Abstract
Introduction
Gene therapy have recently attracted much attention as a curative therapeutic option for inherited single gene disorders such as hemophilia. Hemophilia is a hereditary bleeding disorder caused by the deficiency of clotting activity of factor VIII (FVIII) or factor IX (FIX), and gene therapy for hemophilia using viral vector have been vigorously investigated worldwide. Toward further advancement of gene therapy for hemophilia, we have previously developed and validated the efficacy of novel two types of gene transfer technologies using a mouse model of hemophilia A. Here we investigated the efficacy and safety of the technologies in canine model. Especially, validations of technical procedures of the gene transfers for dogs were focused.
Methods
Green fluorescence protein (GFP) gene were transduced into normal beagle dogs by ex vivo and in vivo gene transfer techniques. For ex vivo gene transfer, blood outgrowth endothelial cells (BOECs) derived from peripheral blood of normal dogs were transduced with GFP gene using lentivirus vector, propagated, fabricated as cell sheets, then implanted onto the omentum of the same dogs. For in vivo gene transfer, normal dogs were subjected to GFP gene transduction with non-viral piggyBac vector by liver-targeted hydrodynamic injections.
Results
No major adverse events were observed during the gene transfers in both gene transfer systems. As for ex vivo gene transfer, histological findings from the omental biopsy performed 4 weeks after implantation revealed the tube formation by implanted GFP-positive BOECs in the sub-adipose tissue layer without any inflammatory findings, and the detected GFP signals were maintained over 6 months. Regarding in vivo gene transfer, analyses of liver biopsy samples revealed more than 90% of liver cells were positive for GFP signals in the injected liver lobes 1 week after gene transfers, then the signals gradually declined overtime.
Conclusions
Two types of gene transfer techniques were successfully applied to a canine model, and the transduced gene expressions persisted for a long term. Toward clinical application for hemophilia patients, practical assessments of therapeutic efficacy of these techniques will need to be performed using a dog model of hemophilia and FVIII (or FIX) gene.
Keywords: Gene therapy, Dog, Cell sheet, Hydrodynamic injection, Hemophilia
Abbreviations: BOEC, blood outgrowth endothelial cell; GFP, green fluorescent protein; FVIII, factor VIII; FIX, factor IX
Highlights
-
•
Gene therapy for hemophilia has recently attracted much attentions.
-
•
Two types of gene transfer techniques were developed and tested in canine model.
-
•
No major adverse events were observed in both gene transfer systems.
-
•
Both systems achieved long-term gene expression in the targeted tissues or organs.
-
•
Clinical application of these techniques for hemophilia are anticipated.
1. Introduction
Hemophilia is an X-linked bleeding disorder caused by the deficiency of clotting activity of factor VIII (FVIII) (hemophilia A) or factor IX (FIX) (hemophilia B) [1]. These two coagulation disorders are clinically indistinguishable. However, hemophilia A represents 80% of all human hemophilia cases, occurring in around 1 in 10,000 live male births in the world. Patients with severe hemophilia A have residual FVIII activity <1% of normal, resulting in recurrent spontaneous bleeding events from early in life, in joints (hemarthrosis) and soft tissues (hematoma), but also in closed space such as the brain, leading to increased morbidity and mortality. Currently, treatment of hemophilia A is based on plasma-derived or recombinant FVIII protein replacement therapy to control bleeding or prevent bleeding prior to invasive procedures such as surgical operation. For the past decade, several types of long-acting coagulation factor concentrate have been developed [2]. It is, however, still problematic because of the high costs of the factor concentrate, as well as the limited availability (estimated only 20% of patients worldwide have access to the treatment). Moreover, this type of therapy requires life-long and frequent (1–3 times/week) intravenous injections to maintain safety FVIII levels in plasma. On the other hand, the success of gene therapy for hemophilia B was reported and the early data showed efficient expression of FIX reaching levels of 12% of normal following delivery of an adeno-associated viral (AAV) vector encoding the human factor IX gene for hepatocyte-restricted expression [3]. In addition, recent data showed that long-term expression of FIX in severe hemophilia B patients following a single injection of AAV-FIX at levels 1–6% in a dose dependent manner, and in 4 of 7 patients, prophylactic therapy was discontinued [4,5]. Recently, the efficacy of AAV-based gene therapy has been also investigated for hemophilia A [6,7]. AAV-based gene therapy for hemophilia seems to be promising, but it entails several drawbacks such as the risk of adverse immunological reaction against viral proteins and vector-mediated cytotoxicity [8]. Furthermore, patients who was previously infected with AAV and possess neutralizing antibody against AAV capsid protein are ineligible for the therapy. This means that not all the hemophilia patients can benefit from AAV-based gene therapy, and repetitive vector administration is impractical.
To overcome these issues, we have been engaged in the development of two types of new gene transfer strategies for hemophilia A, including ex vivo gene therapy using autologous cells and non-viral in vivo gene therapy using piggyBac vector [[9], [10], [11], [12]]. As for the ex vivo gene therapy, we previously reported that therapeutic efficacy of transplantation of genetically-modified autologous blood outgrowth endothelial cells (BOECs) for a mouse model of hemophilia A [11]. In this study, BOECs established from peripheral blood of hemophilia A mice were proliferated, transduced with FVIII gene using lentivirus vector, harvested as cell sheet using temperature-responsive culture dishes, then transplanted into the subcutaneous place of hemophilia A mice. As a result, significant and sustainable increase of plasma FVIII levels (up to 11% of normal) have been achieved. Furthermore, tail-clipping assay revealed significant improvement of bleeding time in the treated mice. Regarding non-viral in vivo gene therapy, we previously utilized gene delivery system based on piggyBac DNA transposon to transfer the full-length FVIII cDNA [12]. We tested the efficiency of this new vector system in human 293 T cells and induced pluripotent stem (iPS) cells, and confirmed the expression and secretion of FVIII. Hydrodynamic injection of the piggyBac vectors into hemophilia A mice resulted in stable production of circulating FVIII for over 300 days. Tail-clipping assay also demonstrated significant improvement of bleeding tendency in the treated mice.
