Abstract
Plasticizers are commonly used in different consumer goods and personal care products to provide flexibility, durability and elasticity to polymers. Due to their reported toxicity, the use of several plasticizers, including phthalates has been regulated and/or banned from the market. Di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH) is an alternative plasticizer that was introduced to replace toxic plasticizers. Increasing global demand and lack of toxicity data and safety assessment of DINCH have raised the concern to human and animal health. Hence, in the present study, we investigated the adverse effects of DINCH (at concentrations ranging from 0.01 to 10 μM) in early developmental stages of zebrafish using different endpoints such as hatching rate, developmental abnormalities, lipid content, behavior analysis and gene expression. We found that DINCH caused hatching delay in a dose-dependent manner and altered the expression of genes involved in stress response. Lipid staining using Oil Red O stain showed a slight lipid accumulation around the yolk, brain, eye and neck with increasing concentration. Genes associated with lipid transport such as fatty acid synthesis, β-oxidation, elongation, lipid transport were significantly altered by DINCH. Genes involved in cholesterol biosynthesis and homeostasis were also affected by DINCH indicating possible developmental neurotoxicity. Behavioral analysis of larvae demonstrated a distinct locomotor activity upon exposure to DINCH. The present data shows that DINCH could induce physiological and metabolic toxicity to aquatic organisms. Hence, further analyses and environmental monitoring of DINCH should be conducted to determine its safety and toxicity levels.
Keywords: Obesity, Fatty acid, Stress response, Plasticizer, Toxicity
obesity, fatty acid, stress response, plasticizer, toxicity
1. Introduction
Plasticizers are multifunctional chemicals that provide flexibility, durability and elasticity to polymers by reducing their glass transition temperature, melt viscosity and elastic modulus (Bui et al., 2016; Wypych, 2004). Phthalate plasticizers are widely used in consumer goods and personal care products and comprise more than 80% of the global plasticizer market of polyvinyl chloride plastic production (Chiellini et al., 2013; Crinnion, 2010). Phthalates do not have covalent interaction with the products, hence, they leach out and contaminate the environment (Crinnion, 2010). Their high and widespread use has resulted in the detection of their metabolites in urine samples of the general population from the U.S., Europe and Canada (de Renzy-Martin et al., 2014; Mínguez-Alarcón et al., 2016; Saravanabhavan et al., 2013; Zota et al., 2014). Phthalates show many adverse health effects, including carcinogenesis, cardiotoxicity, hepatotoxicity, nephrotoxicity, neurotoxicity and reprotoxicity (Braun et al., 2013; Diamanti-Kandarakis et al., 2009; Dodge et al., 2015; Gore et al., 2014; Messerlian et al., 2016; Miodovnik et al., 2014). Based on the reported toxicity, several phthalates have been regulated or their use in various products is banned in Europe (Fontelles and Clarke, 2005), U.S. (CPSIA, 2008), and Canada (CCPSA, 2020). Increasing demand for safer and environmentally friendly plasticizers has led the industry to investigate and produce non-phthalate plasticizers. Several alternative plasticizers are now in the market and their production is gradually increasing (Bui et al., 2016).
Di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH) was introduced in the market with a trade name of Hexamoll DINCH in 2002 as a safer alternative plasticizer (EFSA, 2007). The use of DINCH in different food contact items was approved by the European Food Safety Authority in 2007 (EFSA, 2007). It is also used in many polyvinyl chloride (PVC) products such as children toys and medical devices (EFSA, 2007). The global demand for alternative plasticizers is increasing and in parallel with this, the production and consumption rate of DINCH is on the rise (BASF, 2014). In the European market, the production volume of DINCH is more than 10000 tons per year, making it one of the most used plasticizers (ECHA, 2021).
DINCH was found to be the most abundant non-phthalate plasticizer in Swedish preschool dust with geometric mean level of 73 μg/g dust (Larsson et al., 2017). DINCH is metabolized to the corresponding monoester cyclohexane-1,2-dicarboxylic acid monoisononyl ester (MINCH), and further to monoester derivatives (Koch et al., 2013). DINCH metabolites, cyclohexane-1,2-dicarboxylic acid-monocarboxy isooctyl ester (MCOCH) and cyclohexane-1,2-dicarboxylic acid-mono (hydroxy-isononyl) ester (MHiNCH) were not detected in urine samples of adult population from Germany and USA (Schütze et al., 2014; Silva et al., 2013). However, the concentrations of MINCH metabolites, OH-MINCH, cx-MINCH and oxo-MINCH, increased over the years and were detected to be 2.09, 0.86 and 1.81 μg/L, respectively in urine samples of the German Environmental Specimen Bank (Schütze et al., 2014). Moreover, several other studies have also detected DINCH metabolites in urine samples (Fromme et al., 2016; Kasper-Sonnenberg et al., 2019; Mínguez-Alarcón et al., 2016; Ramos et al., 2016; Schwedler et al., 2020; Urbancova et al., 2019). The tolerable daily intake is estimated to be 1 mg/kg bw/day based on the rat data (EFSA, 2006). The oral reference dose for DINCH was estimated to be 0.7 mg/kg bw/day. This dose was based on a human equivalent 10% benchmark response level of 21 mg/kg-day for the thyroid growth status observed in rats (Bhat et al., 2014).
Several in vivo and in vitro toxicological studies have analyzed possible adverse effects of DINCH and contradictory results were obtained (Campioli et al., 2015; Campioli et al., 2019; Campioli et al., 2017; David et al., 2015; EFSA, 2007; Eljezi et al., 2019; Engel et al., 2018; Nardelli et al., 2017; Vasconcelos et al., 2019). The in vivo toxicological studies of DINCH on rats have shown no effect on behavior, organ weight, serum chemistry (David et al., 2015), and no evidence of reproductive toxicity or endocrine disruptive properties (EFSA, 2007). However, higher doses (300–1000 mg/kg body weight/day) resulted in thyroid hyperplasia and renal toxicity (EFSA, 2007). It has been suggested that one of the metabolites, MINCH, is a potent PPAR-α agonist and has a potential metabolic disrupting effect that alters fat storage in adipocytes resulting in obesity (Campioli et al., 2015). The same research group also showed that DINCH alters gene expression in rat liver at a dose of 1 mg/kg bw/day (Campioli et al., 2019), affects Leydig cell function, and impairs liver metabolic capacity upon in utero exposure of rats (Campioli et al., 2017). In another study, higher incidence of hemorrhagic testes was observed in the offspring of timed-pregnant Sprague-Dawley rats that were gavaged with 30 and 300 mg/kg/day of DINCH (Nardelli et al., 2017). In a study, it has been shown that DINCH did not have any effect on the activity of human nuclear receptors ERα, ERβ, AR, PPARα and PPARγ in HEK293 cell line, while its metabolites, M2NCH, MINCH, OH-MINCH, oxo-MINCH, and cx-MINCH, were shown to activate these receptors (Engel et al., 2018). Taken together, there is still a lack of information regarding toxicity and safety assessment of DINCH. Moreover, the available data are debatable whether DINCH is of concern to human health, hence, further research should be conducted to reveal its potential risks and toxic effects on population.
Zebrafish (Danio rerio) has become an important vertebrate model system for biomedical and genetic research such as development, lipid metabolism and behavior (Ho et al., 2016; Kalueff et al., 2016; Santoro and Metabolism, 2014; Seth et al., 2013). It offers several advantages including easy handling, small size, and transparent body that allows for continuous visualization of developmental changes (Bailey et al., 2013). The latter characteristic is particularly important to assess structural integrity of zebrafish as well as its functional ability that can be used to determine the impact of chemicals on behavior (Bailey et al., 2013; Fitzgerald et al., 2020). Besides, it shares a higher genetic similarity with human, as around 70% of human genes have at least one ortholog in zebrafish (Howe et al., 2013). Several important genes associated with lipid metabolism in mammals such as genes involved in fatty acid transport and acyl-CoA synthesis have been found in zebrafish (Seth et al., 2013).
The present study aimed to analyze the adverse effects of DINCH at early developmental stages of zebrafish through assessment of different endpoints such as hatching rate, developmental abnormalities, lipid metabolism, behavior and gene expression. The results reveal that DINCH has negative effects on zebrafish embryonic development and provide insights into molecular mechanisms of DINCH toxicity.
2. Materials and methods
2.1. Chemicals
DINCH (CAS No. 166412-78-8; molecular formula: C6H10(CO2C9H19)2) was purchased from Sigma (purity ≥95 %). The physical chemical properties of DINCH are as follows: vapour pressure (mm Hg) < 0.000001 hPa, water solubility <0.02 mg/L, partitioning coefficient n-octanol/water (log Pow) = 10. The stock solutions were prepared in dimethylsulfoxide (DMSO; Sigma).
2.2. Zebrafish maintenance
The wild type zebrafish (ORU strain) were maintained at 25 ± 1 °C with 14 hr light/10 hr dark cycle. Artemia salina and flake food (Tetrarubin) were used as food source and fish were fed twice a day. To get eggs, 4–5 couples were kept in a spawning container during the evening hours and the eggs were collected the following morning. The ethical permit for zebrafish handling was approved by the Swedish Ethical Committee (Permit 5.2.18–4065/18).
