Abstract
Wild canids are hosts to a wide range of parasites and can play a role in transmission of zoonoses. As many parasites are transmitted through food webs, and wild canids are at high trophic levels, parasite prevalence and diversity in wild canids can serve as excellent indicators of ecosystem health. Our main objectives were to update knowledge on the composition of gastrointestinal helminths in wild canids from Québec, Canada, and to describe differences in parasite prevalence and diversity among canid species and regions. Hunters and trappers provided whole carcasses of red foxes (Vulpes vulpes) (N = 176), and intestinal tracts of coyotes (Canis latrans) (N = 77) and gray wolves (Canis lupus) (N = 23) harvested for non-research purposes over the winter of 2016–2017. A modified Stoll's centrifugation sucrose flotation on feces of 250 wild canids was used, and eggs of one family and eight genera of parasitic helminths were recovered: diphyllobothriids, Taenia/Echinococcus spp., Capillaria spp., Toxascaris sp., Toxocara sp., Trichuris sp., Uncinaria sp., and Metorchis sp. Adult Taenia spp. cestodes were recovered from 61 of 276 (22%) canids. Six different species (T. hydatigena, T. twitchelli, T. crassiceps, T. polyacantha, T. krabbei, and T. pisiformis-“like”) were differentiated based on DNA sequenced from 65 individual adult cestodes using primers for the nicotinamide adenosine dinucleotide dehydrogenase subunit 1 (ND1) and cytochrome c oxidase subunit 1 (CO1) mitochondrial DNA loci. Alaria sp. trematodes infected 89 of 276 canids (32%). A subset were identified as A. americana at the CO1 locus. The marine trematode Cryptocotyle lingua was reported for the first time in foxes in the province of Québec. These results help us understand more fully the predator-prey relationships within this group of canids. This baseline data in regional parasite prevalence and intensity is critical in order to detect future changes following ecological disturbances due to climate and landscape alterations.
Keywords: Canids, Wildlife, Parasites, Cestodes, Nematodes, Trematodes
Graphical abstract
Highlights
-
•
Combining gross examination and fecal flotation improve detection of helminths.
-
•
Cestode prevalence is underestimated when diagnosis is based only on eggs in feces.
-
•
Foxes had significantly more nematodes and fewer cestodes than wolves and coyotes.
-
•
Diets and trophic relationships influence gastrointestinal parasite communities.
-
•
Cryptocotyle lingua is reported for the first time in foxes from Québec, Canada.
1. Introduction
Wild canids, such as red foxes (Vulpes vulpes), coyotes (Canis latrans) and gray wolves (Canis lupus), are reservoirs of several zoonoses, including parasites, that can spillover to humans and domestic animals (Aguirre, 2009). Despite the widespread distribution of wild canids in Québec, little information is available on their parasite communities or the prevalence and intensity of particular species in this region. This type of baseline data is necessary to monitor ecological changes. Parasite surveillance is thus an important tool to assess the population health of wildlife, particularly in canid populations in close contact with human settlements (McCallum and Dobson, 1995).
Among the factors that influence the gastrointestinal parasite communities of wild canids are their diets and trophic relationships. For example, red foxes feed mainly on voles and other small rodents, but also forage on a variety of other prey species, with dietary patterns influenced by season and prey availability (Larivière and Pasitschniak-Arts, 1996). The broad distribution and diverse diet of this opportunistic predator expose it to diverse parasites. In Canada, nine cestode, 14 nematode, and 11 trematode parasites have been reported in red foxes. It includes nematodes Ancyclostoma caninum (hookworm) and Toxascaris leonina (roundworm), as well as parasites that pose potential threats to domestic animals or humans, such as Toxocara canis (roundworm), Dirofilaria immitis (canine heartworm), and Echinococcus multilocularis (a zoonotic tapeworm that causes liver disease in dogs and people) (Curtis et al., 1988; Robbins, 2018; Schurer et al., 2018).
Coyotes are considered opportunistic and generalist predators and scavengers, capable of exploiting many habitats, including urban areas (Watts et al., 2015; Breck et al., 2019). Their diet consist mainly of rodents and lagomorphs, and the scavenged remains of larger wildlife (Wells and Bekoff, 1982). Previous surveys of parasites in coyotes in Canada reported several zoonotic species, including Echinococcus spp. cestodes (Schurer et al., 2018), Toxocara canis (Bridger et al., 2009), and the intestinal trematodes Alaria arisaemoides and Alaria americana (Luong et al., 2018).
Gray wolves are broadly distributed across Canada, with the exception of the Maritime provinces and Newfoundland (Mech and Boitani, 2004). The main prey of gray wolves are large ungulates, but their diet also includes secondary prey species, such as hares, foxes, beavers, small rodents, and birds (Hénault and Jolicoeur, 2003; Chester, 2016). At least 25 helminth species have been reported in gray wolves in Canada. These commonly include Taenia spp. and Echinococcus spp. cestodes, Uncinaria stenocephala (northern hookworm) and Toxascaris leonina nematodes, and several species of Alaria (Craig and Craig, 2005).
In addition to host trophic ecology, the transmission and distribution of canid parasites is affected by ecological disturbances, such as rapid urbanization, global warming, or habitat destruction, with climate change being recognized as one of the top individual drivers (Patz et al., 2000; Semenza et al., 2016). Many factors driven by climate change could potentially affect parasite distribution in wildlife. These include biodiversity loss and latitudinal and altitudinal host range shifts, such as wild canids moving northward (Jenkins et al., 2013), as well as decreased habitat connectivity (Cable et al., 2017). In Canada, climate has been, and continues to be, impacted by human activity. Canada has a rate of surface warming more than twice the global rate (Bush and Lemmen, 2019). As a consequence, this may alter seasonal and geographic patterns of parasite transmission, as well as the development and survival of environmental stages of parasites (Jenkins et al., 2011). Climate-driven mismatch between prey and host may also add variability to the epidemiological outcomes of parasite transmission (Altizer et al., 2013). Landscape changes, such as deforestation, may also favor paratenic and intermediate hosts of parasites, such as rodents. These changes may increase risk of transmission of helminth parasites through carnivory (Romig et al., 2006).
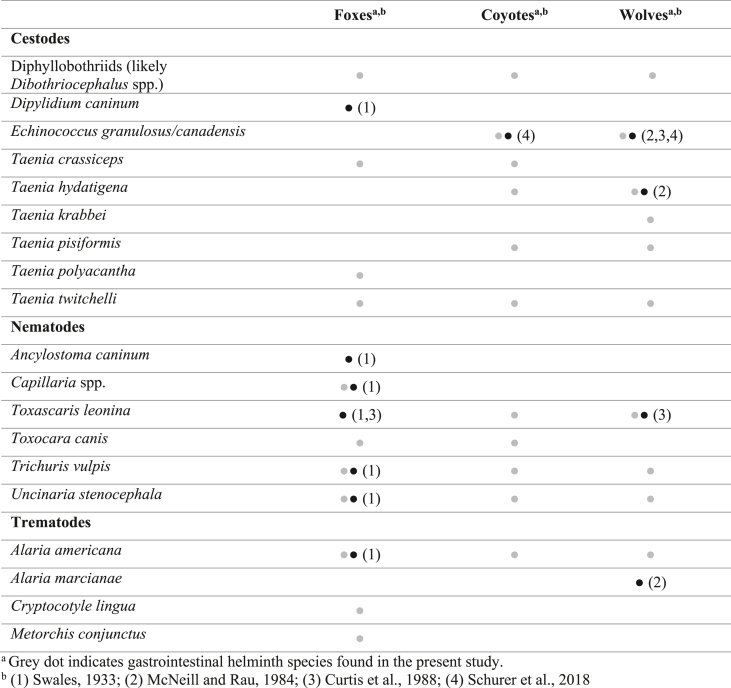
Despite the ecological and distributional differences among wild canid species, as high-trophic-level predators and scavengers throughout eastern Canada, these hosts share many parasites through similar habitats and prey species. Parasite communities in these hosts can serve as indicators of intact trophic relationships for indirectly transmitted parasites, one example being the important zoonotic cestodes of the genus Echinococcus. Urbanization impacts ecosystems, affecting the gut microbiota of generalist canid species following consumption of anthropogenic food, resulting in higher parasite susceptibility (Sugden et al., 2020). Still, studies describing parasite diversity and prevalence in wild canids from the province of Québec are lacking (Table 1). There are very few reports on helminths in red foxes (Swales, 1933; Curtis et al., 1988; Schurer et al., 2018), no published studies on parasite diversity in coyotes, and only a few studies of gray wolves, the latter including reports of Echinococcus granulosus/E. canadensis, Taenia hydatigena, Taenia krabbei, Taenia pisiformis, Alaria spp., and Dioctophyme renale (giant kidney worm) (McNeill and Rau, 1984; Curtis et al., 1988; Hénault and Jolicoeur, 2003; Schurer et al., 2018). With unprecedented climate and landscape change in Canada, and with little baseline data in Québec, we address a pressing need to update knowledge on parasites hosted by wild canids and their distribution to better understand risk to human and animal populations. Initially driven by the need for Echinococcus surveillance in wild canids (published previously in Schurer et al., 2018), here we describe the composition of other gastrointestinal helminths among foxes, coyotes, and wolves, as well as differences in parasite prevalence and diversity based on gross examination and fecal flotation in canids in two different climate regions of Québec (QC), Canada.
Table 1.
List of gastrointestinal helminth species recorded from foxes (Vulpes vulpes), coyotes (Canis latrans), and wolves (Canis lupus) observed by gross examination and/or fecal flotation in Québec, Canada, including the present study and references.
