Abstract
Metabolic syndrome is a group of risk factors including high blood glucose, dyslipidemia, high blood pressure, and high body weight. It can increase the risk of diabetes and cardiovascular disorders, which are the important reasons for death around the world. Nowadays, there are numerous demands for herbal medicine because of less harmful effects and more useful effects in comparison with chemical options. Ginseng is one of the most famous herbs used as a drug for a variety of disorders in humans. The antihyperlipidemia, antihypertension, antihyperglycemic, and anti‐obesity effects of ginseng and its active constituents such as ginsenosides have been shown in different studies. In this review article, the different in vitro, in vivo, and human studies concerning the effects of ginseng and its active constituents in metabolic syndrome have been summarized. According to these studies, ginseng can control metabolic syndrome and related diseases.
Keywords: diabetes, ginseng, herbal medicine, hypertension, metabolic syndrome, Panax ginseng
Metabolic syndrome is a group of risk factors including high blood glucose, dyslipidemia, high blood pressure, and high body weight. Ginseng is one of the most famous herbs used as a drug for a variety of disorders in humans. According to the different in vitro, in vivo, and human studies, ginseng can control metabolic syndrome and related diseases.
1. INTRODUCTION
Metabolic syndrome or syndrome X is the name given to the collection of clinical conditions including overweight, high blood pressure, high blood glucose (or type 2 diabetes mellitus), and hyperlipidemia (Chen et al., 2017).
Metabolic syndrome is the most important risk factor for atherosclerosis in response to chronic inflammation and vascular endothelial dysfunction. Metabolic syndrome increases the risk of cardiovascular diseases. Cardiovascular diseases are the main reasons for mortality around the world (Kang & Park, 2012). The metabolic syndrome is rising due to a sedentary lifestyle. Children, adolescents, and young women with polycystic ovary syndrome are at risk for metabolic syndrome (Vassallo et al., 2016).
Medicine herbs, because of their potential efficacy in improving and holding human health, low cost, and adverse effects, have been the focus of attention. Studies have been shown that several plants and their active constituents can exert beneficial effects on metabolic syndrome. For example, grapes (Vitis vinifera), a source of polyphenol antioxidants, is useful for preventing the risk factors involved in metabolic syndromes such as hyperlipidemia, hypertension, and hyperglycemia (Akaberi & Hosseinzadeh, 2016). Garlic (Allium sativum) has been documented in the treatment of metabolic syndrome as it showed hypoglycemic, hypotensive, and hypolipidemic activities (Hosseini & Hosseinzadeh, 2015). Rosemary (Rosmarinus officinalis L.) is a source of phenolic phytochemicals having considerable anti‐inflammatory, antioxidant, hypoglycemic, hypolipidemic, and hypotensive effects (Hassani et al., 2016).
Panax ginseng belonging to the genus Panax and the family Araliaceae is one of the popular pharmaceutical and perennial plant species. The plant is cultivating in China, Japan, and Korea (Lee et al., 2017).
Its curative function for the first time was seen in the Chinese medicine monograph (Yun, 2001). Ginseng is one of the most famous herbs used as a drug and nutritional supplements for a variety of disorders in humans (Liu et al., 2019b).
“Panax” taken from the word “panacea” in Greek which means “cure‐all.” Ginseng has been proven to have a wide variety of therapeutic effects. Red ginseng and white ginseng are two prevalent products of ginseng. Red ginseng provided during the process of steaming, and dried white ginseng provided by air‐drying (Karmazyn et al., 2011). Red ginseng has shown efficacy for the remedy of a wide range of disorders including hyperglycemia (Nam et al., 2019).
The important bioactive structures in ginseng are ginsenosides, the different types of triterpene saponins including oleanane‐type ones and dammarane‐type ones, that classified according to their chemical skeleton structures (Han et al., 2006). Until now, over than 150 ginsenosides have been purified from ginseng; in Panax ginseng, 40 kinds of ginsenosides have been found (Christensen, 2008). Some of the most active constituents of ginseng are structurally shown in Figure 1. Ginsenosides are the principal group of effective compounds in ginseng. They demonstrate unique biological activity and broad pharmacological properties including anticancer, anti‐inflammatory, antioxidant, and anti‐apoptotic effects (Razgonova et al., 2019).
FIGURE 1.
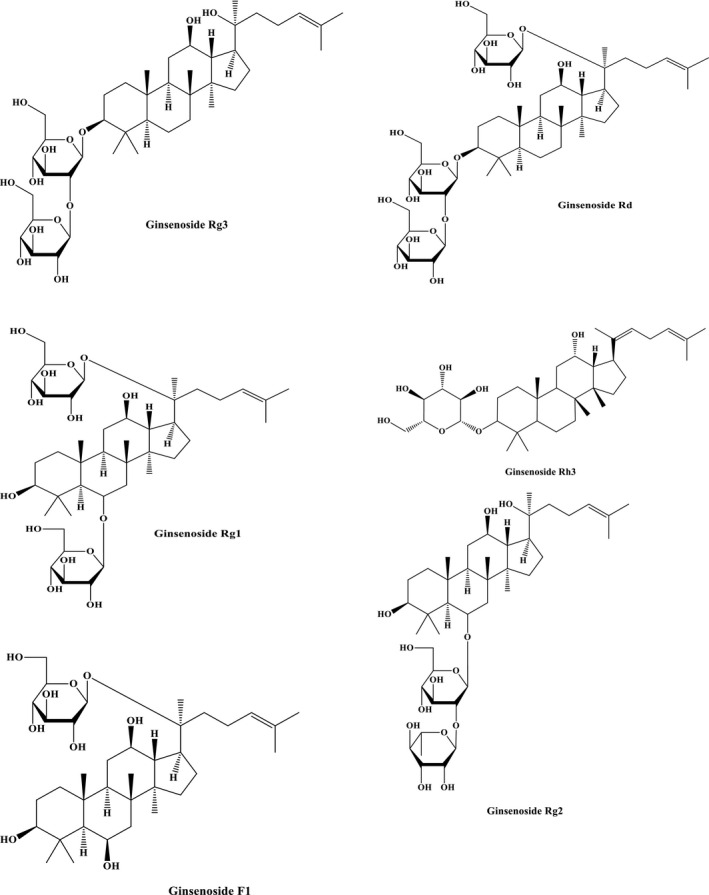
Some of the pharmacologically active constituents of ginseng
According to the modern pharmacological, preclinical, and clinical studies, ginseng has marvelous beneficial effects in multiple neurological and neurodegenerative diseases and it has various biological activities such as antihypertension, antitumor, anti‐anxiety, and immune‐modulatory activities, so it has several protective mechanisms (Liu et al., 2019b). Furthermore, ginseng has wide curative potentials including diminishing blood glucose, modifying blood lipids, and enhancing insulin sensitivity (Imanshahidi & Hosseinzadeh, 2008). Therefore, ginseng is effective in the treatment of different components of metabolic syndrome.
