Introduction
Ticks are important pathogen vectors that are a growing threat owing to expanding habitats and longer active periods (Dennis et al., 1998; Ostfeld and Brunner, 2015; Beard et al., 2016; Eisen et al., 2016a; Eisen et al., 2016b). The incidence of reported tick-borne diseases in the United States has risen significantly over the last decade with cases more than doubling (Rosenberg, 2018). Various issues hinder the ability to combat tick-borne disease including the lack of available vaccines, misdiagnosis and the emergence of antibiotic/antiparasitic resistance (Masters et al., 1998; Aguero-Rosenfeld et al., 2005; Wormser et al., 2006; Stanek et al., 2012; Molins et al., 2017). Much of what is known about tick-borne pathogens is centered around interactions with mammalian hosts, as this is where pathological outcomes are often observed. Comparatively less is understood about pathogen interactions with the arthropod vector.
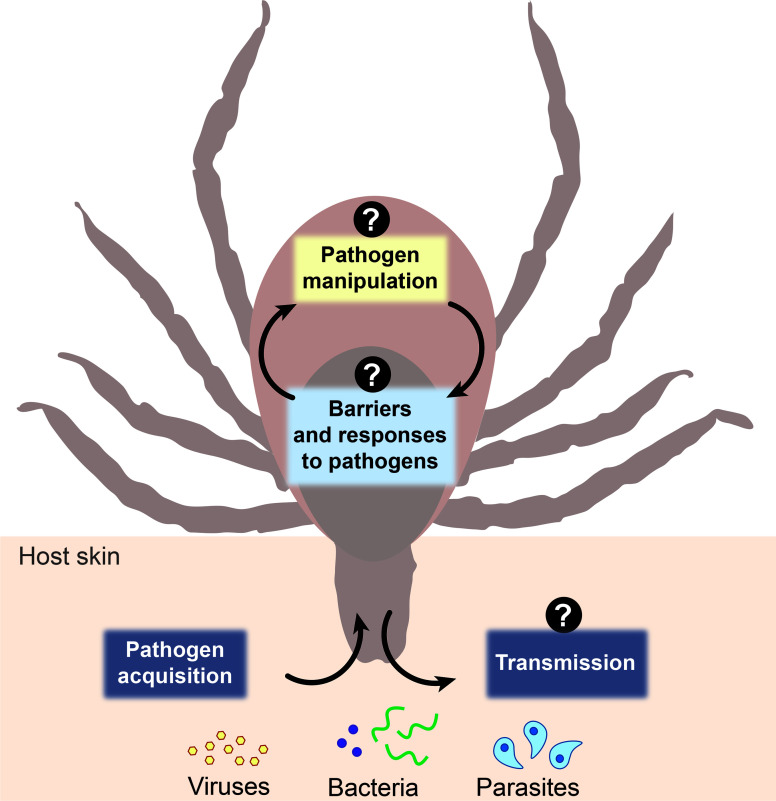
The environmental milieu between vertebrate hosts and arthropods vectors differs significantly with disparities in body temperature, physiological architecture, immunological potential, and nutrient availability. Ticks play a crucial role in not only harboring pathogens, but also priming them for transmission (De Silva and Fikrig, 1995; Ramamoorthi et al., 2005; Fikrig and Narasimhan, 2006; Dunham-Ems et al., 2009; Shaw et al., 2016; Chávez et al., 2019; Oliva Chávez et al., 2021). Herein, we will discuss three aspects of tick-microbe interactions: barriers to colonization, microbial manipulation, and saliva-transmission dynamics. We contend that, rather than a passive vessel, the arthropod is a dynamic environment that shapes pathogen adaptation, selection, and transmission (Figure 1). To advance the field of tick-borne disease, cross-disciplinary approaches are needed to uncover fundamental basics centered on the vector-side of this equation, which may pave the way for novel disease prevention strategies.
Figure 1.
Schematic representation of three aspects of tick-microbe interactions. The vector-side of tick-borne disease is open for discovery. Question marks represent topics in the field that remain incompletely understood including arthropod barriers to colonization and responses to pathogens, how microbes manipulate the arthropod to colonize and survive within the tick, and what the role and composition of salivary secretions are during pathogen transmission dynamics.
Colonization: Overcoming Barriers
There are several barriers that must be overcome for a microbe to successfully colonize the tick. The peritrophic membrane and midgut epithelium are physical barriers that pathogens first encounter when colonizing a vector with an incoming bloodmeal. Different microbes occupy specific niches, but all must eventually cross the midgut and migrate to the salivary glands for transmission to a host. For example, Borrelia burgdorferi will cross the peritrophic membrane and colonize the midgut epithelium where it will remain during the molt, until the tick feeds again (Narasimhan et al., 2014; Narasimhan et al., 2017). In contrast, Anaplasma phagocytophilum rapidly escapes the midgut and colonizes the salivary glands (Hodzic et al., 1998; Liu et al., 2011; Abraham et al., 2017). Strategies used by microbes to respond to stimuli and traverse physical barriers at specific times are not fully understood (Sonenshine and Macaluso, 2017).
A secondary barrier to colonization is the tick immune system (Oliva Chávez et al., 2017; Fogaça et al., 2021). Some immune pathways are described as restricting vector-borne microbes, such as the Immune Deficiency pathway (Rosa et al., 2016; Capelli-Peixoto et al., 2017; Shaw et al., 2017; Carroll et al., 2019), the JAK-STAT pathway (Liu et al., 2012; Smith et al., 2016), the RNAi pathway (Rückert et al., 2014; Schnettler et al., 2014; Grubaugh et al., 2016; Hart and Thangamani, 2021), and phagocytic hemocytes (Coleman et al., 1997; Dunham-Ems et al., 2009; Talactac et al., 2021); however, vectored pathogens are still able to colonize the tick. Whether this is through microbial-mediated immune evasion/suppression or through immunological tolerance from the arthropod is not clearly defined (Sonenshine and Macaluso, 2017; Shaw et al., 2018; Boulanger and Wikel, 2021; Rosche et al., 2021). Prolonged nutrient limitation between bloodmeals and competition with the resident microbiota/endogenous virome are also restrictive forces that must be dealt with (Bell-Sakyi and Attoui, 2013; Shaw et al., 2018; O’Neal et al., 2020; Samaddar et al., 2020; Bonnet and Pollet, 2021; Narasimhan et al., 2021). To what extent these factors limit colonization and/or survival is not clear, but are undoubtedly pressures that must be coped with by the pathogen.
