Abstract
Introduction
Behçet's disease (BD) is an immune-mediated chronic systemic vasculitis, characterized by clinical manifestations that include: mucocutaneous ulcers, ocular involvement, immunological alterations, vascular and neurological implications. The available treatments present limitations such as high cost and side effects, and the search for a low-cost biological treatment with immunomodulatory potential becomes of great value. Platelet rich plasma (PRP) has some characteristics that indicate a possible use as an immunomodulator due to the wide range of secreted cytokines, especially through the participation of TGF-β1 in the differentiation of T regulatory cells (Treg). This study aimed to characterize the PRP poor in leukocytes (P-PRP) of patients with BD and active ulcers and to evaluate its effects as an immunomodulator through a subcutaneous application.
Methods
We selected patients with a diagnosis of BD, with a low dose of prednisone and with no central nervous system or ocular involvement. Platelet and leukocyte count and quantification of 17 cytokines were evaluated in P-PRP. The effects of P-PRP were evaluated by cell frequency of TCD4 +, TCD8 +, Treg, natural killer (NK), and activated NK, as well as by the cytokine profile in patient's plasma, and the clinical manifestations through score and questionnaire. Also, it was evaluated the number and timing of oral ulcer closure. PRP was used as an adjuvant, with 9 applications of 3 mL, over 6 months, with a follow-up of one year.
Results
The results using PRP showed adequate values and no significant inter-and intra-individual variations. The systemic evaluations during the use of PRP showed significant alterations, characterized by the increase in Treg cell frequency (p = 0.0416) and a decrease in activated NK cells (p = 0.0010). However, no clinical correlation was observed through score analysis. The most relevant clinical data was the decrease in the closing time of ulcers throughout the application period.
Conclusion
In a pilot study with BD patients, P-PRP promoted an anti-inflammatory profile characterized by increased Treg cells and decreased activated NK cells and alterations in cytokines. A clinical improvement was observed with a decrease in the number and time of closure of oral ulcers.
Keywords: Immunomodulation, Platelet rich plasma, Behçet disease
1. Introduction
Behçet's disease (BD) is a chronic inflammatory condition characterized by vasculitis, with recurrent attacks of aphthous oral and genital ulcers, skin lesions, and clinical ocular involvement. Other manifestations include the central nervous system (CNS), gastrointestinal tract (GIT), lung, and heart. The disease presents periods of remission and exacerbation and the symptomatology can vary according to the geographic location [[1], [2], [3]].
HLA-B5 histocompatibility antigen is the major genetic factor for BD susceptibility [4]. The involvement of Th1 cells has been demonstrated [3,5], with a predominance of the immune response of Th1 and Th17 cells and increased levels of related cytokines, such as IFN-γ, IL-12, TNF-α, and IL-17. Alteration patterns are observed when the disease is active in comparison to remission and controls, such as increased IL-17 [6,7], a higher frequency of NK cells and activation state with CD69 [8]. T regulatory cells (Treg) play a role in the pathogenesis and active BD patients had significantly higher CD4+CD25+/high T cells [9]. mRNA for Forkhead box p3 (Foxp3) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) showed impaired CD4+CD25+/high T cells in their proliferative responses, suppressing the proliferation of their CD4+CD25-counterparts [9].
The diagnosis of BD is essentially clinical and based on diagnostic criteria of the International Study Group for Behçet Disease (ISGBD) [10].
The conventional treatment is based on drugs that aim to decrease the activity/function of white blood cells and cytokines release. In non-responders, high-cost biological drugs such as IFN-α and TNF-α blocking can be used [11,12]. The search for new treatments, with low cost and fewer side effects, is a challenge and is of great value in clinical practice.
Platelet rich plasma (PRP) is defined as a platelet concentration above the baseline in a small volume of plasma [13,14] containing biomolecules, such as growth factors, immune messengers, enzymes, and other bioactive components [16,17,13]. Platelets also modulate the inflammatory response via interaction with endothelial cells and leukocytes. The major immunomodulators described are PDGF, TGF-β, soluble CD40 ligand (sCD40L) and platelet factor 4 (PF4/CXCL4) [18]. TGF-β is the major immunosuppressant, and Treg differentiation is dependent on TGF-β [19]. PRP has been used in diseases with immune impairment, such as rheumatoid arthritis [15].
This study aimed to characterize the PRP of BD patients with active ulcers, and to evaluate PRP immunomodulatory effects through a subcutaneous application, analyzing TCD4, TCD8, NK, NK activated (CD69), and Treg cells frequency and cytokines dosage.
2. Materials and methods
2.1. Patient selection and ethical aspects of the research
This was a prospective pilot study with 1-year follow-up. All the medical records of the patients with the diagnosis of BD according to the criteria established by ISGBD attended at the Vasculitis Clinic of the Clinical Hospital/Rheumatology Department of the Faculty of Medical Sciences of the University of Campinas (HC/FCM-UNICAMP) were reviewed between February 2014 and August 2015. After signing the informed consent form (CAAE number 27542214.3.0000.5404), the identification data, history of the disease, use of medications, personal and family history, physical examination, and routine laboratory tests (blood counts, determination of HSV/Wintrobe, urine I, creatinine, aminotransferases and serologies for hepatitis B, C, and HIV) were performed for each patient.
