Abstract
Introduction
We aimed to compare annual changes in the bone mineral density (BMD) at the lumbar spine (LS) and the femoral neck (FN) in males with HIV-associated osteoporosis treated with either zoledronate (ZOL) or denosumab (Dmab).
Methods
In this open label, 12-month, prospective, multicenter, cohort study, 23 male people living with HIV (PLWH) under antiretroviral therapy (ART) with low BMD were administered either a single iv infusion of ZOL 5 mg (n = 10) or Dmab 60 mg sc injections biannually (n = 13). Fourteen age-matched male PLWH with normal BMD served as controls. BMD was measured at baseline and at 12 months.
Results
LS-BMD increased within both treatment groups at 12 months (ZOL 5.43% ± 3.60%, p = 0.001; Dmab 5.76% ± 3.44%, p < 0.005) and decreased in controls (−2.58% ± 4.12, p = 0.04). FN-BMD increased in both treatment groups at 12 months (ZOL 7.23% ± 5.46%, p = 0.003; Dmab 3.01% ± 2.46%, p < 0.005), and remained unchanged in controls (1.22% ± 2.09, p = 0.06). LS-BMD changes did not differ between the two treatment groups, but FN-BMD changes were more prominent in the ZOL group (p < 0.05). None of our study cohort sustained new fragility fractures during the 12-month study period, and no case of acute phase response was recorded in the ZOL group.
Conclusions
In male PLWH under ART requiring osteoporosis treatment both ZOL and Dmab are efficient and well tolerated therapeutic options achieving BMD increases at least for the first year of treatment.
Keywords: Denosumab, Zoledronate, Osteoporosis, HIV, Antiretroviral therapy (ART), Acute phase response
Highlights
-
•
Zoledronate and denosumab are efficient therapies for HIV-associated osteoporosis.
-
•
Zoledronate-induced acute phase response may be less frequent with concomitant ART.
-
•
Alternative osteoporosis agent should follow in case of denosumab discontinuation.
-
•
Significant annual BMD loss may occur in male PLWH under long term treatment with ART.
1. Introduction
In people living with HIV (PLWH) the prevalence of low bone mineral density (BMD) is multifold higher than in the general population (Brown and Qaqish, 2006). This prevalence is even higher in PLWH receiving antiretroviral therapy (ART) (Brown and Qaqish, 2006), including nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) (Bedimo et al., 2012; Tebas et al., 2000). Among these agents, the commonly used NRTI tenofovir (TDF) is considered to have the most prominent adverse skeletal effects (Stellbrink et al., 2010). HIV proteins, elevated tumor necrosis factor (TNF)α levels, and ART increase osteoclastic activity and decrease bone formation by promoting osteoblast apoptosis (McComsey et al., 2010; Cotter and Mallon, 2014). The decreased BMD along with the accelerated bone turnover result in increased risk of fractures in PLWH ranging from 1.6 to 3-fold compared to the general population, especially after initiation of ART (Triant et al., 2008; Young et al., 2011; Starup-Linde et al., 2020). Low BMD values do not fully explain the increased fracture risk in PLWH suggesting that osteoporosis treatment may be beneficial even at higher BMD values (Starup-Linde et al., 2020).
Current management of osteoporosis in PLWH follows general osteoporosis guidelines (Starup-Linde et al., 2020; European AIDS Clinical Society (EACS), 2020). Since HIV-associated bone disease is a state of high bone turnover, the administration of antiresorptive agents would be a rational therapeutic approach. Both oral alendronate and intravenous zoledronate (ZOL) resulted in significant BMD increases at the lumbar spine (LS) and total hip (TH) in HIV-associated osteoporosis (Starup-Linde et al., 2020; Pinzone et al., 2014); however, the small study populations and the relatively short study duration do not allow an estimation of the effect of bisphosphonates on fracture risk. Denosumab (Dmab) is a monoclonal antibody that specifically binds the receptor Activator of Nuclear Factor κΒ Ligand (RANKL), a soluble factor essential for osteoclast maturation, activity and survival, thus profoundly suppressing bone resorption.
In women with postmenopausal osteoporosis (Anastasilakis et al., 2018) Dmab has shown superiority over bisphosphonates in terms of BMD increases, but its efficacy has not yet been evaluated in HIV-associated bone disease. To address this issue, we conducted a prospective cohort study in male PLWH requiring treatment for osteoporosis, in whom we evaluated the effect of Dmab versus ZOL treatment in the LS and the femoral neck (FN) BMD values after one year of treatment.
