Abstract
Blastocystis cf. Blastocystis hominis is the most common unicellular parasite found in human and animal intestines. Little is known about the life cycle, transmission and mechanisms of pathogenesis for this parasite. The aim of this study was to obtain new data on the genetic diversity of Blastocystis in selected species of mammals found in the Białowieża Primeval Forest (BPF), Poland, the best preserved lowland forest in temperate Europe. 113 faecal samples were collected in the period 2018–2020 from seven species of wild mammals occurring within the Polish part of BPF and its surroundings. Blastocystis was detected by molecular amplification and sequencing the small subunit rRNA gene. The overall prevalence of Blastocystis in animals was 8.9%. A larger number of stool samples (90) were collected from European bison and only for this species was it possible to calculate the prevalence of infection (5.6%). The isolates obtained from European bison were classified as ST1, ST3, ST5, ST7. Blastocystis was also detected in the grey wolf (Canis lupus) (ST5), wild boar (Sus scrofa) (ST5) and red deer (Cervus elaphus) (ST1). In conclusion, for the first time we have demonstrated the presence of Blastocystis in wild European bison. Preliminary studies have shown that Blastocystis is present in at least 4 species of wild mammals in the BPF area and that it exhibits great genetic diversity.
Keywords: Blastocystis, Sus scrofa, Cervus elaphus elaphus, Bison bonasus, Canis lupus, Białowieża Primeval Forest, Poland
Graphical abstract
Highlights
Blastocystis is present in wild mammals in the Białowieża Primeval Forest
Blastocystis in the Białowieża Primeval Forest exhibits great genetic diversity
Intra-species and interspecific transmission of Blastocystis in Białowieża Primeval Forest is highly probable.
1. Introduction
Blastocystis cf. Blastocystis hominis is a common unicellular protozoan inhabiting the intestinal tracts of humans and many animals, both domestic and wild (Stensvold et al., 2009; Stenzel and Boreham, 1996). The human Blastocystis infection rates range from 10% in developed countries to 100% in developing countries (Cian et al., 2017; El Safadi et al., 2014; Ramírez et al., 2014).
High genetic diversity has been found among isolates from humans and animals. Based on nucleotide differences in small subunit ribosomal RNA genes (SSU rDNA) of Blastocystis sp., 31subtypes (STs) have been found so far, however, the separateness of some of them (STs 18–20 and ST22) raises doubts (Maloney et al., 2021; Stensvold and Clark, 2020). Ten subtypes (STs 1–9 and ST12) have been identified in humans to date (Maloney et al., 2019). Since Blastocystis subtypes from animals include the same subtypes as are found in humans (with the exception of ST9), this indicates a zoonotic potential (Stensvold et al., 2009). There are some reports of the possibility of transmission of this parasite between humans and animals (Salim et al., 1999). Infection with the same subtypes of Blastocystis has been demonstrated in both humans and animals in zoo owners, butchers, and humans using the same water sources as livestock (Lee et al., 2012; Parkar et al., 2010). Transmission of Blastocystis from animals to humans has been described in various ways: directly or through contaminated water or food (Lee et al., 2012). There are reports of the occurrence of Blastocystis in companion and farm animals, but little data on the occurrence of this protist in wild animals.
So far, there have been few studies on the prevalence and genetic diversity of Blastocystis in animals in Poland. The first reports of animal blastocystosis were described in chickens in 2014 (Bobusia and Gaweł, 2014) and two years later by (Lewicki et al., 2016). In 2018, Kaczmarek and Salamatin detected Blastocystis in hedgehogs for the first time in the world (Kaczmarek and Sałamatin, 2018), and in 2019 Wesołowska et al. published the results of a study of Blastocystis in mammals from the zoo in Wrocław (Wesołowska et al., 2019).
The aim of the present study was to obtain new data on genetic diversity of Blastocystis in selected species of mammals found in the Białowieża Primeval Forest (BPF). BPF (52°30′–53°00′ N, 23°30′–24°15′ E) is the best-preserved lowland forest in temperate Europe. BPF covers 1500 km2, of which 580 km2 is located in Poland, the rest belongs to Belarus. About 30% of the forests of the BPF are of natural origin and they are spatially connected with many forests in eastern Poland as well as western and central Belarus. The BPF is inhabited by 61 species of mammals, representing about 60% of terrestrial species found in Poland (Dietz et al., 2018; Diserens et al., 2020; Jędrzejewska and Wójcik, 2004). To the best of our knowledge, there is a lack of data on occurrence of Blastocystis in wild animals living in Poland, and data from Europe are sparse.