In this review, different relevant studies to realize the role of ginseng and its active components in metabolic syndrome risk factors including hypertension, hyperglycemia, hyperlipidemia, and obesity have been explained.
2. METHODOLOGY
This review was carried out by the means of the databases of Scopus, PubMed, and Web of Science. All the articles in this review were collected from 2009 to 2019. The search keywords contain “metabolic syndrome”, hypertension, “blood pressure”, hypotensive, antihypertensive, hypertensive, diabetes, hyperglycemia, insulin, hypoglycemic, antihyperglycemic, antidiabetic, “blood glucose”, dyslipidemia, hyperlipidemia, "high cholesterol", "high triglyceride", hypercholesterolemia, hypertriglyceridemia,atherogenic, atherosclerosis, obesity, overweight, appetite, anti‐obesity, “weight loss”, “bodyweight”, “food intake”, “feed intake”, ginseng, ginsenoside, and panaxsoide.
3. EFFECT ON DYSLIPIDEMIA
Hyperlipidemia is found as an important risk factor for heart and vessel disorders, which is one of the important reasons for human mortality (Mueller et al., 2011).
Recently, there has been an enhancing interest in the usage of medicinal herbs with more efficiency and lower adverse effects than chemical drugs for a variety of disorders including hyperlipidemia.
According to the studies focused on the plants with hypolipidemic effects, some plants including Allium sativum, Nigella sativa, Curcuma longa, Anethum graveolens, and Commiphora mukul showed the best hypolipidemic effect (Mollazadeh et al., 2019).
Numerous studies have been shown that ginseng could decline total cholesterol (TC), triglyceride (TG), and low‐density lipoproteins (LDL) and increase high‐density lipoprotein (HDL) level.
A study on male and female rats intoxicated with ethanol for two weeks indicated that the administration of 150 mg/kg of ginseng for six weeks improved the serum lipid profiles. Ginseng lowered the serum level of TC, TG, LDL‐C, and atherogenic index and elevated the serum level HDL‐C (Ayaz & Alnahdi, 2018). In another study in mice receiving the alcoholic extract of North American ginseng at 4 and 32 weeks of age, a decrease in hepatic and intestinal lipoprotein secretion and the level of blood lipid has been shown. Treatment by ginseng protected the mice against fatty liver. Moreover, ginseng reduced the expression of genes involved in the adjustment of fatty acid and triglyceride secretion by the lipoproteins. On the other hand, ginseng stimulated lipolysis (Singh et al., 2017). The protective effects of ginseng on dyslipidemia may be related to the increased phosphorylation of AMP‐activated protein kinase (AMPK) and acetyl‐CoA carboxylase. Also, the different gene expressions related to lipolysis and uptake of fatty acids such as peroxisome proliferator‐activated receptor‐α and CD36 were enhanced. These results have shown that ginseng improved hyperlipidemia by stimulating lipolysis through AMPK activation (Yuan et al., 2010). Administration of fermented red ginseng for eight weeks could reduce the levels of ALT, AST, TC, TG, and LDL‐C and hepatic MDA levels in high‐fat diet‐treated rats. Moreover, ginseng improved the HDL‐C level and hepatic SOD, CAT, and GSH‐Px activity induced by a high‐fat diet. These results demonstrated that ginseng modified lipid profiles, prevented lipid peroxidation, and increased antioxidant activities (Kim, Lee, et al., 2016). Also, Saba et al. showed that the administration of aqueous and ethanolic extracts of ginseng decreased the cholesterol and LDL levels in HFD‐treated rats. It also downregulated the important genes responsible for lipogenesis, such as acetyl‐coenzyme A (CoA) acetyltransferase 2, 3‐hydroxy‐3‐methyl‐glutaryl‐CoA reductase, and sterol regulatory element‐binding protein 2 (Saba et al., 2016). According to these studies, it could be concluded that ginseng could modify the lipid profile and inhibit atherosclerosis (Table 1).
TABLE 1.
Effects of ginseng on lipid profile
| Study design | Constituents | Result | References |
|---|---|---|---|
| In vivo, male and female rats (intoxicated with ethanol for two weeks) | 150 mg kg−1 day−1 Ginseng for 6 weeks/P.O |
↓ TG, ↓ TC, ↓ LDL‐C, ↑ HDL‐C |
Ayaz and Alnahdi, (2018) |
| In vivo, Wistar rats, Fed HFD | 0.2 g kg−1 day−1 of Ginseng for 90 days/ P.O | ↓ TC | Akdoğan et al., (2018) |
| In vivo, ETKO obese mouse | 200 mg kg−1 day−1 NAGEE, for 4 weeks / P.O | ↓ HILS, ↓ CLL | Singh et al., (2017) |
| In vivo, Sprague Dawley rats, Fed HFD | 200 mg kg−1 day−1 GWEE, for 4 weeks/ P.O | ↓ TC,↓ LDL‐C | Saba et al., (2016) |
| In vivo, Rats, Fed HFD | Cotreatment with FBR and GE 250 mg kg−1 day−1, for 12 weeks/ P.O | ↓ TG, ↓ TC, ↓ LDL‐C, ↑ HDL‐C, ↑ FEC | Lee et al., 2015) |
| In vivo, Male white rabbits, Fed HFD | RGNK and RG 0.11%(w/w) and NT 0.11%(w/w), P.O / for 8 weeks |
RG: ↓ TG RGNK : ↓ TG, ↓ HC, ↓CETPAL |
Kang et al., (2014) |
| In vivo, C57BL/6J mice, Fed HCD | Cotreatment with UBR and RG, 125 mg kg−1 day−1 for 12 weeks | ↓ TC, ↓ HTL, ↓ LA | Lee, Lee, et al., (2014) |
| In vivo, ICR mice, fed HFD | PPGR, 75, 150, 300 mg/Kg/day, P.O, for 5 weeks | ↓ Hyperlipidemia | Yuan et al., (2012) |
| In vivo, C57BL/6J mice, Fed HFD | GLE, 250 and 500 mg kg−1 day−1, P.O, for 8 weeks | ↓ TG, ↓ TC, ↓ NEFA, ↓ LLD | Yuan et al., (2010) |
