Abbreviations
- CR
complete response
- CRC
colorectal cancer
- CTLA‐4
anti‐cytotoxic T lymphocyte antigen‐4
- DCR
disease control rate
- dMMR
mismatch repair‐deficient
- ICB
immune checkpoint blockade
- MMR
mismatch repair
- MSI
microsatellite instability
- MSI‐H
microsatellite instability‐high
- MSI‐L
microsatellite instability‐low
- MSS
microsatellite stable
- ORR
objective response rate
- OS
overall survival
- PD
progression disease
- PD‐1
programmed cell death protein‐1
- PFS
progression‐free survival
- pMMR
mismatch repair‐proficient
- PR
partial response
- SD
stable disease
- TRAE
treatment‐related adverse event
Dear Editor,
Colorectal cancer (CRC) is a common cancer in China and worldwide [1, 2]. Immune checkpoint blockade (ICB) has been proven effective for DNA mismatch repair‐deficient (dMMR)/microsatellite instability‐high (MSI‐H) CRC [3, 4, 5, 6, 7, 8, 9, 10] but not for mismatch repair‐proficient (pMMR)/microsatellite stable (MSS) CRC in clinical trials [3]. No published data on the real‐world application of ICB in CRC exist, and thus, whether the response to ICB in unselected patients is similar to that in patients from published trials remains unclear. In this study, we reported results from the real‐world application of ICB in off‐trial CRC patients (irrespective of stage), treated at the Sun Yat‐sen University Cancer Center from March 1, 2017, to October 1, 2019. We analyzed the mismatch repair (MMR) and microsatellite instability (MSI) status, demographic characteristics, treatment regimens, response to ICB, and adverse events of CRC patients who received ICB in a real‐world off‐trial, to help devise effective immunotherapy strategies for routine clinical practice.
We identified 69 CRC patients who received ICB using an off‐trial protocol. The detailed methods are provided in the Supplementary Materials. The inclusion and exclusion criteria are shown in Supplementary Figure S1. Fifty‐two patients were classified according to their MMR status; 27 (51.9%) as dMMR and 25 (48.1%) as pMMR. Fifty patients were classified according to their MSI status; 29 (58.0%) as MSI‐H, 2 (4.0%) as MSI‐low (MSI‐L), and 19 (38.0%) as MSS. In this study, dMMR and/or MSI‐H patients were further classified as dMMR/MSI‐H, and patients who were neither dMMR nor MSI‐H were classified as pMMR/MSI‐L/MSS. Finally, 36 (52.2%) patients were classified as dMMR/MSI‐H, and 30 (43.5%) as pMMR/MSI‐L/MSS. The MMR/MSI status of 3 (4.3%) patients was unknown.
The patients’ characteristics are shown in Supplementary Table S1. Their median age was 45 (range, 16‐67) years at ICB initiation. Thirty‐nine (56.5%) patients were male. Fifty‐six (81.2%) patients had stage IV disease when ICB was initiated. The most common primary tumor site was the colon (n = 48; 69.6%). Twenty‐one (30.4%) patients had a family history of CRC.
Before immunotherapy, the number of patients who received chemotherapy, targeted therapy, surgery, and radiotherapy were 55 (79.7%), 30 (43.5%), 38 (55.1%), and 11 (15.9%), respectively (Supplementary Table S1 and S2). In addition, 38 patients underwent surgery and among them, 21 patients had stage II/III disease and 17 patients had stage IV disease when first diagnosed. The 21 patients with stage II/III disease all underwent primary tumor resection, and two also underwent metastasectomy when metastatic disease progression was observed. Among the 17 patients with stage IV disease who underwent surgery, 16 underwent primary tumor resection, and 13 underwent metastasectomy (7 received radical surgery, and 6 received palliative surgery). Three of the 17 patients also underwent metastasectomy when disease progression with new metastasis was observed.
The median time from initial diagnosis to first ICB treatment was 6.2 months (range, 0.2‐124.1 months, Supplementary Figure S2). ICB was administered as neoadjuvant therapy to 13 (18.8%) patients, as first‐line therapy to 16 (23.2%) patients, and as second‐line or later therapy to 40 (58.0%) patients. The median duration of immunotherapy was 68 days (range, 1‐939 days, Supplementary Figure S2), and the median number of immunotherapy cycles was 4 (range, 1‐39). All patients received one or two types of programmed cell death protein‐1 (PD‐1) blockade. The cytotoxic T lymphocyte antigen‐4 (CTLA‐4) inhibitor ipilimumab was administered to only 8 (11.6%) patients (Supplementary Table S3). Twenty‐three (33.3%) patients received only mono‐immunotherapy, and 46 (66.7%) patients received combined therapy, including combined chemotherapy (32, 46.4%), targeted therapy (24, 34.8%), and/or radiotherapy (8, 11.6%). The chemotherapy and targeted therapy agents administered during immunotherapy are listed in Supplementary Table S4.