A comprehensive understanding on this topic is limited by the scope of basic tick biology knowledge. For example, although important morphological and structural descriptions of tick hemocytes have been reported (Dolp, 1970; Brinton and Burgdorfer, 1971; Dolp and Hamdy, 1971; Pereira et al., 2001; Kadota et al., 2003; Matsuo et al., 2004; Borovicková and Hypsa, 2005; Kotsyfakis et al., 2015), the categorization of specific subpopulations and how they function during infection remains undefined. Modern technical advancements may provide insight into the molecular and cellular biology of discrete hemocyte subsets and how they may impact vector competence. Historically, much of what is known about arthropods is informed by the insect model organism Drosophila. This has been and continues to be a powerful system for elucidating important biological concepts, but there are significant physiological and genetic differences between insects and ticks which diverged approximately 450 million years ago (Mans et al., 2016; Beati and Klompen, 2019). For instance, Ixodidae ticks do not have a prophenoloxidase system, which is an important insect defense against infection (Zhioua et al., 1997; Sonenshine and Roe, 2014; Palmer and Jiggins, 2015; Gulia-Nuss et al., 2016). β-1,3-glucan recognition proteins are also not found in ticks, which recognize fungal organisms and induce immune responses in insects (Ferrandon et al., 2007; Bechsgaard et al., 2016). The extent of these differences as they relate to vector competence and microbial colonization remains elusive, but recent findings imply that there is much to learn (Palmer and Jiggins, 2015; Rosa et al., 2016; Capelli-Peixoto et al., 2017; Shaw et al., 2017; Sonenshine and Macaluso, 2017; Carroll et al., 2019). These studies highlight the importance of examining concepts directly in ticks to elucidate what barriers are unique to these non-model organisms and how transmissible microbes are manipulating them.
Microbial Hacking: Manipulating the Arthropod
Microbial strategies to colonize the tick is an understudied area in the field, but likely involve infection determinants that are specific to the arthropod. The overarching goal is to overcome barriers within the tick to enable growth, survival, and transmission to a host. Two categories of microbial molecules that facilitate these goals are surface-localized proteins and secreted effectors. Many microbial surface proteins play essential roles during mammalian infection to enable pathogen adherence, tissue invasion, immune evasion, and dissemination (Sonenshine and Macaluso, 2017). A smaller number of proteins are known to act at the vector-pathogen interface, but those that have been identified perform critical tick-specific functions. For example, B. burgdorferi OspA binds to Tick Receptor for OspA (TROSPA) to facilitate midgut colonization and persistence through the molt (Pal et al., 2004). Post translational modifications of surface proteins can also be critical for survival, as is the case with the A. phagocytophilum O-methyltransferase which modifies Msp4 and is essential for growth in tick cells (Oliva Chávez et al., 2015). The full repertoire of surface proteins used by tick-borne microbes to manipulate interactions with the vector remains largely undefined.
Multiple tick-borne pathogens employ specialized secretion apparatuses to deliver effector molecules. One example is the Type IV Secretion System (T4SS) used by Rickettsial pathogens, including Anaplasma spp., Rickettsia spp. and Ehrlichia spp. (Gillespie et al., 2010; Rikihisa, 2017). During mammalian infections, T4SS-injected effectors manipulate signaling cascades, alter gene transcription, evade cellular defenses, and liberate essential nutrients. For instance, Rickettsia effector Risk1 promotes internalization, phagosomal escape, and autophagosomal escape by modifying phosphoinositides (Voss et al., 2020). A. phagocytophilum and E. chaffeensis effectors Ats1 and Etf1 inhibit host apoptosis and stimulate autophagy to liberate nutrients (Niu et al., 2010; Lin et al., 2016). Most secreted effectors encoded by Rickettsial pathogens are uncharacterized, with only a few that have known functions in mammals and even less with identified roles in the tick.
Although little is known about infection determinants for colonizing the arthropod, evidence suggests that there are protein expression patterns specific for the tick. The cattle pathogen, Anaplasma marginale, differentially transcribes 34 surface protein-encoding genes during growth in tick cells relative to mammalian blood (Hammac et al., 2014), indicating there are arthropod-specific surface modifications yet to be dissected. Secreted effectors can have species-specific roles, as is the case for the non-tick-borne pathogen Legionella pneumophila (Park et al., 2020). Machine learning algorithms predict that A. phagocytophilum encodes up to 48 T4SS-secreted effectors, some of which are differentially expressed during growth within mammalian versus tick cells (Nelson et al., 2008; Esna Ashari et al., 2019). This suggests that the effector repertoire may enable host switching between vertebrate hosts and the arthropod. Identifying and characterizing microbial infection determinants that mediate tick-pathogen interactions will not only define infection strategies used by the pathogen, but also identify pathways that are manipulated within the vector.
Saliva-Transmission Dynamics: A Free Ride or Hijacking the Situation?
Modulation of immune responses, coagulation, and hemostasis at the bite site through the secretion of salivary molecules is a central component of hematophagy (Tirloni et al., 2020a). Likewise, vector-borne pathogens rely on these molecules for transmission and establishment of infection. Several studies have shown that vector transmission promotes pathogen infection and intensifies disease severity (Grasperge et al., 2014; Pingen et al., 2016; Onyango et al., 2020; Karim et al., 2021). This phenomenon is well-known and referred to as saliva-assisted transmission (Nuttall, 2019; Oliva Chavez, 2021). Although some tick saliva molecules that facilitate transmission have been identified, our knowledge of the cellular and molecular mechanisms involved are still underdeveloped.
