigations demonstrated their efficacy and safety in this population.8, 9, 10 Although patients with MPNs were included in these studies, they were not analyzed separately, owing to the relatively small number of cases. The few reports that evaluate treatment outcomes in patients with MPNs have not typically excluded those treated for atrial fibrillation (AF) or those who had been switched to a DOAC from a VKA long after the thrombotic event.11, 12, 13, 14, 15
Given the paucity of prior definitive data, we investigated the incidence of recurrent thrombotic events and hemorrhagic complications in patients with MPN‐associated thrombosis treated with DOACs and VKAs as initial therapy.
2. MATERIALS AND METHODS
We conducted a retrospective cohort study to evaluate treatment outcomes of DOACs and VKAs used as a first‐line treatment for patients with MPN‐associated thrombosis. To identify patients, we used both Looking Glass, an interactive software that integrates demographic and clinical data sets gathered from the Epic electronic medical record system (Epic Systems Corporation, Madison, WI, USA) and lists from patient panels from the hematology practices at Montefiore Medical Center. Inclusion criteria were age ≥18 years, International Classification of Diseases codes for both MPN (PV, ET, or PMF) and arterial or venous thrombosis, and evidence of anticoagulation therapy initiated for the TE during the study period May 2014 to February 2020. We reviewed the charts of all identified patients to confirm that they met the screening criteria. Presence of MPN was considered confirmed if a diagnosis of PV, ET, or PMF was documented by a physician with supporting mutations such as JAK2 V617F, CALR, and MPL or, as in the case of two patients with essential thrombocythemia, who fully met World Health Organization criteria.16 The majority of patients were excluded due to erroneous MPN diagnosis coding (documentation of thrombocytosis or erythrocytosis without confirmation of MPN diagnosis), lack of thrombotic events, and/or lack of treatment with therapeutic anticoagulation. We also excluded patients if there was insufficient documentation of thrombosis and/or anticoagulation therapy (n = 65), if they were treated with a DOAC or VKA for ≤7 days (n = 4), or if they had or developed AF at any point during anticoagulation therapy (n = 5).
Patients’ medical records were reviewed for demographic data, MPN subtype and mutation status; date of MPN diagnosis, date and site of index thrombosis, date and type of anticoagulation initiation, last documented date of therapy, date and site of recurrent thrombosis, changes in therapy, hematologic and coagulation parameters at time of thrombosis, date and type of major bleeding (MB) and clinically relevant nonmajor bleeding (CRNMB) as defined by the ISTH,17 and hematologic and coagulation parameters at the time of the bleeding event.
Since rethrombosis risk decreases with time, only the first anticoagulant class prescribed was included; patients who were switched between VKAs and DOACs were censored at the point of first switching, but patients switching between different DOAC medications were retained. Initial heparin bridging to warfarin was not considered switching in this analysis, and patients were assigned to the VKA therapy. Index date was defined as the date of the thrombotic event prompting initiation of full‐dose anticoagulation.
Primary outcome was the incidence of recurrent thrombotic events during initial anticoagulation therapy. Secondary outcome was the incidence of hemorrhagic events, both CRNMB and MB, during anticoagulation therapy. For time‐to‐event calculation, we used the date of anticoagulation initiation to the date of the first recurrent thrombosis, hemorrhagic event, or last follow‐up, whichever occurred sooner. All data were calculated using medians and interquartile ranges. P values were calculated using Fisher’s exact test for categorical variables and two‐sample t test for continuous variables. Significance was denoted by α = 0.05.
The study was approved by the Montefiore Medical Center and Albert Einstein College of Medicine Institutional Review Board.
3. RESULTS AND DISCUSSION
3.1. Study population
The initial cohort screened was 545 patients, but the final cohort included 30 patients who fully satisfied the criteria. Patient selection details are shown in Figure 1. Twenty‐two (73.3%) of the patients were women. Median age of the total cohort at the index thrombotic event was 70.2 (47.5‐79.7) years old. Twenty‐three (76.7%) patients had ET, 6 (20.0%) had PV, and 1 (3.3%) had unclassified MPNs. There were no patients with PMF in our cohort. Mutation details are displayed in Table 1. Eighteen patients (60.0%) had an MPN diagnosis established within a year before or after the index thrombosis; 10 patients (33.3%) had an MPN diagnosis established more than a year before the index thrombosis; 2 (6.7%) were diagnosed with MPN more than a year after the index thrombosis. Median hemoglobin concentration at the time of the index thrombosis was higher in the DOAC group at 12.8 (11.8‐14.4) g/dL versus 9.6 (9.3‐12.2) g/dL in the VKA group (P = .03). The clinical significance of this finding is uncertain. The median platelet count overall was 554 (427‐775) × 109/L and did not differ between patients treated with DOACs or VKAs (P = .41).