2.2. Ethics approval and consent to participate
The study was approved by Brazilian Law (CEP/CONEP; CAAE number 27542214.3.0000.5404). The participants signed the consent form before any procedure. Trial registration number: RBR-22z3jy and URL: http://www.ensaiosclinicos.gov.br/rg/RBR-22z3jy/.
2.3. Inclusion and exclusion criteria
Inclusion criteria in patients with a confirmed diagnosis of BD comprised a minimum of three episodes of disease activity over the last year, presence of oral or genital ulcers, and a daily maximum of 10 mg prednisone. Exclusion criteria included activity of the disease in the central nervous system, history of neoplasia in the last 5 years, use of anticoagulants, and ocular, hematological, or renal alterations.
2.4. HLA-B typing
HLA locus B genotyping was performed using the Class I locus B specific SSP DNA kit (One Lambda, Thermo Fisher, California), in DNA obtained from a blood sample collected in EDTA tubes.
2.5. P-PRP preparation
Leukocyte-poor PRP (P-PRP) was prepared from six tubes of 8.5 mL of blood with acid citrate dextrose (ACD), as previously described [20,21]. Serum was obtained from blood collected without anticoagulant and centrifuged at 1560×g for 15 min. It was ministered the serum at a rate of 1:6 to P-PRP at the time of application as an activator of P-PRP, which means that we used 0.5 mL of autologous serum for each 2.5 mL of P-PRP.
2.5.1. P-PRP characterization
P-PRP characterization included the platelet and leukocyte counts (in all collections), and cytokine concentration in PRP samples obtained before (basal), 3 and 6 months after the first application. PDGF-AA, VEGF, EGF, TGF-β1 growth factors were measured using Luminex technology (Merck Millipore, United States). Acidification of samples with HCl before TGF-β1 quantification was performed to identify the active form. For all these dosages the manufacturer's protocol was followed. The IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IL-21 interleukins, the cytokines TNF-α, IFN-γ, and CD40L were quantified by the CBA (Cytometric Bead Array, Bencton Dickinson, United States) platform of high (fg/mL) and normal (pg/mL) sensitivity.
2.6. P-PRP applications
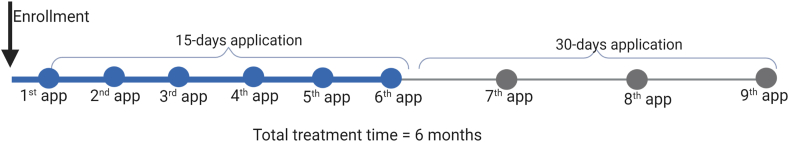
Nine autologous P-PRP applications were performed in each patient during six months of treatment. The first six applications were performed with a 15-day interval, totaling three months of treatment. Subsequently, a further 3 applications were performed, with a 30-day interval, for 3-months, completing the six months of the treatment period (Fig. 1). It was administered 3 mL in each patient (P-PRP + serum) through a periumbilical subcutaneous application.
Fig. 1.
Treatment schedule for BD patients. The patients were treated for 6 months, the first 3 months with a 15-days application, and the last 3 months with 30-days applications. N = 6 patients.
The patients were followed every 3 months up for 12 months after the first application.
2.7. Systemic evaluation
2.7.1. Biologic profile
The quantification of the cytokines was performed on patient plasma samples collected before and every 3 months until 12 months after the first application (baseline, 3, 6, 9, and 12 months). Two tubes of blood were collected in 3.2% citrate, and the methodology was the same used for P-PRP.
2.7.2. Cell quantification
A peripheral blood sample was collected in a heparin tube for cellular quantification by flow cytometry in basal, 3, 6, 9, and 12 months after application. The protocol was standardized for quantification of TCD4 (CD3+ and CD4+), TCD8 (CD3+ and CD8+), NK (double positivity for CD56+ and CD16+ and negativity for CD3-), NK activated (CD3-, CD56+ and CD69+) and Treg cells (CD4+, CD25+ and FoxP3+). All antibodies used were from BD Pharmigen (BD Biosciences, United States).
2.7.3. Evaluation criteria
The clinical criteria were represented by the number and duration of the episodes of activity during the 12 months, evaluated every 3 months using BR-BDCAF (Brazilian Behçet Disease Current Activity Form)10, and life quality short-form (SF-36). Patients recorded the number and ulcers locations on the skin and mucosa and the time between onset and healing.
2.8. Statistical analysis
A frequency table with categorical variables with absolute (n) and percentage (%) frequency values was used, and descriptive statistics of the numerical variables with values of mean, standard deviation, minimum values, and maximum and median.
ANOVA was used to compare the variables between the applications for repeated measures. The data were transformed into ranks due to the absence of normal distribution. The level of significance was 5%.
3. Results
3.1. Casuistic
From February 2014 to August 2015, 77 patients were selected, however, due to inclusion and exclusion criteria, 65 patients were excluded, and 12 patients were considered eligible. The exclusion was due to ocular activity of BD (19.5%), without disease activity (15.5%), higher doses of corticoids (13%), incomplete BD (13%), neurobehçet (6.5%), in use of anticoagulation drugs (6.5%), use of immunobiological (5.2%), did not want to participate (5.2%), as previously reported by Sachetto et al., (2012) [22]. Of these 12 eligible, two dropped out at the time of inclusion, one was excluded for illicit drug use and another patient was pregnant. From the 8 patients included only 6 completed all the follow-up. All patients were taking colchicine 0.5 mg daily, 5 prednisone 10 mg daily and one was taking no medication.
