Abstract
Both empirical and theoretical studies show that an individual's spatial position within a group can impact the risk of being targeted by predators. Spatial positions can be quantified in numerous ways, but there are no direct comparisons of different spatial measures in predicting the risk of being targeted by real predators. Here, we assess these spatial measures in groups of stationary and moving virtual prey being attacked by three-spined sticklebacks (Gasterosteus aculeatus). In stationary groups, the limited domain of danger best predicted the likelihood of attack. In moving groups, the number of near neighbours was the best predictor but only over a limited range of distances within which other prey were counted. Otherwise, measures of proximity to the group's edge outperformed measures of local crowding in moving groups. There was no evidence that predators preferentially attacked the front or back of the moving groups. Domains of danger without any limit, as originally used in the selfish herd model, were also a poor predictor of risk. These findings reveal that the collective properties of prey can influence how spatial position affects predation risk, via effects on predators' targeting. Selection may therefore act differently on prey positioning behaviour depending on group movement.
Keywords: group living, virtual prey, domain of danger, collective behaviour, selfish herd, Gasterosteus aculeatus
1. Introduction
Animals often form groups to lessen their risk of predation [1,2]. The risk of predation, however, is not distributed evenly across the different spatial positions an individual might occupy within the group. Risk can be higher for individuals on the group's edge rather than in the centre (also known as marginal predation [3–5]), for individuals positioned further from their near neighbours [6–8], or for those at the front or back of moving groups [9–11]. The majority of evidence for the different risk afforded by different spatial positions comes from observational studies of real predators targeting real prey, and from how prey respond by changing their position in groups in response to predatory attacks [7,8,12–14].
Measures to define prey position within groups fall into two broad categories: those describing centre-edge positioning and those describing the degree of local crowding. Previous studies have tended to focus on one or the other, even though these measures tend to be correlated as individuals on the edge of groups have reduced local crowding. We are therefore limited in our understanding of which measures are particularly important in determining the predation risk faced by prey in different spatial positions. Such a comparison is challenging because it is difficult to accurately measure prey spatial positions during a sufficient number of (often unpredictable) attacks. Additionally, the spatial position within groups of real prey is known to be determined by a number of additional confounding factors, such as parasite load, boldness and the ability to acquire food [15–20]. While models of virtual predators attacking simulated prey allow for unconfounded investigations of the effect of spatial position on risk (e.g. [21]), these models have to make assumptions about how predators behave [6]. Finally, groups vary in their collective properties, such as whether groups are stationary or moving, and these collective properties can impact predation risk and foraging success [22–24]. However, whether such properties affect which measures of spatial position best predict predation risk is unknown.
To address these challenges, we presented simulations of virtual prey to three-spined sticklebacks (Gasterosteus aculeatus). Prey groups of 20 individuals were either stationary (with only small, uncoordinated ‘jitter’ motion) or had relatively slow directional movement (in addition to the jitter motion). The spatial positions of targeted and non-targeted prey were defined according to different measures previously used in the literature, and the success of each measure in predicting the likelihood of predation (i.e. attack) was assessed with an information criterion model comparison approach. We predicted that centre–edge measures of spatial position would better predict predation risk in moving groups due to the greater encounter rate with moving groups, especially at the front of the prey group [10]. By presenting real predators with virtual prey, the limitations of studies using predators attacking live prey, and simulated predators targeting simulated prey, can be overcome [3,9,25].
2. Material and methods
(a) . Subjects and housing
Three-spined sticklebacks (G. aculeatus) were caught from the River Cary in Somerton, UK (51.069990 latitude, −2.758014 longitude) in November 2017 and were transported by car to the University of Bristol. They were housed in 40 × 70 × 34 cm (width × length × height) glass tanks on a flow-through recirculation system, with plastic shelters and plants for environmental enrichment, and kept at 14°C under a 11 : 13 light : dark cycle. Approximately 40 individuals were housed in each tank. The experiment was run from October to November 2018. Fish were fed with defrosted bloodworms before and throughout the experiment. During the experiment, they were fed after testing each day.
(b) . Experimental set-up
Two-dimensional virtual prey were presented to the fish on the front wall of a testing arena, so that the fish's approach was typically from the third dimension (i.e. perpendicular to the two-dimensional spread of the group). This minimized the bias of the fish to attack individuals on the edge of the group because they are the first to be encountered [3]; Romenskyy et al. [8] showed that for three-dimensional prey groups, information about the spatial position taken from only a two-dimensional plane can predict the likelihood of predation.
