Abstract
Background
Previous studies have demonstrated the preclinical pharmacological and toxicological consistency, and clinical pharmacokinetic equivalence of bevacizumab biosimilar LY01008 with reference bevacizumab (Avastin). This randomized controlled trial aimed to compare the efficacy and safety of LY01008 with Avastin in first‐line treatment of Chinese patients with advanced or recurrent non‐squamous non‐small cell lung cancer (NSCLC).
Methods
Stage IIIB‐IV NSCLC patients with evaluable lesions, good physical status, and adequate organ functions from 67 centers across China were randomized in a ratio of 1:1 to receive LY01008 or Avastin 15 mg/kg intravenously in combination with paclitaxel/carboplatin (combined treatment) for 4‐6 cycles, followed by maintenance monotherapy with LY01008 until disease progression, intolerable toxicity, or death. The primary endpoint was objective response rate (ORR) in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 confirmed by independent radiological review committees (IRRC). Secondary endpoints included disease control rate (DCR), duration of response (DoR), progression‐free survival (PFS), overall survival (OS), and safety. This study was registered in ClinicalTrials.gov (NCT03533127).
Results
Between December 15th, 2017, and May 15th, 2019, a total of 649 patients were randomized to the LY01008 (n = 324) or Avastin (n = 325) group. As of September 25th, 2019 for primary endpoint analysis, 589 patients received ORR evaluation, with a median number of combined treatment cycles of 5 (range 1‐6) and median duration of treatment of 3.0 (range 0.0‐5.1) months. ORR of response‐evaluable patients in the LY01008 and Avastin groups were 48.5% and 53.0%, respectively. The stratified ORR ratio was 0.91 (90% CI 0.80‐1.04, within the prespecified equivalence margin of 0.75‐1.33). Up to May 15th, 2020, with a median follow‐up of 13.6 (range 0.8‐28.4) months, no notable differences in DCR, median DoR, median PFS, median OS, and 1‐year OS rate were observed between the LY01008 and Avastin groups. There were no clinically meaningful differences in safety and immunogenicity across treatment groups.
Conclusions
LY01008 demonstrated similarity to Avastin in terms of efficacy and safety in Chinese patients with advanced or recurrent non‐squamous NSCLC. LY01008 combined with paclitaxel/carboplatin is expected to become a new treatment option for unresectable, metastatic, or recurrent non‐squamous NSCLC patients in the first‐line setting.
Keywords: anti‐angiogenesis, anti‐VEGF monoclonal antibody, avastin, bevacizumab, biosimilar, non‐small cell lung cancer, LY01008, vascular endothelial growth factor
This study demonstrated similarity between LY01008 and reference bevacizumab (Avastin) in terms of efficacy, safety, and immunogenicity in combination with paclitaxel and carboplatin as first‐line treatment in Chinese patients with advanced non‐squamous NSCLC.
Abbreviations
- NSCLC
non–small cell lung cancer
- ECOG
Eastern Cooperative Oncology Group
- IRRC
independent radiological review committee
- ORR
objective response rate
- DCR
disease control rate
- DoR
duration of response
- PFS
progression‐free survival
- OS
overall survival
- VEGF
vascular endothelial growth factor
- EGFR
epidermal growth factor receptor
- TKI
tyrosine kinase inhibitor
- FDA
Food and Drug Administration
- EMA
European Medicines Agency
- NMPA
National Medical Product Administration
- PS
performance status
- RECIST
Response Evaluation Criteria in Solid Tumors
- CR
complete response
- PR
partial response
- SD
stable disease
- FAS
full analysis set
- AEs
adverse events
- TEAEs
treatment‐emergent adverse events
- SAEs
serious adverse events
- AESI
adverse events of special interest
- PRES
posterior reversible encephalopathy syndrome
- CTCAE
Common Terminology Criteria for Adverse Events
- ADAs
anti‐drug antibodies
- NAbs
neutralizing antibodies
- ITT
intent‐to‐treat
- PPS
per‐protocol sets
1. BACKGROUND
Lung cancer was associated with estimates of more than 2.2 million new cases and 1.8 million cancer‐related deaths worldwide, in 2020 [1], of which over one‐third occurred in China [2, 3]. Non‐small cell lung cancer (NSCLC) accounts for about 80%‐85% of the total cases of lung cancer [4, 5]. In the past 20 years, many new strategies that have changed the treatment paradigms for NSCLC have emerged [6, 7, 8]. Among these new strategies, targeted therapy and anti‐angiogenesis therapy are very important. For the former, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) exhibited superiority over cytotoxic therapy as the first‐line treatment in sensitive EGFR‐mutated NSCLC patients, with the objective response rate (ORR) of approximately 60%‐80% versus 30%‐40% and progression‐free survival (PFS) of around 9‐14 months versus 4‐6 months. For the latter, targeting the vascular endothelial growth factor (VEGF) by monoclonal antibodies is a key anti‐angiogenesis strategy [9, 10].
Anti‐angiogenesis has become a valid therapy in anti‐cancer treatment since 1971 [11]. The VEGF signaling pathway plays a dominant role in stimulating angiogenesis, which is the main process promoting tumor growth and metastasis [12, 13]. As an anti‐VEGF humanized monoclonal antibody, bevacizumab can neutralize VEGF‐A's biologic activity through a steric blocking of its binding with the VEGF receptor, and its efficacy has been demonstrated in patients with NSCLC [14, 15, 16].
Bevacizumab (Avastin, Roche, Basel, Switzerland) is the first anti‐angiogenic drug that has been widely used in combination with chemotherapy in several malignant tumors including NSCLC since its approval by the US Food and Drug Administration (FDA) on February 26th, 2004 and the European Medicines Agency (EMA) on January 12th, 2005 [17, 18, 19]. For advanced NSCLC, platinum‐based chemotherapy has yielded a response rate of 20%‐30%, with the median overall survival (OS) shorter than 1 year [20]. The BEYOND study (NCT01364012) confirmed the benefit of carboplatin/paclitaxel plus bevacizumab compared with carboplatin/paclitaxel alone in Chinese non‐squamous NSCLC patients. ORR was improved (54% vs. 26%, P < 0.001) and median OS was prolonged (24.3 vs. 17.7 months, P = 0.015, hazard ratio = 0.68) [16]. On August 1st, 2015, Avastin was approved by the China National Medical Product Administration (NMPA) for the treatment of advanced or recurrent non‐squamous NSCLC. In the era of immunotherapy, results of the IMpower150 study showed that bevacizumab combined with atezolizumab and carboplatin plus paclitaxel could generate survival benefits for advanced non‐squamous NSCLC in the first‐line setting [21].