Tick saliva is a complex and dynamic mix of molecules which change in response to different hosts and over the course of tick feeding (Karim and Ribeiro, 2015; Tirloni et al., 2017; Nuttall, 2019; Tirloni et al., 2020b). Non-protein salivary molecules include lipids, such as Prostaglandin E2 (PGE2) (Bowman et al., 1996), metabolites, like adenosine (Oliveira et al., 2011), and microRNAs (Hackenberg et al., 2017; Malik et al., 2019; Nawaz et al., 2020a). Protein salivary components are the best studied and defined as the “sialome” or “sialoverse” (Mans, 2020). Recently, hundreds of thousands of tick salivary gland transcripts were identified and bioinformatically classified into protein families such as lipocalins, metalloproteases, basic tail secreted proteins, etc. (Ribeiro and Mans, 2020). Relationship analyses indicate that a high rate of evolution and intragenic recombination is driving the expansion of these protein families (Ribeiro and Mans, 2020). Compared to the number of transcriptomic and proteomic studies done in salivary glands and saliva from hard ticks (Ixodidae), only a few soft ticks have been explored and the gene expression dynamics during feeding have only recently been described (Oleaga et al., 2021). The Argasid sialome is comprised of several protein families similar to those identified in hard ticks (Martins et al., 2021; Oleaga et al., 2021). Although many proteins appear to be conserved between tick species, functionality has not been extensively investigated and represents an important knowledge gap. Further, a significant number of expressed genes remain uncharacterized and their role in tick feeding and/or pathogen transmission is therefore unknown (Ribeiro and Mans, 2020; Martins et al., 2021; Oleaga et al., 2021).
Recently, many salivary molecules were discovered to be secreted within extracellular vesicles (Nawaz et al., 2020a; Nawaz et al., 2020b; Zhou et al., 2020; Oliva Chávez et al., 2021), which impact host immune responses, wound healing and feeding success of the tick (Karim et al., 2002; Karim et al., 2005; Alarcon-Chaidez et al., 2009; Villarreal et al., 2013; Shaw et al., 2016; Zhou et al., 2020; Oliva Chávez et al., 2021; Pham et al., 2021). Interestingly, salivary extracellular vesicles can modulate the outcome of vector-borne infections, as is the case for A. phagocytophilum and Francisella tularensis (Oliva Chávez et al., 2021). Extracellular vesicles also facilitate vector-borne virus infection and transmission (Silvas et al., 2016; Vora et al., 2018; Zhou et al., 2018; Reyes-Ruiz et al., 2019; York et al., 2021). For instance, the Haemaphysalis longicornis-borne virus SFTS (Severe Fever with Thrombocytopenia Syndrome) exploits host-derived vesicles to invade uninfected cells and evade antibody defenses (Silvas et al., 2016). Extracellular vesicle cargo can be altered by intracellular vector-borne pathogens, not only by adding pathogen-derived contents but also by changing the composition of host-derived molecules within the vesicles (Regev-Rudzki et al., 2013; Silvas et al., 2016; Vora et al., 2018; Jiang et al., 2020; York et al., 2021). The malaria-causing parasite Plasmodium falciparum commandeers red blood cells exosomes to communicate and exchange genetic material with other parasites during infection (Regev-Rudzki et al., 2013). The mite-borne rickettsia Orientia tsutsugamushi influences serum derived exosome contents during mammalian infection by altering the concentrations of specific miRNAs (Jiang et al., 2020). Similarly, A. phagocytophilum changes the contents of exosomes from infected tick cells (Oliva Chávez et al., 2021). To what extent other tick-borne pathogens alter salivary extracellular vesicles and how this may affect immunomodulatory potential and/or pathogen transmission is an area that remains ripe for discovery.
Concluding Remarks
The distinctive biology of ticks and their long co-evolutionary relationship with the pathogens they vector presents a unique set of challenges to the field. This is partially owing to a lack of genetic tools and reagents available for non-model organisms. Progress on these fronts will benefit from creative approaches and cross-disciplinary interactions/collaborations with colleagues across fields. As noted in Sullivan, 2015, if individuals with training in classic model organisms were to “try their chops” at the genetics, cell biology and biochemistry of ticks and tick-borne microbes it may facilitate the development of novel tools that would lead to breakthroughs in the field.
Despite these unique challenges, we offer the opinion that examining the biology of ticks and interactions with the pathogens they transmit will uncover new paradigms in the arthropod-borne disease triangle that could translate to different systems (Figure 1). For example, microbial effectors that are expressed in the arthropod may be targeting undiscovered cellular processes that dictate vector competence, which may be conserved across arthropod vectors. Likewise, understanding the biology of ticks will yield unique insights into requirements for microbial survival, persistence, and transmission in the face of host and vector immunity. With growing accessibility to technology and improved techniques that increase the limits of detection, pulling back the curtain on non-model organism biology is becoming feasible. This information will improve our fundamental knowledge in vector biology and microbiology and may pave the way for developing innovative approaches to control tick-borne disease.
Author Contributions
JP, AO, and DS designed and wrote this opinion. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Institute of Food and Agriculture (NIFA) United States Department of Agriculture Animal Health grant TEX09902 and Texas A&M University, National Institute of Health grants R21AI151412, R21AI154023, R21AI139772 and R21AI148578 and Washington State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health or the United States Department of Agriculture.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Jennifer L. Gillett-Kaufman (Texas A&M University) for helpful suggestions and proofreading this manuscript.