2. Materials and methods
2.1. Study area
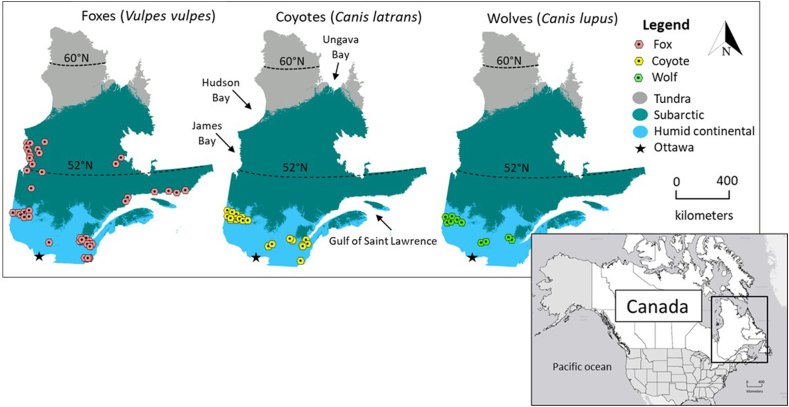
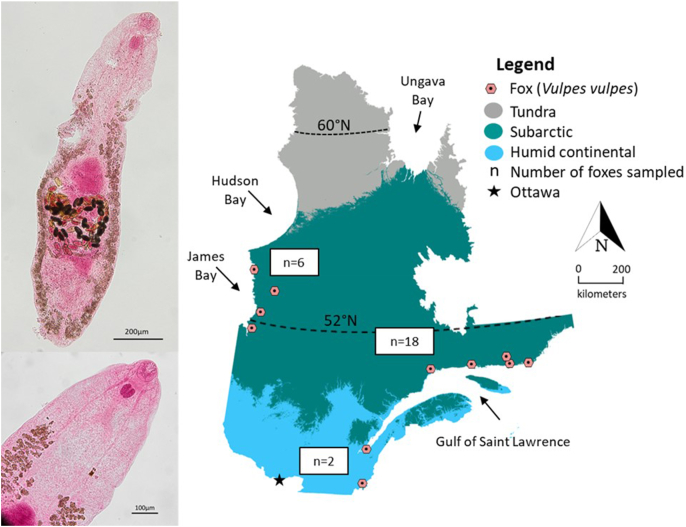
Hunters and trappers in QC provided whole carcasses of red foxes (N = 176), and intestinal tracts of gray wolves (N = 77) and coyotes (N = 23) that were harvested during regular fur-trapping activities of winter 2016–2017. Fox samples were collected in two main climate regions: Subarctic (N = 87) and Humid Continental (N = 89), whereas coyotes and wolves were harvested throughout the Humid Continental climate region (Fig. 1) (Beck et al., 2018). These two regions differ in climate, vegetation, and prey abundance and diversity. The Subarctic climate has long winters, among the coldest in eastern Canada, with warm but short summers. Boreal forest covers most of the southern Subarctic region, with spruce, lichen and moss in the northern part (MFFP, 2019). This climate region supports large numbers of woodland caribou (Rangifer tarandus caribou), as well as moose (Alces alces), beaver (Castor canadensis), Canada lynx (Lynx canadensis), black bears (Ursus americanus), wolves, snowshoe hare (Lepus americanus) and a variety of birds (Bercuson et al., 2021). The Humid Continental climate region includes most major human population centres, and has warm, humid summers and long, cold winters. Deciduous and mixed forest characterise the region, with precipitation abundant throughout the year, in contrast to the Subarctic climate (MFFP, 2019). White-tailed deer (Odocoileus virginianus) thrive in this region, as well as smaller mammals such as squirrels, mink (Neovison vison), raccoons (Procyon lotor), muskrats (Ondatra zibethicus), skunks (Mephitis mephitis), rabbits, groundhogs, mice and moles (Bercuson et al., 2021).
Fig. 1.
Köppen climate regions and sampling distribution of foxes (Vulpes vulpes, N = 176), coyotes (Canis latrans, N = 77), and wolves (Canis lupus, N = 23) collected during winter 2016–2017 by hunters and trappers from Québec, Canada. Arrows indicate major waterways.
2.2. Gross examination
Our research team performed fox necropsies at the Faculté de Médecine Vétérinaire in Saint-Hyacinthe (QC) where intestinal tracts (including feces) of all canids were stored at −20 °C until shipped to the University of Saskatchewan (SK). They were then frozen at −80 °C for at least five days prior to examination to inactivate infectious Echinococcus eggs as per World Health Organization recommendations for safe handling (Eckert et al., 2001). We collected helminths from small intestines by the scraping, filtration, and counting technique (SFCT) (Gesy et al., 2013). Briefly, we divided each small intestine into four equal parts, opened them longitudinally, and placed them in a sealable glass jar with 250 ml of tap water. After shaking vigorously for 1–2 min, we scraped the intestines and washed the contents through a large mesh sieve (one mm pore size, 20.3 cm diameter, USA standard test sieve no. 18, Fisher Scientific Company, Ottawa, Ontario, Canada) prior to counting and identifying adult helminths. Our study team recorded the presence of adult nematodes, primarily ascarids, but did not identify them morphologically, relying on detection of characteristic eggs on flotation for species level identification. We stored cestodes in 90% ethanol prior to morphological examination and molecular analysis. For the trematodes, we morphologically examined the adults, and recorded their presence. We fixed several Alaria specimens (n = 17) in 90% ethanol for molecular identification to species level. After obtaining feces from the rectum, colon, and/or distal ileum, we stored them at −20 °C prior to conducting fecal egg counts (FEC) (Schurer et al., 2014). We identified non-Echinococcus cestodes (Taenia and diphyllobothriids) by morphological examination.
2.3. Modified Stoll's centrifugation sucrose flotation
We thawed 4 g of feces, weighed and mixed them thoroughly in a paper cup with 40 ml of Sheather's sucrose solution (specific gravity of 1.2) to create a homogenized mixture. After sieving the fluid through a cheesecloth into a second cup, we poured 10 ml aliquot (∼25%, representing 1 g of feces) into a test tube then topped it up with ∼5 ml of Sheather's sucrose solution to form a slight convex meniscus. We then placed a coverslip on top, centrifuged the tube at 491 rcf for 10 min after which we lifted the coverslip and placed it on a glass slide. One technician examined one slide per sample from each canid, viewed helminth parasite ova under the microscope at 10–40X objective lens, and identified them based on size and morphology (Dryden et al., 2005). The whole slide was counted. The detection limit per egg count was five eggs per gram (epg) of feces (Nielsen et al., 2010). We categorized fecal infection intensity, defined as the concentration of helminth eggs infecting a host, by using the following semi-quantitative scale: 1+ 1–50 epg; 2 + 51–250 epg; 3 + 251–1000 epg; 4+ >1000 epg. While not ideal, we used a semi-quantitative method based on ordinal measures (1+ to 4+) instead of egg counts to reduce technician time.
2.4. Molecular methods
Our study team identified Taenia cestodes to species level by molecular analysis. Using fine tipped scissors, we macerated approximately 25 mg of tissue from two representative Taenia specimens per host in separate microcentrifuge tubes, from which we extracted DNA using the DNeasy Blood and Tissue Kit as per manufacturer's instructions, except eluting 150 μl of DNA in the final step to increase DNA concentration (Qiagen Inc., Valencia, California, USA). We conducted Polymerase Chain Reaction (PCR) (25 μl, 5 μl of DNA) with two primer sets, previously designed and validated, targeting a 471 base pair (bp) region of the nicotinamide adenosine dinucleotide dehydrogenase subunit 1 (ND1) and a 366 bp region of the cytochrome c oxidase subunit 1 (CO1) mitochondrial DNA (Bowles et al., 1992; Bowles and McManus, 1993). After resolving PCR products by electrophoresis on a 1.5% agarose gel, we purified them using the QIAquick PCR Purification Kit following manufacturer's instruction (Qiagen Inc., Valencia, California, USA), and sent for sequencing (Macrogen Inc., Seoul, Korea). We trimmed forward and reverse sequences, aligned them, and identified them using the BLASTn tool to compare sample to reference sequences in the nucleotide database of GenBank. We used Clustal (https://www.ebi.ac.uk/Tools/msa/clustalo/), a multiple sequence alignment program, for sequences that did not have strong matches to GenBank to look for any similarity, as many had low query coverage but were most similar to the same accession numbers. We recorded mixed infections (i.e. infection with multiple species of taeniid cestodes). Methodology for Echinococcus spp. identification was described by Schurer et al. (2018). For trematodes in the genus Alaria, we extracted DNA using the DNeasy Blood and Tissue Kit as per manufacturer's instruction (Qiagen Inc., Valencia, California, USA). We conducted PCR (25 μl, 5 μl of DNA) with validated primers targeting the ∼366 bp COI region (Moszczynska et al., 2009; Van Steenkiste et al., 2015) on a subset of samples (n = 17) followed by sequencing and comparison with published data through BLASTn searches. Sequences were aligned with those published from other representatives of Alaria using MAFFT (Katoh et al., 2002) and sequence similarity was calculated and visualized in a neighbour-joining tree constructed in Geneious Prime (Biomatters, Auckland, NZ). All PCRs used both negative and positive controls (generated in house).
2.5. Statistical analysis and mapping
Canids were considered infected if we observed (1) parasite adults on SFCT, or (2) ova of helminths, for which wild canids are known definitive hosts, by FEC. We conducted a two-sided Fisher's Exact test to determine if parasite prevalence (percentage of canid host species infected with one or more helminth species) differed between canid host species based on gross examination, and when combined with fecal flotation. The same test was used to determine if parasite prevalence differed between geographic regions (comparing only foxes), when combining gross examination and fecal flotation. Freeman–Halton's extension was used when contingency tables were greater than 2 × 2. We used a one-way ANOVA post hoc test to detect differences in the number of parasite genera between canid host species (defined as parasite genus richness), determined by gross examination and fecal flotation combined. We corrected for multiple pairwise comparisons using Bonferroni correction, with a threshold for significance of 0.05/n (n = 3). We also used one-way ANOVA to determine if there were significant differences in the number of parasite genera between foxes from Subarctic and Humid Continental climate regions. We used the Mann-Whitney U Test to evaluate median infection intensity differences among canid host species, when based on gross examination. Median infection intensity is defined here as the concentration of adult helminths infecting a host. All test were performed at a significant level of 5%. We generated 95% confidence intervals with the Wilson's method using Epitools (Sergeant, 2018). We mapped the distribution of infected and uninfected canids by entering the geographic coordinates (latitude, longitude) of trap sites into QGIS version 3.12.1 (QGIS-Development-Team, 2020). We did not include host sex or age in our analyses because these were known for only a few individuals. Data on Echinococcus spp. reported previously in Schurer et al. (2018), were included in the taeniid analysis. For statistical purposes, we assumed that all Alaria were the same species as the 17 specimens identified by DNA sequence comparison. All statistical tests were performed using SPSS version 26 (IMB Corporation, Armonk, New York, USA).