Bevacizumab has become the standard of care for the treatment of advanced or recurrent non‐squamous NSCLC. In order to make bevacizumab more available, the development of bevacizumab biosimilar is needed [22, 23, 24, 25]. Bevacizumab biosimilar can provide accessibility at low costs with similar efficacy, thus enabling more patients to obtain clinical benefits [24, 26].
LY01008 is a bevacizumab biosimilar developed by Boan Biotechnology Co., Ltd. (Yantai, Shandong, China). Pharmacological and toxicological studies have shown that LY01008 is consistent with Avastin in preclinical pharmacodynamics, pharmacokinetics, and safety (data unpublished). Previous phase I pharmacokinetics similarity study (data unpublished) and a phase III efficacy study were conducted in alignment with the current regulation/recommendations for the development of bevacizumab biosimilars [27, 28, 29, 30]. The clinical pharmacokinetic equivalence of LY01008 with Avastin has been confirmed in a previous phase I study (data unpublished). In this phase III study, we compared the efficacy and safety of LY01008 plus paclitaxel/carboplatin with those of Avastin plus paclitaxel/carboplatin in the first‐line treatment of Chinese patients with advanced or recurrent non‐squamous NSCLC to verify the clinical similarity of the two drugs (ClinicalTrials.gov Identifier: NCT03533127).
2. PATIENTS AND METHODS
2.1. Study design and patients
Patients aged 18 to 75 years old with histologically or cytologically confirmed, unresectable, untreated, metastatic, or recurrent non‐squamous (Stage IIIB‐IV) NSCLC from 67 centers across China were enrolled in this randomized, double‐blinded, phase III study. Major criteria for eligible enrollment included Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0 or 1; at least one measurable lesion as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; adequate bone marrow, hepatic, and renal functions; expected life expectancy ≥6 months. Major exclusion criteria included the history of systemic anti‐cancer therapy for advanced disease or relapsed <6 months after neoadjuvant or adjuvant chemotherapy; mixed with small‐cell histology or squamous cells as the main component; hemoptysis history; tumors invading major blood vessels; symptomatic intracranial metastases (asymptomatic or treated and stable were allowed); major trauma/surgery and radical radiotherapy history in the last 28 days; uncontrolled hypertension; recent therapeutic use of anticoagulants or thrombolytic agents, etc. The smoking status groups included current smoker, former smoker (both defined as the smoking group and the smoking status is “Yes”), and never smoked (defined as the non‐smoking group and the smoking status is “No”). EGFR mutation status identified by EGFR gene‐testing was recorded but was not considered as a criterion for inclusion or exclusion. The protocol was reviewed and approved by all independent institutional review boards and ethics committees of each study center. Written informed consent was obtained from all patients before any study‐related procedures were performed.
2.2. Randomization and blinding
Eligible patients were randomized in a 1:1 ratio by an Interactive Web Response System (the Medidata Solutions, Inc. New York, NY, USA) into an LY01008 or Avastin groups. Patients were stratified based on age, gender, EGFR mutation status. Treatment assignments were blinded to patients and investigators. Only limited independent members of the sponsor's research team and study nurse responsible for preparing the medication were un‐blinded.
2.3. Treatment procedures
Patients were assigned into the two treatment groups and received intravenous (IV) LY01008 (LY01008 group) or Avastin (Avastin group) 15 mg/kg, in combination with carboplatin (area under the curve = 6) and paclitaxel (175 mg/m2), administered every three weeks for 4‐6 cycles (combined treatment) followed by LY01008 maintenance monotherapy, until any of the following events occurred: intolerable toxicity, consent withdrawal, disease progression, loss to follow‐up, study termination or death. Paclitaxel and carboplatin dose reductions were allowed for reducing toxicity, but dose reductions were not permitted for LY01008 or Avastin to ensure the treatment equalization of the two groups. The first, second, and subsequent doses of LY01008 or Avastin were administered over 90, 60, and 30 minutes, respectively, if they were well tolerated.
2.4. Endpoints and assessments
The primary efficacy endpoint was ORR, defined as the rate of the best overall response of either complete response (CR) or partial response (PR) evaluated by an independent radiological review committees (IRRC) using the RECIST version 1.1 during the first 6 cycles in the full analysis set (FAS) population. The secondary efficacy endpoints included disease control rate (DCR), duration of response (DoR), PFS, OS, and safety. DCR was defined as the percentage of patients who achieved CR, PR, or stable disease (SD). DoR was calculated as the time from the first objective response (CR or PR) to disease progression. PFS was defined as the time from randomization until progression or death. OS was defined as the time from randomization to death (for any reason).
During the combined treatment and LY01008 maintenance treatment, imaging examination was performed every 6 weeks (42 ± 7 days) until disease progression, consent withdrawal, loss to follow‐up, death, or the end of the study (defined as 12 months after the last patient was randomized). Telephonic follow‐up was performed every 12 weeks (± 7 days) after disease progression until death, loss to follow‐up, or the end of the study. For patients with brain metastasis, patients whose intracranial progression was observed and recorded prior to the end of the study or patients who received imaging follow‐up until the end of the study with no progression in intracranial or systemic lesions were defined as those who experienced enough intracranial follow‐up.
Safety evaluation mainly involved the assessments of adverse events (AEs) and immunogenicity. Key indicators for AEs included treatment‐emergent AEs (TEAEs), serious adverse events (SAEs), and AEs of special interest (AESI) such as anti‐VEGF‐related AEs. The AESI defined in this study were hypertension, proteinuria or nephrotic syndrome, gastrointestinal perforations or fistula, bleeding/hemorrhage, cardiac disorders, arteriovenous thromboembolic events, posterior reversible encephalopathy syndrome (PRES), and wound‐healing complications. All AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Immunogenicity refers to the incidence of anti‐drug antibodies (ADAs) and neutralizing antibodies (NAbs). A single, sensitive, specific, semi‐quantitative, immuno‐depletion bridging electrochemiluminescence immunoassay was used to detect ADAs. A single, validated, competitive ligand‐binding immunosorbent assay was conducted for NAbs analysis. Only those samples confirmed positive for ADAs were further tested for Nabs.
2.5. Statistical analyses
This study assumed that 42% of patients would achieve objective response in both the LY01008 and Avastin groups. Clinical equivalence was confirmed if 90% confidence interval (CI) of the ORR ratio (LY01008/Avastin) was within the predefined equivalence margin (0.75‐1.33), which was an equivalence threshold based on the China NMPA's technical guidelines for the evaluation of bevacizumab biosimilar [31]. Considering a possible 10% drop rate for patients reaching evaluation for ORR, a sample size of 324 in each group was needed to achieve approximately 80% power for testing the expected equivalence margin at the 0.05 one‐sided significance level.
