Abstract
The COVID-19 pandemic led to a sudden global increase in the production, consumption, and mismanagement of personal protective equipment (PPE). As plastic-based PPE such as disposable face masks and gloves have become widely used, human exposure to PPE-derived pollutants may occur through indirect and direct pathways. This review explores the potential health impacts related to plastic-based PPE through these pathways. Face masks release microplastics, which are directly inhaled during use or transported through the environment. The latter can adsorb chemical contaminants and harbor pathogenic microbiota, and once consumed by organisms, they can translocate to multiple organs upon intake, potentially causing detrimental and cytotoxic effects. However, more research is required to have a comprehensive overview of the human health effects.
Keywords: Personal protective equipment, Plastic pollution, Microplastic, Nanoplastic, Mask, Toxicity
Introduction
Plastics are one of the most ubiquitous materials used across the planet. In the last 60 years, global plastic production has increased 20-fold, reaching 368 million tons in 2019 [1]. However, the improper management of plastic waste and its environmental persistence has resulted in the accumulation of plastics in many environments [2,3]. Plastic debris and particularly microplastics (herein referred to as MP; plastics smaller than 5 mm) are considered ubiquitous pollutants and have been reported in water, soil, air, living organisms [4,5], as well as in processed food and drinking water [6]. Therefore, human exposure to MPs is inevitable, and it is imperative to determine their impacts on human health.
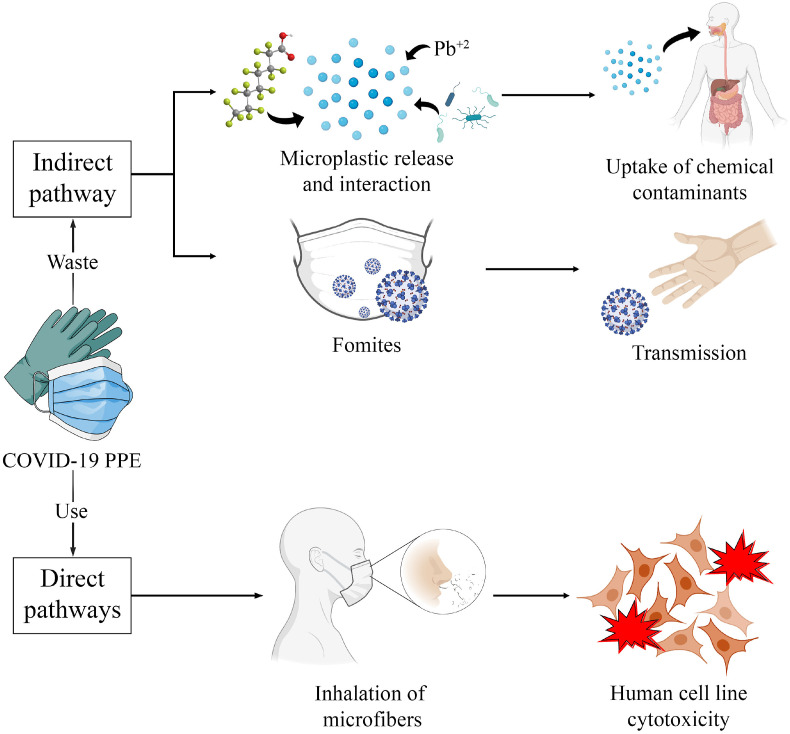
The global immensity and impact of the COVID-19 pandemic were defined by the rapid and effective spread of SARS-CoV-2, the virus responsible for COVID-19. This led to a global pandemic declared by the World Health Organization on March 11, 2020 [7]. The pandemic has resulted in an unprecedented surge in the production and consumption of single-use plastics (SUPs), including personal protective equipment (PPE) [8]. PPE are wearable items that protect the user against infectious diseases, such as SARS-CoV-2, and these items are mostly made from synthetic SUP [9]. The monthly global consumption of face masks and gloves is 129 billion and 65 billion, respectively [10]. This massive consumption of PPE has created an unbearable burden for conventional solid waste management worldwide, leading to the exacerbation of plastic pollution with new types of litter. Exposure to pollutants related to COVID-19 PPE (e.g. MPs, plastic additives, and viruses) may occur through direct and indirect pathways. We define direct pathways as ways in which individuals are immediately exposed to these pollutants during PPE use and management, while indirect pathways result in exposure over extended durations as PPE undergoes different processes. Given the health concerns related to plastic pollution, the unprecedented quantity of PPE being consumed and mismanaged into the environment worldwide, it is necessary to critically analyze the threats of PPE to human health. In this review, we present how PPE pollution is driven by the COVID-19 pandemic and how the direct and indirect exposure pathways of this pollutant can potentially implicate human health (Figure 1).