The testing arena (55 × 40 × 35 cm, L × W x H) was filled to a depth of 25 cm with water from the recirculating system that the fish were housed in. The walls of the back, side and bottom of the arena were covered with opaque white plastic. A screen made from a white translucent film (Rosco gel no. 252) was placed inside the front wall of the tank. When the simulated prey were projected onto this screen, they were visible to the predatory fish, while the predatory fish was also visible through the screen (figure 1). A projector (BenQ MW523) was positioned 82 cm in front and below the bottom level of the tank to minimize the bulb's glare on the glass. Two strip lights were placed outside the tank behind the back wall to illuminate the arena from the rear. This ensured that the projected prey were not visible to the fish on the rear tank wall, while prey were still visible to the fish on the front screen. A camcorder (Panasonic VX870) was positioned directly in front of the tank, behind and above the projector. Videos were recorded at a resolution of 3840 × 2160 pixels at 25 frames per second. The entire experimental set-up was enclosed by black curtains to visually isolate the experiment from the testing room.
Figure 1.
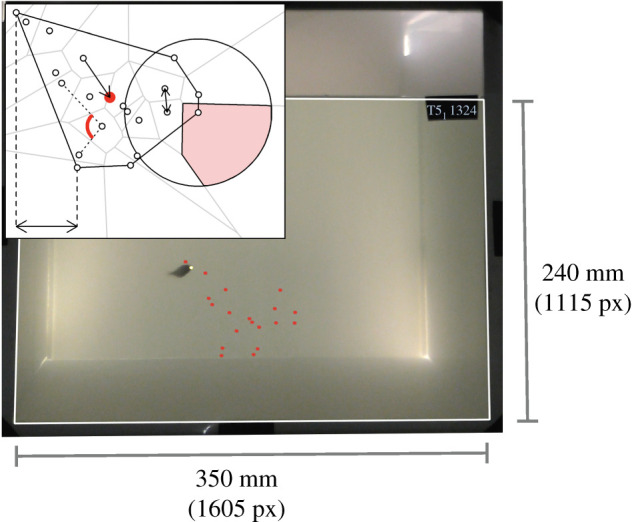
Experimental set-up. Frame from an experimental trial showing the moment a stickleback attacks one of the virtual prey. Virtual prey (red and yellow dots) have been overlaid on this image, which are not visible in the camera footage (see electronic supplementary material, movie S3). The yellow prey indicates which prey was attacked, although all prey were projected as red dots in the trials. The white outline represents the projection area where prey could appear. The black oblong in the top right-hand corner of the arena shows the trial number and the time stamp of the simulation. These time stamps were used to determine the locations of the prey in the calibration videos, where prey were visible. The inset illustrates the measures of spatial position calculated from the prey positions. The grey lines show the Voronoi polygon for each prey. The large circle shows a radius around a prey within which its number of near neighbours is counted, and the shaded area of this circle shows that prey's limited domain of danger. The solid line around the group shows the convex hull. The double-headed arrow between two prey shows the nearest neighbour distance, and the single-headed arrow shows a distance to the group centroid (the red filled circle). The dashed vertical lines and double-headed arrow show a distance to the front of the group (the prey furthest to the left). The dotted lines and red arc show an angle of vulnerability. (Online version in colour.)
(c) . Virtual prey simulations
We generated two types of prey simulations in MatLab 2018b [26] (electronic supplementary material, figure S1): stationary groups and moving groups (electronic supplementary material, movies S1 and S2). Once projected onto the screen, the virtual prey appeared as red dots approximately 3 mm in diameter, a typical size of stickleback invertebrate prey. The prey could appear in an area of 350 × 240 mm and in the moving simulations moved across the screen at 11 mm s−1. The size and speed of prey were set to elicit predatory responses from the fish (e.g. as in [9]). We gave the prey of both types of simulation a small jitter motion, to make them appear more lifelike. See electronic supplementary material for simulation details.
(d) . Prey presentation and identification
Each focal fish was presented with a unique prey simulation playing on a loop. Each loop lasted 40 s, with the first 10 s containing no prey, followed by 20 s of the prey being presented (the prey loomed in at the start over 1.67 s, and then loomed out over 1.67 s at the end of these 20 s) and followed by 10 s without any prey, resulting in 20 s between each prey presentation. Each focal fish was exposed to the loop 15 times (approx. 10 min trials), although we only used the first attack from each trial. A time stamp and playback identification number were shown in the top right corner of the simulations, but this was not visible to the fish as it was masked off from the fish's field of view by black tape (figure 1). To infer which prey had been targeted in the trials, before each trial, we projected the identical simulation the fish was to receive but with prey projected as white dots, and with the strip lights turned off. These ‘calibration’ videos allowed us to subsequently identify the locations of prey, so we could infer which prey had been targeted by matching the time stamps between the calibration video and the prey videos at the time of attack (electronic supplementary material, movie S3).