| In vivo, Sprague Dawley rats, Fed HFD | Ginseng 0.5% and 1%, daily, P.O, for 8 weeks | ↓ TG, ↓ TC, ↓ LDL ‐C, ↑ HDL‐C, ↓ Lipid peroxidation | Kim, Lee, et al., (2016) |
| In vivo, Rat, Fed HFD | Cotreatment 2 ml/kg Nigella sativa oil and 6ml/kg Ginseng, daily, P.O, for two weeks | ↓ BL, ↓Harmful changes in lipids | Arab et al., (2016) |
| In vivo, STZ‐induced diabetic rats | FRG, 100 and 200 mg kg−1 day−1, P.O, for 3 weeks | ↓ TG, ↓ TC | Kim et al., (2010) |
| In vivo, STZ‐induced diabetic rats | PG, 22.5 mg kg−1 day−1, P.O, for 45 days | ↓ TG, ↓ TC, ↓ LDL‐C, ↓ VLDL Cholesterol | El‐Khayat et al., (2011) |
| In vivo, STZ‐induced diabetic rats | PFRG, 300 mg kg−1 day−1, P.O, for 5 weeks | ↓ TC | Park et al., (2012) |
| In vivo, Type 2 diabetic mice (db/db mice) | MEARG, 150 mg kg−1 day−1, IP | ↓ TC, ↓ LDL‐C, ↑ HDL‐C | Yoo et al., (2012) |
| In vivo, HFD and STZ‐induced type 2 diabetic rat | MGR from PG, 50 and 100 mg kg−1 day−1, for 3 weeks | ↓ TG, ↓ TC | Liu et al., (2013) |
| In vivo, Albino rat, Suffering from acute liver diseases and diabetes |
Ginseng (2% and 4%) and cotreatment (2% Ginseng and 0.25% Curcumin) and (4% Ginseng and 0. 5% Curcumin), PO |
↓ TG, ↓ TC, ↓ LDL‐C, ↓ VLDL Cholesterol, ↑ HDL | Arafa (2013) |
| In vivo, C57BL/6N mice, Fed HFD | PG Meyer, 4.0%, PO, for 8 weeks | ↑ Lipid metabolism | Im Chung et al., (2016) |
| In vivo, Type 2 diabetic rat | Baihu ginseng decoction, 37.2 g kg−1 day−1, PO, for 2 weeks | ↓ TG, ↓ TC, ↑ HDL‐C | Hou et al., (2017) |
| In vivo, STZ‐induced diabetic mice | RGPF with probiotics, 150 mg kg−1 day−1, PO, for 8 weeks | Improved serum lipid levels | Jang et al., (2017) |
| In vivo, C57BL/6 Mice, Fed HFD | Ginsenoside Re, 5, 10, and 20 mg kg−1 day−1, for 4 weeks | ↓ TC, ↓ LDL‐C, ↑ HDL‐C, ↓ TG | Kim et al., (2017a) |
| In vivo, STZ‐induced type 2 diabetic C57BL/6 mice, Fed HFD | Ginsenoside Rk3, 10, 30, 60 mg kg−1 day−1, for 4 weeks | ↓ TG, ↓ TC, ↓ LDL ‐C | Liu et al., (2019c) |
| In vivo, High‐fat and high‐sugar induced HIR in rat | Ginsenoside Rg1, 10, 25, 50 mg kg−1 day−1, PO, for 4 weeks | ↓ TC, ↓ LDL‐C, ↑ HDL‐C, ↓ TG | Fan, Zhang, et al., (2019) |
| In vivo, Obese mice, fed HFD | Ginsenoside Rg2, 10 mg kg−1 day−1, PO, for 4 weeks |
↓ FFA ↓ TG |
Liu et al., (2019b) |
| In vivo, Obese mice, Fed HFD | GE, 0.8 and 1.6 (w/w) daily, PO, for 8 weeks |
↓ TG |
Lee et al., (2010) |
| In vivo, Mice, Fed HFD | Ginsenoside Rh2, 20 mg kg−1 day−1, PO, for 4 weeks | ↓ TG | Gu et al., (2013) |
| In vivo, C57BL/6 mice, Fed HFD | Saponins from PG, 125 and 500 mg kg−1 day−1 for 12 weeks | ↓ TG, ↓ TC, ↓ LDL ‐C, ↓ FFA | Chen et al., (2017) |
| In vivo, C57BL/6J mice, Fed HFD | Ginseng‐plus‐Bai‐Hu‐Tang 0.5% (w/w), PO, for 12 weeks | Protected against hyperlipidemia | Lu et al., (2018) |
| In vivo Castrated C57BL/6J mice, Fed HFD | GE, 5% for 8 weeks | ↓ TG, ↓ TC, ↓ HLA | Shin and Yoon (2018) |
| In vivo, Obese C57BL/6 mice, Fed HFD | FGR and FGB for 16 weeks | ↓ Hypercholesterolemia and fatty liver | Li et al., (2018) |
| In vivo, Mice, Fed HFD | Ginsenoside, 0.02% and 0.05% (w/w), for 8 weeks | ↓ Hypertriglyceridemia | Liu et al., (2010) |
| In vivo, Mice, Fed HFD | HGV, 0.8 ml kg−1 day−1 for 8 week | ↓ TG, ↓ LDL‐C, ↑ HDL‐C | Oh et al., (2014) |
| In vivo, Mice, Fed HFD | Cotreatment FG 150 mg kg−1 day−1 and 100 mg kg−1 day−1 of Levan for 11 weeks | ↓ TC | Oh, Lee, et al., (2014) |
| In vitro, 3T3‐L1 adipocytes, Cultured under high cholesterol or fatty acids conditions | Ginsenoside Rb2, 10 μg/ml | ↓TC, ↓ TG | Kim et al., (2009a) |
| Human, type 2 diabetic subject | Cotreatment KGB 6 g and AG 3 g, for 12 weeks | ↓ Lipid concentration | Yang et al., (2018) |
| Human, 45 subjects (men and women) | RG, 20 g/day for 8 weeks |
Improved lipid profile |
Shin et al., (2011) |
Abbreviations: AG, American ginseng; BL, blood lipids; CETPAL, cholesteryl ester transfer protein activity levels; CLL, circulating lipids level; FBR, fermented black raspberry; FCE, fecal cholesterol excretion; FFA, free fatty acid; FG, fermented ginseng; FGB, fermented ginseng berry; FGR, fermented ginseng root; FRG, fermented red ginseng; GE, ginseng extract; GE, ginseng extracts; GLE, ginseng leaf extract; GWEE, ginseng water and ethanol extract; HC, hepatic cholesterol; HCD, high cholesterol diet; HDL‐C, high‐density lipoprotein cholesterol; HFD, high‐fat diet; HGV, hydroponic‐cultured ginseng vinegar; HILS, hepatic and intestinal lipoprotein secretion; HIR, hepatic insulin resistance; HLA, hepatic lipid accumulation; HTL, hepatic total lipid; IP, intraperitoneal; KGB, Konjac‐glucomannan‐based fiber blend; LA, lipid accumulation; LDL‐C, low‐density lipoprotein cholesterol; LLD, liver lipid droplets; MEARG, methanolic extract of American Red Ginseng; MGR, malonyl ginsenosides; NAGEE, North American ginseng ethanol extract; NEFA, nonesterified fatty acids; NT, nattokinase; PFRG, puffed and fermented red ginseng; PG, Panax ginseng; PPGR, pectinase‐processed ginseng radix; RG, red ginseng; RG, red ginseng; RGE, red ginseng extracts; RGNK, RG combined with NT; RGPF, red ginseng powder fermented; STZ, streptozotocin; TC, total cholesterol; TG, triglyceride; UBR, unirpe black raspberry; VLDL, very‐low‐density lipoprotein.