Figure 1.
Schematic representation of the direct and indirect impacts of COVID-19 PPE. PPE, personal protective equipment.
Indirect pathways to exposure
COVID-19 PPE can leak out of waste management systems and pollute different environments through various pathways. In coastal environments, PPE pollution has been attributed to littering during tourist and recreational activities [11,12], incorrect solid waste disposal (e.g. illegal dumping sites) [13], and lack of proper PPE disposal guidelines and infrastructure (e.g. signposts and waste bins) [[14], [15], [16]]. PPE has also been reported in rivers [17] and highly populated urban centers [8,18]. The current evidence puts into perspective the weaknesses of solid waste management around the world and the lack of environmental awareness among the general public.
Once released into the environment, PPE will undergo continuous weathering and mechanical stress from exposure to environmental factors. Stressors such as UV-lighting and/or mechanical abrasion can result in the generation of MPs [19]. This was initially theorized in the early stages of the global pandemic [20,21] and was later confirmed through laboratory experiments [22,23]. Particularly, artificial aging through UV-light irradiation and contact with quartz sand (mimicking marine conditions) promotes MP release from face masks, which is estimated to produce millions of particles per mask [24,25]. Moreover, leachates from commercially available face masks in the UK revealed the presence of potentially hazardous heavy metals (e.g. cadmium and lead), as well as organic chemicals and additives (e.g. plastic oligomers, surfactants, and dye-like molecules) raising the question of what long-term health risks face masks can pose [26]. Similar concerns have arisen with environmental harm for disposable protective gloves (e.g. nitrile, latex, and foil gloves), as they might be a source of plastic additives, such as plasticizers and emulsifiers, and heavy metals [27].
Human uptake of MPs can occur through ingestion, inhalation, and dermal contact. Inhalation is the primary route of biological entry for humans [28], and it is estimated that a person inhales between 26 and 130 MP per day [29]. Common sources of airborne MPs in both indoor and outdoor settings are synthetic fibers shed from clothing and textiles [30] and abraded plastic materials [10]. It should, however, be noted that MP size, density, and hydrophobicity will influence their deposition and absorption in the respiratory system [31]. Prata et al. [29] reported that the elimination of accumulated MPs in the lungs is difficult because of MP polymeric structures and fibrous morphologies that cause lung inflammation. Furthermore, Gasperi et al. [32] theorized that fibrous MPs can evade the lungs' self-cleaning mechanism leading to cytotoxic (toxic to cells) effects in the respiratory system.
It has been increasingly recognized that MPs are chemical pollutants and vectors of microorganisms that can have adverse effects on humans [28]. MPs are vectors of chemical contaminants as they can adsorb heavy metals, polycyclic aromatic hydrocarbons, and pesticides [33]. Furthermore, the surface of MPs is a suitable substrate for biofilm-forming pathogenic bacteria and viruses [34] and can also act as a platform for the propagation of microorganisms [35]. Because the SARS-CoV-2 virus can remain active on inert surfaces for different residency times [36], the non-aerosolized transmission of the virus among humans, via fomites, is a widespread cause of concern [37]. Fomites can exist as a variety of different materials, such as synthetic-based materials. SARS-CoV-2 has been found to remain active on polypropylene surfaces from 3 days [36] to 7 days, with the latter occurring on a face mask [37]. The ability of SARS-CoV-2 to remain active on plastic surfaces can result in the spread of the virus from items like PPE. Liu et al. [38] found that the highest quantities of airborne SARS-CoV-2 were present within healthcare rooms where medical personnel removed their PPE; a potential explanation was that the virus can form aerosolized fomites from contaminated clothing [38]. These non-respiratory particles are aerosolized from contaminated surfaces and have been shown to carry other viruses that infect biota [39].
