Abstract
Foramen magnum dural arteriovenous fistula (FM-DAVF) is a subset of craniocervical junction arteriovenous fistulas. We report a rare case of FM-DAVF with early rebleeding and review the literature. A 50-year-old man experienced 3 episodes of intracranial bleeding from a vessel malformation in the acute stage. We identified an FM-DAVF, supplied by multiple feeding arteries (eg, left ascending pharyngeal artery) that drained into the straight sinus and left superior petrosal sinus. The draining vein had venous varices. We performed transarterial feeder embolization and surgical disconnection of the DAVF. Early rebleeding of FM-DAVF is rare. High-risk patients require risk assessment and appropriate treatment as soon as possible in the acute stage.
Keywords: Foramen magnum dural arteriovenous fistula, Craniocervical junction arteriovenous fistula, Early rebleeding, Ascending pharyngeal artery
Abbreviations: AVM, arteriovenous malformation; CT, computed tomography; DAVF, dural arteriovenous fistula; DSA, digital subtraction angiography; GKS, gamma knife surgery; ICG, indocyanine green; MRI, magnetic resonance imaging; PICA, posterior inferior cerebellar artery; SAH, subarachnoid hemorrhage
Introduction
Foramen magnum dural arteriovenous fistula (FM-DAVF) is a subset of craniocervical junction arteriovenous fistula (CCJ-AVF) with the fistulous point at the foramen magnum. Few relevant reports exist regarding FM-DAVF [1]. CCJ-AVF is rare vascular malformation that may result in subarachnoid hemorrhage (SAH), congestive myelopathy, and brainstem dysfunction [2]. Thus, CCJ-AVF has various clinical features and a spectrum of neuroradiological findings. Diagnosis and appropriate therapy of CCJ-AVF may be delayed because of its low incidence and complex symptomatology [3,4]. However, the rebleeding rate of CCJ-AVFs, including FM-DAVF, is generally very low. Therefore, in patients with SAH, surgery would be performed in the chronic stage [5].
FM-DAVF presenting with rebleeding in the acute stage and a progressive course is rare. To the best of our knowledge, no clinical reports exist on this condition. In this paper, we report a rare case of FM-DAVF manifesting as early rebleeding. We also review the literature and discuss the clinical features of FM-DAVF.
Case presentation
A 50-year-old man, who had a medical history of hypertension and hyperlipidemia, suddenly complained of posterior cervical pain. 6 days after the onset of the initial symptoms, he consulted with his previous physicians and had no apparent neurological deficits. Computed tomography (CT) findings were normal (Fig. 1A), although magnetic resonance imaging revealed a SAH on the bilateral occipital lobes (Fig. 1B, arrows). The next day, his headache worsened. CT revealed bleeding, primarily in the third ventricle (Fig. 1C, arrows). Three-dimensional CT angiography and digital subtraction angiography revealed a vascular malformation. Gamma knife radiosurgery (GKS) (maximum dose, 36 Gy; marginal dose, 18 Gy) was performed to diagnose re-rupture of the posterior fossa arteriovenous malformation (AVM) with arterial supply from the left posterior inferior cerebellar artery. 12 days after the initial bleeding event (ie, 4 days after GKS), severe posterior cervical pain recurred. CT imaging revealed intraventricular rebleeding with ventriculomegaly of the fourth ventricle and the lateral ventricle, which indicated a third bleeding event (Fig. 1D, arrows). The patient was transferred to our hospital for consecutive treatment.