In the present study, to translate these two types of gene transfer technologies into the clinics, we investigated the efficacy and safety of the two technologies in a canine model. As a preliminary step toward the treatment of hemophilia A dogs by introducing FVIII gene, we here focused on the validation of technical procedures of the gene transfers for large animals, in which green fluorescence protein (GFP) gene were transduced into normal beagle dogs by ex vivo and in vivo gene transfer techniques.
2. Methods
2.1. Materials
Vital Signs Monitor for monitoring physiological parameters on dogs was from NIHON KOHDEN (PVM-2701: Tokyo, Japan). Automate injector for hydrodynamic injection was from SHEEN MAN CO.LTD (Zonemaster SR fusion, Osaka, JAPAN). The contrast medium (Iopamiron) was from Bayer (Iomadidol, Leverkusen, GERMANY). The introducer, guide wire and short sheath for image-guided catheter insertion were from COOK (Bloomington, IN, USA). Injection balloon catheters were purchased from MIYANO MEDICAL INSTRUMENTS CO.LTD (Kobe, JAPAN).
2.2. Animals and ethics statement
Beagle dogs were purchased from KITAYAMA LABES CO., LTD. (Nagano, JAPAN). All animal studies were conducted in full compliance with ARRIVE guidelines. These studies are reviewed and approved by Institutional Animal Care and Use Committee at the Nara Medical University.
2.3. Isolation and gene transduction of canine blood outgrowth endothelial cells (BOECs)
Canine BOECs were isolated from venous blood of dogs as previously described. In brief, mononuclear cells were isolated using Histopaque 1077 (Sigma–Aldrich) by centrifuge separation methods. After suspending cells in MCDB131 medium (Gibco) supplemented with endothelial cell growth medium-2 (Clonetics), 2 mM L-gulutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin (ThermoFisher), and 10% Fetal bovine serum (Sigma–Aldrich), we placed them in a bovine type I collagen-coated 12 well plate. Approximately 2–3 weeks later, small colonies of cells were visualized that were confluent within 10 days. The cells had typical endothelial cobblestone appearance and expressed von Willebrand factor (vWF) but not FVIII. The cells had undergone 3–4 cell passages at the time of use for the experiments.
In vitro transduction of lentiviral vector that encodes GFP (green fluorescent protein) under the control of EF1-alpha (EF1α) promoter, were conducted as described previously [10]. In brief, cultured one million (1 × 106) canine BOECs were transduced following single exposure of the Lenti- EF1α-GFP viral vectors. After transduction, cells were further expanded and assessment of GFP gene transduction was checked by fluorescent microscopy and flow cytometry (EPICS ALTRA HSS analyzer, Beckman Coulter).
2.4. Construction of lentiviral vector and piggyBac vectors
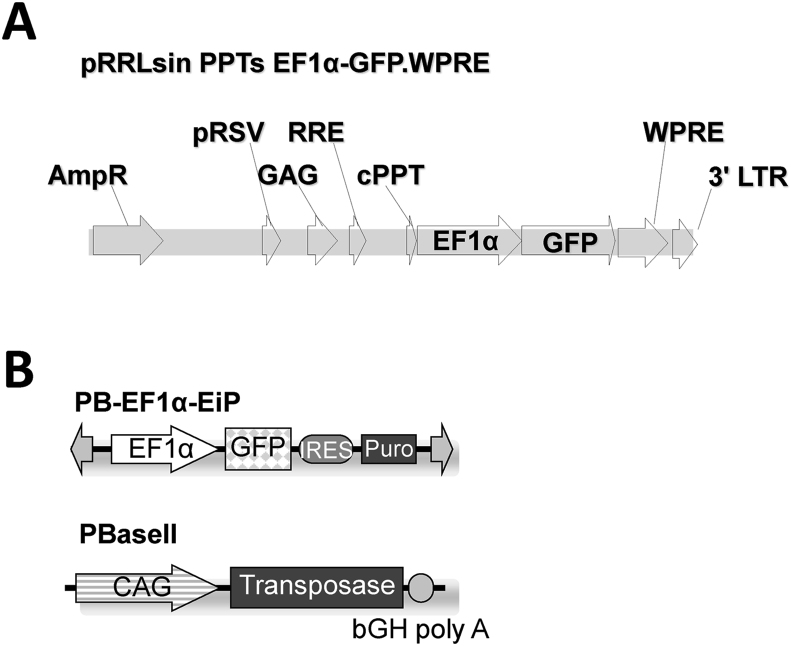
Lentiviral vector and piggyBac transposon vector used in this study were constructed as described previously [11,12], and their schematic representations are shown in Fig. 1.
Fig. 1.
Lentiviral vector and piggyBac transposon vector. Schematic diagram of lentiviral vector (A) and piggyBac transposon vector (B) expressing GFP under the control of the human EF1α promoter. IRES: internal ribosomal entry site.
2.5. Fabrication of canine BOECs sheets
The GFP-transduced BOECs were seeded on 10 cm size (56.7 cm2 of surface area) of temperature-responsive culture dishes (UpCell, CellSeed, Tokyo, Japan) as previously reported [11,13]. When cultured BOECs reached confluency, they were spontaneously detached from the dishes as uniformly connected tissue sheets by lowing the culture temperature to 20 °C for 30min. The obtained BOECs sheet were implanted onto the omentum of the same dog, as described below.