Given the widespread prevalence of plastics to make disposable and reusable PPE, it is feasible that contaminated PPE can pose a human health risk as they can act as potential vectors of SARS-CoV-2 [10,40]. Although pre-pandemic specific protocols existed for managing infectious waste deriving from the healthcare system through sterilization, incineration, and safe disposal (e.g. [41]), such regulations were not widespread for municipal solid waste management across the globe, which currently receive most of the PPE waste produced. This situation could potentially lead to some populations coming into direct contact with contaminated debris. In South Africa, Ryan et al. [42] described the informal waste collectors that are actively ‘breaking open bags of refuse to search for recyclable materials to sell’. Such individuals may come into direct contact with used and contaminated PPE, while using without minimal PPE protection wearing PPE themselves. These interactions with PPE may be reflected for informal waste collectors in various countries.
Despite this potential form of transmission, SARS-CoV-2 primarily spreads through respiratory transmission. In 2020, the Health Expert Statement Addressing Safety of Reusables and COVID-19, which was supported by numerous experts in the healthcare industry, determined that surface contact and successful transmission of COVID-19 were not probable for the general population (see: https://www.greenpeace.org/usa/wpcontent/uploads/2020/06/Health-Expert-Statement_Updated.pdf). In fact, the publication encouraged the use of reusable plastics as opposed to SUP. However, a population that interact more frequently with PPE (e.g. healthcare workers and informal waste collectors) face a higher risk of contacting SARS-CoV-2 exposure from surfaces relative to the general public.
Direct pathways to exposure
Although the use of face masks can protect users from airborne respiratory particles, wearing masks presents a risk of MP inhalation during usage because of the detaching of MPs from their surface [43,44]. Han and He [44] reported the presence of microparticles suspected to be MPs on the inner side of new face masks and N95 respirators. This study highlighted the need to regulate the material integrity and fragmentation of face coverings. The authors suggest that the detected microparticles are part of the face mask structure or maybe the result of contamination during the manufacturing process or from plastic packaging. The prolonged use and disinfection process can damage the structure of face masks, exacerbating the detachment of MPs. Li et al. [43] conducted a breathing simulation with face masks being used, and they found that most of inhalable MPs from masks had a granular and fiber-like form (20–100 μm in size). Disinfection processes have been shown to damage the mask structure in various magnitudes; in particular, alcohol treatments have caused the most damage. In both experiments, the detachment of MPs varied between types of masks and quality of the material, N95 masks had the highest performance as characterized by less MP inhalation relative to the other masks. Face masks made from cloth fabrics may pose a higher risk of releasing MPs as some fabrics, such as velvets and fleeces, may shed nylon, polyester, polyethylene, and polypropylene microfibers [29]. Still, there is very limited knowledge about the quantities and characteristics of MPs released from face masks during usage.
Despite the increasing awareness of inhalation and ingestion of MPs from face masks, studies testing the toxicity of MPs inside the human body toward cells and other biological systems remains unresolved [4,45]. MPs have been reported to enter or be deposited in the central airway and distal lung (e.g. alveoli, alveolar ducts, and terminal bronchioles), and once inhaled, they can cause chronic inflammation, DNA damage, granulomas or fibrosis, cellular damage, secretion of cytokines, and oxidative stress [46]. In vivo and in vitro studies have provided evidence for cellular permeability, teratogenicity, and pulmonary toxicity of airborne MPs (Table 1 ). Similarly, Prata et al. [47] reviewed the human effects of exposure to atmospheric or airborne MPs, which could broadly contribute to immune disorders, neurodegeneration, and inflammations. More concerning, Ragusa et al. [48] observed polypropylene MPs in the amniochorial, maternal, and fetal membranes of human placenta samples collected during vaginal birth of healthy women. The preliminary study shed initial light on the movement of plastics through complex process of reproduction, but more robust work is required. Another important issue regarding inhaled or ingested MPs is the complexity of the chemical makeups of plastics. Chemical toxic additives used in the manufacturing processes of plastic, including plasticizers, phthalates, UV stabilizers, and bisphenol A, have been shown to leach and cause adverse health effects in humans through estrogenic activity [49]. Studies that demonstrate potential human health implications associated with exposure to MPs are provided in Table 1.