2. Materials and methods
2.1. Sample collection
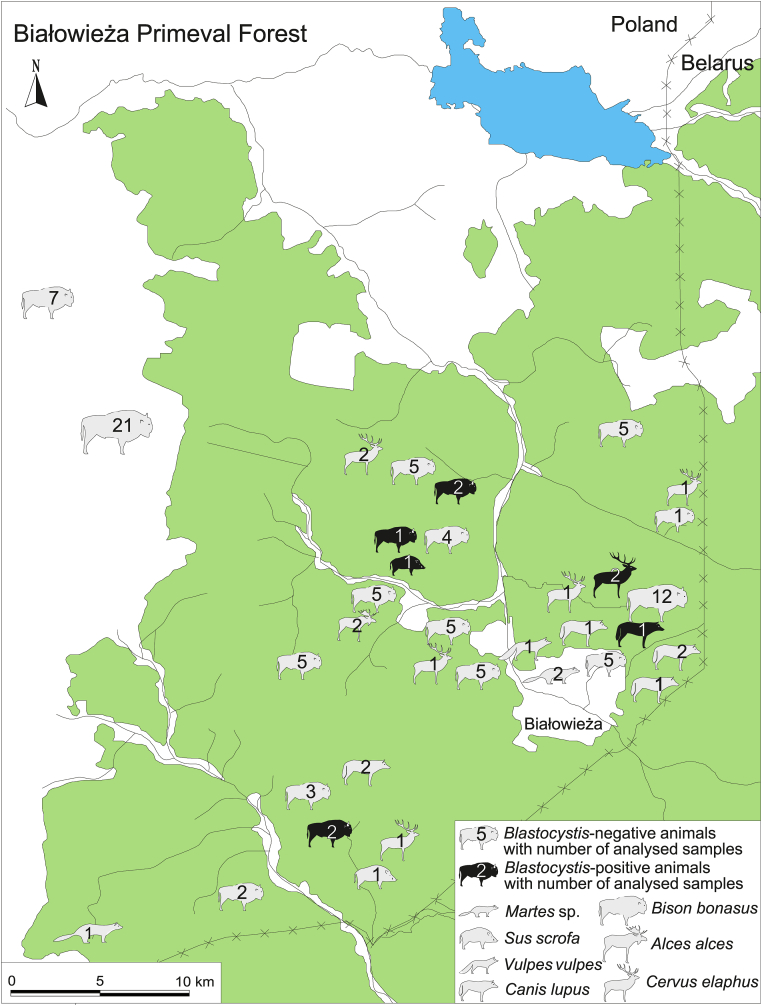
Material for the study consisted of 113 faecal samples obtained in the period 2018–2020 from seven species of wild mammals from the Polish part of Białowieża Primeval Forest (BPF) and its surroundings (see details in Fig. 1 and Table 1).
Fig. 1.
Study area with localization and number of Blastocystis-positive and Blastocystis-negative animals of particular mammalian species.
Table 1.
Animal samples collected from study hosts and subtype results from sequencing positive samples.
| Host | Latin name | Sample number | Infected | ST1 | ST3 | ST5 | ST7 |
|---|---|---|---|---|---|---|---|
| Artiodactyla | |||||||
| Wild boar | Sus scrofa | 2 | 1 | – | – | 1 | – |
| Moose | Alces alces | 2 | 0 | – | – | – | – |
| Red deer | Cervus elaphus elaphus | 8 | 2 | 2 | – | – | – |
| European bison | Bison bonasus | 90 | 5 | 1 | 1 | 2 | 1 |
| Carnivora | |||||||
| Grey wolf | Canis lupus | 7 | 1 | 0 | 0 | 1 | – |
| Red fox | Vulpes vulpes | 1 | 0 | – | – | – | – |
| Martens | Martes martes and/or M. foina | 3 | 0 | – | – | – | – |
| Total | 113 | 9 | 3 | 1 | 4 | 1 |
Faecal samples were collected in the field as fresh, randomly selected from several distant places, by field experienced researchers. From this fresh stool specimens, swabs of samples were collected with a disposable sterile polystyrene-viscose swab with carbon-containing transport medium (Equimed, cat. No. 7.012.211.110). The carbon-containing transport medium protected the material against air access, drying out and other unfavourable conditions that could affect the survival of Blastocystis until the samples were delivered to the laboratory.
2.2. Molecular identification and sequencing
DNA isolation was performed using Genomic Mini kits (A&A Biotechnology, Gdynia, Poland). Fragments of the small subunit rRNA gene were amplified by PCR using the primers Bl18SPPF1 and Bl18SR2PP (Poirier et al., 2011).
The obtained products were visualized by electrophoresis on a 2% agarose gel, the excised bands were purified using the Gel-Out kit (A&A Biotechnology, Gdańsk). Sequencing was performed using the Sanger method at the Institute of Biochemistry and Biophysics of the Polish Academy of Sciences.
