Introduction
Lead- and pacemaker pocket–related complications are of relevance in the adult congenital heart disease population, and a significant number of those subjects present with intracardiac obstacles potentially making transcatheter pacemaker implantation a challenge. The intention of the present report therefore is to aid decision-making when considering leadless pacing in adult individuals with congenital heart disease and intracardiac obstacles.
Case report
We report on an 84-year-old woman who had had interventional closure of a muscular ventricular septal defect (VSD) at the age of 77 years owing to progressive heart failure. Coronary artery disease had been ruled out by coronary angiography prior to interventional VSD closure. An 8 mm Amplatzer VSD occluder (Abbott, Abbott Park, IL) had been implanted at that time with no residual left-to-right shunting. Seven years later, the patient presented with recurrent syncope to our adult congenital heart disease outpatient clinic. While resting electrocardiogram revealed first-degree atrioventricular block with a PR interval of 220 ms without any additional abnormalities, Holter monitoring demonstrated intermittent high-degree atrioventricular block with pauses up to 5.2 seconds associated with dizziness and near-syncope.
According to current guidelines, implantation of a permanent pacemaker was indicated in this patient because of symptomatic bradycardia owing to atrioventricular block.1 As bradycardia was intermittent and frequent ventricular pacing was not expected, decision for implantation of a single-chamber ventricular pacemaker was made.1 In order to avoid lead problems in this 84-year-old woman, who was in otherwise excellent condition, the patient was consented for implantation of a transcatheter pacing system (Micra TPS, Medtronic, Minneapolis, MN). Preprocedurally, 2-dimensional transthoracic echocardiography was performed to visualize the patient’s individual cardiac anatomy and the VSD occluder.
The procedure was performed under general anesthesia, and prior to implantation of the Micra TPS, coronary angiography was performed to rule out coronary artery disease as the underlying cause for intermittent high-degree atrioventricular block. For Micra TPS implantation a right femoral venous access was used. In order to delineate the targeted implantation site within the right ventricle underneath the VSD occluder, angiography of the right ventricle was performed (Figure 1). Right heart catheterization ruled out residual left-to-right shunting. Subsequently, the Micra TPS was implanted according to the manufacturer’s recommendations. During implantation, the Micra TPS delivery system was carefully advanced to the targeted implantation site in order to avoid interference with the VSD occluder. After verification of an adequate device position and after performing a pull-and-hold test showing proper engagement of all 4 tines, the tether and subsequently the delivery system were removed (Figure 2). Device interrogation demonstrated excellent electrical properties with a pacing threshold of 0.63 V @ 0.24 ms and an intrinsic R-wave amplitude of 7.9 mV; electrode impedance was 570 Ω. After conclusion of the procedure the patient was monitored for another 24 hours in hospital. Echocardiography was repeatedly performed to rule out pericardial effusion, and device interrogation prior to discharge confirmed adequate function. The device was programmed in a VVI mode with a lower rate limit of 50/min. After discharge from the hospital, the patient did well and was followed 4 weeks and 3 months after Micra TPS implantation at our outpatient clinic. No new episodes of dizziness or syncope were reported, and performance of the Micra TPS was excellent with good ventricular sensing and a low pacing threshold. Ventricular pacing burden was between 5% and 10% during follow-up.
Figure 1.
Angiography of the right ventricle prior to implantation of the Micra (Medtronic, Minneapolis, MN) transcatheter pacing system device (frontal 17° right anterior oblique, 1° caudal). Target implantation site was the niche underneath the Amplatzer ventricular septal defect occluder (asterisk, dotted line).
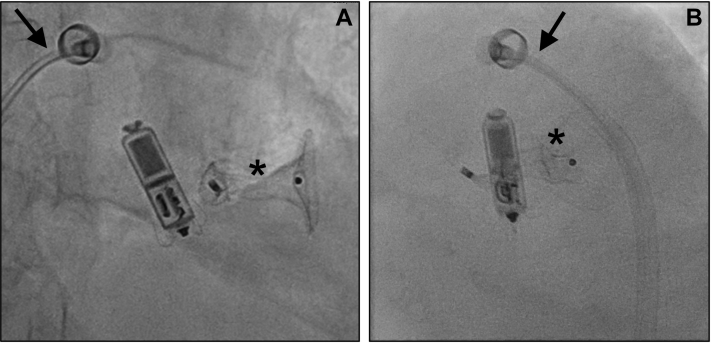
Figure 2.
Micra (Medtronic, Minneapolis, MN) transcatheter pacing system in close proximity to the Amplatzer ventricular septal defect occluder (asterisk) immediately after deployment from the Micra delivery system (arrow; A: frontal 17° right anterior oblique, 1° caudal; B: 41° left anterior oblique).