In accordance with the Canadian Council on Animal Care guidelines, this research was exempt from Animal Research Ethic Board review in Canada because all samples were collected from animals previously harvested for non-research purposes.
3. Results
Examination of small intestinal contents revealed seven helminth parasites: cestodes, including diphyllobothriids (likely Dibothriocephalus spp., the broad fish tapeworm, known previously as Diphyllobothrium spp. (Scholz et al., 2019; Waeschenbach et al., 2017)), Echinococcus canadensis, and Taenia spp.; ascarid nematodes (Toxocara canis and/or Toxascaris leonina); and trematodes, including Alaria sp. and Cryptocotyle lingua (Table 2a). We observed eggs from nine gastrointestinal helminths on fecal flotation, including cestodes diphyllobothriids, Taenia spp. and/or Echinococcus spp.; nematodes T. canis, T. leonina, Trichuris vulpis (canine whipworm), U. stenocephala (northern hookworm), and Capillaria spp. (various species, adults of which can live in the intestine, airways, and bladder); and trematodes Metorchis conjunctus (North American liver fluke) and Alaria sp. Eggs of T. leonina were the most prevalent in foxes and taeniid eggs were most prevalent in coyotes and wolves (Table 3).
Table 2a.
Gastrointestinal helminth prevalence in foxes (Vulpes vulpes), coyotes (Canis latrans), and wolves (Canis lupus) observed by gross examination (N = 276) in Québec, Canada.
| Foxes (N = 176) |
Coyotes (N = 77) |
Wolves (N = 23) |
Difference in parasite prevalence among canid host species (N = 276) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | %d | N | %d | N | %d | N | %d | Pe | |
| Cestodesa | 22 | 13 (8–18) | 49 | 64 (52–73) | 15 | 65 (45–81) | 86 | 31 (26–37) | <0.001 |
| Diphyllobothriids (likely Dibothriocephalus spp.) | 1 | 1 (0–3) | 8 | 10 (5–19) | 1 | 4 (1–21) | 10 | 4 (2–6) | <0.001 |
| Echinococcus canadensisb | 0 | 0 (0–2) | 9 | 12 (6–21) | 8 | 35 (19–55) | 17 | 6 (4–9) | <0.001 |
| Taenia spp. | 13 | 7 (4–12) | 36 | 47 (36–58) | 12 | 52 (33–71) | 61 | 22 (18–27) | <0.001 |
| Nematodesa | 79 | 45 (38–52) | 5 | 7 (3–14) | 0 | 0 (0–14) | 84 | 30 (25–36) | <0.001 |
| Trematodesa | 87 | 47 (40–54) | 15 | 20 (12–30) | 6 | 26 (13–46) | 108 | 38 (33–44) | <0.001 |
| Alaria americana | 68 | 37 (30–44) | 15 | 20 (12–30) | 6 | 26 (13–46) | 89 | 31 (26–37) | 0.008 |
| Cryptocotyle lingua | 26 | 14 (10–20) | 0 | 0 (0–5) | 0 | 0 (0–14) | 26 | 9 (6–13) | <0.001 |
| Overall parasite prevalence after gross examinationc | 134 | 76 (69–82) | 53 | 69 (58–78) | 17 | 74 (54–87) | 204 | 74 (68–79) | 0.46 |
Canids infected with at least one gastrointestinal helminth species from the helminth classes (cestodes, nematodes, trematodes) based on gross examination.
E. canadensis results already published and discussed in Schurer et al. (2018).
Canids infected with at least one gastrointestinal helminth species based on gross examination.
95% confidence intervals are displayed in parentheses.
P values are from Fisher's Exact test.
Table 3.
Gastrointestinal helminth prevalence in foxes (Vulpes vulpes), coyotes (Canis latrans), and wolves (Canis lupus) observed by gross examination and fecal flotation combined (N = 250), and compared by climate regions of Québec, Canada. Fecal infection intensity is categorized using a semi-quantitative scale.
| Subarctic |
Humid Continental |
Difference in parasite prevalence between regions (only foxes, N = 155) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foxes = 74 |
Foxes = 81 |
Coyotes = 73 |
Wolves = 22 |
Difference in parasite prevalence among canid host species in the Humid Continental climate (N = 176) |
||||||||||||||
| N | %b | Ia | N | %b | Ia | N | %b | Ia | N | %b | Ia | N | %b | P | N | %b | P | |
| Cestodes | ||||||||||||||||||
| Diphyllobothriids (likely Dibothriocephalus spp.)c | 4 | 5 (2–13) | 1+ to 2+ | 1 | 1 (0–7) | 1+ | 8 | 11 (6–20) | 1+ | 1 | 5 (1–22) | 0 | 10 | 6 (3–10) | 0.02 | 5 | 3 (1–7) | 0.19 |
| Taeniid (Echinococcus spp. and/or Taenia spp.) | 2 | 3 (1–9) | 1+ | 11 | 14 (8–23) | 0 | 37 | 51 (39–62) | 1+ to 2+ | 14 | 64 (43–80) | 1+ | 62 | 35 (29–43) | <0.001 | 13 | 8 (5–14) | 0.02 |
| Nematodes | ||||||||||||||||||
| Capillaria spp. | 0 | 0 (0–5) | 1+ | 1 | 1 (0–7) | 1+ | 0 | 0 (0–5) | 0 | 0 | 0 (0–15) | 0 | 1 | 1 (0–3) | 1.0 | 1 | 1 (0–4) | 1.0 |
| Toxascaris leonina | 41 | 55 (44–66) | 1+ to 4+ | 10 | 12 (7–21) | 1+ to 4+ | 3 | 4 (1–11) | 1+ | 1 | 5 (1–22) | 1+ | 14 | 8 (5–13) | 0.14 | 51 | 33 (26–41) | <0.001 |
| Toxocara canis | 2 | 3 (1–9) | 1+ | 15 | 19 (12–28) | 1+ to 2+ | 2 | 3 (1–10) | 1+ to 2+ | 0 | 0 (0–15) | 0 | 17 | 10 (6–15) | <0.001 | 17 | 11 (7–17) | 0.002 |
| Trichuris sp. | 16 | 22 (14–32) | 1+ to 3+ | 33 | 41 (31–52) | 1+ to 2+ | 6 | 8 (4–17) | 1+ | 2 | 9 (3–28) | 1+ | 41 | 23 (18–30) | <0.001 | 49 | 32 (25–39) | 0.02 |
| Uncinaria sp. | 7 | 10 (5–18) | 0 | 5 | 6 (3–14) | 1+ | 3 | 4 (1–11) | 1+ to 2+ | 1 | 5 (1–22) | 1+ | 9 | 5 (3–9) | 0.89 | 12 | 8 (5–13) | 0.55 |
| Trematodes | ||||||||||||||||||
| Alaria americana | 29 | 39 (29–51) | 1+ to 2+ | 42 | 52 (41–62) | 1+ to 3+ | 18 | 25 (16–36) | 1+ | 7 | 32 (16–53) | 1+ | 67 | 38 (31–45) | 0.002 | 71 | 46 (38–54) | 0.15 |
| Cryptocotyle lingua | 24 | 32 (23–44) | 0 | 2 | 3 (1–9) | 0 | 0 | 0 (0–5) | 0 | 0 | 0 (0–15) | 0 | 2 | 1 (0–4) | 0.62 | 26 | 17 (12–23) | <0.001 |
| Metorchis sp. | 1 | 1 (0–7) | 1+ | 0 | 0 (0–5) | 0 | 0 | 0 (0–5) | 0 | 0 | 0 (0–15) | 0 | 0 | 0 (0–2) | 1.0 | 1 | 1 (0–4) | 0.48 |
| Overall parasite prevalence after fecal flotationd | 57 | 77 (66–86) | - | 53 | 65 (54–75) | - | 22 | 30 (20–42) | - | 6 | 27 (12–50) | - | 81 | 46 (39–54) | <0.001 | 110 | 71 (63–78) | 0.16 |
| Overall parasite prevalence combining gross examination/fecal flotationd | 67 | 91 (82–95) | - | 71 | 88 (79–93) | - | 53 | 73 (61–82) | - | 16 | 73 (52–87) | - | 140 | 80 (73–85) | 0.05 | 138 | 89 (83–93) | 0.62 |
Fecal infection intensity: 1+ 1–50 eggs per gram of feces (epg)/2 + 51–250/3 + 251–1000/4+ >1000.
95% confidence intervals are displayed in parentheses.
Most likely D. latus (previously Diphyllobothrium latum) or D. dendriticus (previously Diphyllobothrium dendriticum).
Canids infected with at least one gastrointestinal helminth species observed by fecal flotation, or combining gross examination and fecal flotation.
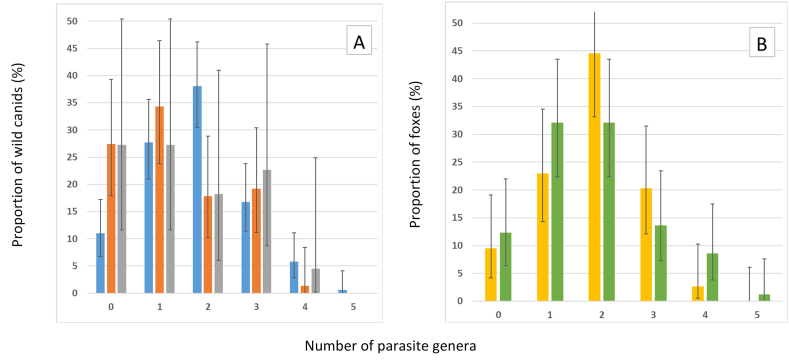
We found that by combining gross examination and fecal flotation, 89% of foxes (138/155, 95% CI:83–93), 73% of coyotes (53/73, 95% CI:61–82), and 73% of wolves (16/22, 95% CI:52–87) were infected with at least one gastrointestinal parasite (Table 3). Parasite genus richness ranged from zero to five for foxes, and zero to four for coyotes and wolves (Fig. 2). Fewer foxes were uninfected than coyotes (p = 0.006). More foxes were infected by two parasite genera than coyotes (p = 0.004). No significant difference was seen in foxes between Subarctic and Humid Continental climate regions and parasite genera.