Table 1.
In vivo and in vitro studies that demonstrate potential human health implications from MP exposure.
| Type of study | MP size | Polymer type | Exposure methods | Dosage and time | Findings | Ref. |
|---|---|---|---|---|---|---|
| MPs translocation from the lung to the placenta | 20 nm | Polypropylene | Intratracheal instillation during gestation | 2.64 × 1014 MPs, 24 h | The exposure resulted in the translocation of MPs to placental and fetal tissues and rendered the fetoplacental unit vulnerable to adverse effects | [57] |
| MPs in human-derived cells | 25–200 μm | Polypropylene | Addition to cultures (media) of somatic cells, blood cells, and murine immune cells | 0.1–4.5 mg per well, 24 h | MPs induced and triggered pro-inflammatory cytokines that caused a local immune response | [51] |
| MPs and various phthalate esters (PAEs) on human lung epithelial cells | 100 nm | Polystyrene | Addition to cells | MPs at 10, 20, 100, 200, 500 or 1000 μg mL−1, 24 h | Cells exhibited changes in viability, oxidative stress, and inflammatory reaction. | [52] |
| MPs on human cells | 1 and 10 μm | Polystyrene | Addition to cells | 0.05–100 μg mL−1, 24, 48, 72, and 96 h |
Exposure significantly retards cell proliferation and triggered morphological changes | [53] |
| MPs in human-derived cells | 5−25 μm, 25–75 μm, and 75–200 μm | Polystyrene | Dispersed in cell culture medium | 1000, 100, and 10 μg mL−1, 1 day and 4 days | MPs increased acute inflammation, cell death by chemical toxicity, and induced cell membrane damage by physical toxicity | [54] |
| MPs on human intestinal epithelial cells | 0.05–0.1 μm and 0.04–0.09 μm | Polystyrene | Exposure in cell culture medium | 1–100 μg mL−1, 24 or 48 h | Cells uptake and internalized MPs, however, no toxic effects were observed. | [55] |
| MPs on human lung epithelial cell | 25 nm and 70 nm | Polystyrene | Dispersion in cell medium | 2.5–300 μg mL−1, 24 h | MPs significantly affected cell viability, activated inflammatory gene transcription, and changed the expression of proteins. | [56] |
Conclusion
The COVID-19 pandemic has led to the increased consumption and mismanagement of SUP. Although the use of PPE has become a global necessity to prevent the transmission of the virus, humans are increasingly exposed via inhalation and ingestion to MPs and their associated chemical contaminants. Under various use and exposure conditions, including the potential of MPs surface to interact with human tissues, MPs have been reported to be able to cause a range of biological responses. Despite the potential risks, knowledge on the concentrations at which MPs are being inhaled or ingested, as well as the effects of their exposures on human health is limited. As plastic production, consumption and exposure to humans are only increasing over time, more studies focusing on the impacts of MPs on human health are required, so that we can better understand exposure pathways and toxicity of MPs to humans on all levels from cellular to the organ.
Credit author statement
Gabriel Enrique De-la-Torre: Conceptualization, Validation, Investigation, Writing – Original Draft, Writing - Review & Editing, Project administration. Carlos Ivan Pizarro-Ortega: Conceptualization, Validation, Investigation, Writing – Original Draft, Writing - Review & Editing. Diana Carolina Dioses-Salinas: Conceptualization, Validation, Investigation, Writing – Original Draft, Writing - Review & Editing. Justine Ammendolia: Conceptualization, Validation, Investigation, Writing – Original Draft, Writing - Review & Editing. Elvis D. Okoffo: Conceptualization, Validation, Investigation, Writing – Original Draft, Writing - Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Gabriel Enrique De-la-Torre would like to thank the Vice-Rectorate for Research of Universidad San Ignacio de Loyola for financial support. The authors also acknowledge the funding provided to Elvis D. Okoffo by the University of Queensland and the Queensland Alliance for Environmental Health Sciences (QAEHS).