The primary endpoint was analyzed in the FAS population. The intent‐to‐treat (ITT) population was defined in this study as those who were randomized and received at least one dose of study drugs. FAS included all randomized ITT patients who received at least one post‐baseline evaluation of response. FAS patients who completed at least 4 treatment cycles or completed the treatment before disease progression, and did not have major protocol deviation were included in per‐protocol sets (PPS), which was also used for the sensitivity analysis of the primary endpoint. The safety set refers to all randomized patients who received at least one dose of study drugs and had data on safety evaluation after administration.
The 90% CIs for risk ratio in ORR and DCR were estimated using a generalized linear model adjusted for stratification factors. Kaplan‐Meier estimates of quartiles and 95% CI are provided for time‐to‐event endpoints. All statistical analyses were performed using the SAS Software (version 9.4, SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Patient baseline characteristics
Between December 25th, 2017, and May 15th, 2019, a total of 649 patients were randomized to the LY01008 group (n = 324) or the Avastin group (n = 325) (Supplementary Table S1). The data cut‐off date for the analysis of the primary endpoint was September 25th, 2019, which was the date the last enrolled evaluable patient received the response evaluation after 18 weeks of the first medication. The cut‐off date for secondary endpoints was May 15th, 2020, which was the date for the end of the study, defined as 12 months after the last patient randomized to group per protocol.
Among the 649 patients, 648 (n = 323 in the LY01008 group and n = 325 in the Avastin group) were included in the ITT population and 589 (n = 293 in the LY01008 group and n = 296 in the Avastin group) in the FAS who had experienced at least one response evaluation. All 648 ITT patients were included in the safety set (Figure 1). Patient baseline characteristics in FAS were comparable in the two treatment groups (Table 1).
FIGURE 1.
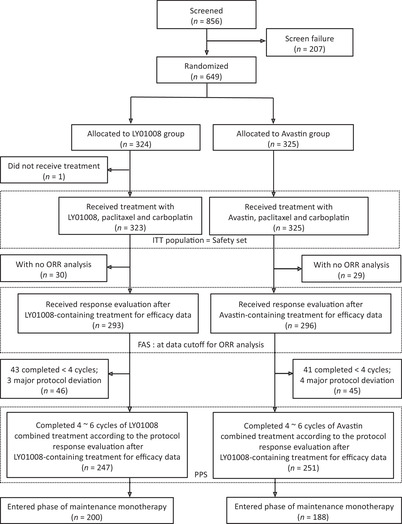
Flowchart of participants’ disposition. Abbreviations: ITT, intention‐to‐treat; ORR, objective response rate; FAS, full analysis set; PPS, per‐protocol set
TABLE 1.
Patient baseline characteristics in full analysis set (N = 589)
| Characteristic | Total (N = 589) | LY01008 group (n = 293) | Avastin group (n = 296) |
|---|---|---|---|
| Age (years) (median) | 58 | 58 | 59 |
| < 65 years (n, %) | 433 (73.5) | 212 (72.4) | 221 (74.7) |
| ≥ 65 years (n, %) | 156 (26.5) | 81 (27.6) | 75 (25.3) |
| Gender (n, %) | |||
| Male | 352 (59.8) | 177 (60.4) | 175 (59.1) |
| Female | 237 (40.2) | 116 (39.6) | 121 (40.9) |
| Nationality (n, %) | |||
| Han | 573 (97.3) | 286 (97.6) | 287 (97.0) |
| Others | 16 (2.7) | 7 (2.4) | 9 (3.0) |
| Weight (kg) (mean±SD) | 61.1±9.6 | 61.1±9.8 | 61.1±9.5 |
| BMI (kg/m2) (median) | 22.8 | 22.9 | 22.6 |
| BSA (m2) (median) | 1.63 | 1.64 | 1.62 |
| ECOG PS (n, %) | |||
| 0 | 209 (35.5) | 94 (32.1) | 115 (38.9) |
| 1 | 380 (64.5) | 199 (67.9) | 181 (61.1) |
| Histology (n, %) | |||
| Adenocarcinoma | 573 (97.3) | 285 (97.3) | 288 (97.3) |
| Large cell carcinoma | 4 (0.7) | 2 (0.7) | 2 (0.7) |
| Mixed adenocarcinoma | 3 (0.5) | 1 (0.3) | 2 (0.7) |
| Others | 9 (1.5) | 5 (1.7) | 4 (1.4) |
| EGFR mutation status (n, %) | |||
| Wild type | 343 (58.2) | 170 (58.0) | 173 (58.4) |
| Mutated | 246 (41.8) | 123 (42.0) | 123 (41.6) |
| Smoking status (n, %) | |||
| No | 314 (53.3) | 153 (52.2) | 161(54.4) |
| Yes | 275 (46.7) | 140 (47.8) | 135 (45.6) |
| Stage (n, %) | |||
| IIIB | 1 (0.2) | 1 (0.3) | 0 (0) |
| IV | 588 (99.8) | 292 (99.7) | 296 (100) |
| Brain metastasis (n, %) | 142 (24.1) | 69 (23.5) | 73 (24.7) |
| Time since initial diagnosis to randomization (month) (median) | 0.5 | 0.5 | 0.5 |
Abbreviations: SD, standard deviation; BMI, body mass index; BSA, body surface area; ECOG, Eastern Cooperative Oncology Group; PS, performance status; EGFR, epidermal growth factor receptor.
3.2. Treatment exposure
Up to primary analysis, the extent of exposure was similar between the LY01008 group and the Avastin group in FAS. The median number of exposure cycles was 5 each, range 1‐6 in both groups. All participants completed at least 1 cycle of combined treatment. Most of them received at least 4 cycles (n = 250 [85.3%] in the LY01008 group vs. n = 255 [86.1%] in the Avastin group; no excluding those with protocol deviation, Figure 1) and some received 6 cycles (n = 128 [43.7%] in the LY01008 group vs. n = 130 [43.9%] in the Avastin group) of treatment. The treatment exposure in the two groups was comparable.