2.3. Exposure
The fertilized eggs were collected and then transferred in E3 medium (580 mg of NaCl, 26.6 mg of KCl, 96 mg of CaCl2, and 163 mg of MgCl2 per liter). DINCH stock solutions were added to E3 medium to obtain final assay concentrations 0.01 (0.0042 mg/L), 0.1 (0.042 mg/L), 1 (0.42 mg/L), 10 (4.2 mg/L) μM. The final assay concentration of DMSO was maintained at 0.1%. For each replicate, 30 embryos (2 h post fertilization; hpf) were exposed in a 6 well plate (Sartstedt). Each exposure was performed in triplicate. The plates were kept at 24.0 ± 1.0 ᴼC. Hundred percent of exposure media was changed every alternate day.
2.4. Hatching delay, mortality and abnormality analysis
Hatching rates, mortality, pigmentation, morphological and developmental abnormalities were monitored and noted each day. Any malformations including spinal defects and pericardial edema were checked under the stereomicroscope (Lumar.V12/Zeiss) connected to AxioVision 4.7.1 software (Zeiss).
2.5. Lipid staining
The 0.5% stock solution of Oil Red O (ORO) dye (Sigma) was prepared in 100% isopropanol and was diluted to the 0.3% working solution using 60% isopropanol. Zebrafish larvae at 6 dpf were collected and fixed using 4% paraformaldehyde for 1 hr at room temperature (RT). The larvae were then washed twice with phosphate-buffer saline (PBS) solution. Subsequently, the larvae were fixed with 60% isopropanol for 30 min and washed again with PBS. The larvae were then stained with ORO working solution for 1 hr at RT. The larvae were washed three times with 60% isopropanol. Images were taken with 4× objective using a bright field microscope (Olympus BX51) and the comparison between the control and DINCH exposure groups were performed through visual observations.
2.6. Behavior analysis
The locomotor response of zebrafish larvae to a light-dark transition was investigated. On day 6, larvae were transferred to 96 well plates containing 250 μl of exposure solution and allowed to acclimatize for 4 hr. Alteration in locomotion was then analyzed using Danio Vision (Noldus). During the recording, temperature was maintained at 25 °C. For stimulation, light to dark transition was used to observe any behavioral change. The parameters consisted of light ON for 10 min (5 min for acclimatization and 5 min for recording), light OFF for 5 min and then light ON for 5 min recording.
2.7. RNA isolation and quantitative real-time PCR (qPCR)
For each exposure group, 8 biological replicates were used and for each biological replicate, 4 larvae at 6 dpf were pooled in a homogenizing tube. The samples were lysed in lysis buffer (Macherey Nagel) and RNA extraction was performed using RNA extraction kit (Macherey Nagel). cDNA was prepared using the cDNA synthesis kit (Quanta Biosciences). The qPCR was carried out on thermocycler (CFX96; BioRad) using SYBR Green (PCR Biosystems). The qPCR cycles consisted of an initial denaturation at 95 °C for 2 min, 40 cycles of 95 °C for 5 s and 60 °C for 30s. The data was normalized using elongation factor (eef1a1) and ΔΔCt method was used to calculate fold change (Schmittgen and Livak, 2008). Primer sequences are listed in Table S1.
2.8. Statistical analysis
To determine if control and DINCH exposed groups were significantly different, one-way analysis of variance (ANOVA) and Dunnett post-hoc test using the GraphPad Prism 8 software (GraphPad Software) were performed. For behavior analysis, Kruskal-Wallis test was used. The differences were considered significant when the p value was <0.05 (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p ≤ 0.001, ∗∗∗∗p < 0.001).
3. Results
3.1. DINCH decreases hatching rates and induces stress
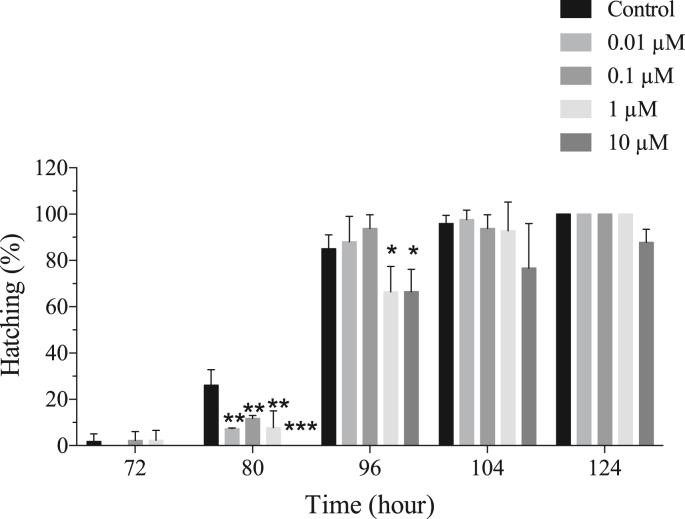
Exposure to DINCH delayed hatching rates in a dose and time-dependent manner. At 80 hpf, all the doses of DINCH significantly reduced hatching rate. The hatching rate was 26.1% in the control group, while it was 7.3%, 11.7% and 7.8% for 0.01, 0.1 and 1 μM of DINCH, respectively. Meanwhile, no hatching was observed for 10 μM of DINCH at 80 hpf. The hatching rates continued to be significantly low for 1 and 10 μM of DINCH at 96 hpf with 66.5% and 66.6%, respectively, while it was 85.0% for the control group. However, no significant difference was observed at 104 and 120 hpf (Figure 1). Exposure to DINCH did not induce any significant change on mortality at analyzed DINCH concentrations (data not shown). DINCH caused slight edema and swelling in yolk region in response to 10 μM, while no other malformation was observed in response to the selected DINCH concentrations.
Figure 1.
DICNH leads to hatching delay. Zebrafish eggs at 2 hpf were exposed to different concentrations of DINCH and hatching delay was recorded until 124 hpf. Statistical analysis was performed using One-way ANOVA followed by Dunnett's post-test. Error bars represent mean ± SD, n = 90.
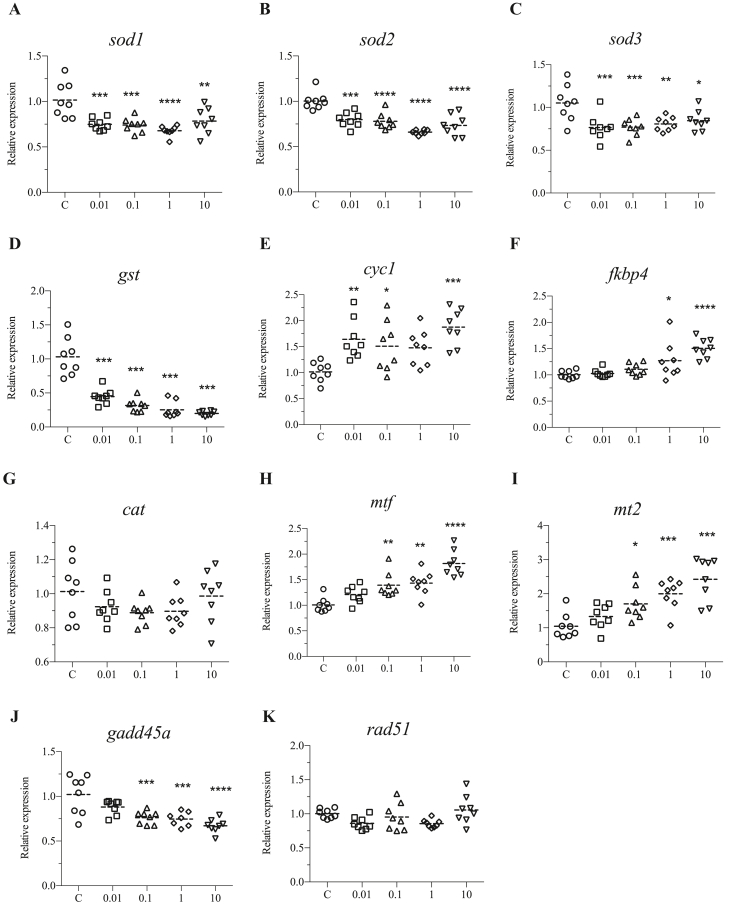
To determine whether DINCH induces stress in zebrafish, expression of stress-related genes was analyzed by qRT-PCR upon exposure to 0.01, 0.1, 1, and 10 μM of DINCH for 120 hpf (0–6 days). The results showed that DINCH can induce oxidative stress response by altering the expression of various genes. Downregulation of superoxide dismutase genes, sod1, sod2 and sod3 was observed in response to all doses of DINCH (Figure 2A, B and C). The glutathione s-transferase (gst) was also downregulated upon exposure to all the doses of DINCH (Figure 2D). The gene involved in mitochondrial oxidative phosphorylation cytochrome c 1 (cyc1) was significantly upregulated by 0.01, 0.1 and 10 μM of DINCH (Figure 2E), while FKBP prolyl isomerase 4 (fkbp4) which is involved in oxidative stress was induced in response to 1 and 10 μM of DINCH (Figure 2F). On the other hand, catalase (cat) did not show any significant change (Figure 2G).