Fig. 2.
A) Parasite genus richness in foxes (Vulpes vulpes, blue), coyotes (Canis latrans, orange), and wolves (Canis lupus, gray) from Québec, Canada, determined by gross examination and fecal flotation combined (N = 250). Fewer foxes were uninfected than coyotes (p = 0.006). More foxes were infected by two parasite genera than coyotes (p = 0.004). B) Parasite genus richness between Subarctic (yellow) and Humid Continental climate (green) in foxes from Québec, Canada, determined by gross examination and fecal flotation combined (N = 155). No significant difference in parasite genera was seen in foxes between Subarctic and Humid Continental climate regions. Parasites counted in both histograms were: diphyllobothriids (likely Dibothriocephalus spp.), Echinococcus spp., Taenia spp., Capillaria spp., Toxascaris sp., Toxocara sp., Trichuris sp., Uncinaria sp., Alaria sp., Cryptocotyle sp., and Metorchis sp. Parasites observed in both fecal and gross examination were only counted once. Bars represent 95% confidence intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Based on gross examination, the median infection intensity of E. canadensis was 120 ± 813 cestodes/coyote (min-max: 6–2378), and 460 ± 1110 cestodes/wolf (min-max: 5–2900), with no significant difference between canid species (p = 0.32). For Alaria americana, the median infection intensity was 12 ± 77 trematodes/fox (min-max: 1–390), 3 ± 9 trematodes/coyote (min-max: 1–30), and 10 ± 35 trematodes/wolf (min-max: 1–80), with more abundant infections in foxes than in coyotes (p = 0.02). Cryptocotyle lingua was found only in foxes, with a median infection intensity of 48 ± 1724 trematodes/fox (min-max: 1–6000). Finally, combined nematode intensity was 5 ± 15 nematodes/fox (min-max:1–120), and 8 ± 13 nematodes/coyote (min-max: 1–33), with no significant difference between both species (p = 0.83). No nematodes were detected in wolves on gross examination. Eggs of T. leonina had the highest fecal infection intensity in foxes (>1000 epg), followed by Trichuris vulpis and A. americana (251–1000 epg). In coyotes, taeniid eggs, Toxocara canis and Uncinaria eggs had moderate fecal infection intensity (51–250 epg) while in wolves, all parasite species recorded had low infection intensity (1–50 epg).
Out of 84 non-Echinococcus adult cestodes, we morphologically identified 65 as Taenia spp. We detected six different species through DNA sequencing: T. hydatigena, T. twitchelli, T. crassiceps, T. polyacantha, T. krabbei, and T. pisiformis-“like” (Table 2b). Of 53 specimens, eight were T. hydatigena (99–100% similarity), ten were T. twitchelli (97–100% similarity), five were T. crassiceps (97–100% similarity), three were T. polyacantha (100% similarity), and three were T. krabbei (99% similarity). The remaining 23 high-quality sequences were 91–92% similar to T. pisiformis and identical to each other. Four other high-quality Taenia sequences had less than 90% similarity to any sequences in Genbank. The nine remaining sequences did not align well in GenBank (low query coverage, ≤ 91%), but six had high similarity to each other after generating multiple alignments of these sequences with the program Clustal. Findings of Echinococcus canadensis genotypes in wolves and coyotes are reported in Schurer et al. (2018).
Table 2b.
Comparison of Taenia spp. in foxes (Vulpes vulpes), coyotes (Canis latrans), and wolves (Canis lupus) based on molecular analyses (N = 65) following gross examination (N = 276) in Québec, Canada.
| Foxes (N = 176) |
Coyotes (N = 77) |
Wolves (N = 23) |
Difference in Taenia prevalence among canid host species (N = 276) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | %b | N | %b | N | %b | N | %b | Pc | |
| Taenia spp. | |||||||||
| T. hydatigena, | 0 | 0 (0–2) | 5 | 6 (3–14) | 3 | 13 (5–32) | 8 | 3 (1–6) | <0.001 |
| T. twitchelli | 5 | 3 (1–6) | 2 | 3 (1–9) | 3 | 13 (3–35) | 10 | 4 (2–7) | 0.08 |
| T. crassiceps | 4 | 2 (1–6) | 1 | 1 (0–7) | 0 | 0 (0–14) | 5 | 2 (1–4) | 1.0 |
| T. polyacantha | 3 | 2 (1–5) | 0 | 0 (0–5) | 0 | 0 (0–14) | 3 | 1 (0–3) | 0.66 |
| T. krabbei | 0 | 0 (0–2) | 0 | 0 (0–5) | 3 | 13 (5–32) | 3 | 1 (0–3) | <0.001 |
| T. pisiformis-“like” | 0 | 0 (0–2) | 21 | 27 (19–38) | 2 | 9 (2–27) | 23 | 8 (6–12) | <0.001 |
| Unidentified | 1 | 1 (0–3) | 11 | 14 (8–24) | 1 | 4 (1–21) | 13 | 5 (3–8) | <0.001 |
| Overall Taenia prevalence after gross examinationa | 13 | 7 (4–12) | 36 | 47 (36–58) | 12 | 52 (33–71) | 61 | 22 (18–27) | <0.001 |
Canids infected with at least one Taenia species based on gross examination.
95% confidence intervals are displayed in parentheses.
P values are from Fisher's Exact test.
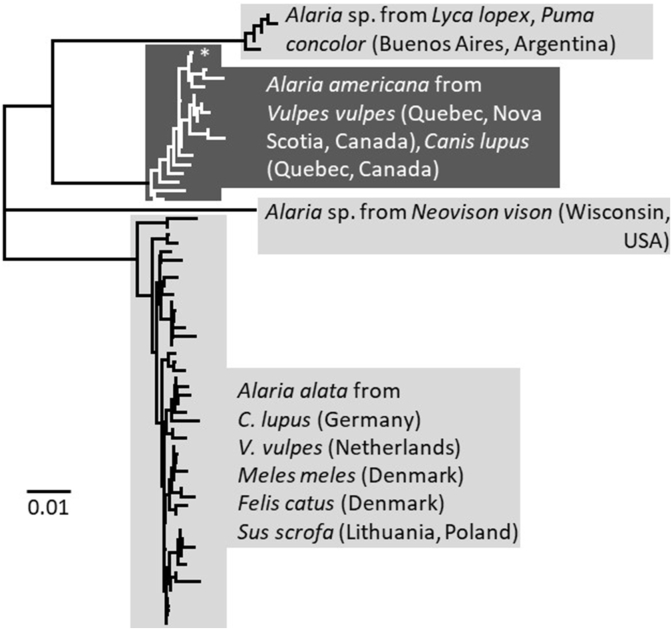
Sequences of CO1 from 17 specimens of Alaria (16 from fox, 1 from wolf; geographic origins: Subarctic 13, Humid Continental 4) were identified as A. americana based on a match with a specimen from a fox in Nova Scotia (MH536507, Locke et al., 2018). Mean CO1 variation in this cluster of 18 sequences of A. americana was 0.81% (range 0–2.51%). By comparison, CO1 mean variation among 49 sequences of Alaria alata sampled across Europe (Fig. 3) was 0.88%, range 0–2.87%, and among five sequences of Alaria sp. in Argentina was 0.3% (range 0–0.8%). Mean interspecific distances among 73 CO1 sequences, including the 17 obtained in the present study, and four species of Alaria (the two named and two unnamed species in Fig. 3) were 8.05% (range 5.96–10.53%).
Fig. 3.
Neighbour-joining tree of Jukes-Cantor distances among sequences of CO1 (alignment 450 bp using all sites) from Alaria available on GenBank as of 7 July 2021. Data from Alaria americana, including data from the present study, indicated by darker shaded cluster and white font. Sequences from A. alata are HM022221-3, KF751233-4, KP123416-20, KP123422-5, KX962374, KX962392, KX962395, KX962397-8, KX962402, KX962406, KX962415, KX962421, KX962433, KX962437, KX962454-5, KX962471-2, KX962481, KX962491, KY012317, MT103215-31; from Alaria sp. in Argentina KF572949, MH892076, MT328804-6; from Alaria sp. in Wisconsin, USA KT223036; from A. americana MZ605217-33 (present study) and MH536507 (indicated with an asterisk).
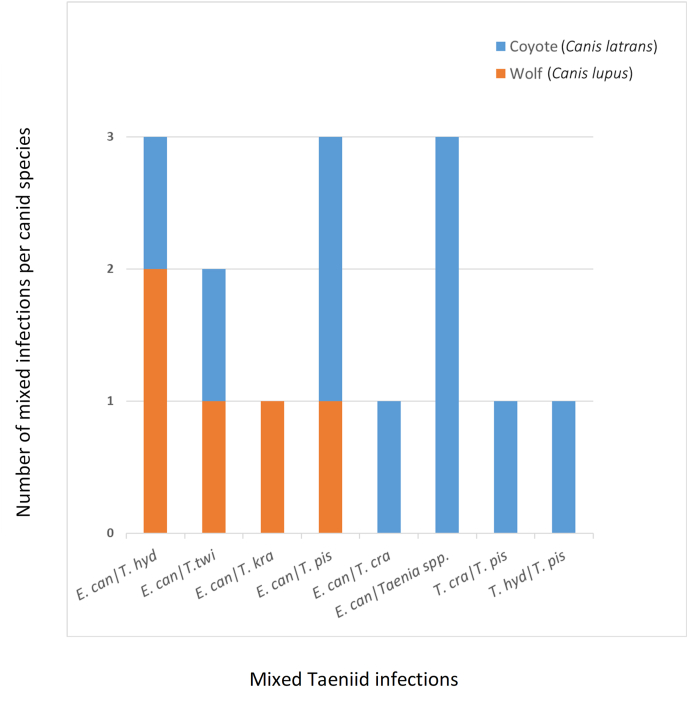
Mixed taeniid infections were present in coyotes and wolves of the Humid Continental region (Fig. 4). In the 13 foxes infected by taeniids, only single taeniid infections were observed (of T. twitchelli, T. crassiceps, or T. polyacantha, Table 2b).