2.6. Preparation of GFP-expressing piggyBac vector
PB-EF1α-EiP and PBase II plasmid vectors were used in this study. Diagram of piggyBac vector expressing GFP under the control of the human EF1α promoter and the PBase II vector expressing piggyBac transposase under the control of CAG promoter were previously reported [12]. Both plasmid vectors were prepared in endotoxin-free vector condition using Qiagen Plasmid Giga Kit (Germantown, MD USA). The vectors were transduced to the dog livers by hydrodynamic injection as described below.
2.7. Animal procedure
Total 4 beagle dogs were used for the experiments. Dogs were anesthetized with an intravenous infusion of propofol and maintained on isoflurane. They were intubated, ventilated and received lactated Ringer's solution throughout the procedure. Various physiological parameters, including body temperature, heart rate, systolic blood pressure, diastolic blood pressure, and oxygen saturation (SpO2), were also monitored throughout the procedure. After the procedure, pain was treated and controlled with buprenorphine for up to 2 weeks.
2.8. Implantation of BOECs sheets onto the omentum of dogs
For cell sheets implantation, an abdominal ventral middle incision was made to exteriorize the greater omentum of dogs. Then, the engineered BOECs sheets consisted of 2.0 ± 0.3 × 106 GFP-positive cells per sheet were implanted onto the surface of omentum using support membrane (Cell Shifter®, Cell Seed, Tokyo, Japan). Five or six cell sheets were implanted at different sites of the omentum. For future biopsy, implanted areas were marked with nylon non-absorbable surgical suture for orientation. After implantation, the omentum was placed back into the abdomen and the incision was closed with suture.
2.9. Hydrodynamic injection of piggyBac vector to the liver of dogs
For hydrodynamic injection, an 18G peripheral catheter was directly inserted into the femoral vein of dogs followed by insertion of 0.035 inch guidewire, a short sheath (5Fr), and an injected balloon catheter. The balloon was inflated just across the edge of hepatic vein of target liver lobe by injecting 1 mL of phase contrast medium. The obstruction of blood flow was verified by injecting small volume of phase contrast medium into the vasculature through the catheter. After preparation, image-guided hydrodynamic injection was conducted. In brief, both vector plasmid (PB-EF1α-EiP 10 mg + PBase II 2.5 mg) were mixed with saline containing with 1% volume of phase contrast medium and diluted up to 2 times volume of liver lobe (total 150 mL). The prepared plasmid mixture was divided into three (50 mL, each), and then injected into the 3 liver lobes, right medial, left medial, and left lateral lobe, respectively, at the speed of 10 mL per second for 5 s. Ten minutes after one injection, balloon clumping was released and moved to the next injection with 20 min interval.
2.10. Evaluations of the dog studies
For the assessment of the tissue damage, blood samples were collected before and 4 h, 24 h, 7 days, and 14 days after hydrodynamic injection. The serum biochemical analyses were performed with clinical chemistry automated analyzers (Dri-Chem 4000sV, Fujifilm, Tokyo, Japan). For the histological analysis of tissue including GFP expression, the tissue samples were collected from the omentum by open biopsy at 4 and 20 weeks after the cell-sheet transplantation procedures, and from the liver by echo-guided biopsy at 1 and 20 weeks after the liver-targeted hydrodynamic injection procedures. For the detecting GFP expression, several parts of the samples were directly frozen and sectioned-specimen were viewed by confocal laser scanning microscope (CLSM). The percentage of GFP positive cells were evaluated at the indicated time points based on CLSM fluorescent images, as previously described [14]. Briefly, percentage of positive cells in a defined area was calculated by each captured image at identical portions using Image Pro Plus image-analyzing computer software version 4.5 (Planetron, Tokyo, Japan). For routine histological analysis, the recovered samples were fixed 4% formalin and embedded in paraffin, and sectioned for hematoxylin and eosin (H&E) staining. Observations were performed on sequential sections.
3. Results
3.1. Lentiviral vector transduction of BOECs in vitro
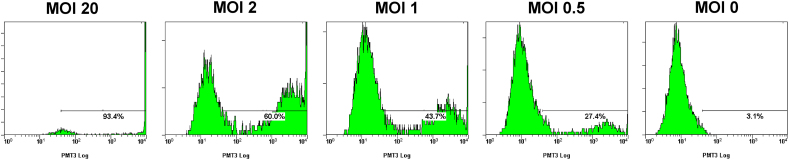
To analyze the efficiency of lentiviral gene transduction in vitro, canine BOECs were transduced with Lenti-EF1α-GFP at various multiplicity of infections (MOIs), and the percentage of BOECs that expressed GFP was calculated by flow cytometry at 3 days after transduction (Fig. 2). At MOIs of 20, 2, 1, and 0.5, 93.4%, 60.0%, 43.7%, and 27.4% of BOECs expressed GFP without any signs of cell toxicity, and the gene expressions persisted for more than 4 weeks. The percentage of GFP-positive cells of non-transduced BOECs was 3.1%. Hence, the BOECs that were transduced at 20 of MOI were used in the subsequent experiments.
Fig. 2.
Lentiviral vector transduction of BOECs in vitro. Canine BOECs were transduced with Lenti-EF1α-GFP at various multiplicity of infections (MOIs), and the percentage of BOECs expressing GFP was calculated by flow cytometry at 3 days after transduction. At MOI of 20, 2, 1, and 0.5, 93.4%, 60.0%, 43.7%, and 27.4% of BOECs expressed GFP. The percentage of GFP-positive cells of non-transduced BOECs was 3.1%.