FIGURE 1.
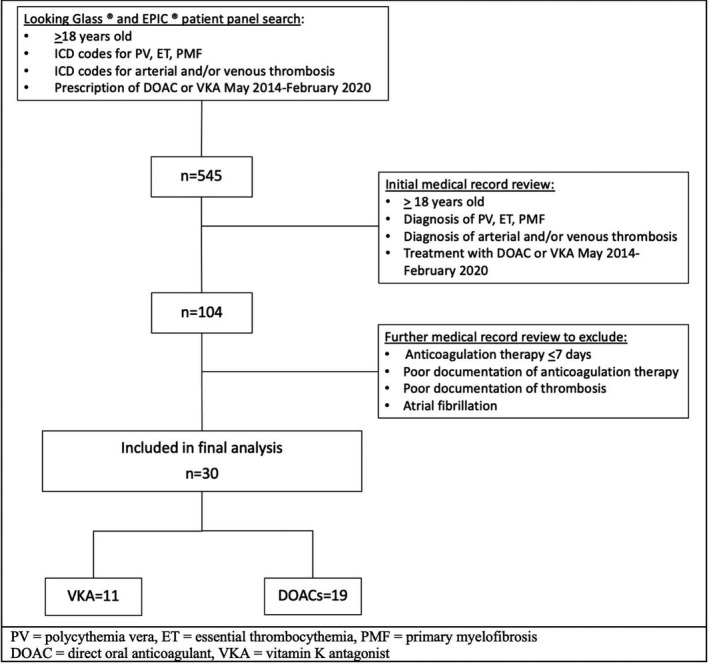
Patient selection diagram
TABLE 1.
Patient characteristics at index thrombosis
| Total (n = 30) | DOAC (n = 19) | VKA (n = 11) | P value | |
|---|---|---|---|---|
| Cohort size, n (%) | 30 (100.0) | 19 (63.3) | 11 (36.7) | |
| Women, n (%) | 22 (73.3) | 15 (78.9) | 7 (63.6) | 0.42 |
| Age at index thrombosis, median (IQR) | 70.2 (47.5‐79.7) | 69.3 (45.8‐77.1) | 74.3 (62.5‐80.0) | 0.50 |
| Days on anticoagulation, median (IQR) | 258 (132‐634) | 251 (137‐694) | 265 (148‐480) | 0.94 |
| MPN subtype and mutation status | ||||
| ET, n (%) | 23 (76.7) | 14 (73.7) | 9 (81.8) | |
| JAK2 V617F+, n | 14 | 8 | 6 | |
| CALR+, n | 6 | 3 | 3 | |
| MPL+, n | 1 | 1 | 0 | |
| No mutations detected, n | 2 | 2 | 0 | 0.25* |
| PV, n (%) | 6 (20.0) | 5 (26.3) | 1(9.1) | |
| JAK2 V617F+, n | 6 | 5 | 1 | |
| PMF, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| MPN, unclassified, n (%) | 1 (3.3) | 0 (0) | 1 (9.1) | |
| JAK2 V617F+, n | 1 | 0 | 1 | |
| MPN diagnosis in relation to index thrombosis | ||||
| MPN diagnosis >1 year before IT, n (%) | 10 (33.3) | 9 (47.4) | 1 (9.1) | |
| MPN diagnosis within 1 year of IT, n (%) | 18 (60.0) | 9 (47.4) | 9 (81.8) | 0.66 |
| MPN diagnosis >1 year after IT, n (%) | 2 (6.7) | 1 (5.2) | 1 (9.1) | |
| Distribution of index thrombosis | ||||
| Arterial, n (%) | 8 (26.7) | 7 (36.8) | 1 (9.1) | 0.10 |
| Venous, n (%) | 20 (66.7) | 10 (52.6) | 10 (91.0) | |
| Mixed, n (%) | 2 (6.7) | 2 (10.5) | 0 (0) | |
| Hematologic parameters at index thrombosis | ||||
| Hgb g/dL, median (IQR) | 12.3 (9.4‐13.8) | 12.8 (11.8‐14.4) | 9.6 (9.3‐12.2) | 0.03 |
| WBC × 109/L, median (IQR) | 10.6 (6.5‐15.0) | 10.3 (8.2‐14.9) | 12.1 (5.7‐14.9) | 0.67 |
| PLT × 109/L, median (IQR) | 554 (427‐775) | 604 (450‐890) | 444 (421‐665) | 0.41 |
| Therapy at time of index thrombosis | ||||
| No therapy, n (%) | 11 (36.7) | 5 (26.3) | 6 (54.5) | |
| ASA, n (%) | 6 (20.0) | 4 (21.1) | 2 (18.2) | |
| HU, n (%) | 3 (10.0) | 3 (15.8) | 0 (0) | |
| ASA+HU, n (%) | 8 (26.7) | 5 (26.3) | 3 (27.3) | |
| ASA+HU+clopidogrel, n (%) | 2 (6.7) | 1 (5.3) | 0 (0) | |
| Therapy after index thrombosis | ||||
| ASA, n (%) | 3 (10.0) | 2 (10.5) | 1 (9.1) | |
| HU, n (%) | 5 (16.7) | 4 (21.1) | 1 (9.1) | |
| ASA+HU, n (%) | 12 (40.0) | 5 (26.3) | 7 (63.6) | |
| HU+clopidogrel, n (%) | 1 (3.3) | 1 (5.2) | 0 (0) | |
| ASA+HU+clopidogrel, n (%) | 4 (13.3) | 4 (21.1) | 0 (0) | |