2. Patients and methods
2.1. Study population
This was an open-label, 12-month, multicenter, prospective cohort study. Previously antiosteoporotic treatment-naïve male PLWH under ART for at least one year, requiring treatment for osteoporosis according to the current Greek guidelines (Makras et al., 2019) were initially considered eligible for participation in the study. In particular, eligible male PLWH had at least one of the following: i) BMD T-score ≤ −2.5 at any skeletal site, and/or ii) a FRAX score advocating treatment according to the Greek PLWH-specific treatment thresholds (10-year probabilities for hip and major osteoporotic fractures equal or exceeding 2.5 and 10%, respectively, under the age of 75; for older patients the relevant thresholds are 5 and 15%, respectively) (Makras et al., 2019; Makras et al., 2017), and/or iii) presence of at least one low energy vertebral fracture (VF) and/or hip fracture and/or two non-vertebral fractures (non-VF). Exclusion criteria were: i) the use of other medications that affect bone metabolism during the last 3 years, except for ART; ii) other metabolic bone diseases, such as Paget's disease of bone; iii) uncontrolled endocrine diseases; vi) creatinine clearance <60 mL/min/1.73 m2; v) liver failure; vi) serum 25-hydroxy vitamin D (25-OHD) concentrations lower than 37.5 nmol/L (15 ng/mL); vii) any type of malignancy during the last 5 years.
Finally enrolled patients were assigned to receive either a single 5 mg i.v. infusion of ZOL (ZOL group) or two 6-monthly subcutaneous injections of Dmab 60 mg (Dmab group), based on the treating physician's choice. From January 2017 osteoporosis outpatient clinics of both participating hospitals offer either ZOL or Dmab as a first line therapeutic strategy in order to ensure adherence to treatment as their relevant unpublished experience from oral osteoporosis treatment was suboptimal, probably due to polypharmacy; however, there is no specific guidance for these two regimens and treating physicians may choose between them according to their regular clinical practice and patients' preference. Age- and BMI-matched male PLWH with normal BMD values, that did not require osteoporosis treatment, served as controls (Control group). All individuals of the three groups received calcium (500 mg twice per day [b.i.d.]) and vitamin D (cholecalciferol 800 IU/day) supplementation during the 12-month study period.
The study was conducted according to the Declaration of Helsinki and its latter amendments, approval was granted in Sep. 2017 by the Ethics Committee of 251 Hellenic Air Force & VA General Hospital (Athens) and AHEPA Hospital (Thessaloniki), and all patients provided written informed consent.
2.2. Study protocol
All participants were evaluated at baseline and at 12 months.
At baseline, a detailed medical history including type and duration of ART and fracture data was obtained, weight and height were measured for body mass index (BMI) calculation, and fasting morning blood samples were acquired for the measurement of serum calcium (Ca), phosphate (P), creatinine, total alkaline phosphatase (ALP), 25 hydroxy-vitamin D (25OHD) within 1 h from blood drawing. Additional samples were centrifuged and stored at -70 °C for the measurement of procollagen type 1 amino-terminal propeptide (P1NP) and carboxy-terminal telopeptide cross-linked type 1 collagen (CTX) in a single batch at the end of the study by electrochemiluminescence immunoassays “ECLIA” on cobase 411 analyzer (Roche Diagnostics, Mannheim, Germany; P1NP intra-assay CV ≤ 2.3%, inter-assay CV ≤ 3.0%; CTX intra-assay CV ≤ 2.5%, inter-assay CV ≤ 4.6%). In case of 25OHD levels between 37.5 and 50 nmol/L (15–20 ng/mL) a single loading dose of 50,000 IU of cholecalciferol was administered orally prior to initiation of the anti-osteoporotic treatment in the 2 treatment-groups. Areal BMD was measured by dual energy X-ray absorptiometry (DXA) at the LS and the FN of the nondominant hip at baseline and at 12 months using a Lunar Prodigy device (Lunar Corporation, Madison, WI, USA); male specific T-scores are reported. Lateral radiographs of the spine were also performed in all study participants at baseline and at 12 months to identify morphometric VFs. All radiographs were examined by a skeletal radiologist who was blinded to the patients' treatment assignment.
2.3. Treatment outcomes
The primary endpoint of the study was to compare the differences in LS-BMD changes between the two treatment groups after 12 months.
Secondary endpoint was the difference in FN-BMD changes between the two treatment groups after 12 months of treatment. The incidence of new vertebral fractures (clinical and morphometric) and other fragility fractures were exploratory endpoints.
2.4. Statistical analysis
The Shapiro–Wilk test was used to assess for normality of distributions. Data are expressed as mean ± standard deviation (S.D.) or median (in case of violation of normality) for continuous and as percentages for categorical variables. The comparison of variables at baseline and examination of homogeneity between groups was performed using the One way ANOVA model. Pairwise comparisons performed using the Bonferroni test. Comparisons of variables during the study period (baseline vs. 12 months) were performed using paired samples t-test. To explore the efficacy of treatments during the observation period, the mean percentage changes after 12 months were calculated. Comparison of percentage changes from baseline to 12 months of BMD and T-scores at different sites between study groups was analyzed using the One way ANOVA model. Pairwise comparisons were performed using the Bonferroni test. Kruskal-Wallis test and Mann-Whitney test were used, as applicable. Using the analysis of covariance model (ANCOVA model) we compared the difference between groups of absolute change of the tested parameters from baseline to 12 months adjusting for baseline measurements (covariates). Statistical analyses of categorical variables were performed using Chi-square (x2) test and Fisher's exact test. All tests are two-sided, statistical significance was set at p < 0.05. All analyses were carried out using the statistical package SPSS vr 21.00 (IBM Corporation, Somers, NY, USA).