Figure 2.
DINCH affects the expression of oxidative stress genes. Zebrafish embryos were exposed to 0.01, 0.1, 1 and 10 μM of DINCH for 6 dpf and qRT-PCR analysis was performed for stress related genes including sod1 (A), sod2 (B), sod3 (C), gst (D), cyc1 (E), fkbp4 (F), cat (G), mtf (H), mt2 (I), gadd45a (J) and rad51 (K). Statistical analysis was performed using One-way ANOVA followed by Dunnett's post-test.
Genes involved in metal stress, metal regulatory transcription factor (mtf) and metallothionein 2 (mt2) were significantly upregulated upon exposure to 0.1, 1 and 10 μM of DINCH (Figure 2H and I). Of the genes involved in cell cycle and DNA damage, growth arrest and DNA-damage-inducible, alpha (gadd45a) was downregulated in response to 0.1, 1 and 10 μM of DINCH (Figure 2J), while RAD51 recombinase (rad51) did not show change at any exposure condition (Figure 2K).
3.2. DINCH alters lipid metabolism
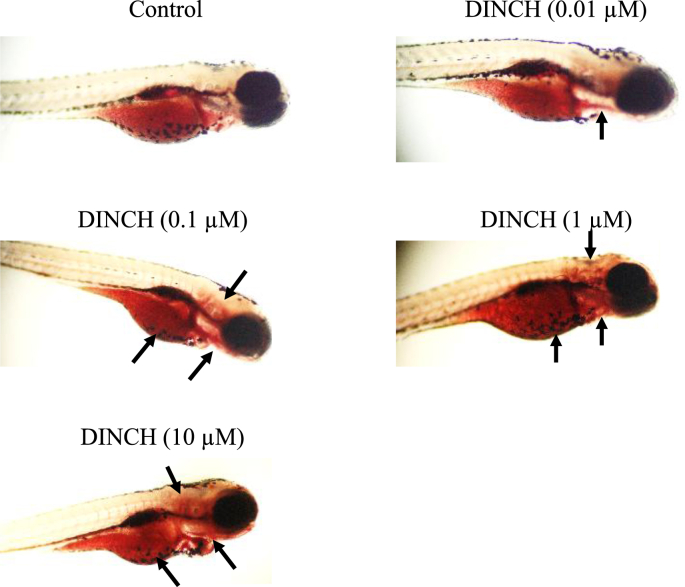
Lipid staining was performed to determine if DINCH affects lipid metabolism in zebrafish larvae. The results showed that there was a modest increase in lipid content as the intensity and localization of ORO stain increased around the yolk region (Figure 3). ORO staining was also localized in the brain, around the eye, neck, and heart in a dose-dependent manner (Figure 3).
Figure 3.
DINCH alters lipid metabolism. Zebrafish embryos were exposed to 0.01, 0.1, 1 and 10 μM of DINCH for 6 dpf and ORO staining was performed. Images were taken with 4X objective using a bright field microscope (Olympus BX51). Arrows indicate the area where differences between the control and exposed group were noticeable. n = 10.
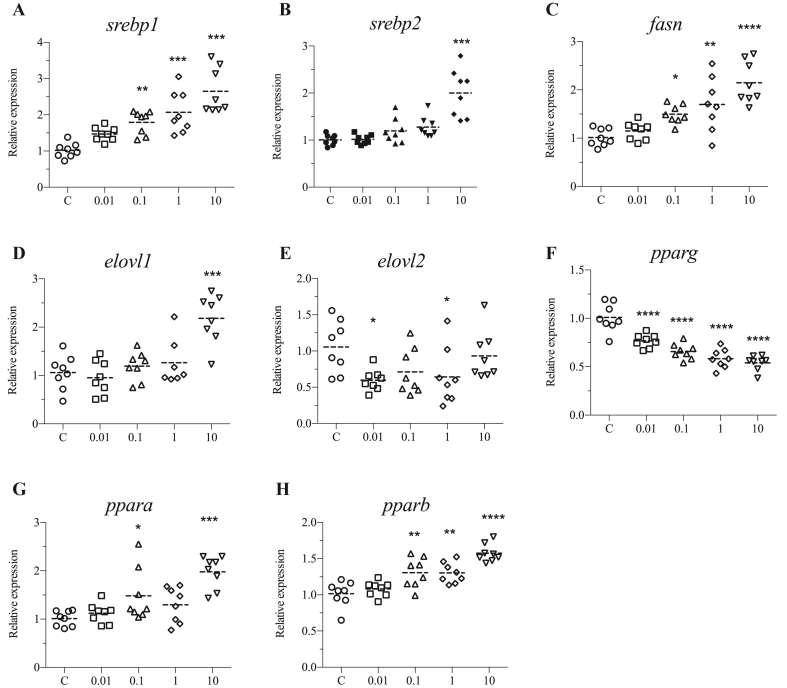
To further understand the reason for lipid metabolism alteration, gene expression analysis was performed. Expression profiles showed that several genes associated with lipid metabolism were regulated upon exposure to DINCH. sterol regulatory element binding transcription factor 1 (srebp1) was significantly upregulated by 0.1, 1, and 10 μM doses (Figure 4A), while srebp2 was only induced by 10 μM (Figure 4B). fatty acid synthase (fasn) was upregulated in response to 0.1, 1, and 10 μM doses (Figure 4C). Other genes including fatty acid elongase 1 (elovl1) was only significantly upregulated by 10 μM (Figure 4D), while elovl2 was repressed by 0.01 and 1 μM of DINCH (Figure 4E). Peroxisome proliferator activated receptor genes were also analyzed. Of these pparg was significantly downregulated in all the exposure groups (Figure 4F), while ppara was upregulated in response to 0.1 and 10 μM doses (Figure 4G). In addition, pparb was induced upon exposure to 0.1, 1, and 10 μM of DINCH (Figure 4H).
Figure 4.
DINCH alters fatty acid synthesis, peroxisome proliferator activated receptor, and fatty acid elongation genes. Zebrafish embryos were exposed to 0.01, 0.1, 1 and 10 μM of DINCH for 6 dpf and qRT-PCR analysis was performed lipid metabolism genes including srebp1 (A), srebp2 (B), fasn (C), srebp1 (A), srebp2 (B), fasn (C), elovl1 (D), elovl2 (E), pparg (F), ppara (G) and pparb (H). Statistical analysis was performed using One-way ANOVA followed by Dunnett's post-test.
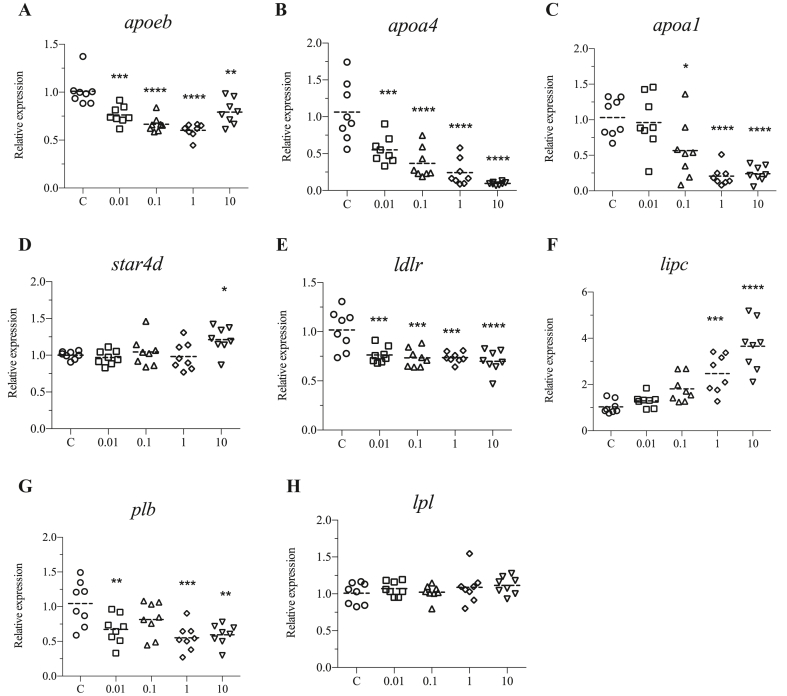
Apolipoprotein genes were also significantly altered. Of these, apoeb and apoa4 were downregulated by all exposure groups (Figure 5A and B), while apoa1 was downregulated by 0.1, 1, and 10 μM doses (Figure 5C). StAR-related lipid transfer domain containing 4 (star4d) which is involved in cholesterol transport was upregulated only by 10 μM DINCH (Figure 5D). Other genes including low density lipoprotein receptor (ldlr) were significantly downregulated by all the exposure concentrations (Figure 5E), while lipase C (lipc) was induced by 1 and 10 μM of DINCH (Figure 5F). Genes involved in fatty acid cleavage including phospholipase B (plb) were downregulated by 0.01, 0.1 and 10 μM of DINCH (Figure 5G), while lipoprotein lipase (lpl) did not show significant change at any exposure condition (Figure 5H).