| No therapy, n (%) | 5 (16.7) | 3 (15.8) | 2 (18.2) | |
Abbreviations: ASA, aspirin; DOAC, direct oral anticoagulant; ET, essential thrombocythemia; Hgb, hemoglobin; HU, hydroxyurea; IT, index thrombosis; MPN, myeloproliferative neoplasm; PLT, platelet; PMF, primary myelofibrosis; PV, polycythemia vera; VKA, vitamin K antagonist; WBC, white blood cell.
*P value refers to MPN subtypes but excluding PMF; does not include for mutations.
The index thrombosis was venous in 20 (66.7%) patients, arterial in 8 (26.7%) patients, and both in 2 (6.7%) patients. There was no significant difference between DOAC and VKA groups with respect to number of arterial versus venous thrombotic events (P = .10). At the time of the index thrombosis, 16 (53.3%) patients were taking aspirin.
Following the thrombotic event, 19 (63.3%) patients were prescribed a DOAC, and 11 (36.7%) patients were prescribed a VKA as their first anticoagulant. The median number of days on first anticoagulation therapy was 258 (132‐634) days for the entire cohort; 251 (137‐694) days for patients on DOACs and 265 (148‐480) days for patients on VKA. At the time of DOAC initiation, aspirin was stopped for 3 of 11 patients who were taking it at the time of the index thrombosis and 2 of 8 patients not previously on aspirin were prescribed it at the time of DOAC initiation. At the time of VKA initiation, aspirin was not stopped for the 5 patients who were taking it; it was started for 3 of 6 of those who had not been on it. Table 1 contains further details on therapy at the time of the index thrombosis and after DOAC and VKA initiation.
The size of the DOAC group in our study is comparable to that of the other single‐institution studies, where it ranges from 18 to 26 patients.13, 15, 18, 19 The predominant MPN subtype in these studies varied, while our cohort predominantly included patients with ET. Mutation distribution was comparable between the studies, JAK2 V617F being the most common. Duration of the follow‐up is difficult to compare, as most studies did not specify whether patients were censored at the time of recurrent thrombotic events or whether the anticoagulation at the time of the outcome event was the initial anticoagulation, as they were in our study. The major difference between these studies and ours is that some include patients with AF, even those without prior thromboembolic disease, and some do not comment on AF history.