4. EFFECT ON HIGH BLOOD PRESSURE
Hypertension is an additional important metabolic risk factor for cardiovascular disease (Organization, 2007). High blood pressure increases the risk of myocardial infarction and cerebrovascular disease as well as heart failure, peripheral vascular disease, stroke, and coronary artery disease (Leong et al., 2013).
Medicinal herbs are accessible, cheap, and useful for both prevention and treatment of hypertension. Furthermore, some medicinal plants such as Ginkgo biloba (Eisvand et al., 2020), Aloe vera (Shakib et al., 2019), Crataegus pinnatifida (Dehghani et al., 2019), Silybum marianum (Tajmohammadi et al., 2018), Capsicum annuum (Sanati et al., 2018), Berberis vulgaris (Tab eshpour et al., 2017), Persea americana (Tab eshpour et al., 2017), Cinnamomum verum (Mollazadeh & Hosseinzadeh, 2016), Crocus sativus L. (saffron) (B. M. Razavi & Hosseinzadeh, 2017), and Nigella sativa (B. Razavi & Hosseinzadeh, 2014) appear to have a great antihypertensive effect.
According to animal and human studies, ginseng can reduce hypertension. In a study, the administration of ginseng to spontaneously hypertensive rats improved endothelium‐dependent vasodilatation. Ginseng treatment for 6 weeks increased the serum NO levels and decreased the mean aortal intima–media width in comparison with control. Furthermore, ginseng mediated the expressions of cyclooxygenase (COX)‐2 in endothelial cells (Park et al., 2014b). Clinical investigations also demonstrated that the daily consumption of capsules of ginseng is helpful for blood flow during exercise. Ginseng reduced peripheral vascular resistance and improved oxygen transfer to activate atrophied muscles (Zaheri & Marandi, 2016). In another animal study, concurrent treatment with ginseng reduced the blood pressure in spontaneously hypertension rat by the inhibition of angiotensin‐I‐converting enzyme and release of NO (Lee, Bae, Park, Park, Lee, 2016). A study on prehypertensive subjects revealed that treatment by ginseng decreased lipoprotein‐associated phospholipase A 2 (Lp‐PLA 2) and lysophosphatidylcholines (lysoPCs) and increased dihydrobiopterin levels, which caused a notable decrease in diastolic and systolic blood pressure (Cha et al., 2016). Besides, the oral administration of ginseng to healthy volunteers for two separate appointments with a 7‐day washout course demonstrated that ginseng extract reduced central and peripheral arterial pressures in healthy adults (Jovanovski et al., 2014). A study on hypertensive diabetic patients showed that 3 g of ginseng for 12 weeks improved arterial stiffness and decreased the systolic blood pressure (Mucalo et al., 2013). Ginseng also showed hypotensive effects in combination therapy in rats, for example, a combination including P. ginseng, P. notoginseng (Burk.), and Ligusticum chuanxiong reduced the expression of cytokines leading to reduce aging and hypertension (Lei et al., 2012). According to the results of a study on hypotension induced by ginseng in spontaneously hypertensive rats, it can be suggested that ginseng is useful in decreasing high blood pressure through eNOS activation and enhanced NO‐releasing (Hong et al., 2012).
Several in vivo and clinical studies reported the antihypertension effect of ginseng and its protection against hypertensive complications such as cardiac hypertrophy (Table 2).
TABLE 2.
Effects of ginseng on high blood pressure
| Study design | Constituents | Result | Reference |
|---|---|---|---|
| In vivo, Spontaneously hypertensive rat | EEG, 50% and 85%, daily, for 6 weeks | ↓ Mean aortal intima–media width | Park et al., (2014b) |
| In vivo, Spontaneously hypertensive Wistar Kyoto rat | Ginseng, 500 and 1,000 mg kg−1 day−1 for 8 weeks | ↓ BP | Lee, Bae, et al., (2016) |
| In vivo, Mice, Fed HFD | Ginseng, 0.5 g kg−1 day−1, for 15 weeks | ↓ BP | Li et al., (2014) |
| Human, Prehypertensive subjects | Ginseng, 5 g, daily for 12 weeks |
↓SBP ↓DBP |
Cha et al., (2016) |
| Human, Athletes men | Ginseng, 2 capsules−200 mg kg−1 day−1, for 4 weeks | Helpful for blood flow during exercise, ↓ Peripheral vascular resistance, improved oxygen transfer to muscles | Zaheri and Marandi (2016) |
| Human, Healthy subjects (male and female) | Ginseng, 400 mg on two separated appointments with a 7‐day washout course |
↓ Central and brachial arterial pressure, ↓Arterial pressure ↓ SBP ↓ DBP |
Jovanovski et al., (2014) |
| Human, Healthy subjects | Ginseng, 100 mg and 300 mg with 2 weeks washout period | ↓ BP after 4 weeks, especially at high dose. Over 8 weeks, the effect is not retained | Rhee et al., (2014) |
| Human, Diabetic patients | Ginseng, 3 g/day for 12 weeks | Improved arterial stiffness and weakened SBP | Mucalo et al., (2013) |
| Human, Hypertensive subject | Ginseng, 3 g/day for 3 months | Ginseng did not progress arterial stiffness in hypertension patients. | Rhee et al., (2011) |
| Human, Type 2 diabetic subject | AG, 3 g/day, for 8 weeks | ↓SBP | Vuksan et al., (2019) |
Abbreviations: AAG, American ginseng; BP, blood pressure; DBP, diastolic blood pressure; EEG, ethanolic extract ginseng; HFD, high‐fat diet; SBP, systolic blood pressure.