This review comes from a themed issue on Plastic Pollution
Edited by Silvia Franzellitti
References
- 1.PlasticsEurope . 2020. Plastics - the facts 2020. [Google Scholar]
- 2.Wang C., Zhao J., Xing B. Environmental source, fate, and toxicity of microplastics. J Hazard Mater. 2021;407:124357. doi: 10.1016/j.jhazmat.2020.124357. [DOI] [PubMed] [Google Scholar]
- 3.De-la-Torre G.E., Dioses-Salinas D.C., Pizarro-Ortega C.I., Santillán L. New plastic formations in the Anthropocene. Sci Total Environ. 2021;754:142216. doi: 10.1016/j.scitotenv.2020.142216. [DOI] [PubMed] [Google Scholar]
- 4.Amato-Lourenço L.F., dos Santos Galvão L., de Weger L.A., Hiemstra P.S., Vijver M.G., Mauad T. An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman S.M.A., Robin G.S., Momotaj M., Uddin J., Siddique M.A.M. Occurrence and spatial distribution of microplastics in beach sediments of Cox's Bazar, Bangladesh. Mar Pollut Bull. 2020;160 doi: 10.1016/j.marpolbul.2020.111587. [DOI] [PubMed] [Google Scholar]
- 6.Barboza L.G.A., Dick Vethaak A., Lavorante B.R.B.O., Lundebye A.-K., Guilhermino L. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar Pollut Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 7.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammendolia J., Saturno J., Brooks A.L., Jacobs S., Jambeck J.R. An emerging source of plastic pollution: environmental presence of plastic personal protective equipment (PPE) debris related to COVID-19 in a metropolitan city. Environ Pollut. 2021;269:116160. doi: 10.1016/j.envpol.2020.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel A., D'Alessandro M.M., Ireland K.J., Burel W.G., Wencil E.B., Rasmussen S.A. Personal protective equipment supply chain: lessons learned from recent public health emergency responses. Heal Secur. 2017;15:244–252. doi: 10.1089/hs.2016.0129. [DOI] [PubMed] [Google Scholar]
- 10.Prata J.C., Silva A.L.P., Walker T.R., Duarte A.C., Rocha-Santos T. COVID-19 pandemic repercussions on the use and management of plastics. Environ Sci Technol. 2020;54:7760–7765. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- 11.Ardusso M., Forero-López A.D., Buzzi N.S., Spetter C.V., Fernández-Severini M.D. COVID-19 pandemic repercussions on plastic and antiviral polymeric textile causing pollution on beaches and coasts of South America. Sci Total Environ. 2021;763:144365. doi: 10.1016/j.scitotenv.2020.144365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De-la-Torre G.E., Rakib Md, Jahan Refat, Pizarro-Ortega C.I., Dioses-Salinas D.C. Occurrence of personal protective equipment (PPE) associated with the COVID-19 pandemic along the coast of Lima, Peru. Sci Total Environ. 2021;774:145774. doi: 10.1016/j.scitotenv.2021.145774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rakib M.R.J., De-la-Torre G.E., Pizarro-Ortega C.I., Dioses-Salinas D.C., Al-Nahian S. Personal protective equipment (PPE) pollution driven by the COVID-19 pandemic in Cox's Bazar, the longest natural beach in the world. Mar Pollut Bull. 2021;169:112497. doi: 10.1016/j.marpolbul.2021.112497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhbarizadeh R., Dobaradaran S., Nabipour I., Tangestani M., Abedi D., Javanfekr F., Jeddi F., Zendehboodi A. Abandoned Covid-19 personal protective equipment along the Bushehr shores, the Persian Gulf: an emerging source of secondary microplastics in coastlines. Mar Pollut Bull. 2021;168:112386. doi: 10.1016/j.marpolbul.2021.112386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okuku E., Kiteresi L., Owato G., Otieno K., Mwalugha C., Mbuche M., Gwada B., Nelson A., Chepkemboi P., Achieng Q., et al. The impacts of COVID-19 pandemic on marine litter pollution along the Kenyan Coast: a synthesis after 100 days following the first reported case in Kenya. Mar Pollut Bull. 2020 doi: 10.1016/j.marpolbul.2020.111840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiel M., de Veer D., Espinoza-Fuenzalida N.L., Espinoza C., Gallardo C., Hinojosa I.A., Kiessling T., Rojas J., Sanchez A., Sotomayor F., et al. COVID lessons from the global south – face masks invading tourist beaches and recommendations for the outdoor seasons. Sci Total Environ. 2021 doi: 10.1016/j.scitotenv.2021.147486. [DOI] [Google Scholar]