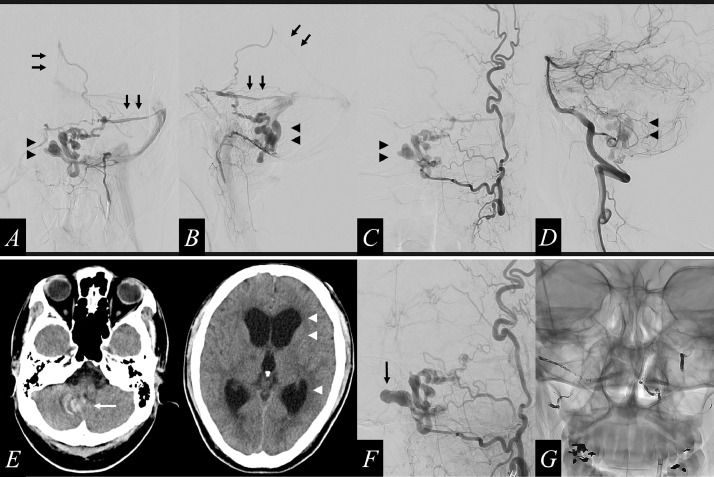
Fig. 1.
Initial computed tomography (CT) displays normal findings (A). The same-day magnetic resonance imaging (MRI) shows a subarachnoid hemorrhage in the bilateral occipital lobe (B, arrows). The next-day CT reveals bleeding, primarily in the third ventricle (C, arrows). 12 days after the initial bleeding event, CT reveals a third intraventricular bleeding event in the fourth ventricle and the lateral ventricle with intracranial ventriculomegaly (D, arrows)
A detailed review of the previous 3-dimensional CT angiography images revealed vascular anomalies fed by meningeal branches around the foramen magnum. A possible DAVF was identified. Re-examination of selective digital subtraction angiography revealed a FM-DAVF fed by the jugular branch of the left occipital artery, the jugular branch and hypoglossal branch of the left ascending pharyngeal artery, the jugular branch and mastoid branch of the right occipital artery, and the posterior meningeal artery from the left vertebral artery. The DAVF drained into a vein of the lateral recess of the fourth ventricle through an arteriovenous shunt on the left lateral margin of the foramen magnum. The draining vein, which was dilated and tortuous with large varices, entered the straight sinus and the left superior petrosal sinus (Figs. 2A-D). No arterial supply existed from the left posterior inferior cerebellar artery or pial feeder.
Fig. 2.
Digital subtraction angiography (DSA) shows a dural arteriovenous fistula (DAVF), which is fed by the jugular branch and hypoglossal branch of the left ascending pharyngeal artery (A and B), the jugular branch of the left occipital artery (C), and the posterior meningeal artery from the left vertebral artery (D) that drains into the straight sinus and the left superior petrosal sinus (A, B; arrows) through the vein of the lateral recess of the fourth ventricle, which is dilated and tortuous with large varices (A-D, arrowheads). 28 days after the initial bleeding event, the CT image shows a radical increase in the size of the varix (E, white arrow) and severe ventriculomegaly (E, white arrowheads). DSA imaging shows a radical increase in the size of the varix (F, black arrow), compared to its previous size. We performed transarterial coil embolization on the main feeders (G)
28 days after the initial bleeding event, he experienced a deterioration in the level of consciousness with a Glasgow Coma Scale score of 10 (E2V3M5). CT imaging revealed severe hydrocephalus due to a radical increase in the size of the varix (Fig. 2E, white arrow and white arrowheads; Fig. 3F, black arrow). Transarterial feeder embolization was immediately performed, after he underwent external ventricular drainage (Fig. 2G).
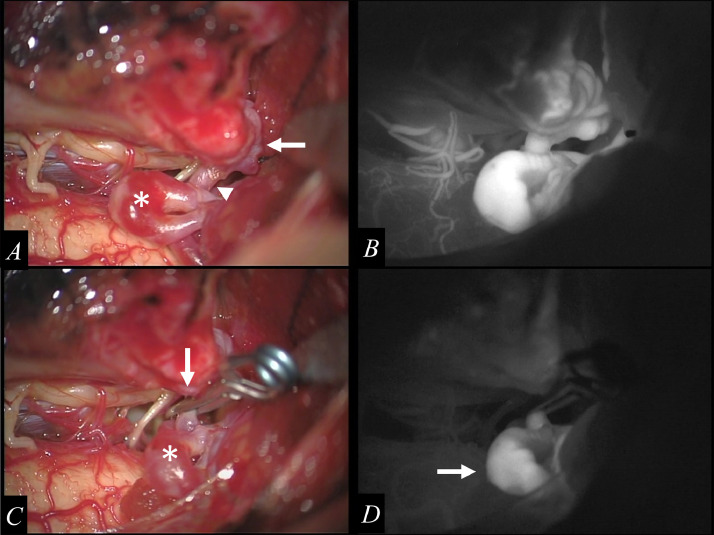
Fig. 3.