(e) . Experimental procedure
Each subject participated in two trials: once in the stationary prey condition and once in the moving prey condition. Fish were randomly assigned to one of two testing groups. One of the testing groups received the stationary prey condition first, and the other group received the moving prey condition first. The two trials for the same individual fish were separated by at least 24 h. Within the moving prey condition, approximately half of the subjects were randomly assigned to receive the group moving from right to left (from the subject's perspective) across the front of the tank, and the other half received prey moving left to right. Trials were generally alternated between the stationary and moving playbacks. Focal fish (n = 126) were given 10 min to acclimatize in the testing tank before any prey were projected.
(f) . Analysis and statistics
Only the first attack of each trial was used in the analysis [9]. An attack was defined when the stickleback orientated towards the screen and pecked (often more than once) at the screen. Where two prey were close to one another and we could not distinguish which of two (and in one trial, three) prey was the target, one prey was selected at random; this occurred in 8 of the 74 trials with an attack.
At the frame of the attack (i.e. when the fish made contact with the screen with its mouth open), we identified the time stamp of the simulation. We then manually tracked the position of all the prey in calibration videos at this time stamp using a bespoke script in MatLab [26]. This gave us the x and y coordinates (in pixels) of all the prey in the group, which were used to calculate the measures of spatial position (figure 1, table 1). All were calculated using R v. 3.6.0 [28]; the R code for these calculations and the statistical analysis are available in the electronic supplementary material. The distribution of, and correlations between, these measures are shown in electronic supplementary material, figure S2 (for stationary groups) and electronic supplementary material, figure S3 (for moving groups).
Table 1.
Measures of spatial position used in our analysis. The distance from front measure was only applied to prey in moving groups.
| measures of local crowding | |
|---|---|
| Voronoi polygon area (i.e. domain of danger) [6] | Area around an individual prey that is closest to that individual and not another individual. The Deldir function in the deldir R package was used. The four corners of the projection area (figure 1) were used as the boundaries. |
| limited domain of danger [27] | As above, although the domains of danger are limited to a maximum distance from each individual prey. |
| nearest neighbour distance | The distance between the focal prey and the closest other prey in the group. |
| number of near neighbours | The number of other prey within a predefined distance. This distance was the radius of a circle centred around each prey. |
| measures of prey position defined by reference to the group centre or periphery | |
| distance to the group centroid | Distance from the mean x and y coordinate of all prey in the group. |
| on the convex hull? | The convex hull is the polygon with minimum area that encloses all prey positions (thus also known as the minimum convex polygon). Calculated using the chull function in R. Each prey was classified as being either on this convex hull perimeter or not. |
| angle of vulnerability [21] | Determined by calculating the angles from the prey as the vertex to all possible pairs of other prey (creating a triad); the largest of these angles where no other prey were located within it was the angle of vulnerability. |
| distance from front | The distance of each prey from the front of the group, where the leading individual is given a distance of zero. |
At the frame of each attack, we also classified whether one-third of the body of the fish (as viewed by the camera) was inside or outside of the perimeter of the prey group, defined by the convex hull (figure 1). This was used as a proxy for the direction that the predator approached the group, where being inside the perimeter suggests an approach perpendicular to the group's two-dimensional plane. The classifications were made blinded to whether the prey were moving or not. We compared whether the prey's movement (i.e. stationary or moving) affected the probability that the fish's body was inside or outside of the group's perimeter using a chi-square test of independence (i.e. whether the approach direction changed depending whether the prey group was moving).
To determine how well each measure of the spatial position predicted the risk of an individual prey being targeted, we used binomial generalized linear models (GLMs) with the default logit link function. The response variable was whether an individual was targeted (1) or not (0), and the explanatory variable was one of the measures of spatial position. A model for each measure of spatial position was constructed, separately for the stationary and moving groups; the data from the two conditions were analysed separately to avoid pseudoreplication, given that we could not keep track of fish identities across the two conditions. The likelihood of each model given the data was compared using the Akaike information criterion corrected for small samples sizes (AICc), as in [9]. A null model without an explanatory variable was also included to test whether the predictive power of models accounting for spatial position exceeded that of a null model after accounting for the extra parameter in these models.