3. Results
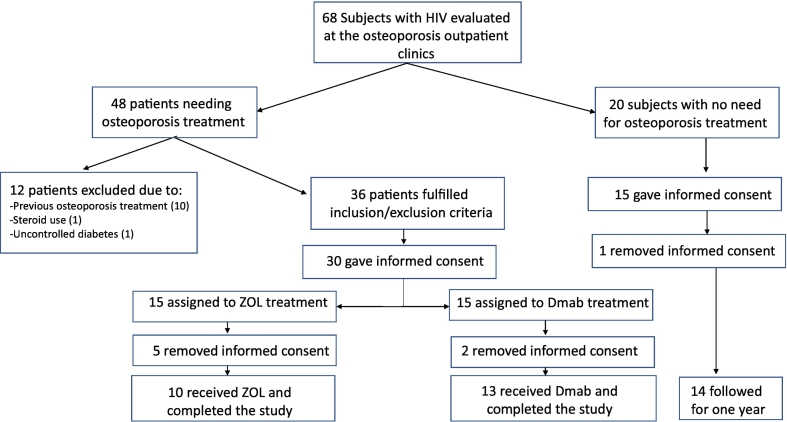
Between October 1, 2017 and December 31, 2018 we invited 36 consecutive male PLWH fulfilling the inclusion/exclusion criteria to participate in the study; thirty of whom agreed and had been assigned to receive either ZOL or Dmab for one year, based on the treating physician's recommendation (Fig. 1). Twenty males PLWH with normal or osteopenic bone mass, not requiring osteoporosis treatment were also invited to participate in the study, as controls and 15 of them signed the informed consent. Five participants from the ZOL group, two from the Dmab group, and one from the Control group withdrew their consent, all before the initiation of the study. Our final study cohort consisted of 10 participants that received ZOL, 13 that received Dmab, and 14 that did not receive any anti-osteoporotic treatment and constituted the control group. All finally enrolled participants completed the study.
Fig. 1.
Study's flow chart.
Demographic and clinical characteristics of our cohort are illustrated in Table 1. With the exception of PIs, duration of treatment, and 25OHD levels at baseline, all other baseline clinical, demographic, and biochemical characteristics were similar between the three groups (Table 1). None of the PLWH assigned to receive anti-osteoporotic treatment had severe osteoporosis based on BMD values and FRAX based 10-years probability for major osteoporotic (MOF) and hip fractures. BMD measurements were comparable between the ZOL and the Dmab group but significantly lower compared to the Control one (Table 2).
Table 1.
Baseline demographics, clinical and laboratory characteristics of study cohort.
| Dmab group (n = 13) |
ZOL group (n = 10) |
Controls (n = 14) |
|
|---|---|---|---|
| Age (years) | 58.31 ± 9.77 | 54.10 ± 13.88 | 50.29 ± 6.59 |
| BMI (kg/m2) | 24.61 ± 4.82 | 23.33 ± 3.49 | 25.46 ± 2.67 |
| ART duration (years) | 9.96 ± 5.86 | 10.75 ± 7.19 | 9.00 ± 6.40 |
| TDF use [n(%)] | 10(76.9%) | 8(80%) | 9(65%) |
| TDF duration (years) | 7.25 ± 5.14 | 6.60 ± 7.55 | 7.07 ± 7.29 |
| NRTIs duration (years) | 9.27 ± 6.31 | 10.75 ± 7.19 | 9.00 ± 6.41 |
| NNRTIs duration (years) | 3.40 ± 5.43 | 3.30 ± 6.14 | 2.01 ± 3.28 |
| PIs duration (years) | 5.04 ± 3.16a | 7.25 ± 6.8.47a | 1.14 ± 4.28 |
| PINP (ng/mL) | 40.94 ± 28.46 | 47.82 ± 28.21 | 49.41 ± 16.56 |
| CTX (ng/mL) | 0.35 ± 0.38 | 0.42 ± 0.26 | 0.37 ± 0.15 |
| Urea (mg/dL) | 36.23 ± 10.81 | 36.30 ± 12.21 | 35.86 ± 18.75 |
| Creatinine (mg/dL) | 1.06 ± 0.18 | 0.95 ± 0.12 | 1.01 ± 0.16 |
| Calcium (mg/dL)d | 9.16 ± 0.38 | 9.12 ± 0.54 | 9.03 ± 0.32 |
| Phosphate (mg/dL) | 2.91 ± 0.49 | 3.13 ± 0.28 | 3.12 ± 0.66 |
| ALP | 92.23 ± 22.19 | 86.50 ± 15.59 | 86.71 ± 27.26 |
| 25(OH)D (nmol/L) | 40.12 ± 9.6 | 70.61 ± 37.69b | 77.24 ± 36.13c |
Parameters did not differ significantly between groups with the exception of those indicated.