Regarding the clinical characteristics: 3 were male and 3 female, 3 black, and 3 caucasian. The mean age was 42.8 ± 11.3 years, the mean age of onset symptoms was 25.5 ± 5.1 years and the mean age of diagnosis was 28.7 ± 7.3 years. One patient presents a familiar history of BD associated with the presence of HLA-B51. Two patients present osteoporosis and one patient hypertension as comorbidities. The clinical signs of BD were verified by oral lesions in all patients, five patients present genital lesions, five patients exhibited stable ocular alterations and three patients showed cutaneous lesions.
HLA locus B genotyping revealed: two B∗08 and B∗44, one B∗14 and B∗48, one B∗15 and B∗35, one B∗15 and B∗53, one B∗15 and B∗53, one B∗38 and B∗53 and one B∗51 and B∗53. The patients evaluated it was not verified the presence of HLA B∗51 in a high incidence.
3.2. P-PRP characterization
All P-PRPs showed platelet counts above 1,000,000/μl, average 1,130,000 (5x the baseline) (Fig. 2A). The number of platelets was homogeneous, with no significant variation in all preparations (p = 0.7440). The average of white blood cells was 1500/μl (0.5 x basal value), with a significant dispersion of the counting values (Fig. 1B) (p = 0.0014). To avoid this high variation and decrease the number of leukocytes, a brake was used in the preparation (Fig. 2B) after the fourth application. It means, that we performed a slow stop of the centrifuge to avoid the mixture of cell layers after the spin, and we were able to produce a PRP poor in leukocytes.
Fig. 2.
Cell yield during the applications. A) Recovery of platelets in P-PRP; B) Recovery of leukocytes in P-PRP. N = 6 patients.
Only TGF-β1 showed a significant increase (p < 0.0001) at 6 months when compared to other periods. The other growth factors showed no significant differences at all times (Table 1).
Table 1.
Median, minimum and maximum of cytokines in baseline, 3 months and 6 months in P-PRP of BD patients (N = 6).
| Cytokines (Median) | Baseline | 3 months | 6 months | P value |
|---|---|---|---|---|
| VEGF (pg/mL) | 1362 (635.8–3764) | 1347 (452.7–3330) | 1376 (786–2465) | n.s |
| EGF (pg/mL) | 1001 (241.6–22,275) | 954.7 (364.8–2963) | 1181 (448–2377) | n.s |
| PDGF (pg/mL) | 51,192 (43,166–104,909) | 89,041 (26,436–288,586) | 50,619 (27,821–106,204) | n.s |
| PF4 (pg/mL) | 82,337 (739.9–1,007,000) | 23,397 (1096–1,390,000) | 91,843 (56,613–92,421) | n.s |
| TGF-β (pg/mL) | 120,935 (76,245–187,411) | 117,292 (83,460–161,593) | 371,765 (194,342–434,320) | <0.0001 |
| CD40L (pg/mL) | 7764 (4377–12,094) | 5645 (3293–14,408) | 4073 (3035–9136) | n.s |
| IL-13 (pg/mL) | 0.0350 (0–0.73) | 0.1850 (0–0.6) | 0.0 (0–0) | n.s |
| IL-17 (pg/mL) | 2.500 (0–5.53) | 0.0 (0–7.89) | 0.0 (0–0.08) | n.s |
| IL-6 (pg/mL) | 1.305 (0–4.6) | 0.6850 (0–7.07) | 1.040 (0–2.72) | n.s |
| TNF-α (fg/mL) | 0.0 (0–829.3) | 0.9750 (0–631) | 9.150 (0–548.8) | n.s |
| IL-1β (fg/mL) | 78.68 (0–578.4) | 15.56 (0–1528) | 166.4 (0–513.1) | n.s |
| IL-2 (fg/mL) | 0.0 (0–41.08) | 0.0 (0–85.96) | 0.0 (0–0) | n.s |
| IL-8 (fg/mL) | 11,208 (8549–20,789) | 11,830 (7020–222,793) | 9322 (779.1–14,880) | n.s |
| IL-10 (fg/mL) | 271,3 (113.5–909.9) | 276,5 (64.51–615.2) | 21,10 (0–436.3) | <0.088 |
The profile of cytokines showed no significant difference between the baseline, 3 and 6 months, except for IL-10 (Table 1). IL-10 concentration was significantly decreased after 6 months when compared to basal levels (p < 0.088).
3.3. Clinical evaluation
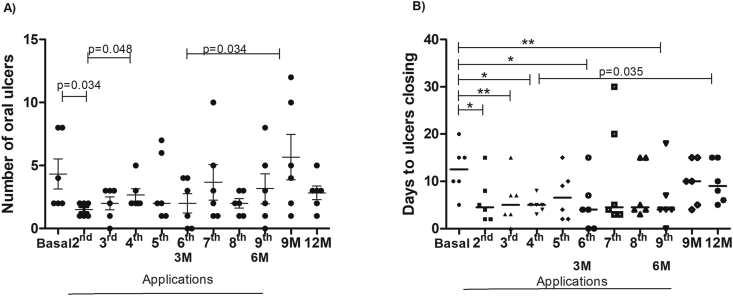
The number of oral ulcers presented a variation throughout the follow-up period. In the second application, the number of ulcers was significantly lower than the basal (p = 0.034). At 9 months, 3 months after stopping P-PRP, a higher number of oral ulcers was observed, significant when compared to the 3 months (p = 0.034). The median number of ulcers in each application is shown in Fig. 3A.