The limited domain of danger (LDOD) and the number of near neighbours both require a maximum radius around the prey to be defined. For the LDOD, this is the distance beyond which a predator will not attack the prey, even if that prey is the closest [27], and for the number of near neighbours, the distance within which near neighbours are counted [21]. We thus included in the AICc comparisons models for the LDOD and number of near neighbours calculated using varying radius sizes, ranging from 10 to 500 pixels in 10-pixel increments. This parameter scan avoided any a priori or post hoc choice of the radius size and allowed us to examine whether the radius size influenced the ability of the LDOD and the number of near neighbours to predict predation risk.
3. Results
The test fish attacked the virtual prey in 35 trials with stationary prey (out of 123 trials) and in 39 trials with moving prey (out of 126 trials). When attacking stationary groups, a substantial proportion of the fish's body was within the group's perimeter in 16 trials and 12 trials when attacking moving prey. Although there was a greater probability of approaching within the group perimeter when attacking stationary prey (0.46 versus 0.31), this difference was not statistically significant (chi-squared test of independence: x2 = 1.17, d.f = 1, p = 0.28).
In stationary groups, the targeted prey was best predicted by the LDOD over a wide range of radius sizes (approx. 50 to 500 pixels); there was only a small range of radius sizes (approx. 50 to 100 pixels) where the number of near neighbours model outperformed it (specifically when LDOD radius sizes were smaller than 50 or greater than 300 pixels) (figure 2a). Greater local crowding was associated with a reduced predation risk (figure 3a). In contrast with the importance of the LDOD in predicting risk, the Voronoi area (i.e. the domain of danger bounded by only the projection area) was a relatively poor predictor of being targeted (figure 2a; electronic supplementary material, table S1). Measures of proximity to the group's edge as predictors of risk were less well supported but were generally greater than 2 AIC units less than the null model [29], suggesting that a prey's angle of vulnerability, whether it is or is not on the convex hull edge, and the distance from the prey to the centroid, still have explanatory power in predicting predation risk (figure 3a; electronic supplementary material, table S1).
Figure 2.
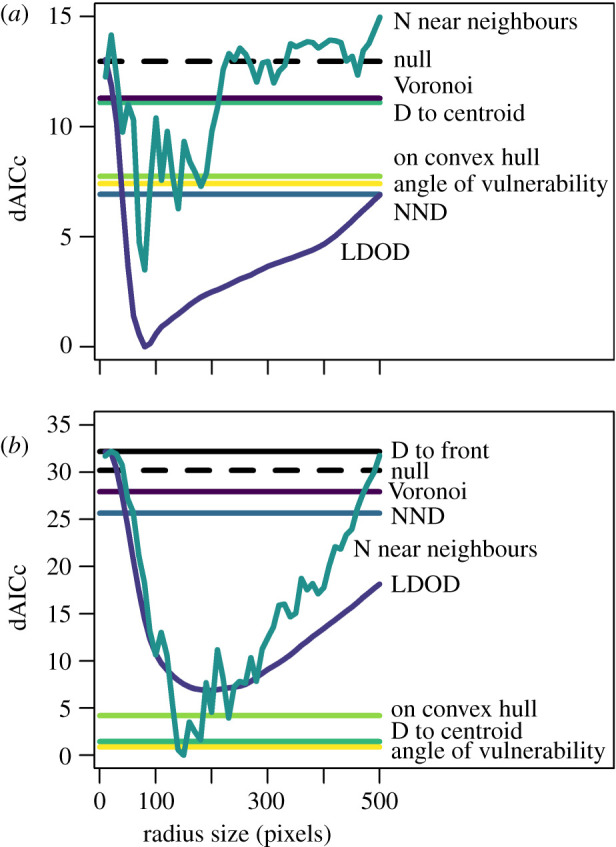
Model comparison results for stationary (a) and moving (b) groups based on the difference in the Akaike information criterion corrected for small sample sizes (dAICc) between the most likely model given the data (0 on the y-axis) and each other model. In the model names, D represents distance, N represents number, LDOD represents limited domain of danger and NND represents nearest neighbour distance. As the dAICc for the limited domain of danger and number of near neighbours models are dependent on the radius size used to calculate these variables, dAICc values are plotted against radius size. For all other measures of spatial position, there is only one model (and hence dAICc value). Line colours for the different models correspond between the panels and figure 3. (Online version in colour.)