Dmab,Denosumab; ZOL, Zoledronate; BMI, body mass índex; ART, anti-retroviral treament; NRTIs, nucleoside analog reverse-transcriptase inhibitors; TDF, tenoforvir; NNRTIs, non-nucleoside analog reverse-transcriptase inhibitors; PIs, protease inhibitors; P1NP, procollagen type 1 amino-terminal CTX, carboxy-terminal telopeptide cross-linked type 1 collagen ALP, alkaline phosphatase.
p < 0.001 vs. Controls.
p = 0.007 vs. Dmab group.
p < 0.001 vs. Dmab group.
Corrected for albumin.
Table 2.
FRAX based 10-years probability for Hip fracture and major osteoporotic fractures, and BMD measurements at baseline of study cohort.
| Dmab group (n = 13) |
ZOL group (n = 10) |
Control (n = 14) |
p-Valued | |
|---|---|---|---|---|
| FRAX Hip (%) | 1.85 ± 0.80a | 1.99 ± 1.24a | 0.38 ± 0.53 | <0.001 |
| FRAX MOF (%) | 6.94 ± 1.64a | 4.74 ± 2.20a | 2.20 ± 0.92 | <0.001 |
| BMD L1-L4 | 1.002 ± 0.156b | 1.000 ± 0.140b | 1.200 ± 0.166 | 0.003 |
| BMD FN | 0.748 ± 0.04a | 0.763 ± 0.04a | 0.963 ± 0.08 | <0.001 |
| T-score L1-L4 | −1.79 ± 1.28b | −1.86 ± 1.17b | −0.29 ± 1.45 | 0.006 |
| T-score FN | −2.44 ± 0.30c | −2.13 ± 0.71c | −0.83 ± 0.66 | <0.005 |
FRAX, fracture risk assessment tool; MOF: major osteoporotic fracture; BMD, bone mineral density; FN, femoral neck.
p < 0.001 vs. Control.
p < 0.05 vs. Control.
p < 0.005 vs. Control.
Comparisons performed between the 3 groups. Non-parametric analysis, Kruskal-Wallis and Mann-Whitney test as applicable (with Bonferroni correction p-value: 0.05/3 = 0.017) were used for the non-normally distributed data of FRAX MOF and T-score L1-L4.
No patient had prevalent VFs or a history of a low trauma non-VF. An 84 years old patient from the ZOL group had a history of a recent hip fracture, two months before the inititation of osteoporosis treatment.
Finally, based on our inclusion criteria 20 patients were treated because of an osteoporotic BMD in at least one skeletal site, 2 patients with osteopenia were treated because of their FRAX-based 10-years probability for a hip fracture (2.5% and 3.2%, respectively), while the above mentioned patient with the recent hip fracture was also treated besides his osteopenic BMD and his FRAX scores that were below the cost effective thresholds.
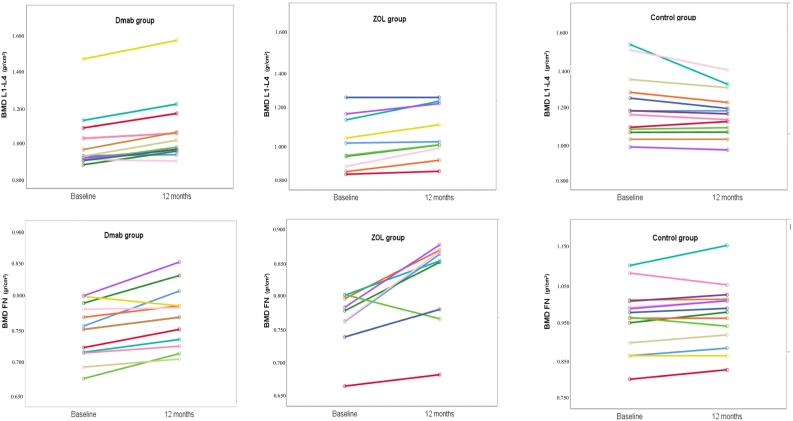
3.1. Bone mineral density
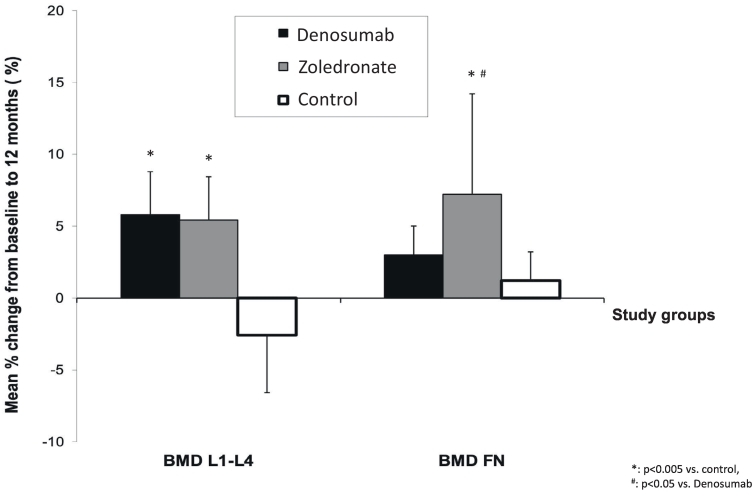
Changes in LS-BMD and FN-BMD during the study are shown in Table 3 and Fig. 2; absolute and percent individual BMD changes are depicted in Fig. 3 and Supplemental Fig. 1, respectively. Compared to baseline, LS-BMD increased in both treatment groups at 12 months (ZOL group 5.43% ± 3.60%, p = 0.001; Dmab group 5.76% ± 3.44%, p < 0.005) while decreased in the Control group (−2.58% ± 4.12, p = 0.044). LS-BMD changes of both treatment groups from baseline to month 12 differed significantly compared to the Control group (both p < 0.005), with no difference between the two treatment groups.