3.3. Primary endpoint
As of the primary endpoint cut‐off date on September 25th, 2019, a total of 142 (48.5%) and 157 (53.0%) in the LY01008 and Avastin groups achieved an objective response (all PR), respectively. The stratified ORR ratio (LY01008 to Avastin) was 0.91, with a 90% CI of 0.80 to 1.04, and was within the prespecified equivalence margin of 0.75 to 1.33 (Table 2). Consistent results were observed in ORR sensitivity analyses, including PPS or ITT‐based stratified and unstratified ORR by IRRC and by investigators in FAS, PPS, and ITT population. These results demonstrated the similarity of efficacy between the LY01008 group and the Avastin group. DCRs from primary analyses were also similar in the two treatment groups.
TABLE 2.
Primary and secondary analyses for efficacy in full analysis set (N = 589)
| LY01008 group (n [%]) | Avastin group (n [%]) | ORR risk Ratio (90% CI) | |
|---|---|---|---|
| Total | 293 | 296 | |
| The primary analyses* | |||
| Complete response | 0 | 0 | |
| Partial response | 142 (48.5) | 157 (53.0) | |
| Stable disease | 143 (48.8) | 129 (43.6) | |
| Disease progression | 8 (2.7) | 10 (3.4) | |
| Objective response rate | 142 (48.5) | 157 (53.0) | 0.91 (0.80, 1.04) |
| Disease control rate | 285 (97.3) | 286 (96.6) | 1.00 (0.98, 1.02) |
| The secondary analyses† | |||
| Complete response | 0 | 0 | |
| Partial response | 164 (56.0) | 174 (58.8) | |
| Stable disease | 116 (39.6) | 103 (34.8) | |
| Disease progression | 13 (4.4) | 18 (6.1) | |
| Not evaluable | 0 | 1 (0.3) | |
| Objective response rate | 164 (56.0) | 174 (58.8) | 0.95 (0.85, 1.07) |
| Disease control rate | 280 (95.6) | 277 (93.6) | 1.03 (0.99, 1.06) |
The data cutoff date was September 25th, 2019, which was the date that the last enrolled evaluable patient completed the response evaluation after 18 weeks of the first medication. Response was evaluated by independent radiological review committees.
The data cutoff date was May 15th, 2020, which was the date for the end of the study, defined as 12 months after the last patient randomized to group per protocol. Response was evaluated by investigators.
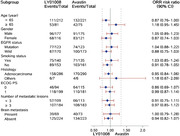
ORR ratios were within the predefined equivalence margins in most subgroups, including patients aged <65 years, male patients, patients with adenocarcinoma, patients with different EGFR mutation status (mutated or wild type), smoking status (yes or no), number of metastases (<3 or ≥3) and brain metastasis status (present or absent) (Figure 2).
FIGURE 2.
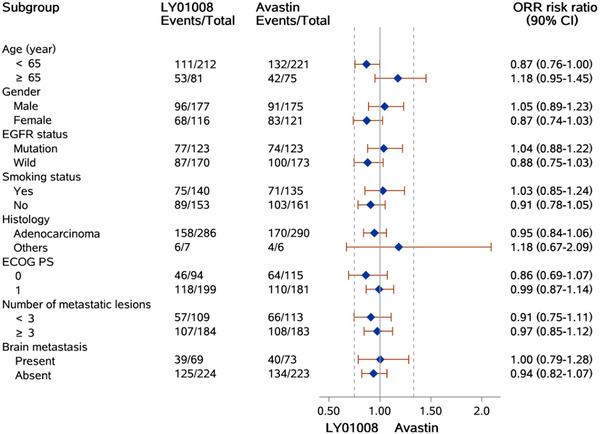
Forest plot for subgroup analysis of objective response rate in the full analysis set. Abbreviations: CI: confidence interval; ECOG: Eastern Cooperative Oncology Group; PS: performance status; EGFR, epidermal growth factor receptor
3.4. Secondary efficacy endpoints
Up to May 15th, 2020, renewed efficacy results were reviewed by the investigators (Table 2), and observed that the ORR was 56.0% in the LY01008 group and 58.8% in the Avastin group in the FAS. ORR ratio was 0.95 (90% CI = 0.85‐1.07). DCR was 95.6% in the LY01008 group and 93.6% in the Avastin group. DCR risk ratio was 1.03 (90% CI = 0.99‐1.06). These data thus confirmed the clinical equivalence between the two treatment groups.
In the FAS, the median follow‐up time was 13.6 (range 0.8‐28.4) months, and 338 patients achieved PR while 416 patients developed disease progression. The median DoR was 5.62 (95% CI = 4.96‐6.87) and 5.72 (95% CI = 4.86‐7.06) months for the LY01008 group and the Avastin group (P = 0.508), respectively. The median PFS was 7.16 (95% CI = 6.87‐8.28) and 7.10 (95% CI = 6.74‐8.21) months for the LY01008 group and the Avastin group, respectively (P = 0.812). The Kaplan‐Meier curves of DoR and PFS are shown in Figure 3A and Figure 3B.
FIGURE 3.
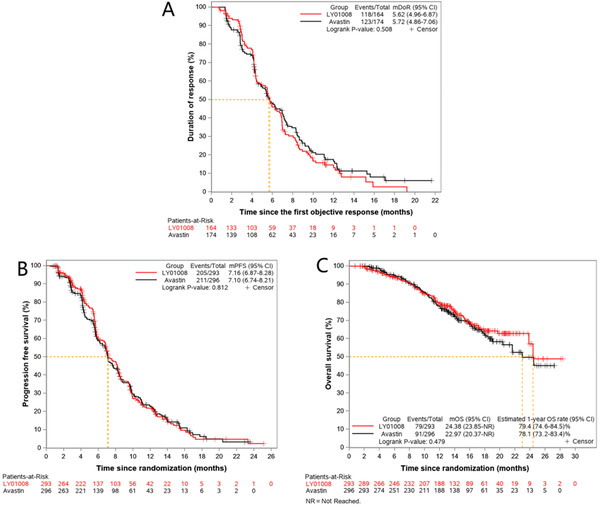
Kaplan‐Meier curves of the LY01008 group and the Avastin group in the full analysis set. A: Percentage rate of patients with a duration of response; B: Progression‐free survival; C: Overall survival. Abbreviations: CI, confidence interval; mDoR, median duration of response; mPFS, median progression‐free survival; mOS, median overall survival; NR, not reached
As of May 15th, 2020, 79 and 91 patients of the FAS in the LY01008 group (27.0%) and the Avastin group (30.7%) died, respectively. The median OS was 24.38 (95% CI = 23.85‐not reached) months and 22.97 (95% CI = 20.37‐not reached) months for the LY01008 group and the Avastin group, respectively (P = 0.479). The 1‐year OS rate was 79.4% (95% CI = 74.6%‐84.5%) in the LY01008 group and 78.1% (95% CI = 73.2%‐83.4%) in the Avastin group (Figure 3C).