Regarding treatment effects, the best overall response was considered as the short‐term treatment effect, while overall survival (OS) and progression‐free survival (PFS) as long‐term effects. Of the 69 patients, an objective response to ICB was noted in 22 (31.9%) patients, including complete response (CR) in 9 (13.0%) and partial response (PR) in 13 (18.8%) patients. Stable disease (SD) was observed in 18 (26.1%) patients. The remaining 29 patients (42.0%) experienced progressive disease (PD), with a median PFS duration of 2.1 (range, 0.3‐30.6) months. The best overall response of all patients, dMMR/MSI‐H patients, and pMMR/MSI‐L/MSS patients are described in Supplementary Tables S5‐7. Univariate analysis showed that MMR/MSI status and immunotherapy setting were significantly associated with the objective response rate (ORR) and disease control rate (DCR). Similar to that reported in clinical trials, in this study, dMMR/MSI‐H patients had a higher ORR and DCR than pMMR/MSI‐L/MSS patients (50.0% vs. 13.3%, P = 0.002; 75.0% vs. 43.3%, P = 0.003) (Supplementary Table S5). In addition to MMR/MSI status, immunotherapy setting was also associated with ORR and DCR. The ORRs in patients who received ICB as neoadjuvant therapy, first‐line therapy, and second‐line or later therapy were 84.6%, 50.0%, and 7.5%, respectively (P < 0.001), and the DCRs were 92.3%, 87.5%, and 35.0%, respectively (P < 0.001). When the two factors were subjected to multivariate analysis, only the immunotherapy setting was independently associated with ORR and DCR (Supplementary Table S5).
We also separately analyzed the best overall response of dMMR/MSI‐H and pMMR/MSI‐L/MSS patients. Patients who received ICB early, as neoadjuvant therapy or first‐line therapy, had higher ORRs and DCRs in both the dMMR/MSI‐H (Supplementary Table S6) and pMMR/MSI‐L/MSS patient groups (Supplementary Table S7).
For all patients, by January 4, 2021, after a median follow‐up time of 15.1 (range, 0.3‐38.0) months, the median OS had not been reached. The median PFS was 6.3 months, and the one‐year OS and PFS rates were 72.2% and 46.4% (Supplementary Table S8, Figure 1A‐B). The OS of each patient is shown in Supplementary Figure S2.
FIGURE 1.
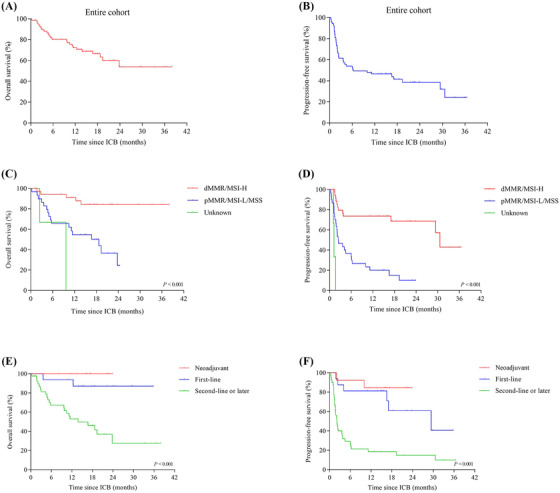
Kaplan‐Meier plots showing the OS and PFS curves for the entire cohort, patients with different MMR/MSI statuses, and patients at different immunotherapy settings. (A) Kaplan‐Meier plot showing the OS for the entire cohort. (B) Kaplan‐Meier plot showing the PFS for the entire cohort. (C) OS comparison among dMMR/MSI‐H patients, pMMR/MSI‐L/MSS patients, and patients with unknown MMR/MSI status (P < 0.001, log‐rank test). (D) Comparison of PFS among dMMR/MSI‐H patients, pMMR/MSI‐L/MSS patients, and MMR/MSI unknown patients (P < 0.001, log‐rank test). (E) Comparison of OS among patients who received immunotherapy as neoadjuvant therapy, first‐line therapy, and second‐line or later therapy (P < 0.001, log‐rank test). (F) Comparison of PFS among patients who received immunotherapy as neoadjuvant therapy, first‐line therapy, and second‐line or later therapy (P < 0.001, log‐rank test). Abbreviations: OS, overall survival; PFS, progression‐free survival; dMMR, mismatch repair‐deficiency; MSI‐H, microsatellite instability‐high; pMMR, mismatch repair‐proficient; MSI‐L, microsatellite instability‐low; MSS, microsatellite stable
Univariate analysis for investigating the association between clinical factors and survival showed that dMMR/MSI‐H status and early immunotherapy use were significantly associated with favorable OS (P < 0.001, P < 0.001) and PFS (P < 0.001, P < 0.001) (Supplementary Table S8, Figure 1C‐F). Multivariate analyses showed that MMR/MSI status was significantly associated with OS and PFS, while the immunotherapy setting was only significantly associated with PFS (Supplementary Table S8).