Chromatograms were analysed using CLC Main Workbench v 20.0.1 software (QIAGEN Aarhus A/S). The obtained sequences without the primer sequences were compared with the sequences deposited in the GenBank database using the BLAST tool (Sayers et al., 2019).
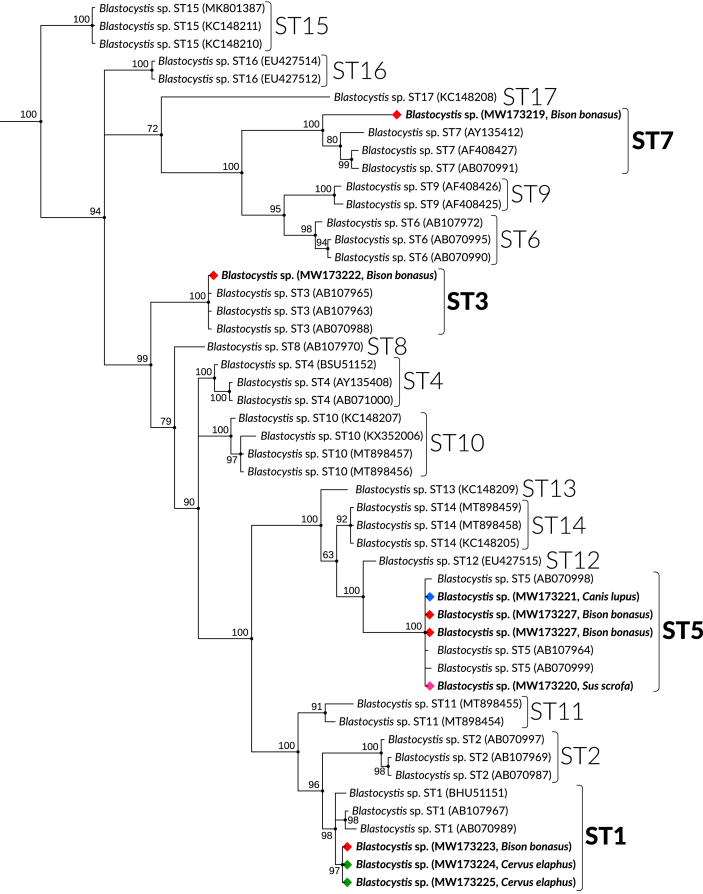
Phylogenetic analysis using Bayesian inference was performed (Huelsenbeck and Ronquist, 2005; Miller et al., 2010) taking into account 41 reference sequences representing the Blastocystis ST1–ST17 subtypes (Kaczmarek et al., 2017; Mattiucci et al., 2016; Rivera, 2008). Proteromonas lacertae (GenBank: U37108) was used as an outgroup. The sequences have been deposited with GenBank (accession numbers: MW173219–MW173229). Blastocystis subtype nomenclature according to Stensvold et al. (2007).
3. Results
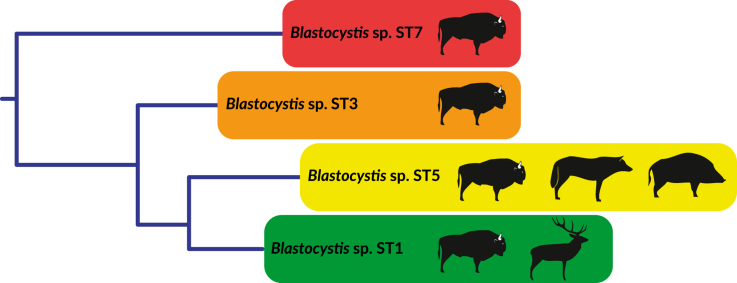
Molecular diagnosis of 113 stool samples revealed Blastocystis infection in 9 samples. Total prevalence of Blastocystis infection was 8.9%.The protozoan was detected in faecal samples from: grey wolf (Canis lupus) (1 positive), wild boar (Sus scrofa) (1 positive), red deer (Cervus elaphus) (2 positive) and European bison (Bison bonasus) (5 positive) (see Table 1). A larger number of faecal samples (90) were collected from European bison and only for this species was it possible to calculate the prevalence of infection, which was 5.6%. Based on the phylogenetic analysis, the obtained isolates were identified as the subtypes ST1, ST3, ST5 and ST7. The ST5 subtype was the most common (Table 1, Fig. 2).
Fig. 2.
Bayesian inference tree based on fragment of sequences obtained from the small subunit rRNA gene (SSU rDNA) of Blastocystis isolates of the present study, performed using MrBayes 3.2.7a. The Bayesian posterior probabilities are shown adjacent to branch nodes.