3.2. Implantation of genetically-modified BOECs sheets onto the omentum of dogs
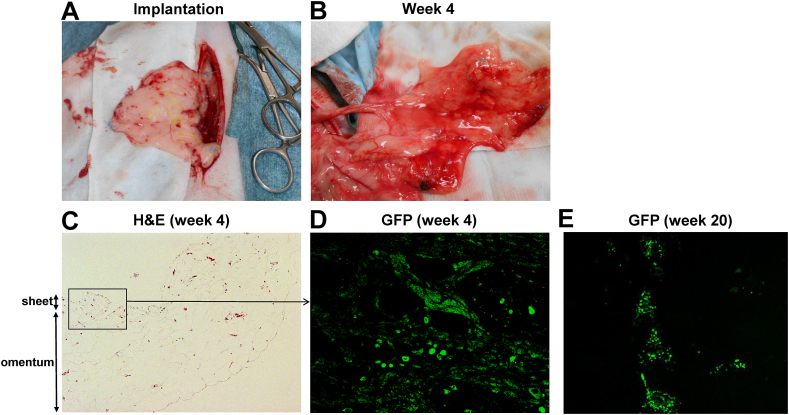
Two normal beagle dogs (Dog 1: female, 6.5 years old, 9 kg of body weight, and Dog 2: male, 3.5 years old, 10 kg of body weight) were implanted with autologous BOECs sheets which were transduced with GFP gene ex vivo, on their omentum, as described in methods. Open biopsies were performed at 4 and 20 weeks after operation on both dogs. Then, Dog 1 and Dog 2 were euthanized at 56 and 72 weeks after initial operations, respectively. The surgical procedures were well-tolerated, and during the observation periods including both the cell-sheets implantation and biopsy, there were no adverse events on physiological parameters. The macroscopic views of the omentum of recipient dogs immediately after cell sheet implantation (Fig. 3A) and 4 weeks after implantation (the timing of first biopsy) (Fig. 3B) were shown. Histological findings from the first omental biopsy at week 4 revealed the tube-like structure formation by implanted GFP-positive BOECs in the sub-adipose tissue layer without any inflammatory findings (Fig. 3C&D). The second biopsy performed 20 weeks after implantation and histological analyses using samples harvested at euthanasia also showed GFP-positive cells at the implantation sites (Fig. 3E). These results suggested that the implanted BOECs could efficiently engrafted, partly differentiated into mature endothelial cells, and formed new blood vessel structures within recipient omentum without reducing expressions of the transduced gene.
Fig. 3.
Implantation of genetically-modified blood outgrowth endothelial cells (BOECs) sheets on the omentum of dogs. Two beagle dogs (dog 1 and 2) were implanted with autologous BOECs sheets which were transduced with GFP gene ex vivo, on their omentum. (A) The macroscopic views of the omentum of recipient dog 1 immediately after cell sheet implantation. For future biopsy, implanted areas were marked with nylon non-absorbable surgical suture (blue color) for orientation. (B) The macroscopic views of the omentum of dog 1 at 4 weeks after implantation (the timing of first biopsy). (C–E) Histological analysis from the ometum biopsy samples. H&E staining (x10) at 4 weeks (C), GFP fluorescence at 4 weeks (x80) (D), and GFP fluorescence at 20 weeks after implantation (x10) are shown. GFP view at 4 weeks was focused on the parts of omentum indicated by open square. The implanted BOECs could efficiently engrafted, partly differentiated into mature endothelial cells and formed new blood vessel structures within recipient omentum without reducing GFP gene expressions.
3.3. Liver-targeted gene transfer by hydrodynamic injection in dogs
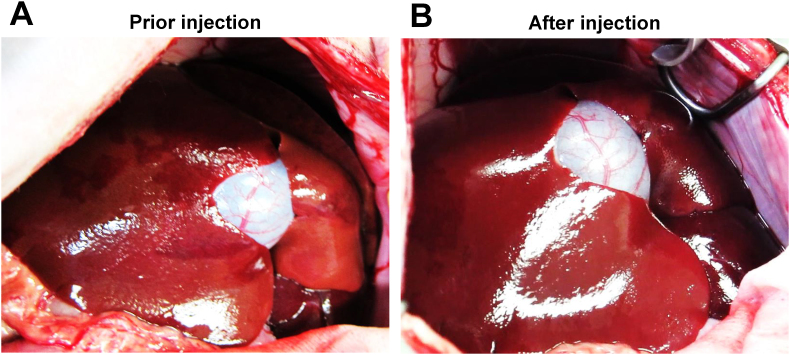
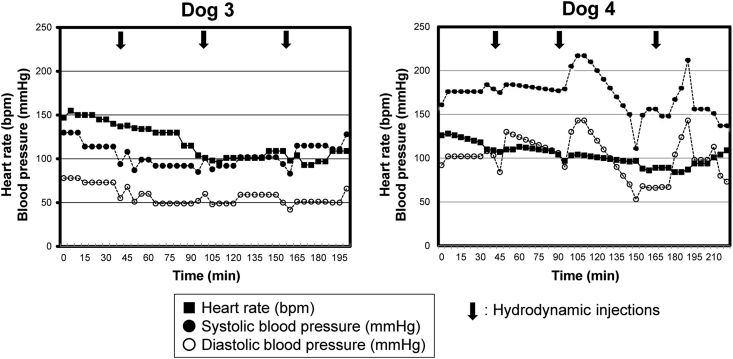
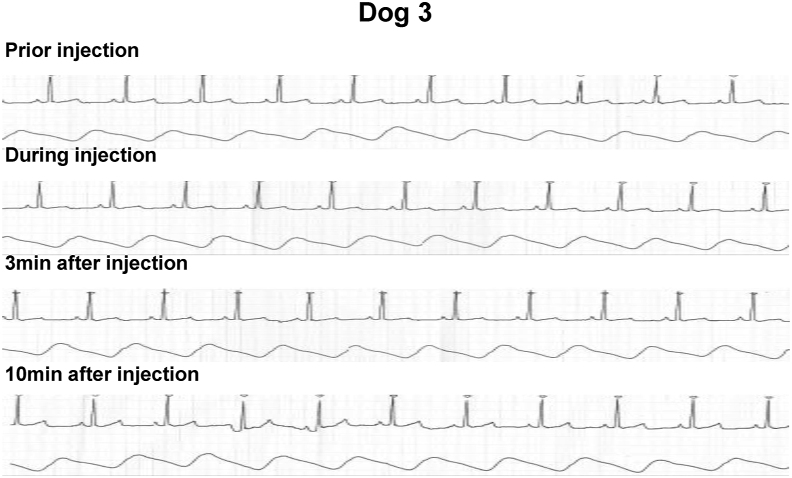
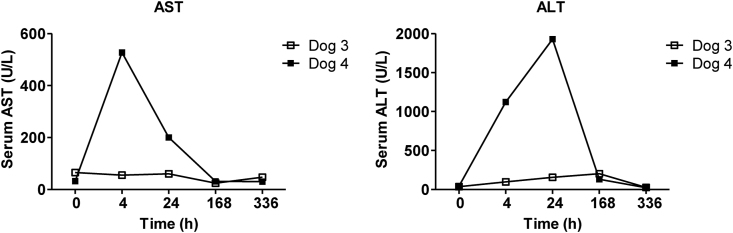
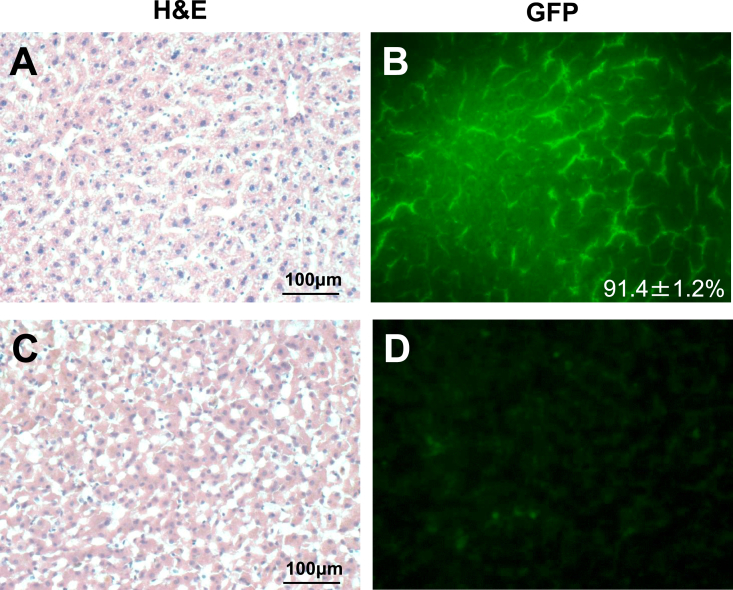