Fig. 3.
A) The median number of oral ulcers in BD patients throughout the follow-up period; B) Median time (in days) for the closure of oral ulcers in the follow-up period ∗ p = 0.036; ∗∗p = 0.031. N = 6 patients. Abbreviation: M−months.
The number of days for ulcer closure was significantly higher at baseline when compared to almost the entire application period of P-PRP. After finalizing P-PRP applications (6 months), there was an increase in the number of days to ulcer closure. The median time in days for the closure of oral ulcers is shown in Fig. 3B.
The other symptoms and quality of life did not differ before and after P-PRP use (data not shown).
3.3.1. Cell frequency of BD patients
NK and CD4 cells were not significantly different throughout the entire period of observation (1 year) (data not shown).
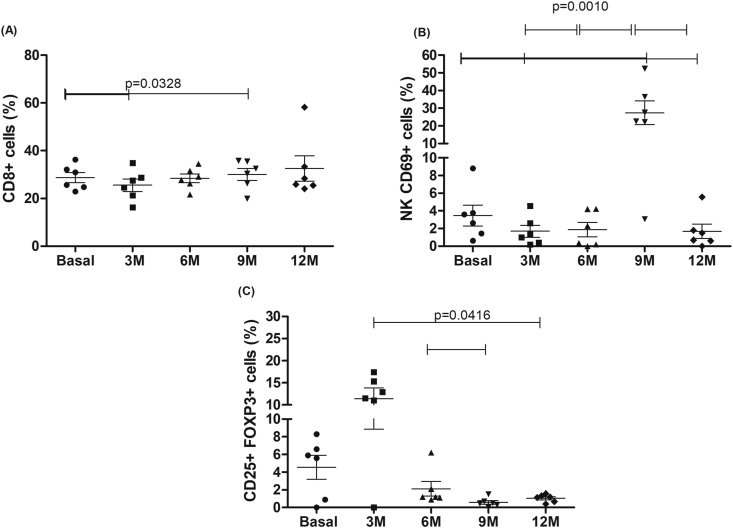
CD8+ cells showed a significant increase at 9 months (p = 0.0329) in relation to baseline (Fig. 4A). The baseline activated NK cells were significantly elevated when compared to the values obtained at 3 and 12 months (Fig. 4B). Elevated values of activated NK cells were observed at 9 months when compared to all other times. A significantly higher frequency of Treg cells was observed at 3 months when compared to 12 months, and 6 months when compared to 9 months, as shown in Fig. 4C.
Fig. 4.
Frequency of different cell populations during the follow-up period. A) TCD8 +, B) NK CD69 + (disease activity); C) regulatory T cells (CD4 +, CD25 +, FoxP3 +). N = 6 patients. Abbreviation: M−months.
3.3.2. Systemic cytokines
The evaluation of the systemic cytokines TGF-β1, PDGF-AA, and EGF showed no significant variation during all times (Table 2).
Table 2.
Median, minimum and maximum values of systemic cytokines concentration during treatment and follow-up of BD patients (N = 6).
| Cytokine | Baseline | 3 months | 6 months | 9 months | 12 months | P value |
|---|---|---|---|---|---|---|
| TGF-β1 (pg/mL) | 5489 (2041–10,072) | 4040 ((961.5–41,101) | 6906 (2415–48,474) | 10,869 (6624–278,552) | 6774 (2976–17,293) | n.s |
| EGF (pg/mL) | 18.3 (9.3–24.98) | 17.3 (0–2936) | 12.1 (0–102.2) | 3.3 (0–55) | 4.3 (0–85) | n.s |
| PDGF-AA (pg/mL) | 324.6 (59.7–2199) | 378.5 (22.1–42,603) | 216.8 (0–326.1) | 208.5 (0–1285) | 119.0 (0–256.6) | n.s |
| VEGF (pg/mL) | 736.5 (452.7–1648) | 542.7 (81–1491) | 512.2 (244.5–874) | 144.6 (21.1–995.3) | 142.8 (100.9–870.2) | 0.0050 |
| IL-6 (pg/mL) | 2.2 (0–3.35) | 1.5 (0–5.6) | 0.4 (0–4.0) | 3.4 (0–8.7) | 3.8 (0.3–5.4) | n.s |
| PF4 (pg/mL) | 1536 (829.9–17,044) | 3106 (1007–18,694) | 1157 (628.7–2670) | 2035 (815.3–3608) | 3968 (2027–5124) | 0.0128 |
| IL-10 (fg/mL) | 170 (95.1–487.4) | 160.1 (56.9–288.2) | 143 (58.5–357.2) | 5.6 (0–154.3) | 9.1 (4.4–67.6) | <0.0001 |
| IL-8 (fg/mL) | 1517 (508.6–4385) | 1943 (749.5–4736) | 1471 (534.9–3103) | 344.8 (52.4–2596) | 904.8 (409–6224) | 0.0459 |
| CD40L (pg/mL) | 22 (1.8–171.2) | 9.9 (0.05–38.8) | 2.2 (0–4.6) | 16.2 (1.7–22.3) | 24.1 (17.3–50.9) | 0.0475 |
VEGF showed a decrease during follow-up, reaching the lowest value at 12 months, being significantly lower when compared to baseline, 3 and 6 months (p = 0.0050). In addition, the baseline value was significantly higher (p = 0.0050) than at 9 months of follow-up. The concentration of PF4/CXCL4 showed a significant decrease at 6 months when compared to 12 months (p = 0.0128) (Table 2).