Figure 5.
DINCH alters lipid transport genes. Zebrafish embryos were exposed to 0.01, 0.1, 1 and 10 μM of DINCH for 6 dpf and qRT-PCR analysis was performed for lipid transport and processing including apoeb (A), apoa4 (B), apoa1 (C), star4d (D), ldlr (E), lipc (F) plb (G) and lpl (H). Statistical analysis was performed using One-way ANOVA followed by Dunnett's post-test.
3.3. DINCH alters behavior
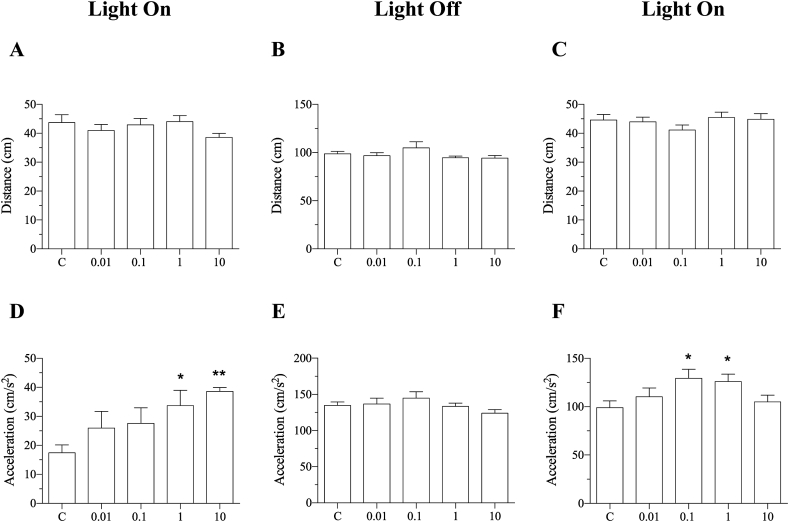
The distance moved did not differ in any exposure groups during both light periods (Figure 6A, B and C). However, 0.1 and 1 μM treated group showed increased acceleration while 10 μM DINCH did not show any difference when the light was switched on again (Figure 6F). Interestingly, the acceleration was found to be significantly lower during the first light period compared to light off and second light period (Figure 6D, E, and F).
Figure 6.
DINCH alters behavior in larvae. Zebrafish embryos were exposed to 0.01, 0.1, 1 and 10 μM of DINCH for 6 dpf and behavior analysis was performed. Distance and acceleration were recorded during light on (A and D), Light off (B and E) and light on (C and F). Kruskal-Wallis test. n = 48.
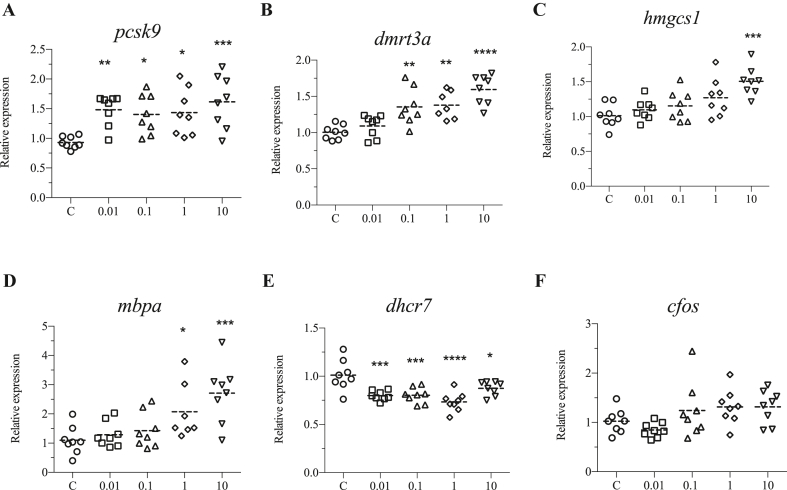
Since behavior was altered following DINCH exposure, the expression of genes involved in behavior were also investigated. proprotein convertase subtilisin/kexin type 9 (pcsk9) was significantly upregulated by all the doses (Figure 7A), while doublesex and mab-3 related transcription factor 3A (dmrt3a) was induced in response to 0.1, 1, and 10 μM of DINCH (Figure 7B). 3-hydroxy-3-methylglutaryl-coa synthase 1 (hmgcs1) were upregulated by only 10 μM of DINCH (Figure 7C). In addition, myelin basic protein a (mbpa) was upregulated upon exposure to 1 and 10 μM of DINCH (Figure 7D), while 7-dehydrocholesterol reductase (dhcr7) was significantly downregulated by all the doses (Figure 7E). However, cfos expression did not change in any exposure condition (Figure 7F).
Figure 7.
DINCH affects behavior, cholesterol biosynthesis and homeostasis genes. Zebrafish embryos were exposed to 0.01, 0.1, 1 and 10 μM of DINCH for 6 dpf and qRT-PCR analysis was performed for genes involved in behavior including pcsk9 (A), dmrt3a (B), hmgcs1 (C), mbpa (D), dhcr7 (E) and cfos (F). Statistical analysis was performed using One-way ANOVA followed by Dunnett's post-test.
3.4. Pathway analysis
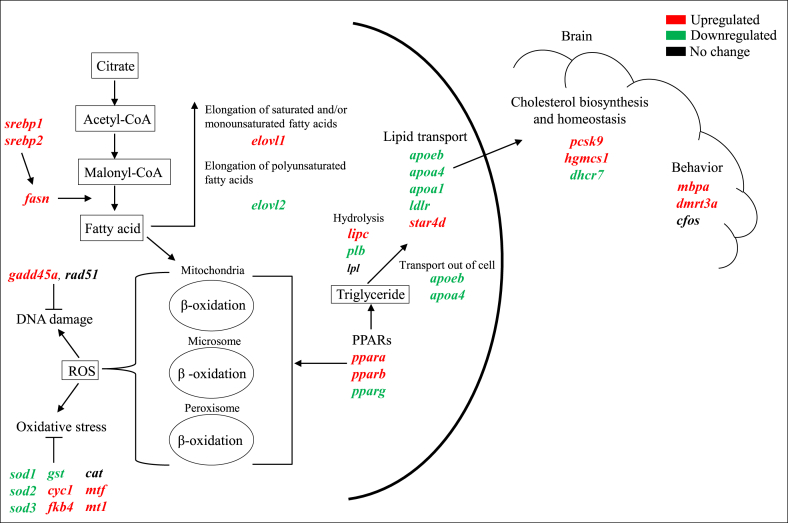
Pathway analysis was performed to determine the mechanism behind DINCH toxicity. It was found that exposure to DINCH affected lipid metabolism through inducing fatty acid synthesis which subsequently resulted in β-oxidation. Altered expression of oxidative and DNA damage related genes may be explained based on these changes. Meanwhile, lipid transport in zebrafish was also decreased in response to DINCH as most of the related transport genes were significantly downregulated. This could contribute to alter behavior and to overcome this stress, zebrafish significantly induced genes involved in cholesterol biosynthesis and homeostasis (Figure 8).
Figure 8.
Overview of the differentially expressed genes in response to DINCH. The schematic diagram shows the regulation and involvement of genes in different signaling mechanisms. Gene involved in lipid synthesis, elongation, hydrolysis and transport were altered in larvae. The stress response and DNA repair genes were also regulated following DINCH exposure. The altered lipid metabolism and transport could also be responsible for the observed cholesterol biosynthesis/homeostasis and brain function alteration in the larvae. The upregulated genes are indicated in red color, the downregulated genes are indicated in green color and the genes that were not regulated are indicated in black color.
4. Discussion
Phthalates are the most widely used and extensively studied plasticizers and are known to cause various detrimental effects such as carcinogenesis, cardiotoxicity, hepatotoxicity, nephrotoxicity, neurotoxicity and reprotoxicity (Braun et al., 2013; Diamanti-Kandarakis et al., 2009; Dodge et al., 2015; Gore et al., 2014; Messerlian et al., 2016; Miodovnik et al., 2014). Epidemiological studies on children from Norway, Sweden and Finland showed a correlation between the plasticizer containing materials at home and asthma (Bornehag et al., 2004; Jaakkola et al., 1999, 2000). Several plasticizers have also been shown to alter fatty acid metabolism and linked to childhood obesity (Kim and Park, 2014; Xia et al., 2018). Increasing demand for safer and environmentally friendly plasticizers has led the industry to investigate and produce phthalate-free plasticizers. DINCH is one such alternative plasticizers that was introduced into the market in 2002 and it is of great importance to determine its biological effects. Taken together, in the present study, we aimed to evaluate the adverse effects of alternative plasticizer, DINCH at the early developmental stages of zebrafish using different endpoints.