Figure 3.
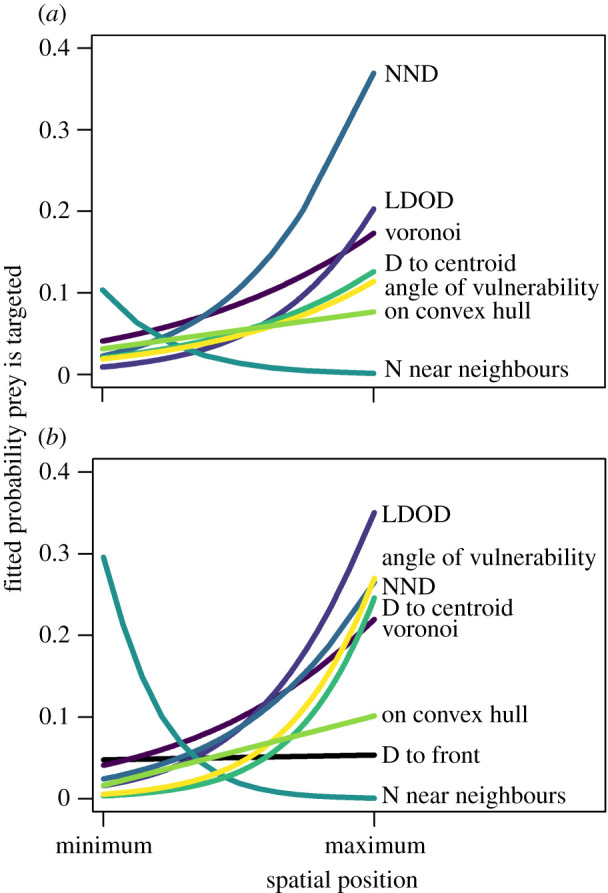
The fitted relationships between the probability that an individual prey in a stationary group (a) and moving group (b) is targeted and their spatial position, calculated using different measures, based on binomial GLMs. The radius size used for the LDOD and N near neighbours measures is the optimal determined by a parameter scan (figure 2): 80 pixels for both measures in stationary groups, and 200 and 150 pixels for LDOD and N near neighbours, respectively, in moving groups. As the scale of each measure of spatial position varies (shown in electronic supplementary material, figures S2 and S3), the x-axis ranges from the minimum to the maximum value of that measure observed in the data. The labels for the different measures of spatial position are arranged from top to bottom in order of the fitted probability of risk at the maximum value of the spatial measures. Colours of the lines of best fit correspond between the panels and figure 2. (Online version in colour.)
For moving groups, the measures of spatial position that best predicted which prey were targeted were not consistent with the results from stationary groups. Although the number of near neighbours was the best predictor of risk when the radius size was optimized at 150 pixels, for much of the range of radius sizes examined, the angle of vulnerability and the distance to the group centroid (both measures of proximity to the group edge) had lower AICc values, and thus were better predictors of risk (figure 2b; electronic supplementary material, table S1). Whether prey were on the convex hull edge was less predictive of risk, but this had a lower AICc value than the remaining measures of local crowding (the LDOD, nearest neighbour distance and Voronoi size (domain of danger)). Prey closer to the edge of the group were more likely to be targeted (figure 3b). The measure of spatial position relative to the front of the group (distance from the front), was a poor predictor of risk as it performed worse than the null model.
4. Discussion
Our results demonstrate that even when comparing two relatively similar prey group types, where one group had directional movement and the other did not, the selection of a target by predators was altered enough that the risk of being targeted was best predicted by different measures of spatial position. Measures of local crowding best-predicted risk in stationary groups, with the LDOD [27] outperforming all other measures. Although similar, the Voronoi area was a relatively poor predictor of risk, which lends substantial support to the more biologically realistic LDOD [27] in contrast with the unlimited domain of danger as originally formulated by Hamilton [6]. In moving groups, the best predictor of risk was the number of near neighbours but only for a small range of radius sizes within which neighbours are counted; more generally, measures of whether the prey were closer to the group edge performed best, rather than measures of local crowding. Although these results are consistent with the predators encountering individuals near the edge of the group more when the prey are moving, we also found that prey's proximity to the front of the group was a poor predictor of risk, unlike a recent study also using sticklebacks [9]. There was also no evidence that whether groups were moving or not affected whether they were more likely to be approached from outside the group. The radius that minimized the AICc for the LDOD and number of near neighbours was substantially larger when groups were moving, possibly because moving objects have higher salience [30], which suggests that the radius size is context specific.