The concentration of IL-10 showed a decrease over the follow-up period, with a significant decrease (p < 0.0001) at 12 months when compared to baseline, 3 and 6 months (Fig. 5A). The concentration of IL-8 showed a significant decrease at 9 months when compared to baseline and 3 months (p = 0.0459) (Fig. 5B). The concentration of sCD40L showed a significant decrease at 6 months, as compared to baseline, 9 and 12 months, as observed in Fig. 5C (p = 0.0475). The other cytokines evaluated were not detectable, even with enhanced sensitivity analysis.
Fig. 5.
Plasma concentration of interleukins and CD40L of BD patients during the follow-up period, measured through CBA of high and normal sensitivity (CD40L). A) VEGF; B) PF4/CXCL4. N = 6 patients. Abbreviation: M−months.
4. Discussion
BD is characterized by important biological changes related to the immune system. Once activated, the release of inflammatory cytokines and interleukins is promoted, explaining the clinical status with periods of remission and exacerbation [1]. Due to BD severity, being chronic, and presenting a heterogeneous response to conventional or biological treatments, the search for therapeutic alternatives is of importance. In this setting, the investigation of PRP as an immunomodulatory product could be remarkably interesting.
It is known that the platelet's role is not just limited to its hemostatic functions. Platelets participate in the inflammation process, releasing different substances that can modulate the inflammatory response via interaction with endothelial cells and leukocytes. The main immunomodulators described are platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), soluble ligand (sCD40L), and platelet factor 4 (PF4) [18]. TGF-β is the main immunosuppressant and evidence suggests that Treg differentiation is dependent on TGF-β. This was suggested when patients with immune thrombocytopenia who have decreased Treg cells and TGF-β, were treated with therapies that increase the number of platelets (eg. Immunoglobulin, dexamethasone), and functional and quantitative restoration of Treg cells was observed [19]. Furthermore, CD154 that is expressed on activated platelets can affect adaptive immunity. CD40L is primarily expressed on activated T cells and platelets and is a transmembrane molecule that plays an important role in the innate and adaptative immune response. Soluble forms of CD40L (sCD40L) can form trimers, which signal various biological processes through binding to receptors, antigen-presenting cells [23]. Platelets are responsible for 95% of soluble CD40L in circulation. Human PF4/CXCL4 is a protein of the CXC chemokine family, which binds to heparin and is secreted from α-granules after platelet activation [24]. Some studies have shown that PF4/CXCL4 aids in T cell trafficking, and other in vitro studies have suggested a role in Treg development [25,26]. Shi et al. (2014) proposed a new role in maintaining Th cell homeostasis, limiting their Th17 cell development and response, thus suggesting that platelets play an important immunological role [27]. No clinical studies have evaluated the immunomodulatory effect of PRP. However, a study in an animal model that used intra-articular PRP for the treatment of experimental rheumatoid arthritis presented some systemic changes that may suggest this role [15]. The authors demonstrated a systemic effect on the concentrations of inflammatory markers, with a reduction in IL-6, VEGF, IGF-1, and IL-1. Protein analysis of PRP revealed a low concentration of IL-1α, which is considered a classic cytokine of pro-inflammatory catabolism, justifying the use of PRP for anti-inflammatory purposes. Thus, there is some evidence to suggest that PRP may have a role as an immunomodulator.
This is the first study of PRP in patients with BD. Considering the great variability of clinical manifestations and the concentration profile of circulating cytokines according to the periods of clinical activity and remission, the establishment of an objective pattern of response to PRP in this heterogeneous disease is a challenge. As we did not know whether the use of PRP could worsen the disease, we decided to only include patients with less serious disease.
We also included only patients with low doses of corticoids to avoid interference with immunomodulation, which explains the low number of patients. In addition, since BD patients present active and very reactive T cells, we used P-PRP to avoid leukocytes in the product and prevent severe inflammatory reactions.
The main results were divided according to the function of each protein/cell: PF4/CXCL4, IL-10, and Treg cells represent anti-inflammatory profile, and sCD40L, VEGF, IL-8, and CD69 + NK cells represent pro-inflammatory profile.
The importance of P-PRP characterization has been emphasized in the literature, enabling the correlation of clinical results and comparison between the studies. Thus, we prospectively measured platelet and leukocyte number, growth factors, cytokines, interleukins, PF4/CXCL4, and sCD40L in plasma and P-PRP.
The standardization of P-PRP was performed according to Amable et al. (2013), and the product achieved the expected quality of the number of platelets and leukocytes in relation to the number observed in the peripheral blood after the technique without the use of brake [20,21].
In this study, we performed a longitudinal analysis of interleukins and cytokines in P-PRP. We could not compare our results with previous studies, since the patients presented other diseases, the analysis was performed only once, transversely, with diverse protocols of PRP preparation. Even the comparison with another study of our research group that included patients with knee osteoarthritis was different, such as lower TGF-β1 at the basal time and after 3 months of application. This can be the result of diverse mechanisms involved in BD etiopathogenesis and osteoarthritis.