3.5. Subgroup analysis
ORR of patients in the EGFR mutated subgroup (61.4%) was numerically higher than those in the wild‐type subgroup (54.5%) in FAS, without statistical significance (P = 0.235). ORR ratio for the LY01008 group to the Avastin group was 1.04 (90% CI = 0.88‐1.22) and 0.88 (90% CI = 0.75‐1.03) in the EGFR mutated subgroup and the wild‐type subgroup, respectively. The 90% CI of the ORR ratio was within the predefined equivalence margin of 0.75 to 1.33. DoR and PFS between patients with different EGFR mutation status were similar in either the LY01008 group or the Avastin group (Supplementary Figure S1). Kaplan‐Meier curves of OS showed that patients with EGFR mutation had a better survival trend than those with EGFR wild‐type. The estimated 1‐year OS rates of patients with EGFR mutation in the LY01008 group and the Avastin group were 85.3% (95% CI = 78.9%‐ 92.3%) and 85.4% (95% CI = 79.0%‐92.3%), respectively, while the estimated 1‐year OS rates for patients with EGFR wild‐type in the LY01008 group and the Avastin group were 75.0% (95% CI = 68.3%‐82.4%) and 72.8% (95% CI = 65.9%‐80.4%), respectively (Supplementary Figure S1).
There were 142 (24.1%) patients in FAS with baseline brain metastases (69/293 [23.5%] patients in the LY01008 group and 73/296 [24.7%] patients in the Avastin group). Among these patients, 39 (56.5%) in the LY01008 group and 40 (54.8%) in the Avastin group achieved PR evaluated by investigators. ORR ratios of the two groups were similar in patients with different baseline brain metastasis status (1.00 [90% CI = 0.79‐1.28] for the brain metastasis subgroup and 0.94 [90% CI = 0.82‐1.07] for the no brain metastasis subgroup, of which the 90% CI both within the equivalence margins) (Figure 2). Median DoR was shorter in the brain metastasis subgroup (4.34 months) compared with no brain metastasis subgroup (6.28 months) in FAS (P = 0.010). Median PFS was shorter in the brain metastasis subgroup (6.83 months) compared with the no brain metastasis subgroup (7.62 months) in FAS (P = 0.006). A comparison of PFS stratified by treatment group and brain metastasis status is shown in Supplementary Figure S2. The 1‐year OS rates in patients with brain metastasis in the LY01008 group and the Avastin group were 70.1% (95% CI = 59.3%‐82.8%) and 73.7% (95% CI = 63.5%‐85.7%), respectively, and for those without brain metastasis was 82.2% (95% CI = 77.0%‐87.8%) in the LY01008 group and 79.5% (95% CI = 74.0%‐85.4%) in the Avastin group (Supplementary Figure S2).
In the 142 patients with baseline brain metastasis, all were non‐target lesions. The median number of intracranial lesions was 1 (range 1‐6). Twenty‐two (15.5%) patients achieved intracranial CR, with 11 patients in each group. Of these patients, 21 (95.5%) had no intracranial radiotherapy history, 10 (14.3%) with EGFR mutation, and 12 (16.7%) with EGFR wild‐type. Forty‐three (30.3%) patients had intracranial PD. Among the 142 patients, 65 (45.8%) experienced enough intracranial follow‐up, and the median intracranial PFS for these 65 patients was 7.00 (range 1.20‐27.40, 95% CI = 5.70‐9.70) months.
3.6. Safety
Safety analysis was conducted on the first analysis cut‐off date of September 25th, 2019. The incidence of TEAEs was 99.1% and 98.5% in the LY01008 and Avastin groups, respectively (Table 3). Most TEAEs were grade 1‐2. In general, 78.3% of patients in the LY01008 group and 82.5% of the Avastin group experienced any TEAE grade ≥3. LY01008‐related and Avastin‐related TEAEs were 73.7% and 76.6%, respectively. Grade ≥3 LY01008‐related and Avastin‐related TEAEs were 37.8% and 41.8%, with 70.9% grade ≥3 chemotherapy drug‐related TEAEs in the LY01008 group and 77.2% in the Avastin group.
TABLE 3.
Summary of adverse events in the safety set (N = 648)
| TEAE category | LY01008 group (n = 323) (n [%]) | Avastin group (n = 325) (n [%]) |
|---|---|---|
| Any TEAE | 320 (99.1) | 320 (98.5) |
| SAE | 126 (39.0) | 125 (38.5) |
| AESI | 145 (44.9) | 156 (48.0) |
| Grade ≥3 TEAE | 253 (78.3) | 268 (82.5) |
| Study drug‐related TEAE | 238 (73.7) | 249 (76.6) |
| Study drug‐related TEAE, grade ≥3 | 122 (37.8) | 136 (41.8) |
| Chemotherapy drug‐related TEAE, grade ≥3 | 229 (70.9) | 251 (77.2) |
| Study drug‐related SAE | 53 (16.4) | 46 (14.2) |
| TEAE leading to transient discontinuation | 125 (38.7) | 121 (37.2) |
| TEAE leading to permanent discontinuation | 45 (13.9) | 46 (14.2) |
| TEAE leading to death | 15 (4.6) | 13 (4.0) |
| Grade ≥3 TEAE * | ||
| Neutropenia | 138 (42.7) | 132 (40.6) |
| Leukopenia | 84 (26.0) | 76 (23.4) |
| Bone marrow failure | 70 (21.7) | 89 (27.4) |
| Anemia | 50 (15.5) | 48 (14.8) |
| Thrombocytopenia | 47 (14.6) | 41 (12.6) |
| Grade ≥3 study drug‐related TEAE * | ||
| Neutropenia | 51 (15.8) | 52 (16.0) |
| Bone marrow failure | 26 (8.0) | 35 (10.8) |
| Incidence ≥1.0% study drug‐related SAE | ||
| Bone marrow failure | 15 (4.6) | 18 (5.5) |
| Thrombocytopenia | 7 (2.2) | 5 (1.5) |
| Grade ≥3 AESI | ||
| Proteinuria | 2 (0.6) | 2 (0.6) |
| Hemorrhage | 4 (1.2) | 5 (1.5) |
| Hypertension | 19 (5.9) | 25 (7.7) |
| Cardiotoxicity | 3 (0.9) | 1 (0.3) |
| Thromboembolic events | 8 (2.5) | 7 (2.2) |
| Posterior reversible encephalopathy syndrome | 0 (0) | 0 (0) |
| Gastrointestinal perforations and fistulae | 1 (0.3) | 0 (0) |
| Surgery and wound healing complications | 0 (0) | 1 (0.3) |
Abbreviations: TEAE, treatment‐emergent adverse event; SAE, serious adverse events; AESI, adverse events of special interest.