- 17.Cordova M.R., Nurhati I.S., Riani E., Nurhasanah, Iswari M.Y. Unprecedented plastic-made personal protective equipment (PPE) debris in river outlets into Jakarta Bay during COVID-19 pandemic. Chemosphere. 2021;268:129360. doi: 10.1016/j.chemosphere.2020.129360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akarsu C., Madenli Ö., Deveci E.Ü. Characterization of littered face masks in the southeastern part of Turkey. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-14099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De-la-Torre G.E., Aragaw T.A. What we need to know about PPE associated with the COVID-19 pandemic in the marine environment. Mar Pollut Bull. 2021;163:111879. doi: 10.1016/j.marpolbul.2020.111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aragaw T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar Pollut Bull. 2020;159:111517. doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci Total Environ. 2020;737:140279. doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22∗.Morgana S., Casentini B., Amalfitano S. Uncovering the release of micro/nanoplastics from disposable face masks at times of COVID-19. J Hazard Mater. 2021 doi: 10.1016/j.jhazmat.2021.126507. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors evaluated the release of MPs from polypropylene fabric used in single-use face masks under shear force (resembling weathering conditions). Their results demonstrated that longer exposure to mechanical deterioration significantly increases the number of MPs (>100 μm released). Moreover, MPs in the range of 0.1–100 μm were quantified by flow cytometry, resulting in a mean of 2.1 ± 1.4 × 1011 MPs per m2 of treated fabric (various shear stress exposure times).
- 23.Shen M., Zeng Z., Song B., Yi H., Hu T., Zhang Y., Zeng G., Xiao R. Neglected microplastics pollution in global COVID-19: disposable surgical masks. Sci Total Environ. 2021;790:148130. doi: 10.1016/j.scitotenv.2021.148130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saliu F., Veronelli M., Raguso C., Barana D., Galli P., Lasagni M. The release process of microfibers: from surgical face masks into the marine environment. Environ Adv. 2021;4:100042. [Google Scholar]
- 25∗.Wang Z., An C., Chen X., Lee K., Zhang B., Feng Q. Disposable masks release microplastics to the aqueous environment with exacerbation by natural weathering. J Hazard Mater. 2021;417:126036. doi: 10.1016/j.jhazmat.2021.126036. [DOI] [PMC free article] [PubMed] [Google Scholar]; The present study evaluated the release of MPs from face masks (polypropylene layer) after undergoing UV light exposure and mechanical stress in the presence of natural sand, which mimicked natural weathering conditions. It was estimated that a single face mask releases 1.5 million MPs to the aquatic environment, exacerbated by natural weathering conditions.
- 26∗.Sullivan G.L., Delgado-Gallardo J., Watson T.M., Sarp S. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks - linked to the COVID-19 pandemic. Water Res. 2021;196:117033. doi: 10.1016/j.watres.2021.117033. [DOI] [PMC free article] [PubMed] [Google Scholar]; The results from the present study evidenced for the first time the presence of heavy metals of concern (e.g., Cd, Cu, and Pb), polymer oligomer, and plastic additives in leachates of commercially available face masks. Additionally, small MPs (0.1–1 μm) were detected scanning electron microscopy. Larger MPs were mostly fibrous-like particles.