5. EFFECT ON OBESITY
Obesity is an important global issue that is characterized by an imbalance between lipogenic and lipolytic processes, which causes the accumulation of excess body fat in the form of triglyceride in adipose tissue and is associated with several diseases including diabetes and heart disease (Langin, 2006).
As the approved anti‐obesity drugs have poorly documented effects, so, there is an immediate need for novel and effective anti‐obesity medicines (Kang & Park, 2012).
The research reported that ginseng exhibited an anti‐obesity effect by different mechanisms. Several studies have shown that ginseng in animals exhibited anti‐obesity effects.
Ginseng decreased adipose tissue mass and obesity in high‐fat diet‐induced obese mice and this effect mediated through the reduction of angiogenesis and extracellular matrix metalloproteinase (MMP) activity (Lee et al., 2013). In high‐fat diet‐induced obese mice, administration of 125 and 500 mg kg−1 day−1 of ginseng for 12 weeks decreased body and liver weight, epidermal adipose tissue weight through the downregulation of PPARγ expression, and upregulation of PPARα, PGC‐1α, UCP‐1, and UCP‐3 genes in adipose tissues (Chen et al., 2017).
The body weight‐lowering effect of ginseng extract (0.8% and 1.6% w/w) on obesity induced by a high‐fat diet in mice was investigated for 8 weeks. A significant decrease in plasma TG levels, body weight gains, and white adipose tissue were observed. The possible mechanism is through the regulation of lipogenesis‐related gene expression in white adipose tissue and delays in intestinal fat absorption (Lee et al., 2010). In a study on obese rats received a high‐fat diet, ginseng significantly reduced epididymal and abdominal adipose tissue mass and total body weight (Lee et al., 2017). In this regard, another experiment revealed that high hydrostatic pressure extract of ginseng (PEG) reduced the protein expression of adipogenic genes such as PPARγ and aP2. The results of this study showed PEG may have more useful than water extract ginseng on obesity and inflammation. This effect is mediated through the increase of fecal triacylglycerol and adjustment of gene expression (Jung et al., 2014). A study on the anti‐obesity effect of ginseng in 3T3‐L1 cells showed that ginsenoside Rg2 decreased adipocyte differentiation and the accumulation of intracellular lipids (Liu et al., 2019b). Ginseng decreased adipose tissue and adipocyte size, triglyceride, cholesterol, and body weight without changing food intake in high‐fat diet mice (Shin & Yoon, 2018).
Based on these studies, the anti‐obesity effect of ginseng and its active constituents is moderate to very strong. Furthermore, ginseng may have important roles in the treatment and prevention of obesity through several mechanisms (Table 3).
TABLE 3.
Effects of ginseng on obesity
| Study design | Constituents | Result | References |
|---|---|---|---|
| In vivo, Dog | GE, 1%, daily for 8 weeks | ↓ BW, ↓ FM | Bae and Oh (2019) |
| In vivo, BALB/c mice, fed HFD | WEWG and WERG, 5.5 ml kg−1 day−1, for 10 weeks | Anti‐obesity effect of white ginseng was stronger than red ginseng | Zhou et al., (2018) |
| In vivo, mice, fed HFD | Mixture of FG (0.25%,0.5%,1%) and two probiotics, Bifidobacterium longum BORI and Lactobacillus paracasei CH88, daily for 9 weeks | ↓ WG, ↓ lipid deposition in the liver and adipose tissues, ↓ Adipocyte size | Kang et al., (2018) |
| In vivo, Obese Sprague Dawley rats, fed HFD | GL, 3.3 mg kg−1 day−1 | ↓ BW, ↓ Epididymal and abdominal adipose tissue mass | Lee, Yoon, et al., (2017) |
| In vivo, Obese rats, Fed HFD | HPEG, 15 g/kg and WEG 15 g/kg for 14 weeks |
PEG: ↓ BW and white adipose tissue mass Anti‐obesity effect of PEG was more than WEG |
Jung et al., (2014) |
| In vivo, Mice, fed HFD | Cotreatment FG150 mg/kg/day and Levan 100 mg kg−1 day−1 for 11 weeks |
↓ BW ↓ FM ↑ food efficiency ratio |
Oh, Kwon, et al., (2014) |
| In vivo, Mice, fed HFD | HGV, 0.8 ml kg−1 day−1 for 8 weeks | ↓ BW, ↓ fat weight, ↓ LW | Oh, Kwon, et al., (2014) |
| In vivo, Diet‐Induced Obese Mice | Ginsenoside Rb1, 20 mg kg−1 day−1, for 3 weeks |
↓ WG ↓ FI |
Lin et al., (2014) |
| In vivo, Mice, fed HFD | BGEE (1%,3%,5%) daily for 12 weeks |
↓ FA in the liver, and white adipose tissues ↑ Total fecal weight ↑ Fecal fat excretion |
Lee, Kim, et al., (2013) |
| In vivo, Obese‐prone C57BL/6J strain mice, Fed HFD | PG extracts, for 10 weeks | ↓ WG | Woo et al., (2011) |
| In vivo, Mice, Fed HFD | Ginsenoside 0.02% and 0.05% (w/w),for 8 weeks |
↓ Obesity ↓ Fatty liver |
Liu et al., (2010) |
| In vivo, Obese insulin‐resistant rat | PG extract 300 mg kg−1 day−1 for 6 weeks |
↓ BW ↓ LW ↓Serum alanine aminotransferase |
Lim et al., (2009) |
| In vivo, obese mice, Fed HFD | Ginsenoside Rb2 40 mg kg−1 day−1 for 9 weeks | ↓ BW | Gu et al., (2019) |
| In vivo, Obese C57BL/6 mice, fed HFD | FGR and FGB for 16 weeks |
↓ WG ↓ FI FGR: ↓ Epididymal fat weight FGR : More potent anti‐obesity effect |