Only adverse events of which incidence over 10% was reported.
Note: study drugs referred to LY01008 or Avastin.
The most common (rate ≥10%) drug‐related TEAEs were similar in the LY01008 group and the Avastin group, including leukopenia (24.5%), neutropenia (22.3%), anemia (19.5%), thrombocytopenia (18.0%), proteinuria (15.2%), bone marrow failure (13.9%), alopecia (12.4%) and nausea (10.8%) for all patients in safety set (data not shown). A total of 126 (39.0%) and 125 (38.5%) patients in the LY01008 group and the Avastin group, respectively, experienced SAEs (Table 3). Among all the SAEs, chemotherapy‐related events accounted for a large proportion of AEs (83.6% in the LY01008 group and 85.8% in the Avastin group) while study drug‐related events made up of 16.4% and 14.2% in the LY01008 group and the Avastin group, respectively. Most study drug‐related SAEs were rare with an incidence rate less than 1.0%, with only the incidence rate of bone marrow failure and thrombocytopenia over 1.0%. A comparison of AESI grade ≥3 in the two groups is presented in Table 3.
ADA and NAb were detected for the study drugs. Of 648 patients in the safety set, 13 (2.0%) were positive for ADA before the first cycle of treatment (7 [2.2%] in the LY01008 group vs. 6 [1.8%] in the Avastin group). Six (0.9%) patients (three in each group) showed at least once ADA‐positivity during the study of combined treatment, which were all transient. One patient showed transient post‐treatment ADA‐positivity. By September 25th, 2019, all ADA‐positive cases had changed to negative. No NAb‐positive cases were found. There were no clinically meaningful differences in immunogenicity across treatment groups. Given the low rate of ADA‐positive data, no obvious effect of immunogenicity on pharmacokinetic and safety was observed.
4. DISCUSSION
This phase III study compared the bevacizumab biosimilar, LY01008, with Avastin in efficacy, safety, and immunogenicity. The results showed that this study met the primary endpoint and verified the bioequivalence of the two treatment groups. To the best of our knowledge, this study enrolled the largest number of patients comparing bevacizumab biosimilar with Avastin in the first‐line treatment of Chinese patients with advanced‐stage non‐squamous NSCLC.
The results showed that the ORR of the LY01008 group was 48.5% and the Avastin group was 53.0%. The stratified ORR ratio was 0.91 (90% CI = 0.80‐1.04), within the prespecified equivalence margin (0.75‐1.33), which indicated that LY01008 was similar in clinical efficacy to Avastin, achieving the primary endpoint of this study. In subgroup analysis, subgroups including age ≥65 years, female, ECOG PS 0, and other types of histology, except adenocarcinoma, were not in the equivalence margins. The reason for this result might be the small number of patients in these subgroups, which could lead to the large variability of their ORR ratios. The equivalence of LY01008 and Avastin was supported by other ORR sensitivity analyses based on the ITT population, investigator's evaluation, unstratified factors, as well as ORR analysis of multiple stratified subgroups, and ORR analysis of updated data during follow‐up time spanning across the maintenance therapy phase. An analysis of secondary endpoints suggested that there was no statistically significant difference between the LY01008 group and the Avastin group in DCR (95.6% vs 93.6%, risk ratio = 1.03, 90% CI = 0.99‐1.06), median DoR (5.62 vs. 5.72 months, P = 0.508), and median PFS (7.16 vs. 7.10 months, P = 0.812). Median OS (24.38 vs. 22.97 months, P = 0.479) and 1‐year OS rate (79.4% vs. 78.1%) were also similar, both of which support the equivalence of the two treatment groups. The safety analysis of LY01008 and Avastin indicated that the safety profile was similar in the two treatment groups. Immunogenicity was also similar in the two treatment groups. Through the comparison results of these clinical features, the bioequivalence of LY01008 and Avastin could be confirmed.
Since it was first approved by FDA in 2004, bevacizumab (Avastin) has been marketed in more than 100 countries and regions for the treatment of more than ten types of cancer, including NSCLC [19, 32]. In China, Avastin has been approved for the first‐line treatment of unresectable, metastatic, or recurrent non‐squamous NSCLC in combination with platinum‐based chemotherapy. The BEYOND study demonstrated that bevacizumab (Avastin) combined with carboplatin/paclitaxel was superior to carboplatin/paclitaxel alone in the first‐line treatment among Chinese non‐squamous NSCLC patients [16]. With the advent of the era of immunotherapy for lung cancer, the IMpower150 study has confirmed that the addition of bevacizumab (Avastin) to atezolizumab plus carboplatin plus paclitaxel could play a more synergistic role [21]. Although the use of bevacizumab can improve the efficacy of cancer treatment, the high price may prevent it from clinical application, failing to translate into the benefits of more patients. Therefore, bevacizumab biosimilars could contribute to reduce the costs of treatment and increase drug availability.
Although there are similar designs and same reference data for the equivalence clinical trials of bevacizumab biosimilars, the results obtained are often different due to the differences in the year of the clinical trial, selected patient population, inclusion and exclusion criteria. Nevertheless, these studies suggest that in patients with non‐squamous NSCLC, ORR of the first‐line treatment of Avastin or its biosimilars in combination with chemotherapy was approximately 44%‐56%. In these studies, Chinese patients tended to have a higher ORR. ORRs of the Avastin group among Chinese patients were 54% in the BEYOND study [16], 56.02% in QL1101 equivalence study [33], 46.4% in IBI305 equivalence study [34], and 53.0% in our study, while ORRs were lower in the studies of predominantly Caucasian patients, including 35% in ECOG 4599 study [14], 41.7% in ABP215 study [35] and 44.6% in PF06439535 study [36]. Safety assessment presented a consistent feature with ORR. The Chinese patients had a higher incidence of grade ≥3 AE in combined treatment than predominantly Caucasian patients (78.3%‐89.8% in our study and IBI305 study vs. 38.8%‐48.1% in PF06439535 study and ABP215 study). Incidence of SAE was also higher in Chinese patients (37.6% in the IBI305 study and 39.0% in our study for the Avastin group; 33.5% in the IBI305 study and 38.5% in our study for the bevacizumab biosimilar group) than predominantly Caucasian patients (22.3% in PF06439535 study and 23.0% in ABP215 study for the Avastin group; 22.8% in PF06439535 study and 26.2% in ABP215 study for the bevacizumab biosimilar group). The incidence of grade ≥3 AESI hypertension resulting from Avastin in Chinese patients (7.7% for the Avastin group and 5.7% for the bevacizumab biosimilar group in our study) was similar to that in Caucasian patients (8.9% in PF06439535 study and 5.5% in ABP215 study for the Avastin group; 9.6% in PF06439535 study and 6.8% in ABP215 study for the bevacizumab biosimilar group).