References
- Abraham N. M., Liu L., Jutras B. L., Yadav A. K., Narasimhan S., Gopalakrishnan V., et al. (2017). Pathogen-Mediated Manipulation of Arthropod Microbiota to Promote Infection. Proc. Natl. Acad. Sci. U. S. A. 114, E781–E790. 10.1073/pnas.1613422114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguero-Rosenfeld M. E., Wang G., Schwartz I., Wormser G. P. (2005). Diagnosis of Lyme Borreliosis. Clin. Microbiol. Rev. 18, 484–509. 10.1128/CMR.18.3.484-509.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon-Chaidez F. J., Boppana V. D., Hagymasi A. T., Adler A. J., Wikel S. K. (2009). A Novel Sphingomyelinase-Like Enzyme in Ixodes scapularis Tick Saliva Drives Host CD4 T Cells to Express IL-4. Parasite Immunol. 31, 210–219. 10.1111/j.1365-3024.2009.01095.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C. B., Eisen R. J., Barker C. M., Garofalo J. F., Hahn M., Hayden M., et al. (2016) Chapter 5: Vector-Borne Diseases. The Impacts of Climate Change on Human Health in the United States: A Scientific Assessment. U.S. In: Global Change Research Program (Washington, DC: ). [Google Scholar]
- Beati L., Klompen H. (2019). Phylogeography of Ticks (Acari: Ixodida). Annu. Rev. Entomol. 64, 379–397. 10.1146/annurev-ento-020117-043027 [DOI] [PubMed] [Google Scholar]
- Bechsgaard J., Vanthournout B., Funch P., Vestbo S., Gibbs R. A., Richards S., et al. (2016). Comparative Genomic Study of Arachnid Immune Systems Indicates Loss of Beta-1,3-Glucanase-Related Proteins and the Immune Deficiency Pathway. J. Evol. Biol. 29, 277–291. 10.1111/jeb.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L., Attoui H. (2013). Endogenous Tick Viruses and Modulation of Tick-Borne Pathogen Growth. Front. Cell. Infect. Microbiol. 10.3389/fcimb.2013.00025 [DOI] [PMC free article] [PubMed]
- Bonnet S. I., Pollet T. (2021). Update on the Intricate Tango Between Tick Microbiomes and Tick-Borne Pathogens. Parasite Immunol. 43, e12813. 10.1111/pim.12813 [DOI] [PubMed] [Google Scholar]
- Borovicková B., Hypsa V. (2005). Ontogeny of Tick Hemocytes: A Comparative Analysis of Ixodes ricinus and Ornithodoros moubata. Exp. Appl. Acarol. 35, 317–333. 10.1007/s10493-004-2209-8 [DOI] [PubMed] [Google Scholar]
- Boulanger N., Wikel S. (2021). Induced Transient Immune Tolerance in Ticks and Vertebrate Host: A Keystone of Tick-Borne Diseases? Front. Immunol. 12:625993. 10.3389/fimmu.2021.625993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A. S., Dillwith J. W., Sauer J. R. (1996). Tick Salivary Prostaglandins: Presence, Origin and Significance. Parasitol. Today 12, 388–396. 10.1016/0169-4758(96)10061-2 [DOI] [PubMed] [Google Scholar]
- Brinton L. P., Burgdorfer W. (1971). Fine Structure of Normal Hemocytes in Dermacentor andersoni Stiles (Acari:Ixodidae). J. Parasitol. 57, 1110–1127. 10.2307/3277874 [DOI] [PubMed] [Google Scholar]
- Capelli-Peixoto J., Carvalho D. D., Johnson W. C., Scoles G. A., Fogaça A. C., Daffre S., et al. (2017). The Transcription Factor Relish Controls Anaplasma Marginale Infection in the Bovine Tick Rhipicephalus microplus. Dev. Comp. Immunol. 74, 32–39. 10.1016/j.dci.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Carroll E. E. M., Wang X., Shaw D. K., O’Neal A. J., Chávez A. S. O., Brown L. J., et al. (2019). P47 Licenses Activation of the Immune Deficiency Pathway in the TickIxodes scapularis. PNAS 116, 205–210. 10.1073/pnas.1808905116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez A. S. O., O’Neal A. J., Santambrogio L., Kotsyfakis M., Pedra J. H. F. (2019). Message in a Vesicle – Trans-Kingdom Intercommunication at the Vector–Host Interface. J. Cell Sci. 132, 1–11. 10.1242/jcs.224212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Gebbia J. A., Piesman J., Degen J. L., Bugge T. H., Benach J. L. (1997). Plasminogen Is Required for Efficient Dissemination of B. burgdorferi in Ticks and for Enhancement of Spirochetemia in Mice. Cell 89, 1111–1119. 10.1016/S0092-8674(00)80298-6 [DOI] [PubMed] [Google Scholar]
- Dennis D. T., Nekomoto T. S., Victor J. C., Paul W. S., Piesman J. (1998). Reported Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 35, 629–638. 10.1093/jmedent/35.5.629 [DOI] [PubMed] [Google Scholar]
- De Silva A. M., Fikrig E. (1995). Growth and Migration of Borrelia burgdorferi in Ixodes Ticks During Blood Feeding. Am. J. Trop. Med. Hyg. 53, 397–404. 10.4269/ajtmh.1995.53.397 [DOI] [PubMed] [Google Scholar]
- Dolp R. M. (1970). Biochemical and Physiological Studies of Certain Ticks (Ixodoidea). Qualitative and Quantitative Studies of Hemocytes. J. Med. Entomol. 7, 277–288. 10.1093/jmedent/7.3.277 [DOI] [PubMed] [Google Scholar]
- Dolp R. M., Hamdy B. H. (1971). Biochemical and Physiological Studies of Certain Ticks (Ixodoidea). Protein Electrophoretic Studies of Certain Biological Fluids of Argas (Argasidae) and Hyalomma (Ixodidae). J. Med. Entomol. 8, 636–642. 10.1093/jmedent/8.6.636 [DOI] [PubMed] [Google Scholar]