The vascular mass suspected of being a “shunted pouch” is confirmed inside of the dura of the left lateral margin of the foramen magnum (A, arrows), which is connected to vein of the lateral recess of the fourth ventricle through a single drainer (A, arrowheads). The inferior portion of the varix is identified (A, asterisk). Intraoperative indocyanine green (ICG) angiography shows retrograde flow to the varix through the single drainer in the early arterial phase (B). The single drainer is obliterated as close as possible to the shunted pouch by using an 11-mm straight clip (C, arrow). The surface of the varix is darker and the variceal wall tension is reduced (C, asterisk). ICG angiography shows stagnation of blood flow in the varix (D, arrow) after the occlusion of the draining vein.
2 days after the feeder embolization, direct surgical interruption of the draining vein was performed by using a 11-mm straight clip. Complete obliteration was achieved (Fig. 3). After he underwent an additional surgery for hydrocephalus, he was transferred to a rehabilitation facility because of mild weakness of the bilateral lower extremities. He returned home without neurological deficits.
At 6 months postsurgery, follow-up angiographic examinations showed no recurrence of the DAVF. No recurrence of intracranial hemorrhage has occurred during 2 years of follow up.
Discussion
CCJ-AVF occurs in 1%-2% of patients with an intracranial or spinal arteriovenous fistula [6]. CCJ-AVF may result in SAH and congestive myelopathy. A diagnosis of CCJ-AVF may be difficult because of its low incidence and variable clinical features [1,4].
FM-DAVF is a subset of CCJ-AVF. However, FM-DAVF has not been clearly defined. A CCJ-AVF can occur anywhere from the foramen magnum to the high cervical spine. Hiramatsu et al. [6] proposed the disease concept of “radiculomeningeal AVF,” which develops along the C-1 or C-2 nerve roots and mostly includes radiculomeningeal arteries from the vertebral artery as the feeding arteries. By contrast, previous reports [3] reveal that a FM-DAVF is primarily fed by the ascending pharyngeal arteries. This finding is consistent with the fact that a part of the foramen magnum is phylogenetically regarded as a somite, which the ascending pharyngeal artery may supply [7].
We searched relevant published articles regarding FM-DAVF with SAH. We found overall 11 corresponding cases among 5 articles (Table 1) [1,[3], [4]–5,8]. 5 patients had an arterial supply from the ascending pharyngeal artery. The other cases included the vertebral artery or occipital artery. Zhao et al. [9] report that most FM-DAVFs were supplied by the meningeal branches of the vertebral artery or by the occipital artery and ascending pharyngeal artery. The 3 arteries have a complicated anastomosis with each other at the dura mater around the foramen magnum [10].
Table 1.
Summary of reports in the literature on FM-DAVF presenting with subarachnoid hemorrhage.
| Reference | Age/Sex | Presentation | Feeder | Drainer | Treatment |
|---|---|---|---|---|---|
| Kinouchi et al. 1998 [5] | 65/M | SAH | VA | MV | Direct surgery |
| 68/M | SAH | VA | MV | Direct surgery | |
| Guo et al. 2010 [8] | 47/M | SAH | VA | MV | Direct surgery |
| 51/M | SAH | VA | MV | Direct surgery | |
| 35/M | SAH | OA, APA | MV, COS | Direct surgery | |
| 40/M | SAH | OA | MV, StS, COS | Without surgery | |
| Nakamura et al. 2017 [4] | 69/F | SAH | VA | MV, IPS | Direct surgery |
| Motebejane and Choi 2017 [3] | 53/M | SAH | APA | MV | IVR with NBCA |
| 42/M | SAH | APA, VA | MV | IVR with NBCA | |
| 51/M | SAH | APA, VA | MV, JB | IVR with NBCA | |
| Kim et al. 2018 [1] | 48/M | SAH | APA | VP, SS | IVR with Onyx |
APA, ascending pharyngeal artery; COS, confluence of sinuses; F, female; FM-DAVF, foramen magnum dural arteriovenous fistula; IPS, inferior petrosal sinus; IVR, interventional radiology; JB, jugular bulb; M, male; MV, medullary vein; NBCA, n-butyl cyanoacrylate glue; OA, occipital artery; SAH, subarachnoid hemorrhage; SS, sigmoid sinus; StS, straight sinus; VA, vertebral artery; VP, venous plexus.