4. Discussion
The results obtained in this study are the first world data on the occurrence of Blastocystis in wild wolves and European bison and the first data on wild boar and red deer in Poland. Moreover, it should be emphasized that these data come from a forest complex, unique in Europe, which is the Białowieża Primeval Forest, inhabited by a diverse community of wild mammals. In our study Blastocystis ST5 was the most commonly identified subtype. In the literature Blastocystis ST5 subtype has been reported worldwide in both humans and animals, especially in farm animals, such as cattle, goats and pigs. In Poland, Blastocystis ST5 subtype was confirmed in zoo animals (no species listed) (Wesołowska et al., 2019), in hens (Kaczmarek et al., 2019), and in pigs (Rudzińska et al., 2020). Pig studies have shown a high Blastocystis infection prevalence of 46.97% (Rudzińska et al., 2020).
The ST1 and ST3 subtypes are the most commonly reported subtypes of Blastocystis in humans. The ST3 is considered typically anthroponotic, although in the literature it was reported from many non-humans host such as rodents and cattle (Alfellani et al., 2013; Ramírez et al., 2014). In Poland, so far ST3 subtype has only been detected in humans. A common feature of the examined wild mammals is their use of space. They mainly live in the forest, but often forage in forest meadows and field crops, which are subject to anthropogenic changes. (Kuijper et al., 2014). It is likely that the infection of wild animals with Blastocystis occurred while foraging in cultivated fields in contact with soil fertilized with manure. Organic fertilizer, such as manure, is a mixture of solid and liquid animal excrements and litter, for improving the chemical and biological properties of the soil. However, if it is poorly composted, it can be a potential source of infection with microorganisms, including parasites (Bintsis, 2018). Wild animals can be reservoirs of pathogens and transport them over long distances. Comprehensive understanding of the transmission of Blastocystis is complicated, given the wide distribution of this protozoan in the environment. The lack or a very small number of studies on the occurrence of Blastocystis in wild living animals should be emphasized, which makes it difficult to assess how this parasite is transmitted.
4.1. Mammalian orders and families infected with Blastocystis
4.1.1. Arctiodactyla
4.1.1.1. Bovidae – European bison(Bison bonasus)
European bison is a poorly studied group of mammals in terms of Blastocystis infection. So far, the ST10 subtype has been detected only in captive European bison from a UK zoo (Betts et al, 2018, 2020). Previously, Alfellani et al. (2013) tested only one sample from European bison from Poland, but they obtained a negative result. Our research showed that the prevalence of Blastocystis infection in the wild European bison population in the BPF is 5.6%.
The Blastocystis sequences obtained as a result of analysis of the European bison faecal samples from BPF belong to four different subtypes – ST1, ST3, ST5 and ST7. The ST1 and ST3 detected in European bison individuals are among the most common of Blastocystis in humans, while ST5 and ST7 are also found in humans, but more often in animals (Kaczmarek et al., 2017). So far, the ST3 subtype has not been detected in animals in Poland. Subtypes ST10 and ST14, other than those found in our population of European bison, were found in the species related to European bison – the American bison from the French zoo in La Palmyre (Cian et al., 2017). It is known that the resettlement of European bison from BPF, resulting from the history of the species and the current management of its populations, may cause transmission of diseases to various parts of Europe (Vadlejch et al., 2017). Every new pathogen found in this vulnerable species may pose a real threat and should be continuously monitored.
4.1.1.2. Suidae – wild boar(Sus scrofa)
The ST5 subtype found in our studies in wild boar populations is characteristic for Suidae; previously ST5 was also detected in wild boar in Spain (Rivero-Juarez et al., 2020), in the same study ST5 subtype (as well as ST1 and ST3) was detected in Black Iberian pig. Blastocystis ST5 is also found in other animals, but it can infect humans as well (Rudzińska et al., 2020; Stensvold et al., 2009).
The study conducted by Ithoi et al. (2011) indicated that the faeces of wild boar and other wild animals were probably the source of Blastocystis contamination in the Malaysia River. We can expect a similar situation in Poland; in the only PCR study of river water conducted so far in Poland, the presence of Blastocystis ST1 and ST3 in north-west Poland was detected (Adamska, 2020). Under the conditions of central and eastern Europe, wild boar is a species living in relatively high densities, which may affect the rapid transmission of diseases and pathogens within groups (Podgórski et al., 2018). However, the transmission of parasites, including Blastocystis, by wild boars can also be favoured by low densities of this species. Such a situation is currently observed in eastern Poland as a result of the high mortality rate caused by African swine fever (ASF) (Morelle et al., 2020). It is fostered by the method of fighting ASF, relying on carcass disposal from the forest (wild boars also feed on carrion). The scarcity of food may increase the mobility of live individuals, which contributes to the spread of diseases and pathogens (Pepin et al., 2020).