Another two normal beagle dogs (Dog 3: female, 6.5 years old, body weight 10 kg, and Dog 4: male, 3.5 years old, body weigh 11 kg) were subjected to in vivo GFP gene transduction with piggyBac vector by liver-targeted hydrodynamic injection as described in methods. Echo-guided liver biopsy were performed 1 and 20 weeks after the procedures on both dogs. Then, Dog 3 and Dog 4 were euthanized at 64 and 52 weeks after the procedures, respectively. Movie of the image-guided and liver-targeted hydrodynamic gene delivery procedure were demonstrated in the supplementary video file. Appropriate catheter placement was ensured, and the distribution of the contrast medium was confirmed before and after injection, then hydrodynamic injections were performed. Immediately following hydrodynamic injection through hepatic vein, backflow was observed in both hepatic artery and portal vein. After injection, the transient overexpansion of the targeted liver lobe was macroscopically observed (Fig. 4A&B). Physical examination data, including electrocardiogram, heart rate, systolic blood pressure and diastolic blood pressure during the perioperative period was shown in Fig. 5, Fig. 6. Although the transient slight decreases of heart rate were observed immediately after each hydrodynamic injection in both dogs, they were spontaneously normalized during the interval (Fig. 5). Slight increases of blood pressures were recorded immediately after hydrodynamic injections, especially in dog 4, but they returned to the baseline during the interval without any medical treatments (Fig. 5). In electrocardiogram, there were no changes in the dynamic ST segments and T waves throughout the studies (Fig. 6). Both dogs kept 100% of SpO2 and no remarkable changes of body temperatures during the studies (data not shown). Gene delivery-related liver toxicity was analyzed using collected plasma samples during the studies at appropriate time points (before and 4, 24, 168, 336 h after the procedures). In dog 4, transient 3 to 40-fold increase in hepatobiliary enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was observed within 24 h after hydrodynamic injection, but these levels were normalized within 168 h without any medical treatments (Fig. 7). Both dogs demonstrated no signs of jaundice. Gene delivery efficiency was assessed by detecting GFP expression of the liver biopsy samples collected at 1 and 20 weeks after injections (Fig. 8). More than 90% of liver cells (91.4 ± 1.2%) such as hepatocytes and sinusoidal endothelial cells in injected liver lobes were positive for GFP signals at 1 week in the samples harvested from right medial liver lobe (injected lobe) (Fig. 8B). On the other hand, GFP signal were scarcely detected in the non-injected liver lobes (right lateral liver lobe) (Fig. 8D), indicating that detected GFP signals were derived from transduced gene and not from autofluorescence of the liver. At week 20, however, the signals have decreased, and 52.7 ± 3.4% of liver were calculated as GFP positive. H&E staining of liver biopsy samples at 1 week after injections showed no significant histological findings such as the deformation of hepatocytes and the expansion of the sinusoids in injected and non-injected lobes (Fig. 8A&C). These results suggested that liver-targeted hydrodynamic gene delivery was efficiently conducted with minimum liver dysfunction and no significant adverse events on physiological parameters such as systemic circulation, respiration and cardiac function during the observation periods.
Fig. 4.