Concerning the cellular analysis of patients, there was a significant change in Treg and activated NK cells (CD69 +). The TCD4+ CD25+ regulatory cells constitute approximately 3–7% of T CD4+ cells in human peripheral blood, thus maintaining self-immune tolerance [28]. Hamzoui et al. (2007) showed that the frequency of T CD4+ cells expressing CD25+high is significantly increased during BD activity when compared to the remission stage and controls. Phenotypically, TCD4+ CD25+high cells express high levels of FoxP3 mRNA and when isolated, had functional characteristics of regulatory T cells [9]. In our study, we found that patients with BD had an extremely low basal frequency of Treg, practically null and that after the application of P-PRP a significant increase in the cellular frequency of this cell population was observed. This could suggest a beneficial response, including the maintenance of interleukin levels during the period of application. However, it is important to note that the cellular function of Treg was not evaluated. Studies of patients with autoimmune diseases, especially rheumatoid arthritis, have shown that CD4+ CD25+ Treg cells were able to suppress the proliferation of effector T cells, but unable to suppress the secretion of proinflammatory cytokines from activated T cells and monocytes [29]. This deficiency of Treg cells from patients with rheumatoid arthritis is associated with a defective expression and function of CTLA-4, a key molecule related to the Treg cell suppressor function. In this sense, as we observed an increase of Treg throughout the applications, the evaluation of the function of these cells would have been interesting to better understand the effect of this finding.
CD69 is a type II transmembrane glycoprotein widely expressed and related to type C lecithins in animals, exhibiting regulated expression in a variety of hematopoietic lineage cells such as neutrophils, monocytes, T and B cells, NK, and platelets. Activation of these cell types results in the induction of CD69 expression on the cell surface [30]. This molecule is selectively expressed in chronic cellular infiltrates that are involved in the pathogenesis of diseases such as rheumatoid arthritis, lupus, sclerosis, and HIV [31,32]. The rapid induction of CD69 expression in T cells suggests activation and/or differentiation, which occurs with the participation of sCD40L. In vitro studies have shown that anti-CD69 monoclonal antibodies stimulate IL-2 production, increasing T-cell proliferation, and TNF-α. The findings of in vitro studies have shown changes that characterize a pro-inflammatory role by direct or indirect mechanisms [33]. In the context of BD, a Japanese group published a study on the NK1/NK2 and NK CD69+ profile in patients with active and inactive diseases during disease. The active disease was defined as a typical BD symptomatology and significantly higher amounts of NK CD69+ when compared to inactive patients and controls8. In our study, we showed a significant variation of activated NK (CD69+) frequency during the follow-up period, with a decrease until the end of PRP application, and an increase at 9 months when compared to other periods. This time was characterized by clinical worsening and the need for higher immunosuppressive therapy. In the literature, the patients treated with corticosteroids alone or with immunosuppressive drugs had a significantly higher level of Treg cells in comparison to patients without corticosteroids treatment or controls for systemic lupus erythematosus (SLE) [34]. The authors suggested that corticosteroids may induce the expansion or generation of Treg cells, and this increase in the number of cells during corticosteroids treatment was reported in other studies with myasthenia gravis and multiple sclerosis [35,36]. In our study, as BD can show periods of remission and activity of the disease and we included patients without severe disease manifestations using a low immunosuppressive dose of corticosteroids, in the presence of any sign of disease activity, more aggressive therapy was required. The increased therapy could have interfered with the values of the markers, clinically observed by worsening in symptoms even at high doses of corticosteroids, this could be due to an unresponsive pattern of glucocorticoids at that dose, as with the use of corticosteroids and decreased numbers of Treg cells and an increase in pro-inflammatory profile; or even because they are without the P-PRP as an adjunctive treatment. We cannot discard other mechanisms involved in the clinical evaluation of the patients; however, we suggest a non-responsive corticosteroids treatment and a possible role of P-PRP applications that is more evident in the period of application as adjunctive therapy.
When evaluating patient evolution and laboratory results, we observed a beneficial effect of autologous P-PRP on the biological profile demonstrated by a significant change in some parameters such as PF4/CXCL4, IL-10, and Treg, favoring anti-inflammatory changes during the period of P-PRP use. Noteworthy, P-PRP was used as an adjuvant to corticosteroid therapy in patients with mucocutaneous ulcerations refractory to conventional therapy. After the completion of the injections, there was a trend towards pattern inversion, with an increase in pro-inflammatory markers. After 9 months (3 months after stopping P-PRP injections) we found a decrease of these markers, probably related to an increase of the dose of the conventional immunosuppressive therapy due to the clinical worsening in this period. We may question whether the frequency of P-PRP injections and the period of action may be determinant for the biological effect. The best results were obtained over 3 months of treatment with a 15-day interval between injections.
Despite not having found a correlation between these markers/Treg and clinical scores/number of oral ulcers, there was a decrease in the number of days for ulcer closure during the entire period of P-PRP applications. This highlights the fact that scores did not always encompass all the details of the clinical findings.
There are no reports in the literature of the deleterious effects associated with the use of PRP; however, 85% of our patients reported pain at the application site, with a duration of 3–4 days. The subcutaneous route could probably explain this adverse event. Patients also reported generalized pain during the week of application, especially at the beginning of the study when the number of leukocytes in P-PRP was higher. One of the mechanisms involved in the noninfectious inflammatory process is the release of molecules associated with damage (DAMPS), as a response to stress [37].