Li, Li, et al., (2018) |
| In vivo Castrated C57BL/6J mice, fed HFD | GE, 5%, for 8 weeks |
↓ BW ↓Adipose tissue mass ↓ Adipocyte size |
Shin and Yoon (2018) |
| In vivo, C57BL/6J mice, fed HFD | Ginsenoside Rg1, 300 and 500 mg kg−1 day−1 for 8 weeks |
↓ BW ↓ LA in white adipocyte tissue |
Li et al., (2018) |
| In vivo, C57BL/6J mice, Fed HFD | Ginseng‐plus‐Bai‐Hu‐Tang 0.5% (w/w) for 12 weeks |
↓ Obesity, ↓ Expansion of adipose tissue, ↓ Adipocyte hypertrophy |
Lu et al., (2018) |
| In vivo, C57BL/6 mice, fed HFD | Saponins from PG, 125 and 500 mg kg−1 day−1 for 12 weeks |
↓ BW ↓ LW ↓ Epididymal adipose tissue weight ↓ Food efficiency |
Chen et al., (2017) |
| In vivo, C57BL/6J mice | Ginseng 5% (w/w) for 15 weeks |
↓ BW ↓ Adipose tissue mass ↓ Adipocyte size |
Lee et al., (2016) |
| In vivo, Rats, fed high‐fat/ high‐cholesterol diet | Cotreatment GBR and GE, 250 mg kg−1 day−1, for 12 weeks | ↓ Obesity | Lee et al., (2015) |
| In vivo, Obese female db/db mice | GE, 4.5 g /kg/day, for 13 weeks |
↓ BW ↓Adipose tissue mass ↓ Size of adipocytes in visceral adipose tissues |
Lee et al., (2014) |
| In vivo, Mice, fed HFD | Ginseng, 0.5 g kg−1 day−1, for 15 weeks |
↓ Body fat mass gain ↓ obesity |
Li et al., (2014) |
| In vivo, Obese mice, Fed HFD | Cotreatment Veratrum nigrum 0.75g kg day−1 and PG 0.75g kg day−1, for 16 week |
↓ fat weight, ↓ BW, ↓ LA |
Park et al., (2013) |
| In vivo, Obese C57BL/6J mice, fed HFD | GE,0.5 and 5 (w/w) daily for 8 weeks |
↓ Adipose tissue mass ↓ Obesity |
Lee, Kim, et al., (2013) |
| In vivo, Mice, Fed HFD | Ginsenoside Rh2, 20 mg kg−1 day−1, for 4 weeks |
↓ Adipogenesis, ↓ Adipocyte differentiation ↓ Body and epididymal fat weight gains |
Gu et al., (2013) |
| In vivo, Obese mice, Fed HFD | GE, 0.8 and 1.6 (w/w) daily for 8 weeks |
↓ White adipose tissue weight ↓ BW |
Lee et al., (2010) |
| In vivo, Obese mice, Fed HFD | Ginsenoside Rg2, 10 mg kg−1 day−1, for 4 weeks | ↓ BW | Liu et al., (2019b) |
| In vivo, Type 2 obese diabetic db/db mice | FSGB, 0.5 g kg−1 day−1, for 5 weeks | ↓ BW | Kim et al., (2012) |
| In vivo, Otsuka Long‐Evans Tokushima fatty rats | PG, 200 mg/ kg/day for 40 weeks |
↓ BW ↓ FM |
Lee et al., (2009) |
| In vivo, Rat, Fed HFD | PG, 200 mg kg−1 day−1, for 18 weeks |
↓ BW ↓ FM |
Lee et al., (2012) |
| In vivo, Albino rat, Suffering in from acute liver diseases and diabetes |
Ginseng (2% and 4%) and cotreatment (2% Ginseng and 0.25% Curcumin) and (4% Ginseng and 0. 5% Curcumin) |
↓ BW ↓ FI ↓ Organs weight |
Arafa (2013) |
| In vivo, Old‐aged, obese, leptin‐deficient mice(B6.V‐Lepob, “ob/ob”) | FRG, 0.5%, and 1.0% FRG for 16 weeks | ↓ BW | Cheon et al., (2015) |
| In vivo, C57BL/6N mice, Fed HFD | Aged ginseng, 4.0%, for 8 weeks | ↓ BW | Im Chung et al., (2016) |
| In vivo, STZ‐induced type 2 diabetic C57BL/6 mice, fed HFD | Ginsenoside Rk3, 10, 30, 60 mg kg−1 day−1, for 4 weeks | ↓ LA | Liu et al., (2019c) |
| In vitro, 3T3‐L1 cells | WEGE | ↓ LA | Park et al., (2015) |
| In vitro, 3T3‐L1 cells | Ginsenosides F2 | ↓ Adipogenesis | Siraj et al., (2015) |
| In vitro, 3T3‐L1 cells | Ginsenoside (95% purity) | ↓ Lipid droplet accumulation, anti‐adipogenic effect | Simu et al., (2017) |
| In vitro, co‐culture system of 3T3‐L1 and RAW264.7 cells | Saponin fraction from ginseng 100 μg/mL | ↓Obesity | Kim et al., (2018) |
| In vitro, 3T3‐L1 cells | Ginsenoside Rg2, 80 μM | ↓ Adipocyte differentiation, ↓Accumulation of intracellular lipids | Liu et al., (2019b) |
| Human, Obese middle‐aged Korean women | Ginseng, 4 g/day for 8 weeks |
↓ Body mass index ↓ BW |
Song et al. (2014) |
Abbreviations: BGEE, black ginseng ethanolic extract; BW, body weight; FBR, fermented black raspberry; FG, fermented ginseng; FGB, fermented ginseng berry; FGR, fermented ginseng root; FI, food intake; FM, fat mass; FRG, fermented Korean red ginseng; FSGB, fermented steam‐dried ginseng berries with Lactobacillus plantarum; GE, ginseng extract; GL, ginseng leaf; HFD, high‐fat diet; HGV, hydroponic‐cultured ginseng vinegar; HPEG, high hydrostatic pressure extract of ginseng; LA, lipid accumulation; LW, liver weight; PG, Panax ginseng; STZ, streptozotocin; WEG, hot water extract of ginseng; WEGE, water and ethanolic ginseng extracts; WERG, water extract of red ginseng; WEWG, water extract of white ginseng; WG, weight gain.
6. EFFECT ON HIGH BLOOD GLUCOSE
Diabetes is known as a metabolic disease that outcomes from failure in insulin action or insulin production or both (Mahadeva Rao & Adinew, 2011). Diabetes is one of the major reasons for human death, morbidity, and hospital cost around the world. According to the universal reports about diabetes, the number of people suffering from diabetes has been over 422 million in 2014 and the number of people with diabetes is increasing every day around the world (Collaboration, 2016). So, diabetes is a serious universal health problem, which is guessed to reach 592 million by 2035 and will be the seventh reason for mortality in 2035 (Das et al., 2014).