3.2. Recurrent thromboembolic events
Both the DOAC and the VKA groups had one patient who suffered a recurrent thrombotic event while receiving anticoagulation therapy; both had ET (Table 2). There were no recurrent thrombotic events among patients with PV. The patient with a thrombotic event on a DOAC had a JAK2 V617F mutation. For this patient, the index thrombotic event was cerebral venous sinus thrombosis, initially treated with intravenous heparin, but switched to apixaban at day 10 of treatment due to development of heparin‐induced thrombocytopenia. After 53 days of therapy with apixaban, the patient’s surveillance imaging revealed a new clot in the same vascular territory while the prior thrombus was less prominent. At the time of recurrent thrombosis, both hemoglobin and platelet counts were at goal. The patient who developed a thrombosis on VKA had a CALR mutation. Both initial and recurrent thrombotic events were cerebrovascular accidents (CVAs) in different arterial territories, with rethrombosis occurring after 604 days of therapy. At the time of the recurrent event, this patient had hemoglobin of 16.7 g/dL, platelet count of 1057 × 109/L, and international normalized ratio (INR) of 3. Both patients were receiving aspirin and hydroxyurea at the time of recurrent event. Due to the limited sample size and low rate of recurrent thrombotic events, it is difficult to draw statistically significant conclusions and to generalize our findings to all patients with MPN.
TABLE 2.
Patient characteristics at recurrent thrombosis and hemorrhagic events
| Total (n = 30) | DOAC (n = 19) | VKA (n = 11) | P value | |
|---|---|---|---|---|
| Total events (thrombotic and hemorrhagic), n (%) | 7 (23.3) | 5 (26.3) | 2 (18.2) | 1.0 |
| Time to any event, d, median (IQR) | 53 (49‐147) | 53 (46‐‐114) | 328 (190‐466) | 0.54 |
| Recurrent thrombotic event, n (%) | 2 (6.7) | 1 (5.3) | 1 (9.1) | 1.0 |
| JAK2 V617F+, n | 1 | 1 | 0 | |
| CALR+, n | 1 | 0 | 1 | |
| Time to recurrence, d | 328 | 53 | 604 | |
| Hematologic parameters at recurrent thrombotic event | ||||
| Hgb g/dL, median | 14.9 | 13.1 | 16.7 | |
| WBC × 109/L, median | 11.9 | 4.9 | 18.8 | |
| PLT × 109/L, median | 689 | 320 | 1057 | |
| Therapy at time of recurrent thrombotic event | ||||
| ASA+HU, n | 2 | 1 | 1 | |
| Bleeding events | ||||
| Clinically relevant nonmajor bleed, n (%) | 5 (16.7) | 4 (21.1) | 1 (9.1) | 0.63 |
| Major bleed, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| Time to bleeding event, d, median (IQR) | 52 (46‐114) | 80 (42‐130) | 52 | |
| MPN subtype and mutation | ||||
| ET, n | 4 | 3 | 1 | |
| JAK2 V617F+, n | 3 | 2 | 1 | |
| CALR+, n | 1 | 1 | 0 | |
| PV, n | 1 | 1 | 0 | |
| JAK2 V617F+, n | 1 | 1 | 0 | |
| Hematologic parameters at bleeding event | ||||
| Hgb g/dL, median (IQR) | 11.0 (10.9‐12.2) | 10.9 (10.0‐12.5) | 11.0 | |
| WBC × 109/L, median (IQR) | 4.1 (3.1‐5.5) | 3.6 (3.0‐5.1) | 5.5 | |
| PLT × 109/L, median (IQR) | 414 (195‐463) | 289 (167‐426) | 576 | |
| Therapy at time of bleeding event | ||||
| HU, n | 2 | 2 | 0 | |
| ASA +HU, n | 3 | 2 | 1 | |
Abbreviations: ASA, aspirin; DOAC, direct oral anticoagulant; ET, essential thrombocythemia; Hgb, hemoglobin; HU, hydroxyurea; PLT, platelet; PV, polycythemia vera; VKA, vitamin K antagonist; WBC, white blood cell.
Prior literature reports variable frequency of recurrent thrombotic events for patients with MPNs treated with DOACs. Three of four studies of comparable size report no recurrent events.13, 18, 19 Ianotto et al15 report one thrombotic event, CVA, but the patient’s history of AF has not been specified even though such patients were included in the study. A multicenter study evaluating the efficacy of DOACs for primary prevention of thrombosis in AF and for secondary prophylaxis in venous thromboembolism demonstrated that the rate of thrombotic events in patients with AF is lower than the rate of those treated for secondary thrombosis prophylaxis, 1.5% versus 4.5% patients per year.14 This highlights AF as a significant confounding factor at evaluating incidence of thrombotic events for patients with MPNs.