Table 3.
Comparison of BMD measurements between groups during the 12-month study period.
| Group | Baseline | 12 months | p-Valuewg | % change SP | Adjusted 12 months | |
|---|---|---|---|---|---|---|
| BMD L1-L4 | Denosumab | 1.002 ± 0.156a | 1.060 ± 0.174 | <0.005 | 5.76% ± 3.44b | 1.125 ± 0.013a |
| Zoledronate | 1.000 ± 0.140a | 1.051 ± 0.138 | 0.001 | 5.43% ± 3.60b | 1.120 ± 0.014a | |
| Control | 1.200 ± 0.166 | 1.162 ± 0.117 | 0.044 | −2.58% ± 4.12 | 1.050 ± 0.013 | |
| p-Valuebg | 0.003 | 0.112 | <0.005 | 0.002 | ||
| BMD FN | Denosumab | 0.748 ± 0.04b | 0.771 ± 0.04b | <0.005 | 3.01% ± 2.46 | 0.856 ± 0.010 |
| Zoledronate | 0.763 ± 0.04b | 0.818 ± 0.06b | 0.003 | 7.23% ± 5.46b, c | 0.889 ± 0.011a | |
| Control | 0.963 ± 0.08 | 0.974 ± 0.09 | 0.063 | 1.22% ± 2.09 | 0.845 ± 0.013 | |
| p-Valuebg | <0.005 | <0.005 | 0.010 | 0.013 |
BMD: bone mineral density; bg: between group; wg: within group; SP: study period, baseline-12 months.
Adjusted for baseline measurements.
p < 0.05 vs. control.
p < 0.005 vs. control.
p < 0.05 vs. Denosumab.
Fig. 2.
Comparison of BMD percent change between groups during the study period of 12 months.
Fig. 3.
Individual BMD changes in all study groups throughout the study period.
Similarly, FN-BMD increased in both treatment groups at 12 months (ZOL group 7.23% ± 5.46%, p = 0.003; Dmab group 3.01% ± 2.46%, p < 0.005), while it remained unchanged in the Control group (1.22% ± 2.09, p = 0.060). FN-BMD change in the ZOL group, but not in the Dmab group, was significantly different compared to the Control group (p < 0.005 and p = NS, respectively). Additionally, FN-BMD changes from baseline to month 12 were different between the two treatment groups (p < 0.05), favoring the ZOL group.
3.2. Fractures
None of the participants in either of the three groups sustained a clinical or morphometric fragility fracture during the study.
3.3. Adverse events
Notably, none of the ZOL group developed any symptoms compatible with a transient acute phase response (APR) which was defined as a rise in axillary temperature above 38o C and report of musculoskeletal pain at any time over the 48 h following the infusion of ZOL. Although subjects were specifically instructed to use paracetamol in case of symptoms as such, no one reported use of any pain or fever reliever. No adverse events were recorded in the Dmab group and no cases of osteonecrosis of the jaw or atypical femoral fracture were observed in the whole cohort.
4. Discussion
Low BMD and increased fracture risk is well described in both treatment naïve and ART-treated PLWH (Triant et al., 2008; Young et al., 2011; Starup-Linde et al., 2020; Womack et al., 2013). As in any chronic disease, the goal is to intervene in a timely manner, before permanent consequences such as osteoporotic VFs occur. In the present study, we found that one year of treatment with either ZOL or Dmab increases BMD both at the LS and FN in male PLWH requiring osteoporosis treatment. In addition, PLWH with normal bone mass at baseline experienced significant decrease in the LS-BMD but not in FN-BMD. As the majority of our subjects were in the 6th decade of their life and presented with mild osteoporosis in terms of BMD and fracture risk, these findings reinforce the still unmet need for early screening, detection, and treatment of osteoporosis in PLWH.