In our patient, determining a correct diagnosis and the appropriate treatment took time, owing to misidentifying the venous varix as the nidus of the AVM. The FM-DAVF with its numerous dilated veins and a large varix mimicked an AVM, although the meningeal branches, including the branches of the ascending pharyngeal artery, provided a clue to the diagnosis of the DAVF. Arterial supply from meningeal branches associated with the ascending pharyngeal artery may aid in making a correct diagnosis of FM-DAVF.
Only a few reports exist regarding early rebleeding of a CCJ-AVF. Zhong et al. [11] report that a recurrence of SAH occurred in 3 (8.3%) patients with CCJ-AVF with SAH before surgical treatment and occurred in one of these patients during the acute stage (ie, 14 days after the initial SAH) and occurred in the other 2 patients 28 days later and 4 years later, respectively. However, no report exists of 2 occurrences of rebleeding in the acute stage, as in our patient, and no report exists concerning especially the rebleeding risk of FM-DAVF. Duffau et al. [12] report that intracranial DAVFs with retrograde cortical venous drainage present a high risk of early rebleeding. In addition, Brown et al. [13] report that lesions of petrosal sinus and straight sinus, a venous varix on the draining vein, and lesions draining into leptomeningeal veins increase the risk of intracranial hemorrhage. These features may also be a risk factor for early rebleeding from CCJ-AVFs or FM-DAVFs. GKS was performed because of the misdiagnosis of the DAVF as an AVM in our patient; however, no significant relationship existed between the third bleeding and GKS. In 1 study [14] investigating changes in an animal model of arteriovenous fistula treated with GKS, the researchers concluded that GKS produced morphological, angiographic, and histological changes in the arteriovenous fistula model as early as 6 weeks after treatment.
Table 1 shows that only 1 patient had venous drainage into the straight sinus, although all patients had superior direct drainage into the intracranial venous systems. The aforementioned patient had a high-flow shunt and venous varices, as did our patient. For a FM-DAVF that includes a high-flow shunt and venous varices, venous drainage into the deep cerebral veins may be rare, which is compatible with the low rebleeding rate. However, a significant number of high-risk patients present with an aggressive clinical course. In our patient, the FM-DAVF drained into the petrosal sinus and straight sinus, and it had venous varices on the draining vein with a high-flow shunt. A FM-DAVF that has venous varices and venous drainage into deep cerebral veins is conceivably associated with a higher risk of bleeding, similar to that of an intracranial DAVF involving the aforementioned risk factors of bleeding.
In general, FM-DAVF patients with SAH can be treated surgically in the chronic stage. By contrast, some patients have an aggressive clinical course such as rebleeding or a radical increase in size of the varix. In these situations, administering curative treatments as soon as possible is desirable [10].
Conclusion
FM-DAVF is a rare disease that is sometimes difficult to distinguish from an AVM. Meningeal branches associated with the ascending pharyngeal artery may aid in making a correct diagnosis. The rebleeding rate of FM-DAVF has been considered as very low. However, high-risk patients exist and would require a risk assessment and appropriate treatment as soon as possible in the acute stage.
Patient consent
The patient provided informed consent for treatment and consent for his data to be published in this report. This study was conducted in line with the principles of the Declaration of Helsinki. This is an observational study. The institution review board of Kyoto Prefectural University Graduate School of Medicine (Kyoto, Japan) has confirmed that no ethical approval is required.