We observed that DINCH delayed hatching rates in a dose and time-dependent manner Although there is no study examining the effect of DINCH on zebrafish hatching, studies on other plasticizers, including di-n-butyl phthalate (DBP), di-(2-ethylhexyl) phthalate (DEHP), and acetyl tributyl citrate (ATBC) have reported delayed hatching (Hu et al., 2020; Muhammad et al., 2018). No significant change in mortality was observed in the present study. Consistent with our observation, no mortality has been determined on rats following intravenous administration of DINCH up to 300 mg/kg body weight/day (David et al., 2015). Several in vitro investigations have addressed the effects of DINCH on cell viability and demonstrated contradictory results (Boisvert et al., 2016; Nardelli et al., 2015). We observed slight edema and swelling in yolk region at higher concentrations of DINCH, but no other malformation. Studies using rats, indicated that exposure to DINCH (up to 100 mg/kg body weight/day) has no effect on fibrosis or direct liver toxicity (Campioli et al., 2019). Another study reported that DINCH (up to 300 mg/kg body weight/day) did not show any effect on organ weight and testicular toxicity (David et al., 2015). However, other studies have demonstrated that DINCH causes impaired liver metabolic capacity in utero exposed rats (Campioli et al., 2017), increases the incidence of hemorrhagic testes in Sprague-Dawley rats gavaged with 30 and 300 mg/kg/day (Nardelli et al., 2017).
Oxidative stress is a crucial indicator in environmental and toxicological risk assessment. We observed downregulation of sod1, sod2 and sod3 by all the doses of DINCH. It has previously been indicated that these genes were affected by several plasticizers such as DEHP, dimethyl phthalate (DMP), and butyl benzyl phthalate (BBP) in zebrafish and medaka fish (Cong et al., 2020; Yang et al., 2018; Zhang et al., 2014). It has also been shown that SOD1 enzyme activity was overexpressed in response to subacute exposure to DINCH at post-natal day 21 of dams, while it was repressed upon in utero exposure to DINCH at post-natal day 60 in a dose-dependent manner (Campioli et al., 2019). We also observed an increased expression of cyc1 and fkbp4 upon exposure to DINCH. cyc1 is involved in mitochondrial oxidative phosphorylation (Chen et al., 2015), while fkbp4 encodes an immunophilin protein that may have a protective role against oxidative stress (Gallo et al., 2011). Taken together, we suggest that altered expression of the genes mentioned above in response to DINCH may result in ROS generation and subsequently cause oxidative stress in zebrafish.
Metallothioneins are proteins that can bind metals and implicated in several biological processes, including metal homeostasis and regulation of oxidative stress (Chen et al., 2004; Dorts et al., 2016; Ruttkay-Nedecky et al., 2013). It has been previously demonstrated that the expression of Mt1a, Mt2a, and Mt1m was significantly induced in postnatal day 60 rats treated in utero with 1 and 100 mg/kg/day of DINCH (Campioli et al., 2019). We observed induced expression of two genes associated with the metallothionein pathway, mtf and mt2 in response to 0.1, 1 and 10 μM of DINCH. This result supports that DINCH may cause oxidative stress and metallothionein related genes were overexpressed to overcome the stress. We have also analyzed the genes involved in cell cycle and DNA damage. Although the expression of rad51 did not show any change, we found significant downregulation of gadd45a that promotes cell cycle arrest and DNA excision repair (Salvador et al., 2013). Altogether, these findings suggest that DINCH exposure induces oxidative stress, which may malfunction cell cycle progression, DNA damage, and hatching delay.
Lipids are essential molecules involved in signaling, membrane composition, and energy production (Anderson et al., 2011). It is indicated that defects in lipid metabolism can cause several disorders such as obesity, diabetes, and atherosclerosis (Joffe et al., 2001; McNeely et al., 2001; Watanabe et al., 2008). The yolk consists of the yolk syncytial layer (YSL), which is a lipid-rich structure. The YSL aids in releasing fatty acids and the synthesis of lipoproteins which transport lipids to the embryo to support larval growth (Anderson et al., 2011; Otis et al., 2015). In the present study, we observed a modest increase in lipid content around the yolk and we speculate that this could be due to reduced lipid transport activity following DINCH exposure. In a previous study, it has been indicated that DINCH did not show any difference in lipid accumulation, while its primary metabolite resulted in lipid accumulation in preadipocytes from epididymal adipose tissue of rats (Campioli et al., 2015). In another study, it has been shown that exposure to plasticizers including di-isononyl phthalate (DINP), which has structural similarity to DINCH and di-iso-decyl-phthalate (DIDP) resulted in lipid accumulation in 3T3-L1 cells (Pomatto et al., 2018).
To further confirm these findings, we performed qRT-PCR for genes in certain lipid metabolism pathways including fatty acid synthesis, PPAR signaling pathway, and lipoprotein transport. SREBPs are transcription factors that regulate the expression of genes involved in the uptake and synthesis of cholesterol and fatty acids (Shimano, 2000). We observed an induced expression of srebp1 and srebp2. The downstream gene of Srebp, fasn was also significantly induced upon exposure to 0.1, 1, and 10 μM of DINCH. fasn converts acetyl-CoA and malonyl-CoA into palmitate, which is then esterified into triacylglycerides and stored in adipose tissue (Tian et al., 2013). From this, we can assume that exposure to DINCH leads to fatty acid synthesis in zebrafish. We also analyzed elovl1 and elovl2, which are key genes in synthesizing long-chain mono and polyunsaturated fatty acids (Ayisi et al., 2018), and overexpression of these genes result in high availability of these fatty acids, which provide an adaptation to chemical exposure. Consistent with our observations, a previous study on rats showed alteration in several genes involved in lipid metabolism in postnatal day 3 and 60 treated in utero with 1 and 100 mg/kg/day of DINCH (Campioli et al., 2019). Similarly, it was also shown that exposure to widely used plasticizers, DEHP and DINP alters the expression of genes involved in the metabolism of lipids and fatty acids in zebrafish (Buerger et al., 2019; Forner-Piquer et al., 2017; Huff et al., 2018).
PPARs are involved in lipid metabolism by regulating the transcription of several genes (la Cour Poulsen et al., 2012). ppara and pparb regulate fatty acid β-oxidation (Varga et al., 2011), while pparg is associated with lipid storage and adipogenesis (Francis et al., 2003). We observed an upregulation of ppara1 and pparb while, pparg was significantly downregulated. In contrast to our finding, a previous study showed increased expression of Pparg2 following DINCH exposure (Campioli et al., 2015). In the same study, one of the DINCH metabolites, MINCH, was shown to be PPARα agonist at 50 μM or above concentrations (Campioli et al., 2015). On the other hand, in an in vitro study, it has been indicated that although DINCH did not alter the reporter gene system, its metabolites induced PPARα- and PPARγ-dependent reporter gene activities in a concentration-dependent manner (Engel et al., 2018). In another study, it has been demonstrated that DINCH-treated post-natal day 21 dams showed a residual PPAR-α overexpression (Campioli et al., 2019). It was also shown that DINP resulted in increased expression of ppara in zebrafish (Forner-Piquer et al., 2017) and PPARγ in 3T3-L1 cells (Zhang et al., 2019).
Lipoproteins play a role in transporting lipid molecules to different tissues in fish (Tocher, 2003). Analysis of lipoprotein genes in our study indicated that DINCH can also affect lipid uptake and transport in zebrafish larvae. We noted a decreased expression of apoeb and apoa4 by all exposure groups, while apoa1 was repressed upon exposure to 0.1, 1, and 10 μM of DINCH. Apolipoproteins are involved in lipid transport to specific tissues through specific binding to lipoprotein receptors (Ayisi et al., 2018). We also observed downregulation of ldlr in response to all exposure concentrations and upregulation of lipc by 1 and 10 μM of DINCH. ldlr is important for delivering essential lipids to maintain cellular functions and concentration of cholesterol-rich lipoproteins in the circulation (Willnow, 1999), while lipc is associated with triglyceride hydrolysis and high-density lipoprotein cholesterol function (Neale et al., 2010). Besides, we observed a significantly repressed expression of plb gene that is involved in fatty acid cleavage. Our data suggest that lipid transport becomes less active after DINCH exposure and to overcome this, the genes for lipid uptake are upregulated.
Behavior is a reflection of multifactorial interactions in organisms and significant changes in behavior in response to environmental exposures can be an indicator of adverse effects (Anderson et al., 2004). In our analysis, only acceleration of the larval fish was reduced following DINCH exposure. The study by Del Pozzo et al., showed that dmrt3a gene knockout zebrafish larvae has reduced movement and acceleration due to loss of coordination in the spinal interneurons (Del Pozo et al., 2020). This suggests that apart from distance, acceleration is also an important parameter to consider for behavioral or neurological changes. The altered swimming behavior following DINCH exposure suggests that DINCH may negatively regulate motor activity and result in altered swimming behavior because of neurotoxicity. Plasticizers including DEHP and di-butyl phthalate (DBP) have been shown to alter locomotor activity in zebrafish larvae by affecting spine and skeletal system development (Qian et al., 2020).