Both ZOL and Dmab are effective in increasing BMD in osteoporotic males, irrespectively of age and gonadal function, while ZOL has also reduced the incidence of VFs and the risk of recurrent fractures following a hip fracture (Boonen et al., 2012; Orwoll et al., 2012; Lyles et al., 2007). We show here that a single iv infusion of ZOL 5 mg in our study cohort significantly increased BMD within one year at the spine at an almost identical percentage (around 5.5%) with the pivotal ZOL study in male osteoporosis while the effect we observed at the FN was considerably higher than the one reported in the pivotal study (around 2.1%) (Boonen et al., 2012). Dmab also increased LS BMD at a similar percentage with that expected from the pivotal ADAMO study (around 5.7%) while the annual FN BMD increase was also somewhat higher than in the ADAMO study (around 2.1%) (Orwoll et al., 2012). We cannot provide a solid explanation for the greater increase in FN BMD in the ZOL group, which was significantly higher compared to the Control and the Dmab group (Table 3). The only difference at baseline between our treatment groups was that of 25OHD serum levels, which were lower in the Dmab group; however, this could not explain the observed difference in the response of FN BMD following treatment. The increase in FN BMD in our ZOL group was considerably higher than the increases reported in non-HIV osteoporotic males (Boonen et al., 2012) and even greater than the response following two annual infusions of ZOL 4 mg among young male PLWH with osteopenia under ART (Bolland et al., 2007). However, considering the small number of ZOL-treated male PLWH in our study and the fact that this study was not a randomized controlled (RCT) one, we believe that this finding should be cautiously interpreted. On the other hand, our results are in line with the proven anti-fracture efficacy of ZOL in male osteoporosis (Boonen et al., 2012; Lyles et al., 2007) and supports the use of ZOL in the clinical management of these cases. Another fact that should be considered is the discontinuation of Dmab which has been adequately investigated among postmenopausal women. Stopping Dmab results in an increase in bone turnover above pretreatment values while a rapid decrease of BMD is typically observed. Moreover, in a few patients multiple vertebral fractures might occur during this period (Tsourdi et al., 2020). Duration of Dmab therapy plays a significant role following discontinuation; therefore, a single year of treatment, as in our study, is not expected to induce a major “rebound phenomenon” (Makras and Anastasilakis, 2021). However, a therapeutic design which will include the use of an alternative agent (preferably an i.v. or oral bisphosphonate) following Dmab discontinuation is mandatory whenever Dmab is administered to a patient, and especially among those after a long term treatment period (Tsourdi et al., 2020; Makras et al., 2021).
In case of suppressed viremia and CD4 + T cell count >200u/L, the use of biologic agents is considered safe in PLWH (Louthrenoo, 2015). In this context Dmab can be safely administered in this population, in which increased production of RANKL has been reported (Moran et al., 2017). With the exception of a case report (Marasco et al., 2021), there are no other published data on the efficacy of Dmab in PLWH. The current study is the first evaluating prospectively the effect of Dmab in a cohort of male PLWH under ART. Our population consisted of males of a relatively young age compared with the usual osteoporosis cohorts, and most of our participants had low fracture risk and did not have severe osteoporosis according to their BMD (T-scores between −2.5 and − 3.0). Therefore, our results cannot be readily extrapolated in the whole population of PLWH. However, given that no adverse events were recorded and the annual BMD response was quite similar with the pivotal study of Dmab in male osteoporosis (Orwoll et al., 2012), it seems that Dmab is a rational, well-tolerated and effective treatment option for PLWH under ART requiring a therapeutic intervention for bone fragility.
A noteworthy finding of our study is the significant bone loss at the LS within 12 months in PLWH with normal bone mass. This population lost an almost 2.5% of their LS BMD despite adequate calcium and vitamin D supplementation and while they had a normal BMD and a minimal fracture risk at baseline. This is in contrast with a previous study by Bolland et al. who reported a 2.6% increase in LS BMD over a 2-years period in placebo-treated male PLWH (Bolland et al., 2007). The main differences between the populations of our study and that of Bolland et al. are: i) the age of participants, which was almost 10 years greater in our study, and ii) the duration of ART which was approximately 9 years in our study compared to the less than 4 years in the study of Bolland et al. Therefore, it seems that a significant annual BMD loss, at least in the LS, could be expected in male PLWH who are older than 50 years old and are under long term treatment with ART. However, our results are too preliminary to guide decision making with regards to a specific duration of ART treatment after which a close monitoring is mandated. As the life expectancy of PLWH is approaching that of the general population (Antiretroviral Therapy Cohort, 2017), the management of a disease associated with aging, such as osteoporosis, is quite challenging and probably should start earlier than in the general population, bearing in mind that the fracture risk assessment in PLWH may be underestimated by the FRAX score (Starup-Linde et al., 2020).
