Introduction
Recent studies have brought the potential role of smartwatches in pulse rate and arrhythmia monitoring to public attention,1, 2, 3 potentially leading to the misconception that the technology provides information of diagnostic quality. While some of the latest smartwatches can now record single-lead electrograms,4 the majority owned by the general public use photoplethysmography (PPG) to measure pulse rates. However, in the presence of arrhythmias causing reduced left ventricular output and hence pulsatile microvasculature blood volume at the periphery, PPG may the underdetect pulse rate, analogous to the well-recognized clinical finding of an apical–radial deficit. We report a case of this occurring in routine clinical practice in a patient with premature ventricular complexes (PVCs).
Case report
A 50-year-old man was evaluated in the emergency department after anxiety associated with a reading of bradycardia on his personal smartwatch. The patient had a known history of paroxysmal atrial fibrillation, managed by regular verapamil. Further, between regular visits to his treating cardiologist the patient used the pulse rate measurement function of his personal smartwatch to passively monitor for episodes of acute arrhythmia.
The patient had experienced palpitations on the night prior to the day of admission, and was awoken from sleep at 4 AM by palpitations and chest pain. The patient sought the measurement of his pulse rate according to his personal smartwatch, which reported a range of 33–43 beats per minute. Fearing imminent cardiac emergency, the patient rushed to the hospital’s emergency department in a state of anxiety.
Complete history and physical examination were conducted within evaluation at the emergency department. The patient was asymptomatic at presentation apart from mild anxiety caused by the pulse rate reading on his smartwatch. The blood pressure was 127/79 mm Hg, and the heart rate 69 beats per minute and irregular. The respiratory rate was 20 breaths per minute and oxygen saturation 95% on room air. However, the patient’s smartwatch was still reporting a pulse rate under 40 beats per minute throughout evaluation, causing the emergency department staff considerable alarm. Accordingly, emergency department staff documented a potential requirement of a pacemaker, and admitted the patient for inpatient cardiology evaluation.
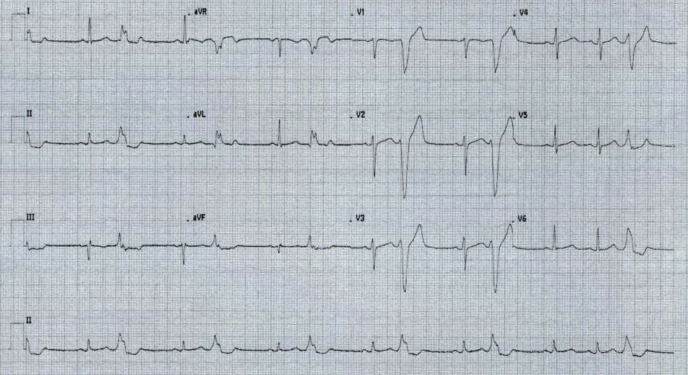
Within inpatient cardiology evaluation, an initial 12-lead electrocardiogram (ECG) was conducted while his smartwatch was registering a pulse rate of below 40 beats per minute. This initial ECG showed frequent monomorphic PVCs with a left bundle branch block pattern and a normal axis (Figure 1). This eliminated other differential diagnoses of sick sinus syndrome and an acute episode of atrial fibrillation as explanations of the patient’s irregular pulse. However, a repeat ECG was conducted 8 minutes later, and showed normal sinus rhythm (Figure 2). Serial blood levels of troponins were all normal. A basic metabolic panel was also conducted, and all components also returned normal.
Figure 1.
Initial 12-lead electrocardiogram showing premature ventricular complexes.
Figure 2.
Repeat electrocardiogram showing sinus rhythm.
The patient was monitored overnight, and the remainder of his admission was uneventful. Upon cardiology evaluation the following morning, the patient was asymptomatic and physical examination was unremarkable. The pulse rate reported by his smartwatch had returned to within normal range. Given that the patient was not experiencing syncope or presyncope, and no other concerns were raised on inpatient cardiology evaluation, he was discharged later that next day.
Upon discharge no changes were made to the management plan for the patient’s known paroxysmal atrial fibrillation. However, given the anxiety that the patient experienced during the described episode, a course of paroxetine was prescribed, to be evaluated by the patient’s primary care physician within the week. The patient was also instructed to follow up with his regular cardiologist within 2 weeks of discharge regarding management of the newly diagnosed PVCs.
The patient did not experience any complications postdischarge and continued the management plan that was being undertaken prior to the described episode. No bradycardia has been detected and the patient has not required a pacemaker.
Discussion
Through hemodynamic compromise of diastolic filling, PVCs transiently decrease stroke volume and cardiac output, underperfusing the microvasculature and creating a clinically appreciable pulse deficit that may not be detected by smartwatches using PPG. As smartwatches gain more attention and patients begin providing readings from these devices alongside their clinical history, it is increasingly important that clinicians be aware of the technology’s limitations and be cautious when called to make decisions from the associated data.