Fig. 4.
Mixed taeniid infections in the Humid Continental climate in coyotes (Canis latrans) and wolves (Canis lupus) from Québec, Canada, following molecular analyses. Abbreviations on x-axis: E. can, Echinococcus canadensis; T. hyd, Taenia hydatigena; T. twi, T. twitchelli; T. kra, T. krabbei; T. pis, T. pisiformis-“like”; T. cra, T. crassiceps.
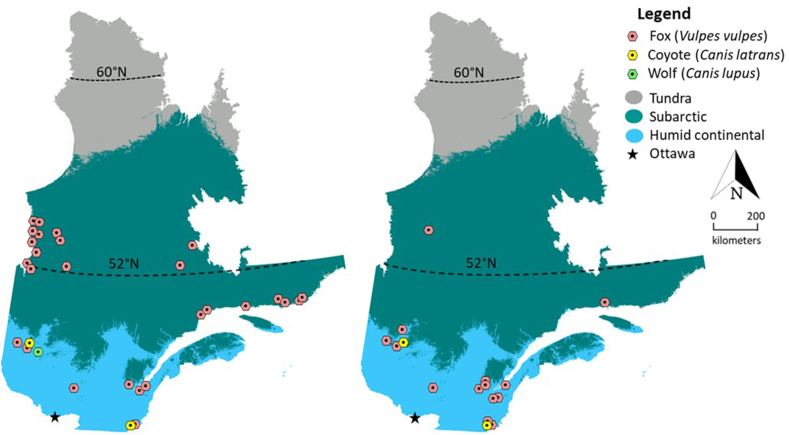
In the Humid Continental climate region, prevalence of T. canis, T. vulpis, and A. americana was higher in foxes compared to other canid hosts (p < 0.05). In coyotes and wolves, taeniid cestodes and diphyllobothriids were more prevalent than in foxes (p < 0.05). In foxes collected in the Subarctic region, T. leonina, T. vulpis, A. americana and Cryptocotyle lingua were the dominant gastrointestinal helminths. We observed multiple foxes (n = 26) infected with Cryptocotyle lingua trematodes, with the majority concentrated along the St. Lawrence estuary (Fig. 5). Finally, Capillaria spp. and M. conjunctus were only observed once in foxes (Table 3).
Fig. 5.
On the left, whole mounted Cryptocotyle lingua adult trematode stained with borax carmine (credit: Brent Wagner). On the right, distribution of foxes (Vulpes vulpes) infected with C. lingua in the Subarctic (samples (n) collected along James Bay and the St Lawrence estuary) and Humid Continental climate collected during winter 2016–2017 by trappers from Québec, Canada. Arrows indicate major waterways.
We found significant geographic differences in parasite prevalence in foxes for taeniids (p = 0.02), Toxascaris leonina (p < 0.001), Toxocara canis (p = 0.002), Trichuris (p = 0.02), and Cryptocotyle lingua (p < 0.001) (Table 3). Foxes from more southerly areas had significantly higher prevalence of Toxocara canis than those from the north (p = 0.002), whilst the contrary applied for Toxascaris leonina, which was less prevalent in southern than northern latitudes (p < 0.001) (Fig. 6) (Table 3). Overall parasite prevalence in foxes was similar between both regions (no significant difference on fecal flotation (p = 0.16) and combined with gross examination (p = 0.62)) (Table 3).
Fig. 6.
Distribution of foxes (Vulpes vulpes), coyotes (Canis latrans), and wolves (Canis lupus) infected with Toxascaris leonina (left, N = 55) and Toxocara canis (right, N = 19) in the Subarctic and Humid Continental climate collected during winter 2016–2017 by hunters and trappers from Québec, Canada.
4. Discussion
We encountered regional and host-specific differences in gastrointestinal parasitism in wild canids from two climate regions of Québec, Canada, combining adult parasite recovery and fecal flotation with morphological and molecular methods to improve detection and maximize taxonomic resolution. Using combined methods was especially important for cestodes in coyotes and wolves, for which prevalence is underestimated when diagnosis is based solely on eggs in feces (Schurer et al., 2016).
4.1. Parasitism, host diet and ecology
4.1.1. Cestodes
Host diet can play a key role in shaping gastrointestinal helminth diversity. Our findings of T. twitchelli, T. crassiceps, and T. polyacantha in foxes highlight predation on rodents (lemmings, voles, and mice) and lagomorphs (Stien et al., 2009). Coyotes can hunt and scavenge larger wildlife such as cervids, accounting for findings of T. hydatigena and E. canadensis (Schurer et al., 2018). Recovery of Taenia species using rodents and lagomorphs as intermediate hosts (e.g., T. twitchelli, T. pisiformis-like, T. crassiceps, and T. polyacantha) from coyotes are consistent with the higher proportion of small mammals in coyote diets compared to wolves (Keith et al., 1986; Rausch and Fay, 1988). Detection of T. hydatigena and T. krabbei in wolves highlight the importance of big game such as moose, deer and caribou in the diet of wolves, which serve as intermediate hosts for these parasites. Beavers, an important secondary prey of wolves, can also harbor T. hydatigena (Gable et al., 2018; Spickler, 2020). Our findings of T. twitchelli and a T. pisiformis-like species also reinforce observations that wolves prey on lagomorphs and porcupines (Erethizon dorsatum), which serve as intermediate hosts for these cestodes (Holmes and Podesta, 1968; Craig and Craig, 2005). Also, our results on mixed infection in coyotes and wolves support laboratory-based studies demonstrating that complete protective immunity against recurrent taeniid infection with different species is unlikely in canids (Jenkins and Rickard, 1985). As well, mixed infections indicate broad ranges of dietary sources for individual wild canids, with each host harbouring a potentially unique community of trophically transmitted parasites.
The higher prevalence of adults and eggs of diphyllobothriids in coyotes (Tables 2a-3) suggests a diet that includes more piscine intermediate and paratenic hosts containing plerocercoids (Ching, 1984; Jenkins et al., 2013; Reid et al., 2018). Given the known geographic distribution of diphyllobothriids, and the distance of collected specimens from marine environments, the most likely identification for these cestodes in our data set are the freshwater species Dibothriocephalus latus (previously Diphyllobothrium latum) or D. dendriticus (previously Diphyllobothrium dendriticum) (Jenkins et al., 2013).
4.1.2. Nematodes
Trichuris vulpis, which was more common in foxes (Table 3), has a direct life cycle with no paratenic hosts. Canids acquire T. vulpis by ingesting larvated eggs that persist for years in the environment (Kirkova and Dinev, 2005). The higher prevalence in foxes versus coyotes and wolves may reflect higher density of foxes in urban areas due to smaller home range (Goszczyński, 2002), and more potential for contact with environments contaminated with feces from stray and domestic dogs in peri-urban settings in southern regions (Traversa, 2011). We did not examine large intestines (the infection site of adult T. vulpis), and consequently our data may underestimate overall prevalence. In addition, some T. vulpis eggs observed may have originated from ingested prey species. Eggs of Uncinaria, another potentially directly transmitted nematode, were found in all canid species. This parasite can be acquired through consumption of hatched third stage larvae (L3) in the environment, indirectly through consumption of L3 in paratenic hosts, or, rarely compared to Ancylostoma, through cutaneous invasion of L3.
4.1.3. Trematodes
All three trematodes detected in Québec canids, Alaria, Metorchis, and Cryptocotyle, are acquired from aquatic environments. Alaria sp. requires aquatic snails and amphibians as intermediate hosts, and can also be transmitted by paratenic hosts such as snakes and rodents (house and deer mouse), which are all prey items of wild canids (Möhl et al., 2009). Eggs of Metorchis conjunctus were only detected in one fox from the Subarctic climate. Multiple Cryptocotyle lingua were detected on gross examination of small intestines in 26 foxes, mostly from the northeastern shore of the St. Lawrence estuary in the Subarctic climate region. Foxes may become infected with this fluke by feeding on fish remains, particularly euryhaline and diadromous species, along the shorelines of marine waters, rivers and ponds (Gibson, 1996; Saeed et al., 2006).
These findings illustrate relationships between trophically transmitted parasites and diet in wild canids (cestodes and trematodes) and between directly transmitted parasites such as T. vulpis and host ecology and habitat use. This is expected as many helminth species are acquired through the ingestion of infective larval stages, from the environment or from infected prey serving as intermediate or paratenic hosts (Lafferty, 1999). In the present study, foxes had significantly more parasite species richness compared to coyotes. Although smaller in body size, this could be explained by a greater dietary breadth than coyotes (Dodd and Whidden, 2018). Other features such as environmental factors, population density, and geographical range, can also play a role as determinants of parasite species richness (Kamiya et al., 2014; Villalobos-Segura et al., 2020).
4.2. Cryptic species and molecular challenges
In the present study, molecular methods were critical for many parasite identifications. For example, even though species of Taenia can be distinguished through rostellar hook morphometrics, freeze-thaw cycles impede recovery of intact worms, making scolices difficult to recover, and strobila alone have limited utility for morphological identification. Molecular methods are therefore a useful alternative. Previously reported in wolves from northern and western Canada (Schurer et al., 2016), we demonstrated coinfection of coyotes and wolves with multiple taeniid genera and species. Following molecular analyses, we found mixed infections of E. canadensis – Taenia (10%) and Taenia species (3%) in coyotes, as well as E. canadensis – Taenia (22%) in wolves. As we did not conduct PCR on all taeniid cestodes, and no coyotes and wolves were harvested in the Subarctic region, our molecular approach likely underestimated the true prevalence of mixed infections. Our results also highlight the need for morphologically confirmed identifications in molecular databases, and the utility and limitations of widely used loci such as the CO1 mtDNA gene. We found 23 isolates with CO1 91–92% similar to T. pisiformis, suggesting that these specimens belong to a closely related and as-yet-unsequenced species of Taenia circulating in wild canids in North America. Future work could include genetic comparisons of putative T. pisiformis from across its global distribution. We also recovered six identical sequences from Taenia spp. specimens that did not align well (query coverage ≤91%) with any sequences in GenBank; this could also be an existing Taenia sp. for which sequence has not yet been published, or a new species. Many Taenia were often observed in gross examination, and molecular identification was not conducted in all specimens because of the costs and resources necessary for sequencing. A multiplex PCR or restriction fragment length polymorphism PCR could be an alternative for future large-scale studies (Al-Sabi and Kapel, 2011; Huttner et al., 2009).