Footnotes
Acknowledgments: The authors received no funding for this work.
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Kim H, Lee Y-S, Kang H-J, Lee M-S, Suh S-J, Lee J-H. A rare case of subarachnoid hemorrhage caused by ruptured venous varix due to dural arteriovenous fistula at the foramen magnum fed solely by the ascending pharyngeal artery. J Cerebrovasc Endovasc Neurosurg. 2018;20:120–126. doi: 10.7461/jcen.2018.20.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129:3150–3164. doi: 10.1093/brain/awl220. Pt 12. [DOI] [PubMed] [Google Scholar]
- 3.Motebejane MS, Choi IS. Foramen magnum dural arteriovenous fistulas: clinical presentations and treatment outcomes, a case-series of 12 patients. Oper Neurosurg (Hagerstown) 2018;15:262–269. doi: 10.1093/ons/opx229. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Miyazaki T, Shinozaki N, Izumi M, Itabashi T. Clinical characteristics of craniocervical junction arteriovenous fistulas. No Shinkei Geka. 2017;45:879–888. doi: 10.11477/mf.1436203611. Japanese. [DOI] [PubMed] [Google Scholar]
- 5.Kinouchi H, Mizoi K, Takahashi A, Nagamine Y, Koshu K, Yoshimoto T. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg. 1998;89:755–761. doi: 10.3171/jns.1998.89.5.0755. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu M, Sugiu K, Ishiguro T, Kiyosue H, Sato K, Takai K. Angioarchitecture of arteriovenous fistulas at the craniocervical junction: a multicenter cohort study of 54 patients. J Neurosurg. 2018;128:1839–1849. doi: 10.3171/2017.3.JNS163048. [DOI] [PubMed] [Google Scholar]
- 7.Lasjaunias P, Moret J, Theron J. The so-called anterior meningeal artery of the cervical vertebral artery. Normal radioanatomy and anastomoses. Neuroradiology. 1978;17:51–55. doi: 10.1007/BF00345270. [DOI] [PubMed] [Google Scholar]
- 8.Guo L-M, Zhou H-Y, Xu J-W, Wang G-S, Tian X, Wang Y. Dural arteriovenous fistula at the foramen magnum presenting with subarachnoid hemorrhage: case reports and literature review. Eur J Neurol. 2010;17:684–691. doi: 10.1111/j.1468-1331.2009.02895.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Xu F, Ren J, Manjila S, Bambakidis NC. Dural arteriovenous fistulas at the craniocervical junction: a systematic review. J Neurointerv Surg. 2016;8:648–653. doi: 10.1136/neurintsurg-2015-011775. [DOI] [PubMed] [Google Scholar]
- 10.Rhoton AL., Jr The foramen magnum. Neurosurgery. 2000;47(3):S155–S193. doi: 10.1097/00006123-200009001-00017. Suppl. [DOI] [PubMed] [Google Scholar]
- 11.Zhong W, Zhang J, Shen J, Su W, Wang D, Zhang P, Wang Y. Dural arteriovenous fistulas at the craniocervical junction: a series case report. World Neurosurg. 2019;122:e700–e712. doi: 10.1016/j.wneu.2018.10.124. [DOI] [PubMed] [Google Scholar]
- 12.Duffau H, Lopes M, Janosevic V, Sichez JP, Faillot T, Capelle L. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg. 1999;90:78–84. doi: 10.3171/jns.1999.90.1.0078. [DOI] [PubMed] [Google Scholar]
- 13.Brown RD, Jr, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg. 1994;81:531–538. doi: 10.3171/jns.1994.81.4.0531. [DOI] [PubMed] [Google Scholar]
- 14.Kashba SR, Patel NJ, Grace M, Lee VS, Raoufi-Rad N, Raj JV. Angiographic, hemodynamic, and histological changes in an animal model of brain arteriovenous malformations treated with gamma knife radiosurgery. J Neurosurg. 2015;123:954–960. doi: 10.3171/2014.10.JNS1435. [DOI] [PubMed] [Google Scholar]