Liver-targeted gene transfer by hydrodynamic injection on dogs. Two beagle dogs (Dog 3 and 4) were subjected to in vivo GFP gene transduction with piggyBac vector by liver-targeted hydrodynamic injection. The prepared plasmid mixture was divided into three (50 mL, each), and then injected into the 3 liver lobes, right lateral, right medial, and left lateral lobe, respectively, at the speed of 10 mL per second for 5 s. Macroscopic views of the liver of dog 3 before (A) and immediately after (B) three times of injections are shown. An obvious overexpansion of liver lobes was observed after hydrodynamic injections.
Fig. 5.
Change of physiological parameters during the liver-targeted gene transfer by hydrodynamic injection on dogs. Heart rate (filled square), systolic blood pressure (filled circle), and diastolic blood pressure (open circle) of dog 3 and 4 were monitored during the hydrodynamic injection procedures. Filled arrows indicate the timing of hydrodynamic injections. The slight elevations of blood pressures accompanied with bradycardia were recorded immediately after hydrodynamic injections but returned to the baseline during the interval without any medical treatments.
Fig. 6.
Electrocardiogram during the liver-targeted gene transfer by hydrodynamic injection on dogs. Electrocardiogram of dog 3 prior, during, 3 min, and 10 min after hydrodynamic injection to the liver lobe are shown. No changes in the dynamic ST segments and T waves were observed.
Fig. 7.
Assessments of liver function by serum biochemistry during the liver-targeted gene transfer by hydrodynamic injection on dogs. Blood samples were collected from the cephalic veins of dog 3 and 4 before, and 4, 24, 168, 336 h after hydrodynamic injections. Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured. Open squares and filled squares indicate dog 3 and 4, respectively. Transient increases of AST and ALT were observed within 24 h after hydrodynamic injection, especially in dog 4, but these levels were normalized within 168 h without any medical treatments.
Fig. 8.
Histological findings from the liver biopsy of dogs after liver-targeted gene transfer by hydrodynamic injection. Gene delivery efficiency was assessed by detecting GFP expression of the liver biopsy samples collected at 1 and 20 weeks after the hydrodynamic injections. Representative photomicrographs of liver histology of dog 3 obtained 1 week after injections were shown. Hematoxylin and eosin (H&E) staining (x20) (A&C) and GFP expressions (x20) (B&D) of right medial liver lobe (injected lobe) (A&B) and right lateral liver lobe (non-injected lobe) (C&D) were shown. Approximately over 90% of liver cells in injected liver lobes were positive for GFP signals, whereas GFP signal were scarcely detected in non-injected lobes. HE staining demonstrated no significant histological findings such as the deformation of hepatocytes and the expansion of the sinusoids.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2021.08.009.
The following is the supplementary data related to this article:
Video 1Movie of the image-guided and liver-targeted hydrodynamic gene delivery procedure were demonstrated. Appropriate catheter placement was ensured, and the distribution of the contrast medium was confirmed before and after injection, then hydrodynamic injections were performed.
4. Discussion
In this study, we demonstrated the efficacy and safety of two types of novel gene transfer techniques for ex vivo and in vivo gene therapy in canine models. Sustainable expression of transduced GFP gene were observed both in gene-transduced BOECs sheets which were implanted onto the omentum, and in the liver which were transduced with the piggyBac vector transgene by modified hydrodynamic injection technique.
An ultimate goal for the treatment of inherited single gene disorder including hemophilia is the permanent expression of the deleted protein. Bleeding tendency of hemophilia patients persists for a lifetime, indicating the patients face a critical bleeding risk at all time. Replacement therapy using coagulation factor concentrates can control bleeding events and improve the patient's quality of life. However, this type of therapy requires life-long and frequent intravenous injections. In this context, development of novel curative measures free from factor concentrate injection are highly anticipated. Under these circumstances, in vivo gene therapy using AAV vector have been developed for hemophilia [[3], [4], [5], [6], [7]]. For further advancing the hemophilia gene therapy, we previously developed two types of novel gene transfer techniques for hemophilia, and validated the therapeutic efficacy using a mouse model of hemophilia A [11,12]. In this study, we succeeded in modifying these gene transfer techniques to apply for a dog model.
Ex vivo gene therapy, in which autologous cells transduced with target gene ex vivo are transplanted to the patients, have an advantage over conventional in vivo gene therapy in term of avoiding direct viral toxicity. On the other hand, to achieve long-term therapeutic effects, stable and persistent gene transduction to the cells and technologies for efficient and durable cell engraftment are required. Furthermore, it is desirable that autologous cells are obtained less-invasively without major surgery. In this context, tissue engineering approach using lentivirally-modified BOEC sheets may be one of the unique and effective treatment options that fulfill all the above-mentioned requirements. BOECs are desirable cell source for this purpose, because they can be sorted from peripheral whole blood, be expanded in culture condition while retaining its cell characteristics as endothelial progenitors, and be efficiently transfected with lentivirus vector [10]. BOECs have been also demonstrated to stably express von Willebrand factor (VWF), a glycoprotein which serves as a carrier protein of FVIII and protects FVIII from proteolysis in the blood [10]. To achieve durable cell engraftment, we utilized cell sheet technology for transplanting the prepared cells [11,13]. Cell sheet technology allows harvesting the cultured cells in monolithic layer format (cell sheet) by using temperature-responsive culture dishes that are covalently grafted with temperature-responsive polymer poly N-isopropylacrylamaide) (PIPAAm) on their surfaces at nanometer thickness [[15], [16], [17], [18]]. PIPAAm coating allows conventional cell culturing at the regular culture temperature (37 °C), but the cultured cells cannot adhere to the surface below 32 °C because of rapid hydration and swelling of the grafted PIPAAm. This results in the natural detachment of the cells from the surface of culture dishes as a viable monolayer cell sheet format. Of note, this technology needs no enzymatic digestion to harvest the cells, enabling us to prepare transplantable tissue constructs preserving intact cell–cell contacts and extracellular matrices, enabling efficient and durable cell engraftment. This technology has been applied for fabricating various types of tissue constructs, and several clinical trials have been practically performed and succeeded [[16], [17], [18]]. In this present study, as a large animal study for cell sheet therapy, we fabricated GFP gene-transduced canine BOECs sheets, transplanted them onto the omentum of the same dogs. We targeted the omentum as a transplantation site, expecting that the abundant blood supply from the recipients enable the transplanted cell sheets to efficiently engraft and survive for a long period. Actually, Ozelo et al. previously demonstrated the feasibility of omentum as a cell implantation site in dogs [19]. As another transplantation site, subcutaneous spaces would be attractive because cell sheet can be transplanted less invasively only by skin incision [11,20]. Liver surface site will be also a candidate because the main organ responsible for the production of coagulation factors, including FVIII and FIX, is the liver. By transplanting the cell sheet onto the liver surface and interacting the cells with the host liver tissues, efficient production of coagulation factors could be expected [[21], [22], [23]]. Optimization of the cell sheet transplantation sites should be conducted before clinical application.