4.3. Distribution ranges and limits of distribution
In our study, T. canis was detected in 11% (17/155) of foxes, mostly in the Humid Continental region, and 3% (2/73) of coyotes following fecal flotation. The highest latitude at which T. canis was detected was 53°43′60.0″N, in a fox in the Subarctic climate region (Fig. 6). In addition to decreasing prevalence when moving north, this finding supports the hypothesis that T. canis does not thrive at northern latitudes (Jenkins, 2020). The eggs of T. canis have low freeze-tolerance but can persist for many years at temperatures of 10–30 °C, which are required for the eggs to fully embryonate (Azam et al., 2012). Antibodies to T. canis have been reported at low prevalence (3.9%) of people in Subarctic QC, and the parasite has been reported in dogs, but is not well documented in QC wildlife (Cameron et al., 1940; Messier et al., 2012). Several factors may increase risks of T. canis infection in northern latitudes with current and future climate change, including growth in human and domestic dog populations, increasingly close contact with wildlife such as foxes, northward shifts in red fox distribution, lack of veterinary services, and enhanced egg survival in warmer and wetter weather (Jenkins et al., 2013). Co-infection with both T. canis and Toxascaris leonina is well documented in wild canids (Okulewicz et al., 2012). Within our study, T. leonina was found in both climate regions and in all canid species, with a higher prevalence in foxes from the Subarctic climate (Fig. 6). This is consistent with earlier coprological studies from arctic fox and in wild canids in general in Canada (Aguirre et al., 2000; Meijer et al., 2011; Elmore et al., 2013; Jenkins, 2020). The higher prevalence of T. leonina in foxes in the Subarctic (55%) compared to the Humid Continental climate region (8%) could be explained by increased competition with T. canis at more southern latitudes (Okulewicz et al., 2012), a higher reliance on rodent paratenic hosts as part of fox diet in subarctic regions, and negative effects of urbanization on rodent-transmitted parasites in southern regions (Reperant et al., 2007).
Prevalence of T. vulpis was higher in foxes from the south (p = 0.02), with no infections north of 53°45′16.4″N in the Subarctic climate. This may reflect the higher temperature and moisture requirements of eggs of T. vulpis (Spindler, 1929; Dubin et al., 1975; Miterpáková et al., 2009). Hookworm eggs, most likely Uncinaria stenocephala given the geographic context, were seen at relatively similar prevalence in foxes (8%) and other canids (4%). Ancylostoma caninum (southern hookworm) is usually found in temperate, tropical, and subtropical regions, and is rarely seen in Canada (Craig and Craig, 2005). Uncinaria is an ancyclostomid occupying a similar niche in northern latitudes, with reports in arctic foxes from Europe, Iceland and Greenland (Meijer et al., 2011).
Finally, Alaria americana, identified in a subset of samples, has previously been considered a synonym of A. marcianae (Locke et al., 2018), which may have led to underreporting of A. americana in eastern Canada. Another trematode, Cryptocotyle lingua, has been reported in foxes from the eastern Canadian provinces of Prince Edward Island, New Brunswick, and Nova Scotia (Smith, 1978; Wapenaar et al., 2013; Robbins, 2018). This is the first report of C. lingua in foxes from QC, where this parasite has been recorded once in a great black-backed gull (Larus marinus) near Ungava Bay (Ferguson et al., 2012).
4.4. Zoonotic potential
Although rarely associated with clinical disease, diphyllobothriid cestodes are zoonotic. People become infected the same way as wild canids, by eating contaminated raw fish. The nematode Toxocara canis is another helminth with zoonotic potential, and is the agent of human toxocariasis, responsible for visceral larva migrans, ocular larva migrans and neurotoxocariasis (Macpherson, 2013). Commonly found in wild canids from temperate regions, T. canis has been reported as the most common parasite in dogs from urban animal shelters across Canada (Villeneuve et al., 2015). Toxascaris leonina, another ascarid nematode but with very limited zoonotic potential, has also been detected. Uncinaria is thought to have much lower zoonotic potential and animal health significance than Ancylostoma, the more pathogenic species in canids (Bowman et al., 2010). Cases of human intraocular infection with Alaria have been demonstrated in a few cases, likely caused by ingestion of undercooked contaminated frog legs (Otranto and Eberhard, 2011). As for Metorchis conjunctus, canids and people acquire this parasite from ingestion of metacercaria in catostomid fishes (suckers). Human infections caused by parasitic helminths remain of particular importance given their possible gravity and the close proximity between people, domestic animals, and wild canids.
4.5. Methodological limitations
Among limitations of our study were sampling that was uneven regionally and in terms of host species, especially in the Subarctic climate region. Given the constraints of sample acquisition and the scale of our study, our methods of parasite detection and identification were generally robust and multidisciplinary, and our data likely reflect real biological patterns, but deficiencies were nonetheless unavoidable. As we did not target or record presence of adult hookworm nematodes from small intestines, as well as for large intestines, we likely underestimated infection levels. Fecal floats were not always conducted, as fecal matter was sometimes absent. Moreover, fecal floats do not reveal non-patent infections, and freezing at −80 °C can affect egg integrity and detection. Indeed, morphological changes were observed in eggs of T. canis and to a lesser extent T. leonina following freezing at −80 °C, although eggs of both these nematodes were still readily identified. In contrast, Uncinaria stenocephala infection levels are likely underestimated due to small sample sizes and the fragile nature of hookworm eggshells which rupture easily following freezing at −80 °C (Schurer et al., 2014). Finally, eggs of the trematode Metorchis conjunctus were only detected in one fox. As we did not examine bile ducts on necropsy, and eggs of this parasite are dense and may not float well, our record likely underestimates true prevalence.
Necropsies are not always feasible or ethically possible in wildlife; therefore, fecal flotation remains a useful technique (especially for fecund nematodes such as ascarids). Results from flotations are quick, inexpensive, and safer than gross examination when samples are properly handled, including freezing at −80 °C for a few days prior to processing to inactivate eggs of Echinococcus spp. On the other hand, gross examination is more sensitive for cestodes and trematodes which may not reliably shed eggs in feces or may not have eggs that float well in standard flotation solutions, and enables recovery of adult specimens suitable for morphological and/or molecular identification of species, i.e. to distinguish among species of Echinococcus and Taenia (Schurer et al., 2016).
5. Conclusion
In this study, we used a combined morphological and molecular approach to obtain new information on parasite communities of wild canids in Québec, Canada, and provided much needed baseline data on canid parasites that may undergo changes in distribution and prevalence in the rapidly changing climate in Subarctic and Arctic regions of Canada. We report two previously unsequenced Taenia species, describe northern distributional limits for two important canid nematodes, Toxocara canis and Trichuris vulpis, clarified the prevalence of a canid intestinal trematode in northeastern North America (Alaria americana), and reported a new geographic record for the trematode Cryptocotyle lingua in foxes. We observed that foxes had more nematodes and fewer cestodes than wolves and coyotes (Table 2a). Many of the parasites observed are transmitted through prey, which informs our understanding of diets among wild canids. Finally, thinning boundaries between wild and domestic canids, as well as human disturbance, likely impact foraging and use of food resources by wild canids. As a result, this could alter the risk of transmission of these wildlife reservoired parasites, all of which can transmit to dogs, and many to people (Echinococcus, diphyllobothriid cestodes, Toxocara, Metorchis, and Alaria spp.) (Manlick and Pauli, 2020; Sugden et al., 2020). Continued studies of host-parasite interaction and parasite distributions are needed to detect changes in parasite communities as a result of climate and landscape alterations.
Declaration of competing interest
This manuscript has not been published and is not under consideration for publication elsewhere. We have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the Québec trappers and hunters for providing samples, Audrey Simon for helping during necropsies, and Sarah Parker for statistical help. We are grateful to Claude Grenier, as well as the personnel of the Faculté de Médecine Vétérinaire and the Centre québécois sur la santé des animaux sauvages (CQSAS) for storage of carcasses, logistic help, and transport. We thank reviewers for their comments that contributed to strongly improve the manuscript. This work was funded by the US National Center for Veterinary Parasitology, ArcticNet Networks of Centres of Excellence, Natural Sciences and Engineering Research Council of Canada (E. Jenkins), and the US National Science Foundation (1845021, S. Locke).
Footnotes
Note: Nucleotide sequence data reported in this paper are available in GenBankTM at accession numbers MZ605217-33.
Contributor Information
Émilie Bouchard, Email: emb232@mail.usask.ca.
Janna M. Schurer, Email: jschurer@gmail.com.
Temitope Kolapo, Email: tuk781@mail.usask.ca.
Brent Wagner, Email: baw125@mail.usask.ca.
Ariane Massé, Email: ariane.masse@mffp.gouv.qc.ca.
Sean A. Locke, Email: sean.locke@upr.edu.
Patrick Leighton, Email: patrick.a.leighton@umontreal.ca.
Emily J. Jenkins, Email: ejj266@mail.usask.ca.