4.1.1.3. Cervidae – red deer(Cervus elaphus elaphus)
Blastocystis has already been detected in red deer - the ST4 and ST10 subtype in Great Britain (Betts et al., 2018), the ST4 subtype in Australia (Roberts et al., 2013), and the ST10 subtype in China and Malaysia (Li et al., 2020; Rauff-Adedotun et al., 2020; Zhao et al., 2017, Zhao et al., 2018). The present study found infection with the ST1 Blastocystis subtype in BPF red deer. In our opinion, among all the mammals in which we detected Blastocystis in the Białowieża Primeval Forest, the red deer is the species with the lowest possibility of transmitting diseases and pathogens over long distances. The unpublished results of the hunting inventory indicate that its density has not changed significantly in recent times. However, some authors show that red deer contribute to the transmission of certain diseases (Vikøren et al., 2019).
4.1.2. Carnivora
4.1.2.1. Canidae – Grey wolf(Canis lupus)
So far, the only subtype of Blastocystis detected in wolves is ST3 in an individual from a French zoo (Cian et al., 2017). Until now, no one has described Blastocystis in wolves in stool samples derived from wild animals. Thus, this study is the first in the world to confirm the Blastocystis infection in this group of Canidae (ST5). Wolves can migrate long distances, being an important reservoir of this pathogen in the environment. In recent years, wolves have been colonizing new areas in Europe (Szewczyk et al., 2019), which may contribute to the spread of Blastocystis across Europe. In the literature, parasitological studies of wolves have been reported (Great Britain, China, Basque Country), but no Blastocystis infection was found (Betts et al., 2020; Zhao et al., 2017, Zhao et al., 2018). In the case of samples from zoos it is important to keep in mind the ability to easily transfer various pathogens between different animals. But in nature it cannot be excluded that wolves become infected with Blastocystis from other animals, for example with ST5 after eating bison or wild boar.
Several studies have shown the presence of Blastocystis in Canidae. One case of Blastocystis infection with ST7 has been reported in dog in Poland (Kaczmarek et al., 2020). However Cian et al., based on their research on Blastocystis infection in various groups of animals, suggest that dogs are not natural hosts for this unicellular protozoan (Cian et al., 2017), as do Wang et al. (2013).
5. Conclusion
Białowieża Primeval Forest is the best-preserved forest complex in the European lowlands, characterized by a high diversity of wild mammals and numerous moist habitats. The area of the BPF is also inhabited by people and their pets, therefore intra-species and interspecific transmission of Blastocystis in this area is highly probable. Our preliminary studies have shown that Blastocystis is present in at least 4 species of wild mammals in the area and that it exhibits great genetic diversity. The limited number of tested mammalian species and collected stool samples did not allow for obtaining comprehensive data on the presence of Blastocystis in wildlife of BPF.
Therefore, we believe that research in this field should be continued, covering a wider range of hosts, as well as other regions of Poland in the future.
Declaration of competing interest
None.
References
- Adamska M. First report of Blastocystis sp. subtypes in natural water bodies in north-western Poland: a one-year monitoring. Int. J. Environ. Health Res. 2020:1–8. doi: 10.1080/09603123.2020.1803804. [DOI] [PubMed] [Google Scholar]
- Alfellani M.A., Taner-Mulla D., Jacob A.S., Imeede C.A., Yoshikawa H., Stensvold C.R., Clark C.G. Genetic diversity of Blastocystis in livestock and zoo animals. Protist. 2013;164(4):497–509. doi: 10.1016/j.protis.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Betts E.L., Gentekaki E., Thomasz A., Breakell V., Carpenter A.I., Tsaousis A.D. Genetic diversity of Blastocystis in non-primate animals. Parasitology. 2018;145(9):1228–1234. doi: 10.1017/S0031182017002347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts E.L., Gentekaki E., Tsaousis A.D. Exploring micro-eukaryotic diversity in the gut: co-occurrence of Blastocystis subtypes and other protists in zoo animals. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bintsis T. Microbial pollution and food safety. AIMS Microbiol. 2018;4(3):377–396. doi: 10.3934/microbiol.2018.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobusia K., Gaweł A. In: Aktualne Problemy w Patologii Drobiu Ze Szczególnym Uwzględnieniem Chorób Układu Oddechowego. Wieliczko A., editor. Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu; Wrocław: 2014. Epizootiologia zarażeń pierwotniaczych Histomonas meleagridis, Tetratrichomonas gallinarum i Blastocystis spp. w stadach kur reprodukcyjnych mięsnych oraz indyków rzeźnych; pp. 80–82. [Google Scholar]