Individual prey in groups vary in multiple aspects of how they appear to predators, including their direction and speed, and how variable such parameters are over time and between individuals. Group-level variables are also diverse, such as the group's shape, the spatial arrangement of individuals and the degree to which individuals change position within the group [31–34], which can depend on species and ecological context [35–37] and also vary over time within the same group and context [24,38]. Given our results with groups which differed only with respect to their directional movement, multidimensional variation in individual and collective properties of prey may make it difficult to generalize which measure of spatial position accurately predicts the risk of prey being targeted. Indeed, our findings are likely to have been different had other aspects of the prey group differed, such as the speed of movement. A lack of generalization between groups with different properties could help explain the surprising result in our study of no evidence that prey at the front of a moving group were more at risk, in contrast to other experiments with virtual [9] and real [10] prey. Further differences in the aspects of spatial position important in predicting risk may come from variation between predators, for example, in the attack strategy of different species [21,39], in hunger or experience between individuals of the same species [40], or from consistent personality differences between individuals within the same population [41]. For prey in groups spread over two, rather than three, dimensions, another likely source of variability is whether the predator is attacking in the same, or perpendicular to, the plane of the prey group, which remains a relatively neglected question in predator–prey interactions [5].
Our findings, where the predictive success of spatial measures differed for attacks on stationary groups and groups with directional movement, suggest that there may be no single best measure of spatial position for describing predation risk. Instead, the spatial measure that most predicts risk might be dependent upon the prey's collective behaviour. This has implications for the selection pressure acting on prey in terms of the movement rules they should follow when locating themselves within the group [13,42]. For example, our results suggest that when a group is relatively still, such as when they are resting or foraging within a patch, individuals should occupy areas which are more crowded to minimize their LDOD, regardless of whether this area falls near the edge of the group. However, occupying the optimal spatial position within the group may be constrained by cognitive abilities in determining where to move to, and the behaviour of other individuals in the group [42–44].
Supplementary Material
Ethics
All procedures were approved by the University of Bristol Ethical Review Group (UIN UB/16/047).
Data accessibility
The data are provided in the electronic supplementary material [45].
Authors' contributions
P.J.L.: investigation, methodology, project administration, writing-original draft; J.E.H.-R.: conceptualization, data curation, methodology, project administration, software, supervision, writing-review and editing; C.C.I.: conceptualization, formal analysis, funding acquisition, project administration, resources, supervision, visualization, writing-review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Natural Environment Research Council Independent Research Fellowship NE/K009370/1 and Leverhulme Trust Grant no. RPG-2017-041 V awarded to C.C.I. J.E.H.-R. was supported by the Whitten Lectureship in Marine Biology and a Swedish Research Council grant: 2018-04076.
References
- 1.Ioannou C. 2017Grouping and predation. In Encyclopedia of evolutionary psychological science (eds Shackelford TK, Weekes-Shackelford VA), pp. 1-6. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 2.Ward A, Webster M. 2016Sociality: the behaviour of group-living animals, 1st edn. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 3.Duffield C, Ioannou CC. 2017Marginal predation: do encounter or confusion effects explain the targeting of prey group edges? Behav. Ecol. 28, 1283-1292. ( 10.1093/beheco/arx090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stankowich T. 2003Marginal predation methodologies and the importance of predator preferences. Anim. Behav. 66, 589-599. ( 10.1006/anbe.2003.2232) [DOI] [Google Scholar]