References
- Aguirre A.A. Wild canids as sentinels of ecological health: a conservation medicine perspective. Parasites Vectors. 2009;2(Suppl. 1) doi: 10.1186/1756-3305-2-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A.A., Angerbjorn A., Tannerfeldt M., Morner T. Health evaluation of arctic fox (Alopex lagopus) cubs in Sweden. J. Zoo Wildl. Med. 2000;31:36–40. doi: 10.1638/1042-7260(2000)031[0036:HEOAFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Al-Sabi M.N., Kapel C.M. Multiplex PCR identification of Taenia spp. in rodents and carnivores. Parasitol. Res. 2011;109:1293–1298. doi: 10.1007/s00436-011-2373-9. [DOI] [PubMed] [Google Scholar]
- Altizer S., Ostfeld R.S., Johnson P.T., Kutz S., Harvell C.D. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341:514–519. doi: 10.1126/science.1239401. [DOI] [PubMed] [Google Scholar]
- Azam D., Ukpai O.M., Said A., Abd-Allah G.A., Morgan E.R. Temperature and the development and survival of infective Toxocara canis larvae. Parasitol. Res. 2012;110:649–656. doi: 10.1007/s00436-011-2536-8. [DOI] [PubMed] [Google Scholar]
- Beck H.E., Zimmermann N.E., McVicar T.R., Vergopolan N., Berg A., Wood E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data. 2018;5:180214. doi: 10.1038/sdata.2018.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercuson D.J., Krueger R.R., Hall R.D., Morton W.L., Nicholson N.L. Encyclopedia Britannica. 2021. Canada.https://www.britannica.com/place/Canada Accessed. [Google Scholar]
- Bowles J., Blair D., McManus D.P. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol. Biochem. Parasitol. 1992;54:165–173. doi: 10.1016/0166-6851(92)90109-w. [DOI] [PubMed] [Google Scholar]
- Bowles J., McManus D.P. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int. J. Parasitol. 1993;23:969–972. doi: 10.1016/0020-7519(93)90065-7. [DOI] [PubMed] [Google Scholar]
- Bowman D.D., Montgomery S.P., Zajac A.M., Eberhard M.L., Kazacos K.R. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010;26:162–167. doi: 10.1016/j.pt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Breck S.W., Poessel S.A., Mahoney P., Young J.K. The intrepid urban coyote: a comparison of bold and exploratory behavior in coyotes from urban and rural environments. Sci. Rep. 2019;9:2104. doi: 10.1038/s41598-019-38543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger K.E., Baggs E.M., Finney-Crawley J. Endoparasites of the coyote (Canis latrans), a recent migrant to insular Newfoundland. J. Wildl. Dis. 2009;45:1221–1226. doi: 10.7589/0090-3558-45.4.1221. [DOI] [PubMed] [Google Scholar]
- Bush E., Lemmen D.S. Government of Canada; Ottawa, ON): 2019. Canada's Changing Climate Report; p. 444. [Google Scholar]
- Cable J., Barber I., Boag B., Ellison A.R., Morgan E.R., Murray K., Pascoe E.L., Sait S.M., Wilson A.J., Booth M. Global change, parasite transmission and disease control: lessons from ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron T.W.M., Parnell I.W., Lyster L.L. The helminth parasites of sledge-dogs in northern Canada and Newfoundland. Can. J. Res. 1940;18d:325–332. [Google Scholar]
- Chester S. Princeton University Press; Princeton, New Jersey: 2016. The Arctic Guide: Wildlife of the Far North. [Google Scholar]
- Ching H.L. Fish tapeworm infections (diphyllobothriasis) in Canada, particularly British Columbia. Can. Med. Assoc. J. 1984;130:1125–1127. [PMC free article] [PubMed] [Google Scholar]
- Craig H.L., Craig P.S. Helminth parasites of wolves (Canis lupus): a species list and an analysis of published prevalence studies in Nearctic and Palaearctic populations. J. Helminthol. 2005;79:95–103. doi: 10.1079/joh2005282. [DOI] [PubMed] [Google Scholar]
- Curtis M.A., Rau M.E., Tanner C.E., Prichard R.K., Faubert G.M., Olpinski S., Trudeau C. Parasitic zoonoses in relation to fish and wildlife harvesting by Inuit communities in northern Quebec, Canada. Arctic Med. Res. 1988;47(Suppl. 1):693–696. [PubMed] [Google Scholar]
- Dodd R.W., Whidden H.P. Dietary analysis of red foxes, gray foxes, and coyotes in eastern Pennsylvania. J. Pa. Acad. Sci. 2018;92:36–52. [Google Scholar]
- Dryden M.W., Payne P.A., Ridley R., Smith V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet. Therapeut. 2005;6:15–28. [PubMed] [Google Scholar]
- Dubin S., Segall S., Martindale J. Contamination of soil in two city parks with canine nematode ova including Toxocara canis: a preliminary study. Am. J. Public Health. 1975;65:1242–1245. doi: 10.2105/ajph.65.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J., Gemmell M., Meslin F.-X., Pawlowski Z. Organization for Animal Health and World Health Organization; 2001. WHO/OIE Manual of Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern. [Google Scholar]
- Elmore S.A., Lalonde L.F., Samelius G., Alisauskas R.T., Gajadhar A.A., Jenkins E.J. Endoparasites in the feces of arctic foxes in a terrestrial ecosystem in Canada. Int. J. Parasitol. Parasites Wildl. 2013;2:90–96. doi: 10.1016/j.ijppaw.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J.A., Locke S.A., Font W.F., Steinauer M.L., Marcogliese D.J., Cojocaru C.D., Kent M.L. Apophallus microsoma N. SP. from chicks infected with metacercariae from coho salmon (Oncorhynchus kisutch) and review of the taxonomy and pathology of the genus Apophallus (Heterophyidae) J. Parasitol. 2012;98:1122–1132. doi: 10.1645/GE-3044.1. [DOI] [PubMed] [Google Scholar]
- Gable T.D., Windels S.K., Romanski M.C., Rosell F. The forgotten prey of an iconic predator: a review of interactions between grey wolves Canis lupus and beavers Castor spp. Mamm Rev. 2018;48:123–138. [Google Scholar]
- Gesy K., Pawlik M., Kapronczai L., Wagner B., Elkin B., Schwantje H., Jenkins E. An improved method for the extraction and quantification of adult Echinococcus from wildlife definitive hosts. Parasitol. Res. 2013;112:2075–2078. doi: 10.1007/s00436-013-3371-x. [DOI] [PubMed] [Google Scholar]
- Gibson D.I. Vol. 124. Canadian Special Publication of Fisheries and Aquatic Sciences; 1996. Guide to the parasites of fishes of Canada. Part IV. Trematoda; pp. 1–373. [Google Scholar]
- Goszczyński J. Home ranges in red fox: territoriality diminishes with increasing area. Acta Theriol. 2002;47:103–114. [Google Scholar]
- Hénault M., Jolicoeur H. Quebec Wildlife and Parks Agency, Laurentian Wildlife Management Department and Department of Wildlife Development; 2003. Les loups au Québec : Meutes et mystères. Société de la faune et des parcs du Québec, Direction de l’aménagement de la faune des Laurentides et Direction du développement de la faune (translation: Wolves in Quebec : Packs and mystery; p. 129pp. [Google Scholar]
- Holmes J.C., Podesta R. The helminths of wolves and coyotes from the forested regions of Alberta. Can. J. Zool. 1968;46:1193–1204. [Google Scholar]
- Huttner M., Siefert L., Mackenstedt U., Romig T. A survey of Echinococcus species in wild carnivores and livestock in East Africa. Int. J. Parasitol. 2009;39:1269–1276. doi: 10.1016/j.ijpara.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Rickard M.D. Specific antibody responses to Taenia hydatigena, Taenia pisiformis and Echinococcus granulosus infection in dogs. Aust. Vet. J. 1985;62:72–78. doi: 10.1111/j.1751-0813.1985.tb14142.x. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J. In: Advances in Parasitology. Bowman D.D., editor. Academic Press; 2020. Chapter thirty-one - Toxocara spp. in dogs and cats in Canada; pp. 641–653. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Castrodale L.J., de Rosemond S.J.C., Dixon B.R., Elmore S.A., Gesy K.M., Hoberg E.P., Polley L., Schurer J.M., Simard M., Thompson R.C.A. In: Advances in Parasitology. Rollinson D., editor. Academic Press; 2013. Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland; pp. 33–204. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Schurer J.M., Gesy K.M. Old problems on a new playing field: helminth zoonoses transmitted among dogs, wildlife, and people in a changing northern climate. Vet. Parasitol. 2011;182:54–69. doi: 10.1016/j.vetpar.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Kamiya T., O'Dwyer K., Nakagawa S., Poulin R. What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts. Biol. Rev. Camb. Phil. Soc. 2014;89:123–134. doi: 10.1111/brv.12046. [DOI] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith I.M., Keith L.B., Cary J.R. Parasitism in a declining population of snowshoe hares. J. Wildl. Dis. 1986;22:349–363. doi: 10.7589/0090-3558-22.3.349. [DOI] [PubMed] [Google Scholar]
- Kirkova Z., Dinev I. Morphological changes in the intestine of dogs, experimentally infected with Trichuris vulpis. Bulg. J. Vet. Med. 2005;8:239–243. [Google Scholar]
- Lafferty K.D. The evolution of trophic transmission. Parasitol. Today. 1999;15:111–115. doi: 10.1016/s0169-4758(99)01397-6. [DOI] [PubMed] [Google Scholar]
- Larivière S., Pasitschniak-Arts M. Vulpes vulpes. Mamm. Species. 1996:1–11. [Google Scholar]
- Locke S.A., Van Dam A., Caffara M., Pinto H.A., Lopez-Hernandez D., Blanar C.A. Validity of the diplostomoidea and diplostomida (digenea, Platyhelminthes) upheld in phylogenomic analysis. Int. J. Parasitol. 2018;48:1043–1059. doi: 10.1016/j.ijpara.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Luong L.T., Chambers J.L., Moizis A., Stock T.M., St Clair C.C. Helminth parasites and zoonotic risk associated with urban coyotes (Canis latrans) in Alberta, Canada. J. Helminthol. 2018;94:e25. doi: 10.1017/S0022149X1800113X. [DOI] [PubMed] [Google Scholar]