The incidence of recurrent thrombotic events for patients with MPNs treated with VKAs varies across the literature, from 8.2% in the large multicenter study by De Stefano et al12, 19 to 61.5% in the smaller‐scale study (comparable to ours) by Huenerbein et al.12, 19 Given such wide variation, it is difficult to draw definitive conclusions.
3.3. Bleeding events
There were no major bleeding events in the DOAC or the VKA group (Table 2). Four (21.1%) patients on a DOAC had CRNMB events on days 30, 46, 114, and 180 (median, 80 days) with median duration of treatment for the whole cohort of 251 (137‐694) days. One (9.1%) patient in the VKA group had a CRNMB event on day 52 with median duration of treatment for the whole cohort of 265 (148‐480) days. None of the events required red blood cell transfusions or hospitalizations.
Of the four patients who developed hemorrhagic complications on a DOAC, three had ET and one had PV; two had JAK2 V617F and two had CALR mutations. The patient who developed bleeding on a VKA had ET with a JAK2 V617F mutation.
At the time of the bleeding event, median hemoglobin and white blood cell concentrations were comparable between the DOAC and the VKA groups, 10.9 (10.0‐12.5) g/dL versus 11.0 g/dL and 3.6 (3.0‐5.1) × 109/L versus 5.5 × 109/L. The patient with a bleeding event on a VKA had a poorly controlled platelet count of 576 × 109/L, while the median platelet count for patients with bleeding events in the DOAC group was 289 (167‐426) × 109/L. For the patient on warfarin, INR at the time of bleeding was 2.1.
Of those who bled, two patients in the DOAC group as well as the one patient in the VKA group were also treated with aspirin and hydroxyurea in addition to full‐dose anticoagulation. The two remaining patients in the DOAC group were treated only with hydroxyurea in addition to full‐dose anticoagulation.
When compared to other studies of similar scale, the incidence of bleeding in our study’s DOAC group falls within the reported range, 21.1% versus 8.3% to 36.4%, respectively.13, 15, 19 The incidence of bleeding events in patients on VKAs in our study (9.1%) was slightly higher than that of patients in the multicenter study reported by De Stefano et al12, 19 (6.5%) but was significantly lower than that reported by Huenerbein et al (63.3%).12, 19 Huenerbein also reported that time to bleed was longer in the VKA group as compared to the DOAC group, 1.6 versus 0.5 years respectively, but their patients on VKAs were followed for much longer.19 In our study, more patients in the DOAC group experienced bleeding, but the one hemorrhagic event in the VKA group occurred earlier after therapy initiation.
The leading limitation of our study is the sample size, which partially reflects infrequency of MPN diagnosis, with even the rarer event of MPN‐associated thrombosis; only 104 patients with confirmed MPN‐associated thrombosis were identified in our medical center. Thereafter, the size of the initial cohort was dramatically reduced by careful consideration of exclusion criteria sought to minimize the number of confounding factors. The majority of patients were excluded due to suboptimal documentation, as significant gaps in records can lead to missed thrombotic or bleeding events, ultimately compromising the outcomes data. Patients with AF were excluded, albeit only five, largely due to difficulties in attributing thrombotic events solely to prothrombotic physiology of MPN. Our sample size is therefore small but with fewer confounding issues. Despite these limitations, our study is unique, as it aims to provide unbiased data on the incidence of recurrent thrombotic events for MPN‐associated thrombosis treated with DOACs and VKAs.
4. CONCLUSION
Quality data on efficacy and safety of DOACs as compared to traditionally used VKAs is needed for patients with MPN‐associated thrombosis. Our study did not demonstrate a significant difference in incidence of recurrent thromboembolism or hemorrhagic complications in patients treated with DOACs and VKAs for MPN‐associated thrombosis. A large‐scale prospective comparative study is required to further delineate and guide treatment practices in the MPN population.
RELATIONSHIP DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: KF, HHB, SG, and MK. Data collection: KF, and SG. Analysis, interpretation, and statistical analysis of data: KF, HHB, SG, and MK. Drafting manuscript: KF. Critical revision of the manuscript: HHB, SG, and MK. Study supervision: HHB.