Baseline EGFR mutation status was an important factor influencing the survival outcomes of patients. Several studies used EGFR mutation as an exclusion criterion [34, 36]. As some Chinese patients are unwilling to wait for the results of EGFR gene test before making first‐line treatment decisions and some unwilling to receive EGFR‐TKI treatment for various reasons, our study did not measure EGFR mutation status as the mandatory exclusion criteria. Of the enrolled patients in our study, 41.8% had EGFR mutation. The results showed that there was no significant difference in ORR, DoR, and PFS between EGFR mutated and wild‐type patients in each group. Kaplan‐Meier curves of OS showed that patients with EGFR mutation had a better survival trend than those with EGFR wild‐type. The better survival prognosis of EGFR mutated patients could be related to the subsequent EGFR‐TKI therapy.
Concomitant intracranial metastasis status was an important factor for survival outcome. Some earlier studies considered intracranial metastasis as an exclusion criterion or only permitted patients with intracranially treated and stable brain metastases to be included to avoid the uncertain risk of cerebral hemorrhage that could be caused by bevacizumab [14, 16, 35, 36]. For the baseline brain metastasis status, our study included patients with untreated asymptomatic brain metastases according to the current consensus [37]. Therefore, 142 (24.1%) patients with baseline brain metastases were enrolled. Among the 142 patients, ORR and CR rates were similar in the LY01008 group and the Avastin group. The DoR of patients without brain metastases was longer than that of patients with brain metastases. The results confirmed the aforementioned consensus on the efficacy and safety of bevacizumab in brain metastasis [37]. A recent exploratory analysis of brain metastases in IMpower150 suggested that the treatment groups of regimen containing bevacizumab had a lower incidence of new brain metastases than the treatment group without bevacizumab, indicating that bevacizumab contributed to delaying the brain metastasis progression [38]. The IMpower150 study further provided more evidence for the use of bevacizumab in non‐squamous NSCLC patients with brain metastases.
5. CONCLUSIONS
This study demonstrated the similarity between LY01008 and Avastin in terms of efficacy, safety, and immunogenicity in combination with paclitaxel/carboplatin as first‐line treatment in Chinese patients with advanced non‐squamous NSCLC. LY01008 combined with paclitaxel/carboplatin can be considered as a new treatment option for unresectable, metastatic, or recurrent non‐squamous NSCLC patients in the first‐line setting.
DECLARATIONS
AUTHORS’ CONTRIBUTIONS
Yuankai Shi was the leading principal investigator of this study, contributed to study conception, study design, data analysis and interpretation, manuscript drafting, and editing. Yuankai Shi, Kaijian Lei, Yuming Jia, Bingqiang Ni, Zhiyong He, Minghong Bi, Xicheng Wang, Jianhua Shi, Ming Zhou, Qian Sun, Guolei Wang, Dongji Chen, Yongqian Shu, Lianke Liu, Zhongliang Guo, Yong Liu, Junquan Yang, Ke Wang, Ke Xiao, Lin Wu, Tienan Yi, Debin Sun, Mafei Kang, Tianjiang Ma, Yimin Mao, Jinsheng Shi, Tiegang Tang, Yan Wang, Puyuan Xing, Dongqing Lv, Wangjun Liao, Zhiguo Luo, Bin Wang, Xiaohong Wu, Xiaoli Zhu, Shuhua Han, QiSen Guo, Rongyu Liu, Zhiwei Lu, Jianyong Zhang, Jian Fang, Changlu Hu, Yinghua Ji, Guolong Liu, Hong Lu, Dedong Wu, Junhong Zhang, Shuyang Zhu, Zheng Liu, Wensheng Qiu, Feng Ye, Yan Yu, Yanqiu Zhao, Qinhong Zheng, Jun Chen, Zhanyu Pan, Yiping Zhang, Wenjuan Lian, Bo Jiang, Bo Qiu, Guojun Zhang, Hua Zhang, Yanju Chen, Yuan Chen, Hongbing Duan, Manxiang Li, Shengming Liu, Lijun Ma, Hongming Pan, Xia Yuan, Xueli Yuan, and Yulong Zheng were involved in patient recruitment and data acquisition. Emei Gao did the literature search, research data analysis, and interpretation, and drafted the manuscript. Li Zhao did the data statistical analysis. Shumin Wang and Can Wu were involved in the literature search, data acquisition, analysis, and interpretation. All authors had full access to all the data in the study and contributed to the writing of the manuscript, reviewed it for intellectual content, and approved the submitted version.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by all independent institutional review boards and ethics committees of each study center. Written informed consent was obtained from all patients before any study‐related procedures performed. Both the collection of all data in this study and the adoption of the materials herein comply with the Declaration of Helsinki and the relevant provisions of the guidelines.
CONSENT FOR PUBLICATION
The authors declare that informed consent can be provided for the publication of any associated data and accompanying images.
CONFLICT OF INTEREST STATEMENT
The tested drug LY01008 in this study is the product of Luye Pharma Group Ltd., which provided main funding to this study. Emei Gao, Li Zhao, Shumin Wang, and Can Wu are employees of the Clinical Research Center of Luye Pharma Group Ltd., Luye Life Sciences Group, Beijing, China. All other authors declare that they have no conflict of interests.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This study was mainly supported by the Clinical Research Center of Luye Pharma Group Ltd., Luye Life Sciences Group and the Chinese Academy of Medical Sciences. The authors also appreciate the contributions from all the investigators, the patients, and their families. Finally, the authors thank Dr. Yu Zhou and Dr. Haizhu Chen (National Cancer Centre/National Clinical Research Centre for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, China) for their medical writing assistance. This study was funded by Luye Pharma Group Ltd., Luye Life Sciences Group, Beijing, China, China National Major Project for New Drug Innovation (Grant no. 2017ZX09304015), and the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS) (Grant no. 2016‐I2M‐1‐001).