An interesting and clinically important finding of our study was the lack of APR symptoms in our ZOL treatment group. It is well known that APR is quite common following the first ZOL infusion (in up to 49–81.3% of osteoporotic patients), as we have previously reported in postmenopausal osteoporosis (Makras et al., 2021; Makras et al., 2011). Higher 25(OH)D levels appear to be protective against ZOL-induced APR while the role of previous oral BPs treatment still needs further investigation. In our study all patients were vitamin D repleted and even those with a 25(OH)D level between 37.5 and 50 nmol/L (15–20 ng/mL) received a single loading dose before treatment; this might have played a role in the absence of APR in our ZOL treated patients. Additionally, all of them were osteoporosis treatment naïve and, therefore, prior treatment cannot explain the lack of APR in our study. In the report by Bolland et al. only about 10% (2 out of 21) of ZOL treated patients experienced an APR which led them to discontinue the study (Bolland et al., 2007). This low percentage is clearly lower than the one reported in the general population and it is in line with the absence of such an event in our study. This finding is of clinical importance, as APR is by far the most common reason for osteoporotic patients to opt out from ZOL treatment. The immunoregulatory action of ZOL, which is not related to its effect on bone resorption but might be associated with its reported clinically significant extraskeletal outcomes, is still under discussion (Giusti et al., 2020); therefore, it may be important to investigate whether ART treatment prevents the appearance or alleviates the symptoms of APR. In any case, ZOL-induced APR is related to the activation and increased proliferation of γδ T cells, which can be potentially affected by both ART and HIV-infection itself (Biradar et al., 2020).
Limitations of our study include i) the small number of participants and the observational nature of the study, ii) the short period of follow up, iii) the relatively low baseline fracture risk of our cohort, and iv) the lack of bone turnover markers measurements at other time points besides baseline, that could show the comparative effects of ZOL and Dmab on bone turnover in PLWH. Given that this trial was not an RCT we can only provide the treatment effects; randomized control studies with a longer follow-up in PLWH and increased fracture risk are urgently needed to prove the efficacy and establish the regular use of both Dmab and ZOL in this population.
In conclusion, both ZOL and Dmab are efficient and well-tolerated therapeutic options that increase BMD, at least for the first year of treatment, and can be safely used in male PLWH under ART in order to prevent or in most cases reverse bone loss.
The following is the supplementary data related to this article.
Individual BMD percent changes in all study groups throughout the study period. Footnote: b,c refer to the comparison of mean percent changes of study groups; red horizontal lines represents the mean values while black horizontal lines corresponds to SEM.
CRediT authorship contribution statement
Conception and design of the work: PM, MPY. Acquisition, analysis, or interpretation of data: PM, PP, ADA, Ar.·K, AK, OT, SM, MPY. Drafting the work: PM, ADA, MPY. Revising the work critically for important intellectual content: PM, PP, ADA, MPY. Final approval of the submitted version: PM, PP, ADA, Ar.·K, AK, OT, SM, MPY. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: PM, PP, ADA, SM, MPY.
Grant support
This work was not supported by any grant, fund, or institution.
Declaration of competing interest
P. Makras reports honoraria for lectures and research grants from Amgen and Gilead; A.D. Anastasilakis reports lecture fees from Amgen; P. Petrikkos, A. Kolynou, A. Katsarou, O. Tsachouridou, S. Metallidis, and M.P. Yavropoulou have nothing to declare.
References
- Anastasilakis A.D., Polyzos S.A., Makras P. Therapy of endocrine disease: denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur. J. Endocrinol. 2018;179:R31–R45. doi: 10.1530/EJE-18-0056. [DOI] [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort C. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedimo R., Maalouf N.M., Zhang S., Drechsler H., Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26:825–831. doi: 10.1097/QAD.0b013e32835192ae. [DOI] [PubMed] [Google Scholar]
- Biradar S., Lotze M.T., Mailliard R.B. The unknown unknowns: recovering Gamma-Delta T cells for control of human immunodeficiency virus (HIV) Viruses. 2020;12(12):1455. doi: 10.3390/v12121455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland M.J., Grey A.B., Horne A.M., Briggs S.E., Thomas M.G., Ellis-Pegler R.B., Woodhouse A.F., Gamble G.D., Reid I.R. Annual zoledronate increases bone density in highly active antiretroviral therapy-treated human immunodeficiency virus-infected men: a randomized controlled trial. J. Clin. Endocrinol. Metab. 2007;92:1283–1288. doi: 10.1210/jc.2006-2216. [DOI] [PubMed] [Google Scholar]
- Boonen S., Reginster J.Y., Kaufman J.M. Fracture risk and zoledronic acid therapy in men with osteoporosis. N. Engl. J. Med. 2012;367:1714–1723. doi: 10.1056/NEJMoa1204061. [DOI] [PubMed] [Google Scholar]