Many animal and human studies have shown useful effects of phytotherapy for the treatment of diabetes (Ghorbani, 2013a, 2013b).
Nowadays, the identification of suitable healthcare approaches, such as medicinal herbs, with fewer adverse effects is more appropriate, especially with attention to the undesirable side effects of chemical drugs. Avocado is a popular source of vitamins, minerals, carotenoids, phenolics, and fatty acids. The antidiabetic effects of avocado have been shown in several studies (Tab eshpour, Razavi, et al., 2017). Nigella sativa and its active component, thymoquinone, have been documented to show hypoglycemic properties (Razavi & Hosseinzadeh, 2014). Flavonoids such as rutin are useful in the treatment of many diseases such as diabetes (Hosseinzadeh & Nassiri‐Asl, 2014). The results of studies revealed that grape polyphenols reduce significantly the level of blood glucose (Akaberi & Hosseinzadeh, 2016).
Several mechanisms have been involved in the treatment of diabetes by phytochemicals. For example, reducing glucose absorption from the intestine, preventing glucose making in the liver, enhancing tissues glucose uptake, and increasing beta cell insulin secretion (Kamyab et al., 2010; Shafiee‐Nick et al., 2011, 2012).
Among different antidiabetic herbs, ginseng is one of the important accepted plants. The antidiabetic effects of ginseng and its active components have been described in numerous studies. For example, the extract of P. ginseng roots (120 mg/ kg) significantly decreased blood glucose level and improved glucose tolerance after 4 days of treatment in diabetic rats. These results suggested that ginseng extract has hypoglycemic effects on diabetic male rats (Liu et al., 2009).
The antidiabetic effect of ginseng was shown in a study on fatty mice, performed by Lee et al. This study demonstrated that ginseng upregulated the expression of genes involved in the activation of AMPK and increased mitochondrial biogenesis and glucose consumption in skeletal muscles (Lee et al., 2009). The ethanolic extract of ginseng significantly decreased the levels of fasting plasma glucose, HbAlc, and insulin resistance. On the other hand, the expression of phospho‐AMPK and glucose transporter 4 (GLUT4) were increased in liver and skeletal muscle in db/db mice (Do Yeon Kim et al., 2009). In another study, the oral administration of fermented red ginseng extract (100 and 200 mg/kg) for 3 weeks was able to significantly decrease the blood glucose level in streptozotocin‐diabetic rats (Kim et al., 2010). The daily administration of ginseng leaf extract (250 and 500 mg/kg) for 8 weeks in C57BL/6J mice, significantly reduced the plasma glucose level but increased the phosphorylation of AMP‐activated protein kinase (AMPK) and its substrate, acetyl‐CoA carboxylase. Moreover, phosphoenolpyruvate carboxykinase gene expression was reduced. These results suggest that ginseng leaf extract improved hyperglycemia by preventing gluconeogenesis and activating lipolysis, by AMPK stimulation (Yuan et al., 2010). In another study, the probable effect of ginseng on high blood glucose and related diseases was investigated. The findings of this study demonstrated that the modulatory effect on tumor necrosis factor‐alpha (TNF‐α) and interleukin‐6 (IL‐ 6) and liver antioxidants may be involved in the improvement of high blood glucose by ginseng in rats (El‐Khayat et al., 2011). Another study on the C2C12 skeletal muscle cells showed that ginseng (ginsenoside Rb1) increased glucose uptake and improved insulin sensitivity. The results showed that glucose uptake was mediated by leptin receptor activation. According to this study, the leptin receptor plays a great role in the ginseng effects on glucose uptake and insulin sensitivity in skeletal muscle cells (Tab andeh et al., 2017). A randomized double‐blind, placebo‐controlled, clinical trial study suggested that 8‐week supplementation with hydrolyzed ginseng extract (HGE) in 23 subjects demonstrated that fasting plasma glucose and postprandial glucose were significantly reduced in the HGE group in contrast to the placebo group (Park et al., 2014b). Randomized double‐blind cross over clinical trial on 24 subjects (F:M = 11:13; age = 64 ± 7 year; BMI = 27.8 ± 4.6 kg/m2; HbA1c = 7.1 ± 1.2%) for 8 weeks indicated that 3 g/day ginseng improved fasting blood glucose (−0.71 mmol/L; p = .008) and HbA1c (−0.29%; p = .041) (Vuksan et al., 2019).
In summary, ginseng can be suggested for the treatment of diabetic patients because it can decrease blood glucose levels with several effective mechanisms, such as insulin sensitivity improvement, the enhancement of tissues glucose uptake, and the reduction of insulin resistance and glucose tolerance. Numerous studies regarding the antidiabetic effect of the ginseng have been conducted on animals (mice and rats); thus, to demonstrate this effect on humans, we need more clinical research projects (Table 4).
TABLE 4.