Shi Y, Lei K, Jia Y, Ni B, He Z, Bi M, et al. Bevacizumab biosimilar LY01008 compared with bevacizumab (Avastin) as first‐line treatment for Chinese patients with unresectable, metastatic, or recurrent non‐squamous non–small‐cell lung cancer: A multicenter, randomized, double‐blinded, phase III trial. Cancer Commun. 2021;41:889–903. 10.1002/cac2.12179
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 3.Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun (Lond). 2020;40(5):205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non‐small‐cell lung cancer: recent developments. Lancet. 2013;382(9893):709–19. [DOI] [PubMed] [Google Scholar]
- 5.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128(1):452–62. [DOI] [PubMed] [Google Scholar]
- 6.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non‐small‐cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31(8):1039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crinò L, Metro G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur Respir Rev. 2014;23(131):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non‐small cell lung cancer. Clin Cancer Res. 2015;21(5):976–84. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, Jiang S, Shi Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). J Hematol Oncol. 2020;13(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinese Association for Clinical Oncologists and Medical Oncology Branch of Chinese International Exchange, Promotion Association for Medical and Healthcare . Clinical practice guideline for stage IV primary lung cancer in China(2021 version). Chin J Oncol. 2021;43(1):39–59. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133(2):275–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9(Suppl 1):2–10. [DOI] [PubMed] [Google Scholar]
- 14.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med. 2006;355(24):2542–50. [DOI] [PubMed] [Google Scholar]
- 15.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first‐line therapy for nonsquamous non‐small‐cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–34. [DOI] [PubMed] [Google Scholar]
- 16.Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: a randomized, double‐blind, placebo‐controlled, multicenter, phase III study of first‐line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non‐small‐cell lung cancer. J Clin Oncol. 2015;33(19):2197–204. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first‐line treatment of advanced/metastatic recurrent nonsquamous non‐small cell lung cancer. Oncologist. 2007;12(6):713–8. [DOI] [PubMed] [Google Scholar]
- 18.Zahn MO, Linck D, Losem C, Gessner C, Metze H, Gaillard VE, et al. AVAiLABLE NIS ‐ AVASTIN® in lung cancer treatment in routine oncology practice in Germany. BMC Cancer. 2019;19(1):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [DOI] [PubMed] [Google Scholar]
- 20.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non‐small‐cell lung cancer. N Engl J Med. 2002;346(2):92–8. [DOI] [PubMed] [Google Scholar]
- 21.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. [DOI] [PubMed] [Google Scholar]
- 22.Cherny N, Sullivan R, Torode J, Saar M, Eniu A. ESMO European Consortium Study on the availability, out‐of‐pocket costs and accessibility of antineoplastic medicines in Europe. Ann Oncol. 2016;27(8):1423–43. [DOI] [PubMed] [Google Scholar]
- 23.Monk BJ, Lammers PE, Cartwright T, Jacobs I. Barriers to the access of bevacizumab in patients with solid tumors and the potential impact of biosimilars: a physician survey. Pharmaceuticals (Basel). 2017;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang S, Shi Y. The development of biosimilars in oncology. Chin J Oncol. 2017;39(10):721–5. [DOI] [PubMed] [Google Scholar]
- 25.Goulart B, Ramsey S. A trial‐based assessment of the cost‐utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non‐small cell lung cancer. Value Health. 2011;14(6):836–45. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CL, Chen B, Hermanson T, Wyatt MD, Schulz RM, Georgantopoulos P, et al. Regulatory and clinical considerations for biosimilar oncology drugs. Lancet Oncol. 2014;15(13):e594–e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Medicines Agency . Guideline on similar biological medicinal products 2005 [cited 2021 01‐01]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003517.pdf
- 28.Food and Drug Administration . Biologics Price Competition and Innovation Act of 2009 2009 [cited 2021 01‐01]. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/UCM216146.pdf.
- 29.World Health Organization . Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs) 2009 [cited 2021 01‐01]. Available from: https://www.who.int/biologicals/areas/biological_therapeutics/BIOTHERAPEUTICS_FOR_WEB_22APRIL2010.pdf.
- 30.Center for Drug Evaluation (CDE) of National Medical Products Administration (NMPA) . Technical Guidelines for the research and development and Evaluation of Biosimilars (Tentative) (Guideline) 2015 [cited 2021 01‐01]. Available from: http://www.cde.org.cn/zdyz.do?method=largePage&id=2f41e8f3c64fedad.
- 31.Center for Drug Evaluation (CDE) of National Medical Products Administration (NMPA) . Guidelines for Clinical Trials in biosimilars of bevacizumab injection 2020 [updated 2020‐12‐01; cited 2021 01‐01]. Available from: http://www.cde.org.cn/zdyz.do?method=largePage&id=c8754eb6cede1c7e.
- 32.Hou Y, Wu B. Atezolizumab plus bevacizumab versus sorafenib as first‐line treatment for unresectable hepatocellular carcinoma: a cost‐effectiveness analysis. Cancer Commun (Lond). 2020;40(12):743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han B, Li K, Chu T, Bi M, Zhang H, Yu Y, et al. A multi‐center, randomized, double‐blind, parallel, two‐group phase III trial on the efficacy and safety of QL1101 or bevacizumab in combination with paclitaxel and carboplatin in first‐line treatment of non‐squamous non‐small cell lung cancer. Ann Oncol. 2019;30(Supplement 2):II62. [Google Scholar]
- 34.Yang Y, Wu B, Huang L, Shi M, Liu Y, Zhao Y, et al. Biosimilar candidate IBI305 plus paclitaxel/carboplatin for the treatment of non‐squamous non‐small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thatcher N, Goldschmidt JH, Thomas M, Schenker M, Pan Z, Paz‐Ares Rodriguez L, et al. Efficacy and safety of the biosimilar ABP 215 compared with bevacizumab in patients with advanced nonsquamous non‐small cell lung cancer (MAPLE): a randomized, double‐blind, phase III study. Clin Cancer Res. 2019;25(7):2088–95. [DOI] [PubMed] [Google Scholar]
- 36.Reinmuth N, Bryl M, Bondarenko I, Syrigos K, Vladimirov V, Zereu M, et al. PF‐06439535 (a bevacizumab biosimilar) compared with reference bevacizumab (Avastin(®)), both plus paclitaxel and carboplatin, as first‐line treatment for advanced non‐squamous non‐small‐cell lung cancer: a randomized, double‐blind study. BioDrugs. 2019;33(5):555–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi Y, Sun Y, Yu J, Ding C, Ma Z, Wang Z, et al. China experts consensus on the diagnosis and treatment of brain metastases of lung cancer (2017 version). Zhongguo Fei Ai Za Zhi. 2017;20(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Federico C, Martin R, Mark AS, Tony M, Robert MJ, Gene GF, et al. IMpower150: exploratory analysis of brain metastases development. J Clin Oncol. 2020;38(15):9587. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