REFERENCES
- 1.Rumi E, Cazzola M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms. Blood. 2017;129(6):680‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176‐2184. [DOI] [PubMed] [Google Scholar]
- 3.Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms? A systematic review and meta‐analysis. Am J Hematol. 2014;89(6):581‐587. [DOI] [PubMed] [Google Scholar]
- 4.Casini A, Fontana P, Lecompte TP. Thrombotic complications of myeloproliferative neoplasms: risk assessment and risk‐guided management. J Thromb Haemost. 2013;11(7):1215‐1227. [DOI] [PubMed] [Google Scholar]
- 5.Hultcrantz M, Bjorkholm M, Dickman PW, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population‐based cohort study. Ann Intern Med. 2018;168(5):317‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng DL, Gan GG, Chai CS, et al. Comparing quality of life and treatment satisfaction between patients on warfarin and direct oral anticoagulants: a cross‐sectional study. Patient Prefer Adherence. 2019;13:1363‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benzimra M, Bonnamour B, Duracinsky M, et al. Real‐life experience of quality of life, treatment satisfaction, and adherence in patients receiving oral anticoagulants for atrial fibrillation. Patient Prefer Adherence. 2018;12:79‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer‐associated venous thromboembolism. N Engl J Med. 2018;378(7):615‐624. [DOI] [PubMed] [Google Scholar]
- 9.Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT‐D). J Clin Oncol. 2018;36(20):2017‐2023. [DOI] [PubMed] [Google Scholar]
- 10.Agnelli G, Becattini C, Bauersachs R, et al. Apixaban versus dalteparin for the treatment of acute venous yhromboembolism in patients with cancer: the Caravaggio study. Thromb Haemost. 2018;118(9):1668‐1678. [DOI] [PubMed] [Google Scholar]
- 11.Kaifie A, Kirschner M, Wolf D, et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): analysis from the German SAL‐MPN‐registry. J Hematol Oncol. 2016;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Stefano V, Ruggeri M, Cervantes F, et al. High rate of recurrent venous thromboembolism in patients with myeloproliferative neoplasms and effect of prophylaxis with vitamin K antagonists. Leukemia. 2016;30(10):2032‐2038. [DOI] [PubMed] [Google Scholar]
- 13.Curto‐Garcia N, Doyle AJ, Breen KA, et al. Outcomes of patients receiving direct oral anticoagulants for myeloproliferative neoplasm‐associated venous thromboembolism in a large tertiary centre in the UK. Br J Haematol. 2020;189(3):e79‐e81. [DOI] [PubMed] [Google Scholar]
- 14.Barbui T, Stefano VD, Carobbio A, et al. Direct oral anticoagulants for myeloproliferative neoplasms (MPN‐DOACs): results from an international study on 442 patients. Blood. 2020;136(Suppl 1):42‐43. 10.1182/blood-2020-139229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ianotto JC, Couturier MA, Galinat H, et al. Administration of direct oral anticoagulants in patients with myeloproliferative neoplasms. Int J Hematol. 2017;106(4):517‐521. [DOI] [PubMed] [Google Scholar]
- 16.Barbui T, Thiele J, Gisslinger H, Finazzi G, Vannucchi AM, Tefferi A. The 2016 revision of WHO classification of myeloproliferative neoplasms: clinical and molecular advances. Blood Rev. 2016;30(6):453‐459. [DOI] [PubMed] [Google Scholar]
- 17.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Subcommittee on Control of Anticoagulation. Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119‐2126. [DOI] [PubMed] [Google Scholar]
- 18.Doyle A, Breen F, McLornan DP, et al. Outcomes of patients receiving direct oral anticoagulants for myeloproliferative neoplasm associated venous thromboembolism. Blood. 2019;134:4183. [DOI] [PubMed] [Google Scholar]
- 19.Huenerbein K, Sadjadian P, Becker T, et al. Direct oral anticoagulants (DOAC) for prevention of recurrent arterial or venous thromboembolic events (ATE/VTE) in myeloproliferative neoplasms. Ann Hematol. 2021;100(8):2015‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fedorov K, Goel S, Kushnir M, Billett HH. Thrombosis in myeloproliferative neoplasms: Treatment outcomes of direct oral anticoagulants and vitamin K antagonists. Res Pract Thromb Haemost. 2021;5:e12574. 10.1002/rth2.12574
Handling Editor: Dr Suzanne Cannegieter
Funding information
This study was unfunded
Contributor Information
Kateryna Fedorov, Email: kfedorov@montefiore.org.
Henny H. Billett, @hhbillett.