Despite being a pilot study with a small number of patients, the longitudinal analysis of innumerable systemic and cellular markers during and after the use of P-PRP could contribute to the greater knowledge of BD characterization.
We emphasize that the patients had a significant decrease in the frequency and time of healing of the oral aphthous lesions, often difficult to control with the current therapy. The use of P-PRP is safe during the 12 months in patients with DB without active severe manifestations and may be an option for patients with recurrent and refractory ulcers.
One limitation of this study was the use of immunosuppressive drugs during P-PRP applications, which can reduce the transcription of pro-inflammatory cytokines such as IL-8, TNF-α, IL-1β, as described with colchicine in BD patients [38]. In this setting, we included only patients using low doses of these drugs, to avoid interference with the response to P-PRP. Furthermore, the use of self-report questionnaires represents a limitation, as the analysis is subjective, however, this is currently the only validated evaluation tool for BD.
5. Conclusions
In this pilot study, including 6 patients with BD, we showed the systemic profile of cell frequency and cytokines during the use of a well-characterized P-PRP. The significant alterations were the increase in the frequency of Treg cells during the first three months and a stable anti-inflammatory cytokine pattern during the application period, suggesting a protective effect. After completion, this changed to a pro-inflammatory state, with a significant increase of NK69 and the lowest number of Treg cells. Clinically we demonstrated an objective decrease in the closing time of the ulcers during the entire period of P-PRP application. These findings suggest a beneficial effect of P-PRP in patients with BD and oral ulcers.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors acknowledge the Ambulatory of Vasculitis of HC - Unicamp for all the support to patient's selection and the nurse of Hemocenter, Marcos Pereira dos Santos for the help in the patient's applications. The source(s) of support in the form of grants or industrial support: The authors acknowledge the financial support from FAPESP and CNPq for the development of this study.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Yazici H., Seyahi E., Hatemi G., Yazici Y. vol. 14. Nature Publishing Group; 2018. pp. 107–119. (Behçet syndrome: a contemporary view. Nat Rev Rheumatol). [Internet] [DOI] [PubMed] [Google Scholar]
- 2.Hirohata S., Kikuchi H. Behçet's disease. Arthritis Res Ther. 2003;5:139–146. doi: 10.1186/ar757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harman E., Sayarlioglu M., Harman M., Sayarlioglu H. The evaluation of coagulation parameters and vessel involvement in Behcet's s disease. A clinical experience of Behcet's s disease: study of 152 cases. Acta Med Iran. 2013;51:215–223. [PubMed] [Google Scholar]
- 4.Gül A. Behçet’s disease: an update on the pathogenesis. Clin Exp Rheumatol. 2001;19:S6–S12. [PubMed] [Google Scholar]
- 5.Alireza Khabbazi, Nadereh Rashtchizadeh, Amir Ghorbanihaghjo, Mehrzad Hajialiloo, Mortasa Ghojazadeh, Taei Ramin K.S. The status of serum vitamin D in patients with active Behcet's disease compared with controls. Int J Rheum Dis. 2014;17:430–434. doi: 10.1111/1756-185X.12153. [DOI] [PubMed] [Google Scholar]
- 6.Sugita S., Kawazoe Y., Imai A., Kawaguchi T., Horie S., Keino H. Role of IL-22- and TNF- -producing Th22 cells in uveitis patients with behcet's disease. J Immunol. 2013;190:5799–5808. doi: 10.4049/jimmunol.1202677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neves F.S., Spiller F. Possible mechanisms of neutrophil activation in Behçet’s disease. Int Immunopharmacol. Elsevier B.V. 2013;17:1206–1210. doi: 10.1016/j.intimp.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi Y., Takahashi H., Satoh T., Okazaki Y., Mizuki N., Takahashi K. Natural killer cells control a T-helper 1 response in patients with Behçet’s disease. Arthritis Res Ther. 2010;12:80–89. doi: 10.1186/ar3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamzaoui K., Hamzaoui A., Houman H. CD4 + CD25 + regulatory T cells in patients with Behçet ’ s disease. Clin Exp Rheumatol. 2006;5:S71–S78. [PubMed] [Google Scholar]
- 10.Neves F.S., Moraes J.C.B., Kowalski S.C., Goldenstein-Schainberg C., Lage L.V., Gonãalves C.R. Cross-cultural adaptation of the Behçet's disease current activity form (BDCAF) to the Brazilian Portuguese language. Clin Rheumatol. 2007;26:1263–1267. doi: 10.1007/s10067-006-0484-y. [DOI] [PubMed] [Google Scholar]
- 11.Deuter C.M.E., Zierhut M., Möhle A., Vonthein R., Stübiger N., Kötter I. Long-term remission after cessation of interferon-α treatment in patients with severe uveitis due to Behçet’s disease. Arthritis Rheum. 2010;62:2796–2805. doi: 10.1002/art.27581. [DOI] [PubMed] [Google Scholar]
- 12.Samru Onal, Kazokoglu Haluk K.A., Akman Mehmet, Tayfun Bavbek, Direskeneli Haner Y.S. Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory Behcet uveitis. Arch Ophthalmol. 2011;129:288–294. doi: 10.1001/archophthalmol.2011.3. [DOI] [PubMed] [Google Scholar]