- 5.Romey WL, Walston AR, Watt PJ. 2008Do 3-D predators attack the margins of 2-D selfish herds? Behav. Ecol. 19, 74-78. ( 10.1093/beheco/arm105) [DOI] [Google Scholar]
- 6.Hamilton WD. 1971Geometry for the selfish herd. J. Theor. Biol. 31, 295-311. ( 10.1016/0022-5193(71)90189-5) [DOI] [PubMed] [Google Scholar]
- 7.Quinn JL, Cresswell W. 2006Testing domains of danger in the selfish herd: sparrowhawks target widely spaced redshanks in flocks. Proc. R. Soc. B 273, 2521-2526. ( 10.1098/rspb.2006.3612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romenskyy M, Herbert-Read JE, Ioannou CC, Szorkovszky A, Ward AJW, Sumpter DJT. 2020Quantifying the structure and dynamics of fish shoals under predation threat in three dimensions. Behav. Ecol. 31, 311-321. ( 10.1093/beheco/arz197) [DOI] [Google Scholar]
- 9.Ioannou CC, Rocque F, Herbert-Read JE, Duffield C, Firth JA. 2019Predators attacking virtual prey reveal the costs and benefits of leadership. Proc. Natl Acad. Sci. USA 116, 8925-8930. ( 10.1073/pnas.1816323116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bumann D, Rubenstein D, Krause J. 1997Mortality risk of spatial positions in animal groups: the danger of being in the front. Behaviour 134, 1063-1076. ( 10.1163/156853997X00403) [DOI] [Google Scholar]
- 11.Krause J, et al. 2017Injury-mediated decrease in locomotor performance increases predation risk in schooling fish. Phil. Trans. R Soc. B 372, 20160232. ( 10.1098/rstb.2016.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parrish JK. 1989Re-examining the selfish herd: are central fish safer? Anim. Behav. 38, 1048-1053. ( 10.1016/S0003-3472(89)80143-5) [DOI] [Google Scholar]
- 13.De Vos A, O'Riain MJ. 2013Movement in a selfish seal herd: do seals follow simple or complex movement rules? Behav. Ecol. 24, 190-197. ( 10.1093/beheco/ars153) [DOI] [Google Scholar]
- 14.Viscido SV, Wethey DS. 2002Quantitative analysis of fiddler crab flock movement: evidence for ‘selfish herd’ behaviour. Anim. Behav. 63, 735-741. ( 10.1006/anbe.2001.1935) [DOI] [Google Scholar]
- 15.Hirsch B. 2007Costs and benefits of within-group spatial position: a feeding competition model. Q. Rev. Biol. 82, 9-27. ( 10.1086/511657) [DOI] [PubMed] [Google Scholar]
- 16.Barber I, Huntingford FA. 1996Parasite infection alters schooling behaviour: deviant positioning of helminth-infected minnows in conspecific groups. Proc. R. Soc. Lond. B 263, 1095-1102. ( 10.1098/rspb.1996.0161) [DOI] [Google Scholar]
- 17.Bevan PA, Gosetto I, Jenkins ER, Barnes I, Ioannou CC. 2018Regulation between personality traits: individual social tendencies modulate whether boldness and leadership are correlated. Proc. R. Soc. B 285, 20180829. ( 10.1098/rspb.2018.0829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrell LJ, Romey WL. 2008Optimal individual positions within animal groups. Behav. Ecol. 19, 909-919. ( 10.1093/beheco/arn050) [DOI] [Google Scholar]
- 19.Piyapong C, Morrell LJ, Croft DP, Dyer JRG, Ioannou CC, Krause J. 2007A cost of leadership in human groups. Ethology 113, 821-824. ( 10.1111/j.1439-0310.2007.01382.x) [DOI] [Google Scholar]
- 20.Rayor LS, Uetz GW. 1990Trade-offs in foraging success and predation risk with spatial position in colonial spiders. Behav. Ecol. Sociobiol. 27, 77-85. ( 10.1007/BF00168449) [DOI] [Google Scholar]
- 21.Hirsch BT, Morrell LJ. 2011Measuring marginal predation in animal groups. Behav. Ecol. 22, 648-5622. ( 10.1093/beheco/arr026) [DOI] [Google Scholar]
- 22.Wood AJ, Ackland GJ. 2007Evolving the selfish herd: emergence of distinct aggregating strategies in an individual-based model. Proc. R. Soc. B 274, 1637-42. ( 10.1093/beheco/arr026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ioannou CC, Guttal V, Couzin ID. 2012Predatory fish select for coordinated collective motion in virtual prey. Science 337, 1212-1215. ( 10.1126/science.1218919) [DOI] [PubMed] [Google Scholar]
- 24.MacGregor HEA, Herbert-Read JE, Ioannou CC. 2020Information can explain the dynamics of group order in animal collective behaviour. Nat. Commun. 11, 2737. ( 10.1038/s41467-020-16578-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobbinson KE, Skarratt PA, Morrell LJ. 2020Computerized stimuli for studying oddity effects. Behav. Ecol. 31, 176-183. ( 10.1093/beheco/arz174) [DOI] [Google Scholar]