While some smartwatches can now record single-lead electrograms through active engagement from the patient,4 the majority owned by the general public with pulse rate measurement capability use PPG. PPG is an optical measurement technique that noninvasively detects blood volume changes in the microvasculature.5 When employed at the wrist, such as within smartwatches, this allows detection and measurement of the wearer’s peripheral pulse.6 Accordingly, phenomena that alter the blood volume in the microvasculature lead to corresponding change in the detection of “pulse” by the PPG technology. Recent studies have investigated the potential use of PPG in passive detection and monitoring of arrhythmia1, 2, 3; however, the current evidence base is insufficient to prompt implementation within accepted guidelines.
PVCs may manifest as an irregular pulse or as an incidental finding on ECG, and can appear as bradycardia when assessed on palpable pulse alone.7 By reducing the time for diastolic filling, PVCs transiently decrease stroke volume and cardiac output, leading to microvasculature underperfusion and a clinically appreciable pulse deficit.8
This insufficiency is compounded by the misconception that smartwatches using PPG technology provide reliable arrhythmia monitoring. Generally, ECG monitoring of PVC burden and structural evaluation via echocardiogram over 24 hours are recommended as part of the management plan for PVCs.7 In instances such as our case where a patient relies on their smartwatch for continuous monitoring of a known arrhythmia, false readings of pulse rate can cause considerable anxiety and distress as the data suggest the possibility of impending cardiac emergency. Further, as highlighted in our report, reliance on the data by the treating clinical team unnecessarily complicates evaluation and increases the risk of erroneous management being pursued. Rather than relying solely on PPG technology, single-lead electrograms or more recently available 6-lead electrogram technology9 should be used where possible.
Conclusion
Smartwatches are likely to continue to gain attention for the detection and monitoring of arrhythmia, and investigation in this field is to be encouraged. However, as presenting patients begin to provide readings from these devices alongside their clinical history, it is increasingly important that clinicians be aware of the technology’s limitations and be cautious when called to make decisions from the associated data.
Key Teaching Points.
-
•
Photoplethysmography is an optical measurement technique that noninvasively detects blood volume changes in the microvasculature, and when employed at the wrist, such as within smartwatches, this allows detection and measurement of the wearer’s peripheral pulse.
-
•
By reducing the time for diastolic filling, premature ventricular complexes transiently decrease stroke volume and cardiac output, leading to microvasculature underperfusion and a clinically appreciable pulse deficit that may not be detected by photoplethysmography.
-
•
There are issues with reliance on presently available smartwatches for arrhythmia monitoring. In instances such as our case where a patient relies on their smartwatch for continuous monitoring of a known arrhythmia, false readings of pulse rate can cause considerable anxiety and distress as the data suggest the possibility of impending cardiac emergency; reliance on the data by the treating clinical team unnecessarily complicates evaluation and increases the risk of erroneous management being pursued.
Footnotes
Financial support: None.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Perez M.V., Mahaffey K.W., Hedlin H. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tison G.H., Sanchez J.M., Ballinger B. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018;3:409–416. doi: 10.1001/jamacardio.2018.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bumgarner J.M., Lambert C.T., Hussein A.A. Smartwatch algorithm for automated detection of atrial fibrillation. J Am Coll Cardiol. 2018;71:2381–2388. doi: 10.1016/j.jacc.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Spaccarotella C.A.M., Polimeni A., Migliarino S. Multichannel electrocardiograms obtained by a smartwatch for the diagnosis of ST-segment changes. JAMA Cardiol. 2020;5:1176–1180. doi: 10.1001/jamacardio.2020.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1–R39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z., Pi Z., Liu B. TROIKA: a general framework for heart rate monitoring using wrist-type photoplethysmographic signals during intensive physical exercise. IEEE Trans Biomed Eng. 2015;62:522–531. doi: 10.1109/TBME.2014.2359372. [DOI] [PubMed] [Google Scholar]
- 7.Marcus G.M. Evaluation and management of premature ventricular complexes. Circulation. 2020;141:1404–1418. doi: 10.1161/CIRCULATIONAHA.119.042434. [DOI] [PubMed] [Google Scholar]
- 8.Cohn K., Kryda W. The influence of ectopic beats and tachyarrhythmias on stroke volume and cardiac output. J Electrocardiol. 1981;14:207–218. doi: 10.1016/s0022-0736(81)80001-5. [DOI] [PubMed] [Google Scholar]
- 9.Albert D. A six-lead heart monitor on your smartphone: an interview with David Albert. Future Cardiol. 2020;16:9–11. doi: 10.2217/fca-2019-0078. [DOI] [PubMed] [Google Scholar]