- Dunham-Ems S. M., Caimano M. J., Pal U., Wolgemuth C. W., Eggers C. H., Balic A., et al. (2009). Live Imaging Reveals a Biphasic Mode of Dissemination of Borrelia burgdorferi Within Ticks. J. Clin. Invest. 119, 3652–3665. 10.1172/JCI39401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen R. J., Eisen L., Ogden N. H., Beard C. B. (2016. b). Linkages of Weather and Climate With Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), Enzootic Transmission of Borrelia burgdorferi, and Lyme Disease in North America. J. Med. Entomol. 53, 250–261. 10.1093/jme/tjv199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen R. J., Eisen L., Beard C. B. (2016. a). County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol. 53, 349–386. 10.1093/jme/tjv237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esna Ashari Z., Brayton K. A., Broschat S. L. (2019). Prediction of T4SS Effector Proteins for Anaplasma phagocytophilum Using OPT4e, A New Software Tool. Front. Microbiol. 10, 1391. 10.3389/fmicb.2019.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D., Imler J.-L., Hetru C., Hoffmann J. A. (2007). The Drosophila Systemic Immune Response: Sensing and Signalling During Bacterial and Fungal Infections. Nat. Rev. Immunol. 7, 862–874. 10.1038/nri2194 [DOI] [PubMed] [Google Scholar]
- Fikrig E., Narasimhan S. (2006). Borrelia burgdorferi–Traveling Incognito? Microbes Infect. 8, 1390–1399. 10.1016/j.micinf.2005.12.022 [DOI] [PubMed] [Google Scholar]
- Fogaça A. C., Sousa G., Pavanelo D. B., Esteves E., Martins L. A., Urbanová V., et al. (2021). Tick Immune System: What Is Known, the Interconnections, the Gaps, and the Challenges. Front. Immunol. 12, 628054. 10.3389/fimmu.2021.628054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. J., Brayton K. A., Williams K. P., Diaz M. A. Q., Brown W. C., Azad A. F., et al. (2010). Phylogenomics Reveals a Diverse Rickettsiales Type IV Secretion System. Infect. Immun. 78, 1809–1823. 10.1128/IAI.01384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasperge B. J., Morgan T. W., Paddock C. D., Peterson K. E., Macaluso K. R. (2014). Feeding by Amblyomma maculatum (Acari: Ixodidae) Enhances Rickettsia parkeri (Rickettsiales: Rickettsiaceae) Infection in the Skin. J. Med. Entomol. 51, 855–863. 10.1603/me13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubaugh N. D., Rückert C., Armstrong P. M., Bransfield A., Anderson J. F., Ebel G. D., et al. (2016). Transmission Bottlenecks and RNAi Collectively Influence Tick-Borne Flavivirus Evolution. Virus Evol. 2. 10.1093/ve/vew033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulia-Nuss M., Nuss A. B., Meyer J. M., Sonenshine D. E., Roe R. M., Waterhouse R. M., et al. (2016). Genomic Insights Into the Ixodes scapularis Tick Vector of Lyme Disease. Nat. Commun. 7, 10507. 10.1038/ncomms10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenberg M., Langenberger D., Schwarz A., Erhart J., Kotsyfakis M. (2017). In silico Target Network Analysis of De novo-Discovered, Tick Saliva-Specific microRNAs Reveals Important Combinatorial Effects in Their Interference With Vertebrate Host Physiology. RNA 23, 1259–1269. 10.1261/rna.061168.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammac G. K., Pierlé S. A., Cheng X., Scoles G. A., Brayton K. A. (2014). Global Transcriptional Analysis Reveals Surface Remodeling of Anaplasma marginale in the Tick Vector. Parasit. Vectors 7, 193. 10.1186/1756-3305-7-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. E., Thangamani S. (2021). Tick-Virus Interactions: Current Understanding and Future Perspectives. Parasite Immunol. 43, e12815. 10.1111/pim.12815 [DOI] [PubMed] [Google Scholar]
- Hodzic E., Fish D., Maretzki C. M., De Silva A. M., Feng S., Barthold S. W. (1998). Acquisition and Transmission of the Agent of Human Granulocytic Ehrlichiosis by Ixodes scapularis Ticks. J. Clin. Microbiol. 36, 3574–3578. 10.1128/JCM.36.12.3574-3578.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- iang L., Belinskaya T., Zhang Z., Chan T. C., Ching W. M., Chao C. C. (2020). Regulation of Serum Exosomal MicroRNAs in Mice Infected With Orientia tsutsugamushi. Microorganisms 9. 10.3390/microorganisms9010080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota K., Walter S., Claveria F. G., Igarashi I., Taylor D., Fujisaki K. (2003). Morphological and Populational Characteristics of Hemocytes of Ornithodoros moubata Nymphs During the Ecdysial Phase. J. Med. Entomol. 40, 770–776. 10.1603/0022-2585-40.6.770 [DOI] [PubMed] [Google Scholar]
- Karim S., Essenberg R. C., Dillwith J. W., Tucker J. S., Bowman A. S., Sauer J. R. (2002). Identification of SNARE and Cell Trafficking Regulatory Proteins in the Salivary Glands of the Lone Star Tick, Amblyomma americanum (L.). Insect Biochem. Mol. Biol. 32, 1711–1721. 10.1016/s0965-1748(02)00111-x [DOI] [PubMed] [Google Scholar]
- Karim S., Kumar D., Budachetri K. (2021). Recent Advances in Understanding Tick and Rickettsiae Interactions. Parasite Immunol. 43, e12830. 10.1111/pim.12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S., Miller N. J., Valenzuela J., Sauer J. R., Mather T. N. (2005). RNAi-Mediated Gene Silencing to Assess the Role of Synaptobrevin and Cystatin in Tick Blood Feeding. Biochem. Biophys. Res. Commun. 334, 1336–1342. 10.1016/j.bbrc.2005.07.036 [DOI] [PubMed] [Google Scholar]