Long‐term survival analysis for dMMR/MSI‐H patients (Supplementary Table S9) showed that the OS and PFS of patients who received ICB early were longer than those who received ICB as second‐line or later therapy, although no significant differences were observed. In pMMR/MSI‐L/MSS patients (Supplementary Table S10), early ICB and combined therapy were associated with longer OS (P = 0.020, P = 0.011) and PFS (P = 0.004, P < 0.001). When the top two factors were subjected to multivariate analysis, immunotherapy setting and combination therapy were independently associated with PFS but not with OS.
Treatment‐related adverse events (TRAEs) were reported for 65 (94.2%) patients (Supplementary Table S11). Eleven (15.9%) patients experienced grade 3 TRAEs, and five patients (7.2%) experienced grade 4 TRAEs. All the TRAEs were successfully treated. Only two patients discontinued ICB treatment due to the TRAEs.
Despite the study limitations (small cohort and non‐uniform ICB regimens), to our knowledge, this is the first study to explore the efficacy and safety of immunotherapy in a real‐world cohort of CRC patients. Our results demonstrated that ICB was more effective for dMMR/MSI‐H CRC than pMMR/MSI‐L/MSS, as previously reported in clinical trials, and the earlier use of ICB resulted in better tumor response, especially in pMMR/MSI‐L/MSS CRC patients. In real‐world settings, combined therapy could prolong the OS and PFS of pMMR/MSI‐L/MSS CRC patients but not dMMR/MSI‐H CRC patients.
DECLARATION
AUTHORS’ CONTRIBUTIONS
Study concept and design: CJZ, WWX, YHG. Data acquisition: CJZ, TJ, RZL. Data analysis and interpretation: CJZ, WHX, XXH, YY, QXW, HC. Manuscript writing: CJZ, WWX. Critical revision: All authors. Final approval of manuscript: All authors.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Ethics Committee of Sun Yat‐sen University Cancer Center (No. B2020‐134‐01). Informed consent was waived as this was a noninterventional study using routinely collected data.
FUNDING
This study was funded by the National Natural Science Foundation of China (81672987, 82073329) and the Natural Science Foundation of Guangdong Province (2020A1515011286).
CONFLICT OF INTEREST
The authors declare there are no conflicts of interest.
DATA AVAILABILITY STATEMENT
The raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the RDD number RDDA2021001951 and the datasets used in this study are publicly available.
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors wish to thank the medical staff and patients for their contribution to this study.
Contributor Information
Weiwei Xiao, Email: xiaoww@sysucc.org.cn.
Yuanhong Gao, Email: gaoyh@sysucc.org.cn.
REFERENCES
- 1.Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang TJ, Wang F, Wang YN, Hu JJ, Ding PR, Lin JZ, et al. Germline mutational profile of Chinese patients under 70 years old with colorectal cancer. Cancer Commun (Lond). 2020;40(11):620‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD‐1 Blockade in Tumors with Mismatch‐Repair Deficiency. N Engl J Med. 2015;372(26):2509‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Neil BH, Wallmark JM, Lorente D, Elez E, Raimbourg J, Gomez‐Roca C, et al. Safety and antitumor activity of the anti‐PD‐1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12(12):e0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science. 2017;357(6349):409‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair‐Deficient/Microsatellite Instability‐High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36(8):773‐9. [DOI] [PubMed] [Google Scholar]
- 8.Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, et al. Phase II Open‐Label Study of Pembrolizumab in Treatment‐Refractory, Microsatellite Instability‐High/Mismatch Repair‐Deficient Metastatic Colorectal Cancer: KEYNOTE‐164. J Clin Oncol. 2020;38(1):11‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite‐Instability‐High Advanced Colorectal Cancer. N Engl J Med. 2020;383(23):2207‐18. [DOI] [PubMed] [Google Scholar]
- 10.Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR‐proficient and MMR‐deficient early‐stage colon cancers. Nat Med. 2020;26(4):566‐76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Data Availability Statement
The raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the RDD number RDDA2021001951 and the datasets used in this study are publicly available.