To understand the altered behavioral activity, we analyzed the expression of genes associated with behavior. Cholesterol plays a crucial role in maintaining neuronal physiology. It has been indicated that the level of cholesterol in the brain is critical for proper brain function and defect in brain cholesterol metabolism is associated with different nervous system problems, including Alzheimer's disease, Huntington's, disease and Parkinson's diseases (Zhang and Liu, 2015). We observed significantly altered expression of several genes involved in cholesterol biosynthesis and homeostasis such as pcsk9, hgmcs1 and dhcr7 (Korade et al., 2013; Mathews et al., 2014; O'Connell and Lohoff, 2020). Cholesterol plays an essential role in development of myelin in central and peripheral nervous systems (Saher et al., 2011). In line with this, we found an upregulation of mbpa that encodes myelin basic protein. Expression of dmrt3a is associated with spinal cord development and fate specification of dorsal interneurons 6 that coordinates locomotion in animals (Andersson et al., 2012). In a recent study, it was demonstrated that dmrt3a-knockout zebrafish has altered behavior with decreased movement, and acceleration (Del Pozo et al., 2020). We observed an increase in acceleration and parallel with this, significantly induced expression of dmrt3a. Altogether, this suggests that DINCH alters larval behavior by altering key genes involved in locomotion and neural function. Analysis of other genes could provide further insights into the mechanisms of behavior toxicity.
5. Conclusion
DINCH is one of the alternative plasticizers whose use is increasing in the European market. There is limited information on animal and human exposure to DINCH and the available data is conflicting as some studies have suggested that DINCH is not toxic and it cannot regulate lipid metabolism like the other plasticizers. We showed that DINCH can alter lipid metabolism and stress response genes in zebrafish larvae. Our data suggest that DINCH can have detrimental effects on aquatic organisms and higher eukaryotes. Taken together, this study indicates that DINCH alters lipid metabolism in zebrafish larvae and this alteration could also lead to changes in other physiological processes including brain functions. Further analysis on mammalian species will help to understand the risk factors associated with DINCH exposure to humans. In conclusion, DINCH is not completely safe, hence, its use, presence in the environment and negative effects on humans and animals should be carefully monitored.
Declarations
Author contribution statement
Noha Saad: Performed the experiments; Analyzed and interpreted the data.
Ceyhun Bereketoglu: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ajay Pradhan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Knowledge Foundation Sweden, Helge Ax:son Johnsons Foundation, Längmanska Culture Foundation, Magnus Bergvall's Foundation, Örebro University, the Scientific and Technological Research Council of Turkey (TÜBİTAK, Grant No: 120Z748), and Iskenderun Technical University.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Table
References
- Anderson G.L., Cole R.D., Williams P.L. Assessing behavioral toxicity with Caenorhabditis elegans. Environ. Toxicol. Chem. 2004;23:1235–1240. doi: 10.1897/03-264. [DOI] [PubMed] [Google Scholar]
- Anderson J.L., Carten J.D., Farber S.A. Zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol. 2011;101:111–141. doi: 10.1016/B978-0-12-387036-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L.S., Larhammar M., Memic F., Wootz H., Schwochow D., Rubin C.J., Patra K., Arnason T., Wellbring L., Hjälm G., Imsland F., Petersen J.L., McCue M.E., Mickelson J.R., Cothran G., Ahituv N., Roepstorff L., Mikko S., Vallstedt A., Lindgren G., Andersson L., Kullander K. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488:642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayisi C.L., Yamei C., Zhao J.-L. Genes, transcription factors and enzymes involved in lipid metabolism in fin fish. Agri Gene. 2018;7:7–14. [Google Scholar]
- Authority E.F.S. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to the 12th list of substances for food contact materials. EFSA J. 2006;4:395. [Google Scholar]
- Bailey J., Oliveri A., Levin E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. C Embryo Today. 2013;99:14–23. doi: 10.1002/bdrc.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2014. Badische Anilin und Soda Fabrik.https://www.basf.com/se/en/media/news-releases/2014/05/p-14-231.html Available: [Google Scholar]
- Bhat V.S., Durham J.L., Ball G.L., English J.C. Derivation of an oral reference dose (RfD) for the nonphthalate alternative plasticizer 1,2-cyclohexane dicarboxylic acid, di-isononyl ester (DINCH) J. Toxicol. Environ. Health B Crit. Rev. 2014;17:63–94. doi: 10.1080/10937404.2013.876288. [DOI] [PubMed] [Google Scholar]
- Boisvert A., Jones S., Issop L., Erythropel H.C., Papadopoulos V., Culty M. In vitro functional screening as a means to identify new plasticizers devoid of reproductive toxicity. Environ. Res. 2016;150:496–512. doi: 10.1016/j.envres.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Bornehag C.G., Sundell J., Weschler C.J., Sigsgaard T., Lundgren B., Hasselgren M., Hagerhed-Engman L. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ. Health Persp. 2004;112:1393–1397. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J.M., Sathyanarayana S., Hauser R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013;25:247. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger A.N., Schmidt J., Chase A., Paixao C., Patel T.N., Brumback B.A., Kane A.S., Martyniuk C.J., Bisesi J.H., Jr. Examining the responses of the zebrafish (Danio rerio) gastrointestinal system to the suspected obesogen diethylhexyl phthalate. Environ. Pollut. 2019;245:1086–1094. doi: 10.1016/j.envpol.2018.11.032. [DOI] [PubMed] [Google Scholar]
- Bui T.T., Giovanoulis G., Cousins A.P., Magnér J., Cousins I.T., de Wit C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 2016;541:451–467. doi: 10.1016/j.scitotenv.2015.09.036. [DOI] [PubMed] [Google Scholar]
- Campioli E., Duong T.B., Deschamps F., Papadopoulos V. Cyclohexane-1, 2-dicarboxylic acid diisononyl ester and metabolite effects on rat epididymal stromal vascular fraction differentiation of adipose tissue. Environ. Res. 2015;140:145–156. doi: 10.1016/j.envres.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 2008. Consumer Product Safety Improvement Act of 2008. Public Law 110–314 14.https://www.cpsc.gov/s3fs-public/pdfs/blk_pdf_cpsia.pdf Avaliable: [Google Scholar]
- Campioli E., Lau M., Papadopoulos V. Effect of subacute and prenatal DINCH plasticizer exposure on rat dams and male offspring hepatic function: the role of PPAR-α. Environ. Res. 2019;179:108773. doi: 10.1016/j.envres.2019.108773. [DOI] [PubMed] [Google Scholar]
- Campioli E., Lee S., Lau M., Marques L., Papadopoulos V. Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-11325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Xiao Y., Gai Z., Li R., Zhu Z., Bai C., Tanguay R.L., Xu X., Huang C., Dong Q. Reproductive toxicity of low level bisphenol A exposures in a two-generation zebrafish assay: evidence of male-specific effects. Aquat. Toxicol. 2015;169:204–214. doi: 10.1016/j.aquatox.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.Y., John J.A., Lin C.H., Lin H.F., Wu S.C., Lin C.H., Chang C.Y. Expression of metallothionein gene during embryonic and early larval development in zebrafish. Aquat. Toxicol. 2004;69:215–227. doi: 10.1016/j.aquatox.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Chiellini F., Ferri M., Morelli A., Dipaola L., Latini G. Perspectives on alternatives to phthalate plasticized poly (vinyl chloride) in medical devices applications. Prog. Polym. Sci. 2013;38:1067–1088. [Google Scholar]
- Cong B., Liu C., Wang L., Chai Y. The impact on antioxidant enzyme activity and related gene expression following adult zebrafish (Danio rerio) exposure to dimethyl phthalate. Animals. 2020;10(4):717. doi: 10.3390/ani10040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinnion W.J. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assists environmental medicine physicians. Alternative Med. Rev. 2010;15(2):101–109. [PubMed] [Google Scholar]
- Consumer Product Safety Program Annual Surveillance Report: 2020. https://www.canada.ca/en/health-canada/services/publications/product-safety/consumer-product-safety-surveillance-report/2020.html Avaliable:
- David R.M., White R.D., Larson M.J., Herman J.K., Otter R. Toxicity of Hexamoll((R)) DINCH((R)) following intravenous administration. Toxicol. Lett. 2015;238:100–109. doi: 10.1016/j.toxlet.2015.07.013. [DOI] [PubMed] [Google Scholar]