Discussion
The advent of leadless pacing revolutionized pacemaker therapy, mitigating many risks and problems associated with pacemaker leads and device pockets. Currently, the Micra TPS (Medtronic) is the only approved and commercially available leadless pacing system.2,3 Whereas evidence on the benefits of leadless pacing in the adult population with structurally normal heart is constantly growing, data on leadless pacing in adult congenital heart disease are sparse and restricted to single case reports or small case series.4 We report, for the first time, implantation of a leadless pacing system in a patient with congenital heart disease who harbored a VSD occluder within the interventricular septum. The challenge in the present case was implantation of the Micra TPS within the interventricular septum after transcatheter VSD closure with an Amplatzer VSD occluder. Issues to consider preprocedurally were (1) firm fixation of the Micra TPS tines within the right ventricular (RV) septum in close proximity to the VSD occluder, having limited space compared to a structurally normal RV septum; and (2) electrical interference of the leadless pacing system with the VSD occluder (eg, noise resulting in ventricular oversensing). With respect to preprocedural imaging, we performed transthoracic echocardiography only, as this sufficed to delineate RV anatomy and the VSD occluder. However, in patients with less favorable transthoracic imaging conditions, one should not hesitate to perform transesophageal echocardiography, a computed tomography scan, or cardiac magnetic resonance imaging in order to identify a possible implantation site. We aimed for deploying the Micra TPS in the niche underneath the VSD occluder, as this site was assumed to result in a firm fixation of the Micra TPS tines within the right ventricle, avoiding interference with the VSD occluder. However, we finally implanted the Micra TPS at a more basal site than initially attempted (Figure 2), as it turned out to be rather time and fluoroscopy consuming to direct the Micra TPS to a more apical position. In contrast, the finally chosen Micra TPS position could be reached effortlessly, electrical properties of the Micra TPS were adequate, and both devices did not touch each other. Even if the tines of the Micra TPS touched the VSD occluder, the likelihood of electrical interference would be low, as the electrically active parts of the Micra TPS are located at the very tip (cathode) and at the proximal end of the device body (band-shaped anode). It is important to remember that the tines of the Micra TPS itself are electrically inactive and that contact of the tines with the VSD device (or any other intracardiac device) is unlikely to result in electrical interference.
It might have been of advantage for implantation of the Micra TPS at its present site that the right ventricular disc of the VSD occluder was not fully deployed (Figures 1 and 2). If implantation of the Micra TPS within the basal septum beneath the VSD occluder had been impossible, a more superior implantation site in a midseptal/proximal outflow tract location could be considered as alternative implantation site. Implantation of Micra TPS within the RV outflow tract has recently been reported to be safe and feasible.5 The straightforward implantation procedure without complications and the excellent electrical properties of the Micra TPS during follow-up confirmed our approach.
In conclusion, implantation of a Micra TPS was safely performed in a patient after percutaneous implantation of a VSD occluder. The adult congenital heart disease population is constantly growing and a significant number of those patients will require permanent pacing in the future. As device pocket–related and lead-related complications are common in this population,6 the use of leadless pacing systems may reduce the incidence of pacemaker-related complications.
Key Teaching Points.
-
•
Intracardiac devices might represent obstacles interfering with transvenous implantation of a leadless pacemaker system.
-
•
Issues to be considered prior to the procedure are identification of a suitable implantation site and device-device interference.
-
•
Implantation of a leadless pacemaker can be safely performed in patients with congenital heart disease already harboring intracardiac devices.
Footnotes
Funding sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors acknowledge support by the Open Access Publication Funds of the Göttingen University. Conflict of interest: UK: none; DZ: none; TP: none; MJM: none.
References
- 1.Kusumoto F.M., Schoenfeld M.H., Barrett C. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. Circulation. 2019;140:e333–e381. doi: 10.1161/CIR.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 2.Tjong F.V., Reddy V.Y. Permanent leadless cardiac pacemaker therapy: a comprehensive review. Circulation. 2017;135:1458–1470. doi: 10.1161/CIRCULATIONAHA.116.025037. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds D., Duray G.Z., Omar R. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 4.Russell M.R., Galloti R., Moore J.P. Initial experience with transcatheter pacemaker implantation for adults with congenital heart disease. J Cardiovasc Electrophysiol. 2019;30:1362–1366. doi: 10.1111/jce.13961. [DOI] [PubMed] [Google Scholar]
- 5.Garweg C., Vandenberk B., Foulon S. Leadless pacing with Micra TPS: a comparison between right ventricular outflow tract, mid-septal, and apical implant sites. J Cardiovasc Electrophysiol. 2019;30:2002–2011. doi: 10.1111/jce.14083. [DOI] [PubMed] [Google Scholar]
- 6.Krause U., Müller M.J., Wilberg Y. Transvenous and non-transvenous implantable cardioverter-defibrillators in children, adolescents, and adults with congenital heart disease: who is at risk for appropriate and inappropriate shocks? Europace. 2019;21:106–113. doi: 10.1093/europace/euy219. [DOI] [PubMed] [Google Scholar]