In the field of in vivo gene therapy for hemophilia, many clinical studies using AAV vector have been conducted worldwide, and several studies have yielded promising results without any adverse events. However, there still remain concerns over the safety including immunological reactions and vector-mediated cytotoxicity. In this context, developments of non-viral gene delivery methods which allows efficient and durable gene expression are highly anticipated. For that, we focused on liver-targeted hydrodynamic injections with the piggyBac vector transgene. Hydrodynamic injection is a one of the promising technologies that is used to achieve sufficient transgene expression in vivo. In mouse models, a large volume (2–3 mL; volume equal to 8–10% of murine body weight) of plasmid DNA solution are intravenously injected within 5–10 s [24]. The transgene expressions by hydrodynamic injection are usually observed in the liver. Kamimura et al. applied this technique to large animal models such as dogs and pigs, and proved its safety by image-guided targeting to the specific liver lobes [25,26]. However, it is generally recognized that gene expressions achieved by naked DNA injection do not persist for long-period, which would be a critical drawback for hemophilia therapy. To overcome this issue, we utilized the piggyBac transposon vector transgene instead of naked DNA for liver-targeted hydrodynamic injection, and succeeded in long-term GFP expression in the targeted liver lobes. Of note, although transient increase of AST and ALT were observed in dog 4, no severe adverse events were occurred during and after the procedures. Regarding the difference of transaminase increase between dog 3 and 4, several reasons are assumed. In a previous report by Hyland et al., the authors also performed liver-targeted hydrodynamic injection to the dogs [27]. They injected 200 mL of solution to the specific liver lobes of dogs (BW 5–6 kg) at the speed of 20–40 mL/s and observed a gene transduction together with transient increase of ALT and AST in all dogs. On the other hand, we used bigger dogs (BW 10–11 kg) and injected less volume of solution (150 mL). It would be reasonable to speculate that these differences led to less increase of transaminase in dog 3. That is, relative volume of liver lobes of dog 3 was bigger, and enough increase of intravascular pressure which could lead to efficient gene transduction and transient liver injury was not achieved. If we used smaller dogs or injected more volume of solution, the results might be different. Although conventional plasmid vector systems are inefficient at stably integrating into the target genome, transposon vectors have emerged as attractive gene-delivery tools because of their ability to stably integrate into the genome and achieve efficient and prolonged transgene expression both in vitro and in vivo. Especially, the piggyBac DNA transposon, which was originally isolated from the cabbage looper moth Trichoplusia ni., have higher transposition activity than other widely used transposon vector systems, such as Sleeping Beauty or Tol2 [[28], [29], [30], [31]]. By combining this piggyBac DNA transposon system with hydrodynamic gene transfer, we previously succeeded in achieving stable and sustained FVIII expression in hemophilia A mice [12].
The rationale that we used a canine model as a large animal mode is the existence of a dog model of hemophilia [32]. Importantly, the models are spontaneous model (i.e. not transgenic) and closely represent similar bleeding symptom as hemophilia patients. This model has been historically utilized as an animal model of preclinical study for the development of novel therapeutics toward hemophilia. On the other hand, transgenic porcine model of hemophilia has been recently developed [33]. This model might be also a large animal model of hemophilia in the future.
Although more technical improvements are necessary to apply to a human clinical trial, the present results of large animal study shown here would be an important milestone for advancing ex vivo and in vivo gene therapy for hemophilia.
5. Conclusions
Two types of gene transfer techniques were successfully applied to a canine model, and the transduced gene expressions persisted for a long term. Toward clinical application for hemophilia patients, assessments of practical therapeutic efficacy of these techniques for hemophilia have to be performed by introducing FVIII or FIX gene to hemophilia dogs. The research on these topics is now ongoing in a canine model of hemophilia A.
Funding statements
The study was partly supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (grant number 15K09662, 25293237, and 24591559), the Bayer Hemophilia Awards Program, and SENSHIN Medical Research Foundation.
Declaration of competing interest
Teruo Okano, Ph.D. is a stockholder of CellSeed Inc. which has licenses for certain cell sheet-related technologies and patents from Tokyo Women's Medical University. Other authors have no interests to declare.