- Cian A., El Safadi D., Osman M., Moriniere R., Gantois N., Benamrouz-Vanneste S., Delgado-Viscogliosi P., Guyot K., Li L.-L., Monchy S., Noël C., Poirier P., Nourrisson C., Wawrzyniak I., Delbac F., Bosc S., Chabé M., Petit T., Certad G., Viscogliosi E. Molecular epidemiology of Blastocystis sp. in various animal groups from two French zoos and evaluation of potential zoonotic risk. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0169659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz M., Brombacher M., Erasmy M., Fenchuk V., Simon O. Bat community and roost site selection of tree-dwelling bats in a well-preserved European lowland forest. Acta Chiropterol. 2018;20(1):117–127. doi: 10.3161/15081109ACC2018.20.1.008. [DOI] [Google Scholar]
- Diserens T.A., Churski M., Bubnicki J.W., Stępniak K., Pekach A., Selva N., Kuijper D.P.J. A dispersing bear in Białowieża Forest raises important ecological and conservation management questions for the central European lowlands. Glob. Ecol. Conserv. 2020;23 doi: 10.1016/j.gecco.2020.e01190. [DOI] [Google Scholar]
- El Safadi D., Gaayeb L., Meloni D., Cian A., Poirier P., Wawrzyniak I., Delbac F., Dabboussi F., Delhaes L., Seck M., Hamze M., Riveau G., Viscogliosi E. Children of Senegal River Basin show the highest prevalence of Blastocystis sp. ever observed worldwide. BMC Infect. Dis. 2014;14 doi: 10.1186/1471-2334-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. Statistical Methods in Molecular Evolution. Springer-Verlag; New York: 2005. Bayesian analysis of molecular evolution using MrBayes; pp. 183–226. [DOI] [Google Scholar]
- Ithoi I., Jali A., Mak J.W., Wan Sulaiman W.Y., Mahmud R. Occurrence of Blastocystis in water of two rivers from recreational areas in Malaysia. J. Parasitol. Res. 2011;2011 doi: 10.1155/2011/123916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jędrzejewska B., Wójcik J.M., editors. Essays on Mammals of Białowieża Forest. Mammal Research Institute, Polish Academy of Sciences; 2004. [Google Scholar]
- Kaczmarek A., Gołąb E., Żarnowska-Prymek H., Rawska A., Jańczak D., Lewicki A., Wesołowska M., Rożej-Bielicka W., Cielecka D., Sałamatin R. Genetic diversity of Blastocystis hominis sensu lato isolated from humans in Poland = Zróżnicowanie genetyczne Blastocystis hominis sensu lato wyizolowanych od ludzi w Polsce. Przegl. Epidemiol. 2017;71(4):539–546. [PubMed] [Google Scholar]
- Kaczmarek A., Lewicki A., Dziedzic K., Sulecki K., Rożej-Bielicka W., Wesołowska M., Gołąb E., Sałamatin R. A survey of Blastocystis in domestic chickens from Poland and Madagascar. Ann. Parasitol. 2019;65(Supplement):s30. [Google Scholar]
- Kaczmarek A., Rocka A., Wesołowska M., Gołąb E., Sałamatin R. Blastocystis isolates from a dog and their owners presenting with chronic diarrhoea. Dogs as reservoirs of Blastocystis: research in Poland and worldwide. Ann. Parasitol. 2020;66(4):573–579. doi: 10.17420/ap6604.300. [DOI] [PubMed] [Google Scholar]
- Kaczmarek A., Sałamatin R. 14th International Congress of Parasitology – ICOPA 2018 at: EXCO, Daegu, Korea. ICOPA; Daegu, Korea: 2018. First record of Blastocystis cf. hominis (Eukaryota: Stramenopiles) in European hedgehog (Erinaceus europaeus) from Poland; p. 1. P.1-105. [Google Scholar]
- Kuijper D.P.J., Verwijmeren M., Churski M., Zbyryt A., Schmidt K., Jędrzejewska B., Smit C. What cues do ungulates use to assess predation risk in dense temperate forests? PloS One. 2014;9(1) doi: 10.1371/journal.pone.0084607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Chye T., Karmacharya B., Govind S. Blastocystis sp.: waterborne zoonotic organism, a possibility? Parasites Vectors. 2012;5 doi: 10.1186/1756-3305-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki A., Rożej-Bielicka W., Sałamatin R. Blastocystis hominis s. l. ST6 – parasite of chickens – new zoonotic agent in Poland. Ann. Parasitol. 2016;62(Supplement):203. [Google Scholar]