- Macpherson C.N.L. The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int. J. Parasitol. 2013;43:999–1008. doi: 10.1016/j.ijpara.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Manlick P.J., Pauli J.N. Human disturbance increases trophic niche overlap in terrestrial carnivore communities. Proc. Natl. Acad. Sci. U. S. A. 2020;117:26842–26848. doi: 10.1073/pnas.2012774117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H., Dobson A. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- McNeill M.A., Rau M.E. Helminths of wolves (Canis lupus L.) from southwestern Quebec. Can. J. Zool. 1984;62:1659–1660. [Google Scholar]
- Mech L.D., Boitani L. In: Status Survey and Conservation Action Plan. Sillero-Zubiri C., Hoffmann M., Macdonald D.W., editors. IUCN/SSC Canid Specialist Group; Gland, Switzerland and Cambridge, UK: 2004. Canids: foxes, wolves, jackals and dogs; p. 430. [Google Scholar]
- Meijer T., Mattsson R., Angerbjörn A., Osterman-Lind E., Fernández-Aguilar X., Gavier-Widén D. Endoparasites in the endangered Fennoscandian population of arctic foxes (Vulpes lagopus) Eur. J. Wildl. Res. 2011;57:923–927. [Google Scholar]
- Messier V., Lévesque B., Proulx J.F., Rochette L., Serhir B., Couillard M., Ward B.J., Libman M.D., Dewailly E., Déry S. Seroprevalence of seven zoonotic infections in Nunavik, Quebec (Canada) Zoonoses Public Health. 2012;59:107–117. doi: 10.1111/j.1863-2378.2011.01424.x. [DOI] [PubMed] [Google Scholar]
- MFFP . 2019. Zones de végétation et domaines bioclimatiques du Québec, Ministère des Forêts, de la Faune et des Parcs, Gouvernement du Québec.https://mffp.gouv.qc.ca/forets/inventaire/inventaire-zones-carte.jsp Accessed. [Google Scholar]
- Miterpáková M., Hurníková Z., Antolová D., Dubinský P. Endoparasites of red fox (Vulpes vulpes) in the Slovak Republic with the emphasis on zoonotic species Echinococcus multilocularis and Trichinella spp. Helminthologia. 2009;46:73. [Google Scholar]
- Möhl K., Grosse K., Hamedy A., Wüste T., Kabelitz P., Lücker E. Biology of Alaria spp. and human exposition risk to Alaria mesocercariae-a review. Parasitol. Res. 2009;105:1–15. doi: 10.1007/s00436-009-1444-7. [DOI] [PubMed] [Google Scholar]
- Moszczynska A., Locke S.A., McLaughlin J.D., Marcogliese D.J., Crease T.J. Development of primers for the mitochondrial cytochrome c oxidase I gene in digenetic trematodes (Platyhelminthes) illustrates the challenge of barcoding parasitic helminths. Mol. Ecol. Resour. 2009;9(Suppl. s1):75–82. doi: 10.1111/j.1755-0998.2009.02634.x. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Vidyashankar A.N., Andersen U.V., Delisi K., Pilegaard K., Kaplan R.M. Effects of fecal collection and storage factors on strongylid egg counts in horses. Vet. Parasitol. 2010;167:55–61. doi: 10.1016/j.vetpar.2009.09.043. [DOI] [PubMed] [Google Scholar]
- Okulewicz A., Perec-Matysiak A., Buńkowska K., Hildebrand J. Toxocara canis, Toxocara cati and Toxascaris leonina in wild and domestic carnivores. Helminthologia. 2012;49:3–10. [Google Scholar]
- Otranto D., Eberhard M.L. Zoonotic helminths affecting the human eye. Parasites Vectors. 2011;4:41. doi: 10.1186/1756-3305-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz J.A., Graczyk T.K., Geller N., Vittor A.Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- QGIS-Development-Team QGIS geographic information system. 2020. http://qgis.osgeo.org Open Source Geospatial Foundation Project.
- Rausch R.L., Fay F.H. Postoncospheral development and cycle of Taenia polyacantha leuckart, 1856 (cestoda: taeniidae). First part. Ann. Parasitol. Hum. Comp. 1988;63:263–277. doi: 10.1051/parasite/1988634263. [DOI] [PubMed] [Google Scholar]
- Reid R.E., Gifford-Gonzalez D., Koch P.L. Coyote (Canis latrans) use of marine resources in coastal California: a new behavior relative to their recent ancestors. Holocene. 2018;28:1781–1790. [Google Scholar]
- Reperant L.A., Hegglin D., Fischer C., Kohler L., Weber J.M., Deplazes P. Influence of urbanization on the epidemiology of intestinal helminths of the red fox (Vulpes vulpes) in Geneva, Switzerland. Parasitol. Res. 2007;101:605–611. doi: 10.1007/s00436-007-0520-0. [DOI] [PubMed] [Google Scholar]
- Robbins W.T. Canada. University of Prince Edward Island. Charlottetown; PE: 2018. A Parasitological Study of the Red Fox (vulpes vulpes) with a Citizen Science Approach for Describing Red Foxes on Prince Edward Island. [Google Scholar]
- Romig T., Thoma D., Weible A.K. Echinococcus multilocularis--a zoonosis of anthropogenic environments? J. Helminthol. 2006;80:207–212. doi: 10.1079/joh2006347. [DOI] [PubMed] [Google Scholar]
- Saeed I., Maddox-Hyttel C., Monrad J., Kapel C.M. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006;139:168–179. doi: 10.1016/j.vetpar.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Scholz T., Kuchta R., Brabec J. Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: recent progress and future challenges. Int. J. Parasitol. Parasites Wildl. 2019;9:359–369. doi: 10.1016/j.ijppaw.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurer J., Davenport L., Wagner B., Jenkins E. Effects of sub-zero storage temperatures on endoparasites in canine and equine feces. Vet. Parasitol. 2014;204:310–315. doi: 10.1016/j.vetpar.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Schurer J.M., Bouchard E., Bryant A., Revell S., Chavis G., Lichtenwalner A., Jenkins E.J. Echinococcus in wild canids in Quebec (Canada) and Maine (USA) PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurer J.M., Pawlik M., Huber A., Elkin B., Cluff H.D., Pongracz J.D., Gesy K., Wagner B., Dixon B., Merks H., Bal M.S., Jenkins E.J. Intestinal parasites of gray wolves (Canis lupus) in northern and western Canada. Can. J. Zool. 2016;94:643–650. [Google Scholar]
- Semenza J.C., Lindgren E., Balkanyi L., Espinosa L., Almqvist M.S., Penttinen P., Rocklov J. Determinants and drivers of infectious disease threat events in Europe. Emerg. Infect. Dis. 2016;22:581–589. doi: 10.3201/eid2204.151073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant E.S.G. Epitools epidemiological calculators. Ausvet. 2018. http://epitools.ausvet.com.au Available at:
- Smith H.J. Parasites of red foxes in new Brunswick and Nova Scotia. J. Wildl. Dis. 1978;14:366–370. doi: 10.7589/0090-3558-14.3.366. [DOI] [PubMed] [Google Scholar]
- Spickler A.R. 2020. Taeniasis, Cysticercosis, and Coenurosis.http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php Accessed. [Google Scholar]
- Spindler L.A. A study of the temperature and moisture requirements in the development of the eggs of the dog trichurid (Trichuris vulpis) J. Parasitol. 1929;16:41–46. [Google Scholar]
- Stien A., Voutilainen L., Haukisalmi V., Fuglei E., MØRk T., Yoccoz N.G., Ims R.A., Henttonen H. Intestinal parasites of the Arctic fox in relation to the abundance and distribution of intermediate hosts. Parasitology. 2009;137:149–157. doi: 10.1017/S0031182009990953. [DOI] [PubMed] [Google Scholar]
- Sugden S., Sanderson D., Ford K., Stein L.Y., St Clair C.C. An altered microbiome in urban coyotes mediates relationships between anthropogenic diet and poor health. Sci. Rep. 2020;10:22207. doi: 10.1038/s41598-020-78891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales W.E. A review of Canadian helminthology: II. Additions to part I, as determined from a study of parasitic helminths collected in Canada. Can. J. Res. 1933;8:478–482. [Google Scholar]
- Traversa D. Are we paying too much attention to cardio-pulmonary nematodes and neglecting old-fashioned worms like Trichuris vulpis? Parasites Vectors. 2011;4:32. doi: 10.1186/1756-3305-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steenkiste N., Locke S.A., Castelin M., Marcogliese D.J., Abbott C.L. New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes) Mol. Ecol. Resour. 2015;15:945–952. doi: 10.1111/1755-0998.12358. [DOI] [PubMed] [Google Scholar]
- Villalobos-Segura M.D.C., Garcia-Prieto L., Rico-Chavez O. Effects of latitude, host body size, and host trophic guild on patterns of diversity of helminths associated with humans, wild and domestic mammals of Mexico. Int. J. Parasitol. Parasites Wildl. 2020;13:221–230. doi: 10.1016/j.ijppaw.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve A., Polley L., Jenkins E., Schurer J., Gilleard J., Kutz S., Conboy G., Benoit D., Seewald W., Gagné F. Parasite prevalence in fecal samples from shelter dogs and cats across the Canadian provinces. Parasites Vectors. 2015;8:281. doi: 10.1186/s13071-015-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeschenbach A., Brabec J., Scholz T., Littlewood D.T.J., Kuchta R. The catholic taste of broad tapeworms - multiple routes to human infection. Int. J. Parasitol. 2017;47:831–843. doi: 10.1016/j.ijpara.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Wapenaar W., Barkema H.W., O'Handley R. Fecal shedding of Toxocara canis and other parasites in foxes and coyotes on Prince Edward Island, Canada. J. Wildl. Dis. 2013;49:394–397. doi: 10.7589/2012-04-113. [DOI] [PubMed] [Google Scholar]
- Watts A.G., Lukasik V.M., Fortin M.J., Alexander S.M. Urbanization, grassland, and diet influence coyote (Canis latrans) parasitism structure. EcoHealth. 2015;12:645–659. doi: 10.1007/s10393-015-1040-5. [DOI] [PubMed] [Google Scholar]
- Wells M.C., Bekoff M. Predation by wild coyotes: behavioral and ecological analyses. J. Mammal. 1982;63:118–127. [Google Scholar]