- de Renzy-Martin K.T., Frederiksen H., Christensen J.S., Kyhl H.B., Andersson A.-M., Husby S., Barington T., Main K.M., Jensen T.K. Current exposure of 200 pregnant Danish women to phthalates, parabens and phenols. Reproduction. 2014;147:443–453. doi: 10.1530/REP-13-0461. [DOI] [PubMed] [Google Scholar]
- Del Pozo A., Manuel R., Iglesias Gonzalez A.B., Koning H.K., Habicher J., Zhang H., Allalou A., Kullander K., Boije H. Behavioral characterization of dmrt3a mutant zebrafish reveals crucial aspects of vertebrate locomotion through phenotypes related to acceleration. eNeuro. 2020;7 doi: 10.1523/ENEURO.0047-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J.-P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge L., Williams P., Williams M., Missmer S., Souter I., Calafat A., Hauser R., Team E.S. Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment. Reprod. Toxicol. 2015;58:184–193. doi: 10.1016/j.reprotox.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorts J., Falisse E., Schoofs E., Flamion E., Kestemont P., Silvestre F. DNA methyltransferases and stress-related genes expression in zebrafish larvae after exposure to heat and copper during reprogramming of DNA methylation. Sci. Rep. 2016;6:34254. doi: 10.1038/srep34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA . 2021. European Chemicals Agency – Information on Chemicals.https://echa.europa.eu/substance-information/-/substanceinfo/100.103.017 Available: [Google Scholar]
- EFSA Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to a 16th list of substances for food contact materials. 2007;5:515. [Google Scholar]
- Eljezi T., Pinta P., Nativel F., Richard D., Pinguet J., Roy O., Sautou V., Grimandi G., Moreau E. In vitro cytotoxic effects of secondary metabolites of DEHP and its alternative plasticizers DINCH and DINP on a L929 cell line. Int. J. Hyg. Environ. Health. 2019;222:583–589. doi: 10.1016/j.ijheh.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Engel A., Buhrke T., Kasper S., Behr A.-C., Braeuning A., Jessel S., Seidel A., Völkel W., Lampen A. The urinary metabolites of DINCH® have an impact on the activities of the human nuclear receptors ERα, ERβ, AR, PPARα and PPARγ. Toxicol. Lett. 2018;287:83–91. doi: 10.1016/j.toxlet.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J.A., Könemann S., Krümpelmann L., Županič A., Vom Berg C. Approaches to test the neurotoxicity of environmental contaminants in the zebrafish model–from behavior to molecular mechanisms. Environ. Toxicol. Chem. 2020;40(4):989–1006. doi: 10.1002/etc.4951. [DOI] [PubMed] [Google Scholar]
- Fontelles J., Clarke C. Directive 2005/84/EC of the European Parliament and of the Council of 14 December 2005 amending for the 22nd time Council Directive 76/69/EEC on the approximation of the laws, regulations and administrative provisions of the Men Der States relating to restrictions on the marketing and use of certain dangerous substances and preparations (phthalates in toys and childcare articles) Offic. J. Eur. Union. 2005;48:40–43. [Google Scholar]
- Forner-Piquer I., Maradonna F., Gioacchini G., Santangeli S., Allarà M., Piscitelli F., Habibi H.R., Di Marzo V., Carnevali O. Dose-specific effects of di-isononyl phthalate on the endocannabinoid system and on liver of female zebrafish. Endocrinology. 2017;158:3462–3476. doi: 10.1210/en.2017-00458. [DOI] [PubMed] [Google Scholar]
- Francis G.A., Fayard E., Picard F., Auwerx J. Nuclear receptors and the control of metabolism. Annu. Rev. Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- Fromme H., Schütze A., Lahrz T., Kraft M., Fembacher L., Siewering S., Burkardt R., Dietrich S., Koch H., Völkel W. Non-phthalate plasticizers in German daycare centers and human biomonitoring of DINCH metabolites in children attending the centers (LUPE 3) Int. J. Hyg. Environ. Health. 2016;219:33–39. doi: 10.1016/j.ijheh.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Gallo L.I., Lagadari M., Piwien-Pilipuk G., Galigniana M.D. The 90-kDa heat-shock protein (Hsp90)-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J. Biol. Chem. 2011;286:30152–30160. doi: 10.1074/jbc.M111.256610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A., Crews D., Doan L., La Merrill M., Patisaul H., Zota A. Introduction to endocrine disrupting chemicals (EDCs)—a guide for public interest organizations and policy makers. Endocr. Soc. 2014:1–69. [Google Scholar]
- Ho J.C., Hsiao C., Kawakami K., William K. Triclosan (TCS) exposure impairs lipid metabolism in zebrafish embryos. Aquat. Toxicol. 2016;173:29–35. doi: 10.1016/j.aquatox.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Howe K., Clark M.D., Torroja C.F., Torrance J., Berthelot C., Muffato M., Collins J.E., Humphray S., McLaren K., Matthews L. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Jiang K., Tang X., Liu H., Zhang H., Yang X., Nie X., Luo H. Chronic exposure to di-n-butyl phthalate causes reproductive toxicity in zebrafish. J. Appl. Toxicol. 2020;40:1694–1703. doi: 10.1002/jat.4030. [DOI] [PubMed] [Google Scholar]
- Huff M., da Silveira W.A., Carnevali O., Renaud L., Hardiman G. Systems analysis of the liver transcriptome in adult male zebrafish exposed to the plasticizer (2-ethylhexyl) phthalate (DEHP) Sci. Rep. 2018;8:2118. doi: 10.1038/s41598-018-20266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola J.J., Oie L., Nafstad P., Botten G., Samuelsen S.O., Magnus P. Interior surface materials in the home and the development of bronchial obstruction in young children in Oslo, Norway. Am. J. Publ. Health. 1999;89:188–192. doi: 10.2105/ajph.89.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola J.J., Verkasalo P.K., Jaakkola N. Plastic wall materials in the home and respiratory health in young children. Am. J. Publ. Health. 2000;90:797–799. doi: 10.2105/ajph.90.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe B.I., Panz V.R., Raal F.J. From lipodystrophy syndromes to diabetes mellitus. Lancet. 2001;357:1379–1381. doi: 10.1016/S0140-6736(00)04616-X. [DOI] [PubMed] [Google Scholar]
- Kalueff A.V., Echevarria D.J., Homechaudhuri S., Stewart A.M., Collier A.D., Kaluyeva A.A., Li S., Liu Y., Chen P., Wang J.J. Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquat. Toxicol. 2016;170:297–309. doi: 10.1016/j.aquatox.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Kasper-Sonnenberg M., Koch H.M., Apel P., Rüther M., Pälmke C., Brüning T., Kolossa-Gehring M. Time trend of exposure to the phthalate plasticizer substitute DINCH in Germany from 1999 to 2017: biomonitoring data on young adults from the Environmental Specimen Bank (ESB) Int. J. Hyg. Environ. Health. 2019;222:1084–1092. doi: 10.1016/j.ijheh.2019.07.011. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Park M.J. Phthalate exposure and childhood obesity. Ann. Pediatr. Endocrinol. Metab. 2014;19:69–75. doi: 10.6065/apem.2014.19.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H.M., Schutze A., Palmke C., Angerer J., Bruning T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH((R))) in humans after single oral doses. Arch. Toxicol. 2013;87:799–806. doi: 10.1007/s00204-012-0990-4. [DOI] [PubMed] [Google Scholar]
- Korade Z., Folkes O.M., Harrison F.E. Behavioral and serotonergic response changes in the Dhcr7-HET mouse model of Smith-Lemli-Opitz syndrome. Pharmacol. Biochem. Behav. 2013;106:101–108. doi: 10.1016/j.pbb.2013.03.007. [DOI] [PubMed] [Google Scholar]
- la Cour Poulsen L., Siersbæk M., Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin. Cell Dev. Biol. 2012;23:631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Larsson K., Lindh C.H., Jönsson B.A., Giovanoulis G., Bibi M., Bottai M., Bergström A., Berglund M. Phthalates, non-phthalate plasticizers and bisphenols in Swedish preschool dust in relation to children's exposure. Environ. Int. 2017;102:114–124. doi: 10.1016/j.envint.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Mathews E.S., Mawdsley D.J., Walker M., Hines J.H., Pozzoli M., Appel B. Mutation of 3-hydroxy-3-methylglutaryl CoA synthase I reveals requirements for isoprenoid and cholesterol synthesis in oligodendrocyte migration arrest, axon wrapping, and myelin gene expression. J. Neurosci. 2014;34:3402–3412. doi: 10.1523/JNEUROSCI.4587-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely M.J., Edwards K.L., Marcovina S.M., Brunzell J.D., Motulsky A.G., Austin M.A. Lipoprotein and apolipoprotein abnormalities in familial combined hyperlipidemia: a 20-year prospective study. Atherosclerosis. 2001;159:471–481. doi: 10.1016/s0021-9150(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Messerlian C., Souter I., Gaskins A.J., Williams P.L., Ford J.B., Chiu Y.-H., Calafat A.M., Hauser R. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Hum. Reprod. 2016;31:75–83. doi: 10.1093/humrep/dev292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez-Alarcón L., Souter I., Chiu Y.H., Williams P.L., Ford J.B., Ye X., Calafat A.M., Hauser R. Urinary concentrations of cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester, a metabolite of the non-phthalate plasticizer di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), and markers of ovarian response among women attending a fertility center. Environ. Res. 2016;151:595–600. doi: 10.1016/j.envres.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A., Edwards A., Bellinger D.C., Hauser R. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology. 2014;41:112–122. doi: 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Muhammad S., Zhang Z., Pavase T.R., Guo H. Long-term exposure of two plasticizers di (2-ethylhexyl) phthalate (DEHP) and acetyl tributyl citrate (ATBC): toxic effects on gonadal development and reproduction of zebrafish (“Danio rerio”) Indian J. Mar. Sci. 2018;47(1/2) [Google Scholar]