- Li X.D., Zou Y., Pan J., Liang Q.L., Zeng Z., Meng Y.M., Wang X.L., Wang H.N., Zhu X.Q. Prevalence and subtypes of Blastocystis sp. infection in zoo animals in three cities in China. Parasitol. Res. 2020;119(2):465–471. doi: 10.1007/s00436-019-06571-9. [DOI] [PubMed] [Google Scholar]
- Maloney J.G., Jang Y., Molokin A., George N.S., Santin M. Wide genetic diversity of Blastocystis in white‐tailed deer (Odocoileus virginianus) from Maryland, USA. Microorganisms. 2021;9(6) doi: 10.3390/microorganisms9061343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney J.G., Lombard J.E., Urie N.J., Shivley C.B., Santin M. Zoonotic and genetically diverse subtypes of Blastocystis in US pre-weaned dairy heifer calves. Parasitol. Res. 2019;118(2):575–582. doi: 10.1007/s00436-018-6149-3. [DOI] [PubMed] [Google Scholar]
- Mattiucci S., Crisafi B., Gabrielli S., Paoletti M., Cancrini G. Molecular epidemiology and genetic diversity of Blastocystis infection in humans in Italy. Epidemiol. Infect. 2016;144(3):635–646. doi: 10.1017/S0950268815001697. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Pfeiffer W., Schwartz T. 2010 Gateway Computing Environments Workshop (GCE) IEEE; 2010. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees; pp. 1–8. [Google Scholar]
- Morelle K., Bubnicki J., Churski M., Gryz J., Podgórski T., Kuijper D.P.J. Disease-induced mortality outweighs hunting in causing wild boar population crash after African swine fever outbreak. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkar U., Traub R.J., Vitali S., Elliot A., Levecke B., Robertson I., Geurden T., Steele J., Drake B., Thompson R.C.A. Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Vet. Parasitol. 2010;169(1–2):8–17. doi: 10.1016/j.vetpar.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Pepin K.M., Golnar A.J., Abdo Z., Podgórski T. Ecological drivers of African swine fever virus persistence in wild boar populations: insight for control. Ecol. Evol. 2020;10(6):2846–2859. doi: 10.1002/ece3.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgórski T., Apollonio M., Keuling O. Contact rates in wild boar populations: implications for disease transmission. J. Wildl. Manag. 2018;82(6):1210–1218. doi: 10.1002/jwmg.21480. [DOI] [Google Scholar]
- Poirier P., Wawrzyniak I., Albert A., El Alaoui H., Delbac F., Livrelli V. Development and evaluation of a real-time PCR assay for detection and quantification of Blastocystis parasites in human stool samples: prospective study of patients with hematological malignancies. J. Clin. Microbiol. 2011;49(3):975–983. doi: 10.1128/JCM.01392-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J.D., Sánchez L.V., Bautista D.C., Corredor A.F., Flórez A.C., Stensvold C.R. Blastocystis subtypes detected in humans and animals from Colombia. Infect. Genet. Evol. 2014;22:223–228. doi: 10.1016/j.meegid.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Rauff-Adedotun A.A., Mohd Zain S.N., Farah Haziqah M.T. Current status of Blastocystis sp. in animals from Southeast Asia: a review. Parasitol. Res. 2020;119(11):3559–3570. doi: 10.1007/s00436-020-06828-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera W.L. Phylogenetic analysis of Blastocystis isolates from animal and human hosts in the Philippines. Vet. Parasitol. 2008;156(3–4):178–182. doi: 10.1016/j.vetpar.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Rivero-Juarez A., Dashti A., López-López P., Muadica A.S., Risalde M.D.L.A., Risalde M.D.L.A., Köster P.C., Machuca I., Bailo B., De Mingo M.H., Dacal E., García-Bocanegra I., Saugar J.M., Calero-Bernal R., González-Barrio D., González-Barrio D., González-Barrio D., Rivero A., Briz V., Carmena D. Protist enteroparasites in wild boar (Sus scrofa ferus) and black Iberian pig (Sus scrofa domesticus) in southern Spain: a protective effect on hepatitis E acquisition? Parasites Vectors. 2020;13(1):1–9. doi: 10.1186/s13071-020-04152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T., Stark D., Harkness J., Ellis J. Subtype distribution of Blastocystis isolates from a variety of animals from new South Wales, Australia. Vet. Parasitol. 