- Brown T.T., Qaqish R.B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- Cotter A.G., Mallon P.W. The effects of untreated and treated HIV infection on bone disease. Curr. Opin. HIV AIDS. 2014;9:17–26. doi: 10.1097/COH.0000000000000028. [DOI] [PubMed] [Google Scholar]
- European AIDS Clinical Society (EACS) Guidelines 2020, version 10.1. 2020. https://www.eacsociety.org/guidelines/eacs-guidelines/ (Accessed 17 August 2021)
- Giusti A., Camellino D., Saverino D., Iervasi E., Girasole G., Bianchi G., Papapoulos S.E. Zoledronate decreases CTLA-4 in vivo and in vitro independently of its action on bone resorption. Bone. 2020;138 doi: 10.1016/j.bone.2020.115512. [DOI] [PubMed] [Google Scholar]
- Louthrenoo W. Treatment considerations in patients with concomitant viral infection and autoimmune rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2015;29:319–342. doi: 10.1016/j.berh.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Lyles K.W., Colon-Emeric C.S., Magaziner J.S. Zoledronic acid and clinical fractures and mortality after hip fracture. N. Engl. J. Med. 2007;357:1799–1809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makras P., Anastasilakis A.D. Questions and facts regarding denosumab discontinuation among postmenopausal women. Expert Opin. Drug Saf. 2021;20(5):499–501. doi: 10.1080/14740338.2021.1867102. [DOI] [PubMed] [Google Scholar]
- Makras P., Anastasilakis A.D., Polyzos S.A., Bisbinas I., Sakellariou G.T., Papapoulos S.E. No effect of rosuvastatin in the zoledronate-induced acute-phase response. Calcif. Tissue Int. 2011;88:402–408. doi: 10.1007/s00223-011-9468-2. [DOI] [PubMed] [Google Scholar]
- Makras P., Boubouchairopoulou N., Katsarolis I., Athanasakis K. Cost-effective osteoporosis treatment thresholds for people living with HIV infection in Greece. J. Musculoskelet. Neuronal Interact. 2017;17:292–298. [PMC free article] [PubMed] [Google Scholar]
- Makras P., Anastasilakis A.D., Antypas G. The 2018 guidelines for the diagnosis and treatment of osteoporosis in Greece. Arch. Osteoporos. 2019;14:39. doi: 10.1007/s11657-019-0584-3. [DOI] [PubMed] [Google Scholar]
- Makras P., Appelman-Dijkstra N.M., Papapoulos S.E. The duration of denosumab treatment and the efficacy of zoledronate to preserve bone mineral density after its discontinuation. J. Clin. Endocrinol. Metab. 2021 doi: 10.1210/clinem/dgab321. (2021) [DOI] [PubMed] [Google Scholar]
- Marasco E., Mussa M., Motta F., Bobbio-Pallavicini F., Maserati R., Montecucco C., Bogliolo L. Denosumab for the treatment of HIV-associated osteoporosis with fractures in a premenopausal woman. Reumatismo. 2021;73:54–58. doi: 10.4081/reumatismo.2021.1358. [DOI] [PubMed] [Google Scholar]
- McComsey G.A., Tebas P., Shane E., Yin M.T., Overton E.T., Huang J.S., Aldrovandi G.M., Cardoso S.W., Santana J.L., Brown T.T. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin. Infect. Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C.A., Weitzmann M.N., Ofotokun I. Bone loss in HIV infection. Curr. Treat. Options Infect. Dis. 2017;9:52–67. doi: 10.1007/s40506-017-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwoll E., Teglbjaerg C.S., Langdahl B.L. A randomized, placebo-controlled study of the effects of denosumab for the treatment of men with low bone mineral density. J. Clin. Endocrinol. Metab. 2012;97:3161–3169. doi: 10.1210/jc.2012-1569. [DOI] [PubMed] [Google Scholar]
- Pinzone M.R., Moreno S., Cacopardo B., Nunnari G. Is there enough evidence to use bisphosphonates in HIV-infected patients? A systematic review and meta-analysis. AIDS Rev. 2014;16:213–222. [PubMed] [Google Scholar]
- Starup-Linde J., Rosendahl S.B., Storgaard M., Langdahl B. Management of Osteoporosis in patients living with HIV-A systematic review and meta-analysis. J. Acquir. Immune Defic. Syndr. 2020;83:1–8. doi: 10.1097/QAI.0000000000002207. [DOI] [PubMed] [Google Scholar]
- Stellbrink H.J., Orkin C., Arribas J.R. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin. Infect. Dis. 2010;51:963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- Tebas P., Powderly W.G., Claxton S., Marin D., Tantisiriwat W., Teitelbaum S.L., Yarasheski K.E. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14:F63–F67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant V.A., Brown T.T., Lee H., Grinspoon S.K. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J. Clin. Endocrinol. Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsourdi E., Zillikens M.C., Meier C. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J. Clin. Endocrinol. Metab. 2020 doi: 10.1210/clinem/dgaa756. [DOI] [PubMed] [Google Scholar]
- Womack J.A., Goulet J.L., Gibert C. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin. Infect. Dis. 2013;56:1498–1504. doi: 10.1093/cid/cit056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B., Dao C.N., Buchacz K., Baker R., Brooks J.T., Investigators H.I.V.O.S. Increased rates of bone fracture among HIV-infected persons in the HIV outpatient study (HOPS) compared with the US general population, 2000–2006. Clin. Infect. Dis. 2011;52:1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual BMD percent changes in all study groups throughout the study period. Footnote: b,c refer to the comparison of mean percent changes of study groups; red horizontal lines represents the mean values while black horizontal lines corresponds to SEM.