- 26.MathWorks. 2018MatLab. Natick, MA: The MathWorks Inc. [Google Scholar]
- 27.James R, Bennett PG, Krause J. 2004Geometry for mutualistic and selfish herds: the limited domain of danger. J. Theor. Biol. 228, 107-113. ( 10.1016/j.jtbi.2003.12.005) [DOI] [PubMed] [Google Scholar]
- 28.R Core Team. 2019R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.r-project.org. [Google Scholar]
- 29.Burnham KP, Anderson DR. 2010Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. New York, NY: Springer. [Google Scholar]
- 30.Brunyé TT, Martis SB, Kirejczyk JA, Rock K. 2019Camouflage pattern features interact with movement speed to determine human target detectability. Appl. Ergon. 77, 50-57. ( 10.1016/j.apergo.2019.01.004) [DOI] [PubMed] [Google Scholar]
- 31.Calovi DS, Lopez U, Ngo S, Sire C, Chaté H, Theraulaz G. 2014Swarming, schooling, milling: phase diagram of a data-driven fish school model. New J. Phys. 16, 015026. ( 10.1088/1367-2630/16/1/015026) [DOI] [Google Scholar]
- 32.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. 2002Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1-11. ( 10.1006/jtbi.2002.3065) [DOI] [PubMed] [Google Scholar]
- 33.Hemelrijk CK, Hildenbrandt H. 2012Schools of fish and flocks of birds: their shape and internal structure by self-organization. Interface Focus 2, 726-737. ( 10.1098/rsfs.2012.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viscido SV, Parrish JK, Grünbaum D. 2007Factors influencing the structure and maintenance of fish schools. Ecol. Model. 206, 153-165. ( 10.1016/j.ecolmodel.2007.03.042) [DOI] [Google Scholar]
- 35.Rodriguez-Pinto II, Rieucau G, Handegard NO, Boswell KM. 2020Environmental context elicits behavioural modification of collective state in schooling fish. Anim. Behav. 165, 107-116. ( 10.1016/j.anbehav.2020.05.002) [DOI] [Google Scholar]
- 36.Ginnaw GM, Davidson IK, Harding HR, Simpson SD, Roberts NW, Radford AN, Ioannou CC. 2020Effects of multiple stressors on fish shoal collective motion are independent and vary with shoaling metric. Anim. Behav. 168, 7-17. ( 10.1016/j.anbehav.2020.07.024) [DOI] [Google Scholar]
- 37.Partridge BL, Pitcher T, Cullen JM, Wilson J. 1980The three-dimensional structure of fish schools. Behav. Ecol. Sociobiol. 6, 277-288. ( 10.1007/BF00292770) [DOI] [Google Scholar]
- 38.Ginelli F, Peruani F, Pillot MH, Chaté H, Theraulaz G, Bon R. 2015Intermittent collective dynamics emerge from conflicting imperatives in sheep herds. Proc. Natl Acad. Sci. USA 112, 12 729-12 734. ( 10.1073/pnas.1503749112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrish JK. 1992Do predators ‘shape’ fish schools: interactions between predators and their schooling prey. Netherlands J. Zool. 42, 358-370. ( 10.1163/156854291X00388) [DOI] [Google Scholar]
- 40.Milinski M. 1979Can an experienced predator overcome the confusion of swarming prey more easily? Anim. Behav. 27, 1122-1126. ( 10.1016/0003-3472(79)90060-5) [DOI] [Google Scholar]
- 41.Szopa-Comley AW, Duffield C, Ramnarine IW, Ioannou CC. 2020Predatory behaviour as a personality trait in a wild fish population. Anim. Behav. 170, 51-64. ( 10.1016/j.anbehav.2020.10.002) [DOI] [Google Scholar]
- 42.Morrell LJ, Ruxton GD, James R. 2011Spatial positioning in the selfish herd. Behav. Ecol. 22, 16-22. ( 10.1093/beheco/arq157) [DOI] [Google Scholar]
- 43.Stricklin WR. 1983Matrilinear social dominance and spatial relationships among Angus and Hereford cows. J. Anim. Sci. 57, 1397-1405. ( 10.2527/jas1983.5761397x) [DOI] [PubMed] [Google Scholar]
- 44.Hemelrijk CK. 2000Towards the integration of social dominance and spatial structure. Anim. Behav. 59, 1035-1048. ( 10.1006/anbe.2000.1400) [DOI] [PubMed] [Google Scholar]
- 45.Lambert PJ, Herbert-Read JE, Ioannou CC. 2021The measure of spatial position within groups that best predicts predation risk depends on group movement. FigShare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lambert PJ, Herbert-Read JE, Ioannou CC. 2021The measure of spatial position within groups that best predicts predation risk depends on group movement. FigShare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data are provided in the electronic supplementary material [45].