- Karim S., Ribeiro J. M. (2015). An Insight Into the Sialome of the Lone Star Tick, Amblyomma americanum, With a Glimpse on Its Time Dependent Gene Expression. PloS One 10, e0131292. 10.1371/journal.pone.0131292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsyfakis M., Kopáček P., Franta Z., Pedra J. H. F., Ribeiro J. M. C. (2015). Deep Sequencing Analysis of the Ixodes ricinus Haemocytome. PloS Negl. Trop. Dis. 9, e0003754. 10.1371/journal.pntd.0003754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Liu H., Xiong Q., Niu H., Cheng Z., Yamamoto A., et al. (2016). Ehrlichia Secretes Etf-1 to Induce Autophagy and Capture Nutrients for its Growth Through RAB5 and Class III Phosphatidylinositol 3-Kinase. Autophagy 12, 2145–2166. 10.1080/15548627.2016.1217369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Dai J., Zhao Y. O., Narasimhan S., Yang Y., Zhang L., et al. (2012). Ixodes scapularis JAK-STAT Pathway Regulates Tick Antimicrobial Peptides, Thereby Controlling the Agent of Human Granulocytic Anaplasmosis. J. Infect. Dis. 206, 1233–1241. 10.1093/infdis/jis484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Narasimhan S., Dai J., Zhang L., Cheng G., Fikrig E. (2011). Ixodes scapularis Salivary Gland Protein P11 Facilitates Migration of Anaplasma phagocytophilum From the Tick Gut to Salivary Glands. EMBO Rep. 12, 1196–1203. 10.1038/embor.2011.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik M. I., Nawaz M., Hassan I. A., Zhang H., Gong H., Cao J., et al. (2019). A microRNA Profile of Saliva and Role of miR-375 in Haemaphysalis longicornis (Ixodida: Ixodidae). Parasit. Vectors 12, 68. 10.1186/s13071-019-3318-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans B. J. (2020). Quantitative Visions of Reality at the Tick-Host Interface: Biochemistry, Genomics, Proteomics, and Transcriptomics as Measures of Complete Inventories of the Tick Sialoverse. Front. Cell Infect. Microbiol. 10:574405. 10.3389/fcimb.2020.574405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans B. J., de Castro M. H., Pienaar R., de Klerk D., Gaven P., Genu S., et al. (2016). Ancestral Reconstruction of Tick Lineages. Ticks Tick Borne Dis. 7, 509–535. 10.1016/j.ttbdis.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Martins L. A., Bensaoud C., Kotál J., Chmelař J., Kotsyfakis M. (2021). Tick Salivary Gland Transcriptomics and Proteomics. Parasite Immunol. 43, e12807. 10.1111/pim.12807 [DOI] [PubMed] [Google Scholar]
- Masters E., Granter S., Duray P., Cordes P. (1998). Physician-Diagnosed Erythema Migrans and Erythema Migrans–Like Rashes Following Lone Star Tick Bites. Arch. Dermatol. 134, 955–960. 10.1001/archderm.134.8.955 [DOI] [PubMed] [Google Scholar]
- Matsuo T., Okoda Y., Badgar B., Inoue N., Xuan X., Taylor D., et al. (2004). Fate of GFP-Expressing Escherichia Coli in the Midgut and Response to Ingestion in a Tick, Ornithodoros moubata (Acari: Argasidae). Exp. Parasitol. 108, 67–73. 10.1016/j.exppara.2004.07.014 [DOI] [PubMed] [Google Scholar]
- Molins C. R., Ashton L. V., Wormser G. P., Andre B. G., Hess A. M., Delorey M. J., et al. (2017). Metabolic Differentiation of Early Lyme Disease From Southern Tick-Associated Rash Illness (STARI). Sci. Transl. Med. 9, eaa12717. 10.1126/scitranslmed.aal2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S., Rajeevan N., Liu L., Zhao Y. O., Heisig J., Pan J., et al. (2014). Gut Microbiota of the Tick Vector Ixodes scapularis Modulate Colonization of the Lyme Disease Spirochete. Cell Host Microbe 15, 58–71. 10.1016/j.chom.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S., Schuijt T. J., Abraham N. M., Rajeevan N., Coumou J., Graham M., et al. (2017). Modulation of the Tick Gut Milieu by a Secreted Tick Protein Favors Borrelia burgdorferi Colonization. Nat. Commun. 8, 184. 10.1038/s41467-017-00208-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S., Swei A., Abouneameh S., Pal U., Pedra J. H. F., Fikrig E. (2021). Grappling With the Tick Microbiome. Trends Parasitol. 722–733 10.1016/j.pt.2021.04.004 [DOI] [PMC free article] [PubMed]
- Nawaz M., Malik M. I., Zhang H., Gebremedhin M. B., Cao J., Zhou Y., et al. (2020. a). miRNA Profile of Extracellular Vesicles Isolated From Saliva of Haemaphysalis longicornis Tick. Acta Trop. 212:105718. 10.1016/j.actatropica.2020.105718 [DOI] [PubMed] [Google Scholar]
- Nawaz M., Malik M. I., Zhang H., Hassan I. A., Cao J., Zhou Y., et al. (2020. b). Proteomic Analysis of Exosome-Like Vesicles Isolated From Saliva of the Tick Haemaphysalis longicornis. Front. Cell Infect. Microbiol. 10, 542319. 10.3389/fcimb.2020.542319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. M., Herron M. J., Felsheim R. F., Schloeder B. R., Grindle S. M., Chavez A. O., et al. (2008). Whole Genome Transcription Profiling of Anaplasma phagocytophilum in Human and Tick Host Cells by Tiling Array Analysis. BMC Genomics 9, 1–16. 10.1186/1471-2164-9-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H., Kozjak-Pavlovic V., Rudel T., Rikihisa Y. (2010). Anaplasma phagocytophilum Ats-1 Is Imported Into Host Cell Mitochondria and Interferes With Apoptosis Induction. PloS Pathog. 6, e1000774. 10.1371/journal.ppat.1000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall P. A. (2019). Tick Saliva and its Role in Pathogen Transmission. Wien. Klin. Wochenschr. 10.1007/s00508-019-1500-y [DOI] [PMC free article] [PubMed]
- Oleaga A., Soriano B., Llorens C., Pérez-Sánchez R. (2021). Sialotranscriptomics of the Argasid Tick Ornithodoros moubata Along the Trophogonic Cycle. PloS Negl. Trop. Dis. 15, e0009105. 10.1371/journal.pntd.0009105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva Chavez A. (2021). Saliva-Assisted Transmission. In: Encyclopedia. Available at: https://encyclopedia.pub/8558.