Acknowledgements
We thank Ms. Yumi Yoshida and Ms. Ayuri Nakamura for their technical assistance. We also thank Dr. Junichi Ori (Ori Animal Hospital), Dr. Sunao Ochi and Dr. Masanari Nakayama (Nakayama Veterinary Hospital) for their technical assistance and helpful advices for the dog experiments.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Bolton-Maggs P.H., Pasi K.J. Haemophilias A and B. Lancet. 2003;361:1801–1809. doi: 10.1016/S0140-6736(03)13405-8. [DOI] [PubMed] [Google Scholar]
- 2.Peters R., Harris T. Advances and innovations in haemophilia treatment. Nat Rev Drug Discov. 2018;17:493–508. doi: 10.1038/nrd.2018.70. [DOI] [PubMed] [Google Scholar]
- 3.Nathwani A.C., Tuddenham E.G., Rangarajan S. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathwani A.C., Davidoff A.M., Tuddenham E.G.D. Advances in gene therapy for hemophilia. Hum Gene Ther. 2017;28:1004–1012. doi: 10.1089/hum.2017.167. [DOI] [PubMed] [Google Scholar]
- 5.Nathwani A.C., Reiss U.M., Tuddenham E.G. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntosh J., Lenting P.J., Rosales C. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangarajan S., Walsh L., Lester W. AAV5-Factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 8.Colella P., Ronzitti G., Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev. 2018;8:87–104. doi: 10.1016/j.omtm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsui H. Endothelial progenitor cell-based therapy for hemophilia A. Int J Hematol. 2012;95:119–124. doi: 10.1007/s12185-012-1015-z. [DOI] [PubMed] [Google Scholar]
- 10.Matsui H., Shibata M., Brown B. Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vivo factor VIII expression from lentivirally engineered endothelial progenitors. Stem Cell. 2007;25:2660–2669. doi: 10.1634/stemcells.2006-0699. [DOI] [PubMed] [Google Scholar]
- 11.Tatsumi K., Sugimoto M., Lillicrap D. A novel cell-sheet technology that achieves durable factor VIII delivery in a mouse model of hemophilia A. PloS One. 2013;8 doi: 10.1371/journal.pone.0083280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui H., Fujimoto N., Sasakawa N. Delivery of full-length factor VIII using a piggyBac transposon vector to correct a mouse model of hemophilia A. PloS One. 2014;9 doi: 10.1371/journal.pone.0104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatsumi K., Okano T. Hepatocyte transplantation: cell sheet technology for liver cell transplantation. Curr Transplant Rep. 2017;4:184–192. doi: 10.1007/s40472-017-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui H., Sugimoto M., Mizuno T. Distinct and concerted functions of von Willebrand factor and fibrinogen in mural thrombus growth under high shear flow. Blood. 2002;100:3604–3610. doi: 10.1182/blood-2002-02-0508. [DOI] [PubMed] [Google Scholar]
- 15.Okano T., Yamada N., Sakai H., Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi J., Kikuchi A., Aoyagi T., Okano T. Cell sheet tissue engineering: cell sheet preparation, harvesting/manipulation, and transplantation. J Biomed Mater Res. 2019;107:955–967. doi: 10.1002/jbm.a.36627. [DOI] [PubMed] [Google Scholar]
- 17.Matsuura K., Utoh R., Nagase K., Okano T. Cell sheet approach for tissue engineering and regenerative medicine. J Contr Release. 2014;190:228–239. doi: 10.1016/j.jconrel.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Owaki T., Shimizu T., Yamato M., Okano T. Cell sheet engineering for regenerative medicine: current challenges and strategies. Biotechnol J. 2014;9:904–914. doi: 10.1002/biot.201300432. [DOI] [PubMed] [Google Scholar]
- 19.Ozelo M.C., Vidal B., Brown C. Omental implantation of BOECs in hemophilia dogs results in circulating FVIII antigen and a complex immune response. Blood. 2014;123:4045–4053. doi: 10.1182/blood-2013-12-545780. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi K., Yokoyama T., Yamato M. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 21.Itaba N., Matsumi Y., Okinaka K. Human mesenchymal stem cell-engineered hepatic cell sheets accelerate liver regeneration in mice. Sci Rep. 2015;5:16169. doi: 10.1038/srep16169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto D., Sakai Y., Huang Y. Functional changes of cocultured hepatocyte sheets subjected to continuous liver regeneration stimulation in cDNA-uPA/SCID mouse: differences in transplantation sites. Regen Ther. 2021;18:7–11. doi: 10.1016/j.reth.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagamoto Y., Takayama K., Ohashi K. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068–1075. doi: 10.1016/j.jhep.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Budker V., Zhang G., Knechtle S., Wolff J.A. Naked DNA delivered intraportally expresses efficiently in hepatocytes. Gene Ther. 1996;3:593–598. [PubMed] [Google Scholar]
- 25.Kamimura K., Kanefuji T., Yokoo T. Safety assessment of liver-targeted hydrodynamic gene delivery in dogs. PloS One. 2014;9 doi: 10.1371/journal.pone.0107203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamimura K., Suda T., Xu W., Zhang G., Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyland K.A., Aronovich E.L., Olson E.R. Transgene expression in dogs after liver-directed hydrodynamic delivery of sleeping beauty transposons using balloon catheters. Hum Gene Ther. 2017;28:541–550. doi: 10.1089/hum.2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding S., Wu X., Li G. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Huang X., Guo H., Tammana S. Gene transfer efficiency and genome-wide integration profiling of Sleeping Beauty, Tol2, and piggyBac transposons in human primary T cells. Mol Ther. 2010;18:1803–1813. doi: 10.1038/mt.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibault S.T., Singer M.A., Miyazaki W.Y. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- 31.Wilson M.H., Coates C.J., George A.L., Jr. PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- 32.Yen C.T., Fan M.N., Yang Y.L. Current animal models of hemophilia: the state of the art. Thromb J. 2016;14:22. doi: 10.1186/s12959-016-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashiwakura Y., Mimuro J., Onishi A. Porcine model of hemophilia A. PloS One. 2012;7 doi: 10.1371/journal.pone.0049450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1Movie of the image-guided and liver-targeted hydrodynamic gene delivery procedure were demonstrated. Appropriate catheter placement was ensured, and the distribution of the contrast medium was confirmed before and after injection, then hydrodynamic injections were performed.