- 27.Jędruchniewicz K., Ok Y.S., Oleszczuk P. COVID-19 discarded disposable gloves as a source and a vector of pollutants in the environment. J Hazard Mater. 2021;417:125938. doi: 10.1016/j.jhazmat.2021.125938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman A., Sarkar A., Yadav O.P., Achari G., Slobodnik J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: a scoping review. Sci Total Environ. 2021;757:143872. doi: 10.1016/j.scitotenv.2020.143872. [DOI] [PubMed] [Google Scholar]
- 29.Prata J.C. Airborne microplastics: consequences to human health? Environ Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 30.Dris R., Gasperi J., Saad M., Mirande C., Tassin B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar Pollut Bull. 2016;104:290–293. doi: 10.1016/j.marpolbul.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Rist S., Carney Almroth B., Hartmann N.B., Karlsson T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci Total Environ. 2018;626:720–726. doi: 10.1016/j.scitotenv.2018.01.092. [DOI] [PubMed] [Google Scholar]
- 32.Gasperi J., Wright S.L., Dris R., Collard F., Mandin C., Guerrouache M., Langlois V., Kelly F.J., Tassin B. Microplastics in air: are we breathing it in? Curr Opin Environ Sci Heal. 2018;1:1–5. [Google Scholar]
- 33.Torres F.G., Dioses-Salinas D.C., Pizarro-Ortega C.I., De-la-Torre G.E. Sorption of chemical contaminants on degradable and non-degradable microplastics: recent progress and research trends. Sci Total Environ. 2021;757:143875. doi: 10.1016/j.scitotenv.2020.143875. [DOI] [PubMed] [Google Scholar]
- 34.Rasool F.N., Saavedra M.A., Pamba S., Perold V., Mmochi A.J., Maalim M., Simonsen L., Buur L., Pedersen R.H., Syberg K., et al. Isolation and characterization of human pathogenic multidrug resistant bacteria associated with plastic litter collected in Zanzibar. J Hazard Mater. 2021;405:124591. doi: 10.1016/j.jhazmat.2020.124591. [DOI] [PubMed] [Google Scholar]
- 35.Meng J., Zhang Q., Zheng Y., He G., Shi H. Plastic waste as the potential carriers of pathogens. Curr Opin Food Sci. 2021;41:224–230. [Google Scholar]
- 36.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corpet D.E. Why does SARS-CoV-2 survive longer on plastic than on paper? Med Hypotheses. 2021;146:110429. doi: 10.1016/j.mehy.2020.110429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 39.Asadi S., Gaaloul ben Hnia N., Barre R.S., Wexler A.S., Ristenpart W.D., Bouvier N.M. Influenza A virus is transmissible via aerosolized fomites. Nat Commun. 2020;11:4062. doi: 10.1038/s41467-020-17888-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patrício Silva A.L., Prata J.C., Walker T.R., Campos D., Duarte A.C., Soares A.M.V.M., Barcelò D., Rocha-Santos T. Rethinking and optimising plastic waste management under COVID-19 pandemic: policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci Total Environ. 2020;742:140565. doi: 10.1016/j.scitotenv.2020.140565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.EuropeanCommission . 2020. Waste management in the context of the coronavirus crisis. [Google Scholar]
- 42.Ryan P.G., Maclean K., Weideman E.A. The impact of the COVID-19 lockdown on urban street litter in South Africa. Environ Proc. 2020;7:1303–1312. [Google Scholar]
- 43∗.Li L., Zhao X., Li Z., Song K. COVID-19: performance study of microplastic inhalation risk posed by wearing masks. J Hazard Mater. 2021;411:124955. doi: 10.1016/j.jhazmat.2020.124955. [DOI] [PMC free article] [PubMed] [Google Scholar]; In the present study, MP inhalation from face mask use was investigated using breathing simulation techniques for the first time. Seven different types of face masks were evaluated at 10 (ranging from 2 to 720 h) breathing test durations. Surgical and activated carbon face masks released the highest number of fibrous-like MPs. MP release increased along with test duration. Moreover, disinfection processes (e.g., UV irradiation, alcohol spraying, air blowing, etc.) exacerbated the potential MP inhalation.