- Oliva Chávez A. S., Fairman J. W., Felsheim R. F., Nelson C. M., Herron M. J., Higgins L., et al. (2015). An O-Methyltransferase is Required for Infection of Tick Cells by Anaplasma phagocytophilum. PloS Pathog. 11, e1005248. 10.1371/journal.ppat.1005248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva Chávez A. S., Shaw D. K., Munderloh U. G., Pedra J. H. F. (2017). Tick Humoral Responses: Marching to the Beat of a Different Drummer. Front. Microbiol. 8, 223. 10.3389/fmicb.2017.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva Chávez A. S., Wang X., Marnin L., Archer N. K., Hammond H. L., Carroll E. E. M., et al. (2021). Tick Extracellular Vesicles Enable Arthropod Feeding and Promote Distinct Outcomes of Bacterial Infection. Nat. Commun. 12, 3696. 10.1038/s41467-021-23900-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C. J. F., Sá-Nunes A., Francischetti I. M. B., Carregaro V., Anatriello E., Silva J. S., et al. (2011). Deconstructing Tick Saliva Non-Protein Molecules With Potent Immunomodulatory Properties. J. Biol. Chem. 286, 10960–10969. 10.1074/jbc.M110.205047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal A. J., Butler L. R., Rolandelli A., Gilk S. D., Pedra J. H. (2020). Lipid Hijacking: A Unifying Theme in Vector-Borne Diseases. Elife 9, e61675. 10.7554/eLife.61675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango M. G., Ciota A. T., Kramer L. D. (2020). The Vector - Host - Pathogen Interface: The Next Frontier in the Battle Against Mosquito-Borne Viral Diseases? Front. Cell Infect. Microbiol. 10:564518. 10.3389/fcimb.2020.564518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld R. S., Brunner J. L. (2015). Climate Change and Ixodes Tick-Borne Diseases of Humans. Philos. Trans. R. Soc B 370, 20140051. 10.1098/rstb.2014.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U., Li X., Wang T., Montgomery R. R., Ramamoorthi N., Desilva A. M., et al. (2004). TROSPA, an Ixodes scapularis Receptor for Borrelia burgdorferi. Cell 119, 457–468. 10.1016/j.cell.2004.10.027 [DOI] [PubMed] [Google Scholar]
- Palmer W. J., Jiggins F. M. (2015). Comparative Genomics Reveals the Origins and Diversity of Arthropod Immune Systems. Mol. Biol. Evol. 32, 2111–2129. 10.1093/molbev/msv093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. M., Ghosh S., O’Connor T. J. (2020). Combinatorial Selection in Amoebal Hosts Drives the Evolution of the Human Pathogen Legionella pneumophila. Nat. Microbiol. 5, 599–609. 10.1038/s41564-019-0663-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L. S., Oliveira P. L., Barja-Fidalgo C., Daffre S. (2001). Production of Reactive Oxygen Species by Hemocytes From the Cattle Tick Boophilus Microplus. Exp. Parasitol. 99, 66–72. 10.1006/expr.2001.4657 [DOI] [PubMed] [Google Scholar]
- Pham M., Underwood J., Oliva Chávez A. S. (2021). Changing the Recipe: Pathogen Directed Changes in Tick Saliva Components. Int. J. Environ. Res. Public Health 18. 10.3390/ijerph18041806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingen M., Bryden S. R., Pondeville E., Schnettler E., Kohl A., Merits A., et al. (2016). Host Inflammatory Response to Mosquito Bites Enhances the Severity of Arbovirus Infection. Immunity 44, 1455–1469. 10.1016/j.immuni.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi N., Narasimhan S., Pal U., Bao F., Yang X. F., Fish D., et al. (2005). The Lyme Disease Agent Exploits a Tick Protein to Infect the Mammalian Host. Nature 436, 573–577. 10.1038/nature03812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev-Rudzki N., Wilson D. W., Carvalho T. G., Sisquella X., Coleman B. M., Rug M., et al. (2013). Cell-Cell Communication Between Malaria-Infected Red Blood Cells via Exosome-Like Vesicles. Cell 153, 1120–1133. 10.1016/j.cell.2013.04.029 [DOI] [PubMed] [Google Scholar]
- Reyes-Ruiz J. M., Osuna-Ramos J. F., De Jesús-González L. A., Hurtado-Monzón A. M., Farfan-Morales C. N., Cervantes-Salazar M., et al. (2019). Isolation and Characterization of Exosomes Released From Mosquito Cells Infected With Dengue Virus. Virus Res. 266, 1–14. 10.1016/j.virusres.2019.03.015 [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M. C., Mans B. J. (2020). TickSialoFam (TSFam): A Database That Helps to Classify Tick Salivary Proteins, a Review on Tick Salivary Protein Function and Evolution, With Considerations on the Tick Sialome Switching Phenomenon. Front. Cell Infect. Microbiol. 10, 374. 10.3389/fcimb.2020.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y. (2017). “Role and Function of the Type IV Secretion System in Anaplasma and Ehrlichia Species,” in Type IV Secretion in Gram-Negative and Gram-Positive Bacteria Current Topics in Microbiology and Immunology. Eds. Backert S., Grohmann E. (Cham: Springer International Publishing; ), 297–321. 10.1007/978-3-319-75241-9_12 [DOI] [PubMed] [Google Scholar]
- Rosa R. D., Capelli-Peixoto J., Mesquita R. D., Kalil S. P., Pohl P. C., Braz G. R., et al. (2016). Exploring the Immune Signalling Pathway-Related Genes of the Cattle Tick Rhipicephalus microplus: From Molecular Characterization to Transcriptional Profile Upon Microbial Challenge. Dev. Comp. Immunol. 59, 1–14. 10.1016/j.dci.2015.12.018 [DOI] [PubMed] [Google Scholar]