- Nardelli T.C., Albert O., Lalancette C., Culty M., Hales B.F., Robaire B. In utero and lactational exposure study in rats to identify replacements for di (2-ethylhexyl) phthalate. 7, 1-13. Sci. Rep. 2017;7(1):3862. doi: 10.1038/s41598-017-03979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli T.C., Erythropel H.C., Robaire B. Toxicogenomic screening of replacements for di(2-ethylhexyl) phthalate (DEHP) using the immortalized TM4 sertoli cell line. PloS One. 2015;10 doi: 10.1371/journal.pone.0138421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale B.M., Fagerness J., Reynolds R., Sobrin L., Parker M., Raychaudhuri S., Tan P.L., Oh E.C., Merriam J.E., Souied E. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc. Natl. Acad. Sci. U. S. A. 2010;107:7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell E.M., Lohoff F.W. Proprotein convertase subtilisin/kexin type 9 (PCSK9) in the brain and relevance for neuropsychiatric disorders. Front. Neurosci. 2020;14:609. doi: 10.3389/fnins.2020.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis J.P., Zeituni E.M., Thierer J.H., Anderson J.L., Brown A.C., Boehm E.D., Cerchione D.M., Ceasrine A.M., Avraham-Davidi I., Tempelhof H., Yaniv K., Farber S.A. Zebrafish as a model for apolipoprotein biology: comprehensive expression analysis and a role for ApoA-IV in regulating food intake. Dis. Model Mech. 2015;8:295–309. doi: 10.1242/dmm.018754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomatto V., Cottone E., Cocci P., Mozzicafreddo M., Mosconi G., Nelson E.R., Palermo F.A., Bovolin P. Plasticizers used in food-contact materials affect adipogenesis in 3T3-L1 cells. J. Steroid Biochem. Mol. Biol. 2018;178:322–332. doi: 10.1016/j.jsbmb.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Liu J., Lin Z., Chen X., Yuan L., Shen G., Yang W., Wang D., Huang Y., Pang S. Evaluation of the spinal effects of phthalates in a zebrafish embryo assay. Chemosphere. 2020;249:126144. doi: 10.1016/j.chemosphere.2020.126144. [DOI] [PubMed] [Google Scholar]
- Ramos M.G., Heffernan A., Toms L., Calafat A., Ye X., Hobson P., Broomhall S., Mueller J. Concentrations of phthalates and DINCH metabolites in pooled urine from Queensland, Australia. Environ. Int. 2016;88:179–186. doi: 10.1016/j.envint.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttkay-Nedecky B., Nejdl L., Gumulec J., Zitka O., Masarik M., Eckschlager T., Stiborova M., Adam V., Kizek R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013;14:6044–6066. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G., Quintes S., Nave K.A. Cholesterol: a novel regulatory role in myelin formation. Neuroscientist. 2011;17:79–93. doi: 10.1177/1073858410373835. [DOI] [PubMed] [Google Scholar]
- Salvador J.M., Brown-Clay J.D., Fornace A.J., Jr. Gadd45 in stress signaling, cell cycle control, and apoptosis. Adv. Exp. Med. Biol. 2013;793:1–19. doi: 10.1007/978-1-4614-8289-5_1. [DOI] [PubMed] [Google Scholar]
- Santoro M.M., Metabolism Zebrafish as a model to explore cell metabolism. Trends Endocrinol. Metab. 2014;25:546–554. doi: 10.1016/j.tem.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Saravanabhavan G., Guay M., Langlois É., Giroux S., Murray J., Haines D. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian health measures survey (2007–2009) Int. J. Hyg Environ. Health. 2013;216:652–661. doi: 10.1016/j.ijheh.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schütze A., Kolossa-Gehring M., Apel P., Brüning T., Koch H.M. Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll® DINCH® in 24 h urine samples from the German Environmental Specimen Bank. Int. J. Hyg Environ. Health. 2014;217:421–426. doi: 10.1016/j.ijheh.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Schwedler G., Conrad A., Rucic E., Koch H.M., Leng G., Schulz C., Schmied-Tobies M.I., Kolossa-Gehring M. Hexamoll® DINCH and DPHP metabolites in urine of children and adolescents in Germany. Human biomonitoring results of the German Environmental Survey GerES V, 2014–2017. Int. J. Hyg Environ. Health. 2020;229:113397. doi: 10.1016/j.ijheh.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Seth A., Stemple D.L., Barroso I. The emerging use of zebrafish to model metabolic disease. Dis. Model Mech. 2013;6:1080–1088. doi: 10.1242/dmm.011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H. Sterol regulatory element-binding protein-1 as a dominant transcription factor for gene regulation of lipogenic enzymes in the liver. Trends Cardiovasc. Med. 2000;10:275–278. doi: 10.1016/s1050-1738(00)00079-7. [DOI] [PubMed] [Google Scholar]
- Silva M.J., Jia T., Samandar E., Preau J.L., Jr., Calafat A.M. Environmental exposure to the plasticizer 1, 2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in US adults (2000—2012) Environ. Res. 2013;126:159–163. doi: 10.1016/j.envres.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Wen H., Zeng L.-B., Jiang M., Wu F., Liu W., Yang C.-G. Changes in the activities and mRNA expression levels of lipoprotein lipase (LPL), hormone-sensitive lipase (HSL) and fatty acid synthetase (FAS) of Nile tilapia (Oreochromis niloticus) during fasting and re-feeding. Aquaculture. 2013;400:29–35. [Google Scholar]
- Tocher D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003;11:107–184. [Google Scholar]
- Urbancova K., Lankova D., Sram R.J., Hajslova J., Pulkrabova J. Urinary metabolites of phthalates and di-iso-nonyl cyclohexane-1, 2-dicarboxylate (DINCH)–Czech mothers' and newborns' exposure biomarkers. Environ. Res. 2019;173:342–348. doi: 10.1016/j.envres.2019.03.067. [DOI] [PubMed] [Google Scholar]
- Varga T., Czimmerer Z., Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos A.L., Silva M.J., Louro H. In vitro exposure to the next-generation plasticizer diisononyl cyclohexane-1, 2-dicarboxylate (DINCH): cytotoxicity and genotoxicity assessment in human cells. J. Toxicol. Environ. Health. 2019;82:526–536. doi: 10.1080/15287394.2019.1634376. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Yaginuma R., Ikejima K., Miyazaki A. Liver diseases and metabolic syndrome. J. Gastroenterol. 2008;43:509–518. doi: 10.1007/s00535-008-2193-6. [DOI] [PubMed] [Google Scholar]
- Willnow T.E. The low-density lipoprotein receptor gene family: multiple roles in lipid metabolism. J. Mol. Med. 1999;77:306–315. doi: 10.1007/s001090050356. [DOI] [PubMed] [Google Scholar]
- Wypych G. ChemTec Publishing; 2004. Handbook of Plasticizers. [Google Scholar]
- Xia B., Zhu Q., Zhao Y., Ge W., Zhao Y., Song Q., Zhou Y., Shi H., Zhang Y. Phthalate exposure and childhood overweight and obesity: urinary metabolomic evidence. Environ. Int. 2018;121:159–168. doi: 10.1016/j.envint.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Yang W.K., Chiang L.F., Tan S.W., Chen P.J. Environmentally relevant concentrations of di(2-ethylhexyl)phthalate exposure alter larval growth and locomotion in medaka fish via multiple pathways. Sci. Total Environ. 2018;640–641:512–522. doi: 10.1016/j.scitotenv.2018.05.312. [DOI] [PubMed] [Google Scholar]
- Zhang C., Yang X., He Z., Zhong Q., Guo J., Hu X.J., Xiong L., Liu D. Influence of BBP exposure on nervous system and antioxidant system in zebrafish. Ecotoxicology. 2014;23:1854–1857. doi: 10.1007/s10646-014-1351-2. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 2015;6:254–264. doi: 10.1007/s13238-014-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Sun W., Duan X., Duan Y., Sun H. Promoting differentiation and lipid metabolism are the primary effects for DINP exposure on 3T3-L1 preadipocytes. Environ. Pollut. 2019;255:113154. doi: 10.1016/j.envpol.2019.113154. [DOI] [PubMed] [Google Scholar]
- Zota A.R., Calafat A.M., Woodruff T.J. Temporal trends in phthalate exposures: findings from the national health and nutrition examination survey, 2001–2010. Environ. Health Perspect. 2014;122:235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table
Data Availability Statement
Data included in article/supplementary material/referenced in article.