2013;196(1–2):85–89. doi: 10.1016/j.vetpar.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Rudzińska M., Kowalewska B., Szostakowska B., Grzybek M., Sikorska K., Świątalska A. First report on the occurrence and subtypes of Blastocystis in pigs in Poland using sequence-tagged-site pcr and barcode region sequencing. Pathogens. 2020;9(7):595. doi: 10.3390/pathogens9070595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim H.R., Kumar G.S., Vellayan S., Mak J.W., Khairul Anuar A., Init I., Vennila G.D., Saminathan R., Ramakrishnan K. Blastocystis in animal handlers. Parasitol. Res. 1999;85(12):1032–1033. doi: 10.1007/s004360050677. [DOI] [PubMed] [Google Scholar]
- Sayers E.W., Beck J., Brister J.R., Bolton E.E., Canese K., Comeau D.C., Funk K., Ketter A., Kim S., Kimchi A., Kitts P.A., Kuznetsov A., Lathrop S., Lu Z., McGarvey K., Madden T.L., Murphy T.D., O’Leary N., Phan L., Schneider V.A., Thibaud-Nissen F., Trawick B.W., Pruitt K.D., Ostell J. Database resources of the National Center for Biotechnology information. Nucleic Acids Res. 2019;48(D1):D9–D16. doi: 10.1093/nar/gkz899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C.R., Alfellani M.A., Nørskov-Lauritsen S., Prip K., Victory E.L., Maddox C., Nielsen H.V., Clark C.G. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int. J. Parasitol. 2009;39(4):473–479. doi: 10.1016/j.ijpara.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Stensvold C.R., Clark C.G. Pre-empting Pandora’s box: Blastocystis subtypes revisited. Trends Parasitol. 2020;36(3):229–232. doi: 10.1016/j.pt.2019.12.009. [DOI] [PubMed] [Google Scholar]
- Stensvold C.R., Suresh G.K., Tan K.S.W., Thompson R.C.A., Traub R.J., Viscogliosi E., Yoshikawa H., Clark C.G. Terminology for Blastocystis subtypes – a consensus. Trends Parasitol. 2007;23(3):93–96. doi: 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Stenzel D.J., Boreham P.F.L. Blastocystis hominis revisited. Clin. Microbiol. Rev. 1996;9(4):563–584. doi: 10.1128/cmr.9.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk M., Nowak S., Niedźwiecka N., Hulva P., Špinkytė-Bačkaitienė R., Demjanovičová K., Černá Bolfíková B., Antal V., Fenchuk V., Figura M., Tomczak P., Stachyra P., Stępniak K.M., Zwijacz-Kozica T., Mysłajek R.W. Dynamic range expansion leads to establishment of a new, genetically distinct wolf population in Central Europe. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-55273-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlejch J., Kyriánová I.A., Rylková K., Zikmund M., Langrová I. Health risks associated with wild animal translocation: a case of the European bison and an alien parasite. Biol. Invasions. 2017;19(4):1121–1125. doi: 10.1007/s10530-016-1306-z. [DOI] [Google Scholar]
- Vikøren T., Våge J., Madslien K.I., Røed K.H., Rolandsen C.M., Tran L., Hopp P., Veiberg V., Heum M., Moldal T., das Neves C.G., Handeland K., Ytrehus B., Kolbjørnsen Ø., Wisløff H., Terland R., Saure B., Dessen K.M., Svendsen S.G., Nordvik B.S., Benestad S.L. First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. J. Wildl. Dis. 2019;55(4) doi: 10.7589/2018-10-262. [DOI] [PubMed] [Google Scholar]
- Wang W., Cuttell L., Bielefeldt-Ohmann H., Inpankaew T., Owen H., Traub R.J. Diversity of Blastocystis subtypes in dogs in different geographical settings. Parasites Vectors. 2013;6 doi: 10.1186/1756-3305-6-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesołowska Maria, Paszta W., Michrowska A., Piekarska J., Wesołowska Marta, Gorczykowski M., Kaczmarek A., Sałamatin R. Molecular characterization of Blastocystis subtypes isolated from various mammalian groups living in Wrocław ZOO, Poland. Ann. Parasitol. 2019;65(Supplement):s47. [Google Scholar]
- Zhao G.H., Hu X.F., Liu T.L., Hu R.S., Yu Z.Q., Yang W.B., Wu Y.L., Yu S.K., Song J.K. Molecular characterization of Blastocystis sp. in captive wild animals in Qinling Mountains. Parasitol. Res. 2017;116(8):2327–2333. doi: 10.1007/s00436-017-5506-y. [DOI] [PubMed] [Google Scholar]
- Zhao G.H., Hu X.F., Liu T.L., Hu R.S., Yu Z.Q., Yang W.B., Wu Y.L., Yu S.K., Song J.K. Correction to: Molecular characterization of Blastocystis sp. in captive wild animals in Qinling Mountains. Parasitol. Res. 2018;117(1):343–344. doi: 10.1007/s00436-017-5692-7. [DOI] [PubMed] [Google Scholar]