- 44.Han J., He S. Need for assessing the inhalation of micro(nano)plastic debris shed from masks, respirators, and home-made face coverings during the COVID-19 pandemic. Environ Pollut. 2021;268:115728. doi: 10.1016/j.envpol.2020.115728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poma A., Vecchiotti G., Colafarina S., Zarivi O., Aloisi M., Arrizza L., Chichiriccò G., Di Carlo P. In vitro genotoxicity of polystyrene nanoparticles on the human fibroblast hs27 cell line. Nanomaterials. 2019;9:1299. doi: 10.3390/nano9091299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vethaak A.D., Legler J. Microplastics and human health. Science. 2021;371:672–674. doi: 10.1126/science.abe5041. [DOI] [PubMed] [Google Scholar]
- 47.Prata J.C., da Costa J.P., Lopes I., Duarte A.C., Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci Total Environ. 2020:702. doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- 48.Ragusa A., Svelato A., Santacroce C., Catalano P., Notarstefano V., Carnevali O., Papa F., Rongioletti M.C.A., Baiocco F., Draghi S., et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- 49.Vandenberg L.N., Hauser R., Marcus M., Olea N., Welshons W.V. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Hwang J., Choi D., Han S., Choi J., Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci Total Environ. 2019;684:657–669. doi: 10.1016/j.scitotenv.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 52∗∗.Shi Q., Tang J., Wang L., Liu R., Giesy J.P. Combined cytotoxicity of polystyrene nanoplastics and phthalate esters on human lung epithelial A549 cells and its mechanism. Ecotoxicol Environ Saf. 2021;213:112041. doi: 10.1016/j.ecoenv.2021.112041. [DOI] [PubMed] [Google Scholar]; The authors evaluated the effects of polystyrene MPs (100 nm) in combination with two phthalic acid esters (used as additives in the plastic industry) on human lung epithelial A549 cells. Their results confirm that co-exposure of these contaminants exhibited a reduction in cell viability, triggered oxidative stress and inflammatory reactions. This is the first study to investigate the cytotoxicity of MPs in combination with phthalatic acid esters.
- 53∗∗.Goodman K.E., Hare J.T., Khamis Z.I., Hua T., Sang Q.X.A. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem Res Toxicol. 2021;34:1069–1081. doi: 10.1021/acs.chemrestox.0c00486. [DOI] [PubMed] [Google Scholar]; This study evaluated the cytotoxic effects of polystyrene MPs (1–10 μm) on human lung epithelial A549 cells. Cell viability was not severely affected by MP exposure. However, cell proliferation was significantly reduced. Moreover, phase contrast imaging revealed morphological changes in cells exposed to MPs, as well as the uptake of 1 μm MP.
- 54.Choi D., Bang J., Kim T., Oh Y., Hwang Y., Hong J. In vitro chemical and physical toxicities of polystyrene microfragments in human-derived cells. J Hazard Mater. 2020;400:123308. doi: 10.1016/j.jhazmat.2020.123308. [DOI] [PubMed] [Google Scholar]
- 55.Cortés C., Domenech J., Salazar M., Pastor S., Marcos R., Hernández A. Nanoplastics as a potential environmental health factor: effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells. Environ Sci Nano. 2020;7:272–285. [Google Scholar]
- 56∗∗.Xu M., Halimu G., Zhang Q., Song Y., Fu X., Li Y., Li Y., Zhang H. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci Total Environ. 2019;694 doi: 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]; In the present study, the cytotoxic effect and interactions of polystyrene MPs (25–70 nm) on human lung epithelial A549 cells were evaluated. Interestingly, exposure to the smallest MP fractions resulted in a rapid internalization into the cell cytoplasm. Furthermore, MPs inhibited cell viability, induced cell cycle S phrase arrest, and activated inflammatory gene transcription. The cytotoxic effects of MP were conditioned by exposure time, size, and concentration.
- 57.Fournier Sara, D’Errico Jeanine, Adler Derek, Kollontzi Stamatina, Goedken Michael, Fabris Laura, Yurkow Edward, Stapleton Phoebe. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part Fibre Toxicol. 2020;17:55. doi: 10.1186/s12989-020-00385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]