- 13.Eppley B.L., Pietrzak W.S., Blanton M. Platelet-rich plasma: a review of biology and application in plastic surgery. Plast Reconstr Surg. 2006;118:147e–159e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 14.Marx R.E. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Lippross S., Moeller B., Haas H., Tohidnezhad M., Steubesand N., Wruck C.J. Intraarticular injection of platelet-rich plasma reduces inflammation in a pig model of rheumatoid arthritis of the knee joint. Arthritis Rheum. 2011;63:3344–3353. doi: 10.1002/art.30547. [DOI] [PubMed] [Google Scholar]
- 16.Andia I., Abate M. Platelet-rich plasma: underlying biology and clinical correlates. Regen Med. 2013;8:645–658. doi: 10.2217/rme.13.59. [DOI] [PubMed] [Google Scholar]
- 17.Arnoczky S.P., Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21:180–185. doi: 10.1097/JSA.0b013e3182999712. [DOI] [PubMed] [Google Scholar]
- 18.Cognasse F., Boussoulade F., Chavarin P., Acquart S., Fabrigli P., Lamy B. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–1189. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 19.Semple Jon W., Italiano Joseph E.F.J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–273. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 20.Amable P.R., Teixeira M.V., da Cruz Pacheco I., Correa do Amaral R.J., Granjeiro J.M., Borojevic R., Carias R.B. Platelet-rich paslam preparation for regnerative medicine: optimization and wuatification of cytokines and growth factors. Stem Cell Res Ther. 2013;4(67):1–13. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber S.C., Luiz J., Cunha Júnior R., Montalvão S., Queiroz L., Silva D. In vitro study of the role of thrombin in platelet rich plasma (PRP) preparation: utility for gel formation and impact in growth factors release. J Stem Cells Regen Med. 2016;12:2–9. doi: 10.46582/jsrm.1201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachetto Z., Mahayri N., Ferraz R.H., Costallat L.T.L., Bertolo M.B. Behçet’s disease in Brazilian patients: demographic and clinical features. Rheumatol Int. 2012;32:2063–2067. doi: 10.1007/s00296-011-1921-z. [DOI] [PubMed] [Google Scholar]
- 23.Elgueta R., Benson M.J., de Vries V.C., Wasiuk A., Guo Y., Noelle R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Ver. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luster A.D. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava K., Cockburn I.A., Swaim A., Thompson L.E., Tripathi A., Fletcher C.A. Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe. 2008;4:179–187. doi: 10.1016/j.chom.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C.Y., Battaglia M., Lee S.H., Sun Q.H., Aster R.H., Visentin G.P. Platelet factor 4 differentially modulates CD4+CD25+ (regulatory) versus CD4+CD25- (nonregulatory) T cells. J Immunol. 2005;174:2680–2686. doi: 10.4049/jimmunol.174.5.2680. [DOI] [PubMed] [Google Scholar]
- 27.Shi G., Field D.J., Ko K.A., Ture S., Srivastava K., Levy S. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. J Clin Invest. 2014;124:543–552. doi: 10.1172/JCI71858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baecher-Allan C., Brown J.A., Freeman G.J., Hafler D.A. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 29.Ehrenstein M.R., Evans J.G., Singh A., Moore S., Warnes G., Isenberg D.A. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-tnfα therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natarajan K., Sawicki M.W., Margulies D.H., Mariuzza R.A. Crystal structure of human CD69: a C-type lectin-like activation marker of hematopoietic cells. Biochemistry. 2000;39:14779–14786. doi: 10.1021/bi0018180. [DOI] [PubMed] [Google Scholar]
- 31.González-Amaro R., Cortés J.R., Sánchez-Madrid F., Martín P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol Med. 2013;19:625–632. doi: 10.1016/j.molmed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzio R., Mauël J., Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol. 1999;21:565–582. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 33.Cebrian M., Yague E., Rincon M., Lopez-botet M., Landazuri M.O.D.E., Sanchez-madrid F. Triggering of T cell proliferation through AIM an activation inducer molecule expressed on activated human lymphocytes. J Exp Med. 1988;168:1621–1637. doi: 10.1084/jem.168.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azab N.A., Bassyouni I.H., Emad Y., Abd El-Wahab G.A., Hamdy G. CD4+CD25+ regulatory T cells (TREG) in systemic lupus erythematosus (SLE) patients: the possible influence of treatment with corticosteroids. Clin Immunol. 2008;127:151–157. doi: 10.1016/j.clim.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Fattorossi A., Battaglia A., Buzzonetti A., Ciaraffa F., Scambia G., Evoli A. Circulating and thymic CD4+CD25+ T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunology. 2005;116:134—141. doi: 10.1111/j.1365-2567.2005.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braitch M., Harikrishnan S., Robins R.A., Nichols C., Fahey A.J. Glucocorticoids increase CD4CD25 cell percentage and Foxp3 expression in patients with multiple sclerosis. Acta Neurol Scand. 2009;119:239–245. doi: 10.1111/j.1600-0404.2008.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun A., Wang Y.P., Chia J.S., Liu B.Y., Chiang C.P. Treatment with levamisole and colchicine can result in a significant reduction of IL-6, IL-8 or TNF-α level in patients with mucocutaneous type of Behcet's disease. J Oral Pathol Med. 2009;38:401–405. doi: 10.1111/j.1600-0714.2009.00774.x. [DOI] [PubMed] [Google Scholar]