- Rosche K. L., Sidak-Loftis L. C., Hurtado J., Fisk E. A., Shaw D. K. (2021). Arthropods Under Pressure: Stress Responses and Immunity at the Pathogen-Vector Interface. Front. Immunol. 11, 629777. 10.3389/fimmu.2020.629777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. (2018). Vital Signs: Trends in Reported Vectorborne Disease Cases — United States and Territories 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 67, 496–501. 10.15585/mmwr.mm6717e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückert C., Bell-Sakyi L., Fazakerley J. K., Fragkoudis R. (2014). Antiviral Responses of Arthropod Vectors: An Update on Recent Advances. VirusDis 25, 249–260. 10.1007/s13337-014-0217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaddar S., Marnin L., Butler L. R., Pedra J. H. F. (2020). Immunometabolism in Arthropod Vectors: Redefining Interspecies Relationships. Trends Parasitol. 36, 807–815. 10.1016/j.pt.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnettler E., Tykalová H., Watson M., Sharma M., Sterken M. G., Obbard D. J., et al. (2014). Induction and Suppression of Tick Cell Antiviral RNAi Responses by Tick-Borne Flaviviruses. Nucleic Acids Res. 42, 9436–9446. 10.1093/nar/gku657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. K., Kotsyfakis M., Pedra J. H. F. (2016). For Whom the Bell Tolls (and Nods): Spit-Acular Saliva. Curr. Trop. Med. Rep. 3, 40–50. 10.1007/s40475-016-0072-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. K., Tate A. T., Schneider D. S., Levashina E. A., Kagan J. C., Pal U., et al. (2018). Vector Immunity and Evolutionary Ecology: The Harmonious Dissonance. Trends Immunol. 39, 862–873. 10.1016/j.it.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. K., Wang X., Brown L. J., Chávez A. S. O., Reif K. E., Smith A. A., et al. (2017). Infection-Derived Lipids Elicit an Immune Deficiency Circuit in Arthropods. Nat. Commun. 8:ncomms14401. 10.1038/ncomms14401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvas J. A., Popov V. L., Paulucci-Holthauzen A., Aguilar P. V. (2016). Extracellular Vesicles Mediate Receptor-Independent Transmission of Novel Tick-Borne Bunyavirus. J. Virol. 90, 873–886. 10.1128/jvi.02490-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. A., Navasa N., Yang X., Wilder C. N., Buyuktanir O., Marques A., et al. (2016). Cross-Species Interferon Signaling Boosts Microbicidal Activity Within the Tick Vector. Cell Host Microbe 20, 91–98. 10.1016/j.chom.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine D. E., Macaluso K. R. (2017). Microbial Invasion vs. Tick Immune Regulation. Front. Cell. Infect. Microbiol. 7, 390. 10.3389/fcimb.2017.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine D. E., Roe R. M. (Eds.) (2014). Biology of Ticks. 2 edition Vol. 2 (New York: Oxford University Press; ). [Google Scholar]
- Stanek G., Wormser G. P., Gray J., Strle F. (2012). Lyme Borreliosis. Lancet 379, 461–473. 10.1016/S0140-6736(11)60103-7 [DOI] [PubMed] [Google Scholar]
- Sullivan W. (2015). The Institute for the Study of Non–Model Organisms and Other Fantasies. Mol Biol Cell 26, 387–389. 10.1091/mbc.E14-03-0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talactac M. R., Hernandez E. P., Hatta T., Yoshii K., Kusakisako K., Tsuji N., et al. (2021). The Antiviral Immunity of Ticks Against Transmitted Viral Pathogens. Dev. Comp. Immunol. 119, 104012. 10.1016/j.dci.2021.104012 [DOI] [PubMed] [Google Scholar]
- Tirloni L., Calvo E., Konnai S., da Silva Vaz I., Jr. (2020. a). Editorial: The Role of Saliva in Arthropod-Host-Pathogen Relationships. Front. Cell Infect. Microbiol. 10, 630626. 10.3389/fcimb.2020.630626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L., Kim T. K., Pinto A. F. M., Yates J. R., 3rd, da Silva Vaz I., Jr., Mulenga A. (2017). Tick-Host Range Adaptation: Changes in Protein Profiles in Unfed Adult Ixodes scapularis and Amblyomma americanum Saliva Stimulated to Feed on Different Hosts. Front. Cell Infect. Microbiol. 7, 517. 10.3389/fcimb.2017.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L., Lu S., Calvo E., Sabadin G., Di Maggio L. S., Suzuki M., et al. (2020. b). Integrated Analysis of Sialotranscriptome and Sialoproteome of the Brown Dog Tick Rhipicephalus sanguineus (s.l.): Insights Into Gene Expression During Blood Feeding. J. Proteomics 229, 103899. 10.1016/j.jprot.2020.103899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal A. M., Adamson S. W., Browning R. E., Budachetri K., Sajid M. S., Karim S. (2013). Molecular Characterization and Functional Significance of the Vti Family of SNARE Proteins in Tick Salivary Glands. Insect Biochem. Mol. Biol. 43, 483–493. 10.1016/j.ibmb.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora A., Zhou W., Londono-Renteria B., Woodson M., Sherman M. B., Colpitts T. M., et al. (2018). Arthropod EVs Mediate Dengue Virus Transmission Through Interaction With a Tetraspanin Domain Containing Glycoprotein Tsp29Fb. Proc. Natl. Acad. Sci. U. S. A. 115, E6604–e6613. 10.1073/pnas.1720125115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss O. H., Gillespie J. J., Lehman S. S., Rennoll S. A., Beier-Sexton M., Rahman M. S., et al. (2020). Risk1, a Phosphatidylinositol 3-Kinase Effector, Promotes Rickettsia typhi Intracellular Survival. mBio 11. 10.1128/mBio.00820-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser G. P., Dattwyler R. J., Shapiro E. D., Halperin J. J., Steere A. C., Klempner M. S., et al. (2006). The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43, 1089–1134. 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- York S. B., Sun L., Cone A. S., Duke L. C., Cheerathodi M. R., Meckes D. G., Jr. (2021). Zika Virus Hijacks Extracellular Vesicle Tetraspanin Pathways for Cell-To-Cell Transmission. mSphere 6 (3), e0019221. 10.1128/mSphere.00192-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhioua E., Yeh M. T., LeBrun R. A. (1997). Assay for Phenoloxidase Activity in Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis. J. Parasitol. 83, 553–554. 10.2307/3284434 [DOI] [PubMed] [Google Scholar]
- Zhou W., Tahir F., Wang J. C., Woodson M., Sherman M. B., Karim S., et al. (2020). Discovery of Exosomes From Tick Saliva and Salivary Glands Reveals Therapeutic Roles for CXCL12 and IL-8 in Wound Healing at the Tick-Human Skin Interface. Front. Cell Dev. Biol. 8, 554. 10.3389/fcell.2020.00554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Woodson M., Neupane B., Bai F., Sherman M. B., Choi K. H., et al. (2018). Exosomes Serve as Novel Modes of Tick-Borne Flavivirus Transmission From Arthropod to Human Cells and Facilitates Dissemination of Viral RNA and Proteins to the Vertebrate Neuronal Cells. PloS Pathog. 14, e1006764. 10.1371/journal.ppat.1006764 [DOI] [PMC free article] [PubMed] [Google Scholar]