Effects of ginseng on high blood glucose
| Study design | Constituents | Result | References |
|---|---|---|---|
| In vivo, STZ‐induced diabetic mice | Malonyl ginsenosides, 30, 60, 120 mg kg−1 day−1 |
↓ FBG Improved GT |
Liu et al., (2009) |
| In vivo, Otsuka Long‐Evans Tokushima fatty rats | PG, 200 mg/ kg/day for 40 weeks |
Improved IS preserved GT up to 50 weeks of age |
Lee et al., (2009) |
| In vivo, C57BL mice |
FG, 100, 200mg kg day−1 for 10 week |
↓ FBG, ↓ HbAlc, ↓IR | Do Yeon Kim, Ahn, et al., (2009) |
| In vivo, Otsuka Long‐Evans Tokushima fatty rats | Ginsam, 300 and 500 mg kg−1 day−1 for 6 weeks |
Improved GT ↓ fasting and postprandial glucose concentrations |
Lim et al., (2009) |
| In vivo, Otsuka Long‐Evans Tokushima fatty rats | Ginsenoside, Rg3, 500 mg kg−1 day−1 for 8 weeks | Improved insulin signaling and GU | Kim et al., (2009b) |
| In vivo, STZ‐induced diabetic rats | BGE, 5 mg kg−1 day−1 for 3 weeks | ↓ BG | Kim and Kang (2009) |
| In vivo, STZ‐induced diabetic rats | GR, 400 mg kg−1 day−1 for 6 weeks |
↓ BG ↑ Preservation of β‐cells |
Karaca et al., (2010) |
| In vivo, STZ‐induced diabetic rats | FRG, 100,200 mg kg−1 day−1 for 3 weeks |
↓ BG, ↑ PIL, ↓ Activated of disaccharidases |
Kim et al., (2010) |
| In vivo, C57BL/6J mice, Fed HFD‐induced hyperglycemia and hyperlipidemia |
GLE, 250, 500 mg kg−1 day−1, for 8 weeks |
↓ BG ↓Gluconeogenesis | Yuan et al., (2010) |
| In vivo, STZ‐induced diabetic rats | GR, 400 mg kg−1 day−1, for 6 weeks | ↓ BG | Karaca et al., (2011) |
| In vivo, STZ‐induced diabetic rats | PG, 22.5 mg kg−1 day−1, for 45 days | ↓ BG | El‐Khayat et al., (2011) |
| In vivo, Sprague Dawley rat, fed HFD | PG, 200 mg kg−1 day−1, for 18 weeks |
↑ Insulin signaling ↑ IS |
Lee, Lee, Lee, et al., (2012) |
| In vivo, STZ‐induced diabetic rats | PFRG, 300 mg kg−1 day−1, orally, for 5 weeks | ↓ BG | Park, Kim, et al., (2012) |
| In vivo, C57BL/6J mice, fed HFD | Ginsenoside Re, 5, 10 and 20 mg kg−1 day−1 for 3 weeks | ↓ BG | Quan et al., (2012) |
| In vivo, STZ‐induced diabetic rats | Ginsenoside Re, 40 mg kg−1 day−1 for 8 weeks | ↓ BG | Liu et al., (2012) |
| In vivo, STZ‐induced diabetic mice | PGBE, 100 or 200 mg kg−1 day−1 for 10 weeks |
↓ BG, Improved GT |
Park, Kim, et al., (2012) |
| In vivo, ICR mice, fed HFD | PPGR,75, 150, 300 mg/Kg/day, orally, for 5 weeks | ↓ Hyperglycemia | Yuan et al., (2012) |
| In vivo, Spontaneously diabetic GK rats | KRGWE, 0.2 g kg−1 day−1 for 12 weeks | ↓ BG | Kim and Kim (2012) |
| In vivo, type 2 diabetic mice (db/db mice) | ARGME, 150 mg kg−1 day−1 | ↓ BG | Yoo et al., (2012) |
| In vivo, Wistar rat, fed HFD | Ginsenoside Re | ↓ IR of skeletal muscle | Han et al., (2012) |
| In vivo, STZ‐induced diabetic C57BL/6J mice | KRGE, 25, 100, 1,000 mg kg−1 day−1, for 6 weeks | ↓ BG | Hong, Kim, Lee, et al., (2012) |
| In vivo, STZ‐induced type 2 diabetic rat, fed HFD | PG, 50 and 100 mg kg−1 day−1, for 3 weeks |
↓ FBG ↓ GTT ↓ IR |
Liu et al., (2013) |
| In vivo, Diabetic C57BL mice | FGE, 0.1% (w/w), for 8 weeks |
↓ BG ↓ HbAlc Improved GT |
Jeon et al., (2013) |
| In vivo, Obese C57/L mice, fed HFD | Ginsenoside Rb1, 10 and 20 mg kg−1 day−1, for 2 weeks | Improved GT | Shang et al., (2013) |
| In vivo, Mice, Fed HFD | Combination of RGM (30%, w/w) and brown rice, for 8 weeks |
Improved GM Hypoglycemic effect |
Chung et al., (2014) |
| In vivo, Obese rat, fed HFD | Ginsenoside Rb1, for 5 days |
Improved GT ↑ IS ↓ FBG |
Shen et al., (2015) |
| In vivo, STZ‐induced diabetic rats | PRGP, 0.3%, 0.6%, for 6 weeks | ↓ BG | Shim et al., (2015) |
| In vivo, C57BL/6 mice | GBE, 0.05%, for 24 or 32 weeks | ↑ IS | Seo et al., (2015) |
| In vivo, Multiple low‐dose STZ‐induced diabetic rats | KGE, 100, 200, and 300 mg kg−1 day−1 for 8 weeks |
Improved glucose homeostasis ↑ PIL |
Moon et al., (2015) |
| In vivo, Old‐aged, obese, leptin‐deficient (B6.V‐Lepob, “ob/ob”) mice | FKRG, 0.5%, and FRG, 1.0% for 16 weeks |
Improving IS ↓ BG |
Cheon et al., (2015) |
| In vivo, Type 2 diabetic mice | Ginsenoside Rg3 | ↓ Hyperglycemic | Kim et al., (2015) |
| In vivo, STZ‐induced diabetic mice | BGE, 200 mg kg−1 day−1, for 5 weeks |
↓ Hyperglycemia ↑ Insulin/glucose ratio |
Kim, Pan, et al., (2016) |
| In vivo, C57BL/6N mice, fed HFD | Aged ginseng, 4.0%, for 8 weeks |
Improved glucose metabolism ↓ BG |
Im Chung et al., (2016) |
| In vivo, Type 2 diabetic rat | BGD, 37.2 g kg−1 day−1, for 2 weeks | ↓ BG | Hou et al., (2017) |
| In vivo, Type 2 diabetic rats | Combination of freeze‐dried aronia, red ginseng, ultraviolet‐irradiated shiitake mushroom, and nattokinase, for 12 weeks |
Improved GT ↓ IR |
Yang et al., (2018) |
| In vivo, Type 2 diabetic mice | Aerobic exercise combined with panaxatriol, 0.2%, for 6 weeks | ↓ IR | Takamura et al., (2017) |
| In vivo, Type 2 diabetic mice, fed HFD | Ginsenoside Rb1, 10 mg kg−1 day−1, for 1 week | ↑ IS | Song et al., (2017) |
| In vivo, STZ‐induced diabetic mice | FRG with probiotics, 150 mg kg−1 day−1, for 8 weeks |
↓ FBG |
Jang et al., (2017) |
| In vivo, C57BL/6 Mice, Fed HFD | Ginsenoside Re, 5, 10 and 20 mg kg−1 day−1, for 4 weeks |
↓ Hyperglycemia ↓ FBG ↓ IR |
Kim et al., (2017a) |
| In vivo, Type 2 diabetic mice | GB, 0.05% or 0.1%, for 12 weeks |
↓ BG ↓ Hyperglycemia ↓ IR |
Kim et al., (2017b) |
| In vivo, Diabetes‐prone biobreeding rat | Diol‐GF, 1 mg g−1 day−1, for 8 weeks | Antidiabetogenic effect | Ju et al., (2019) |
| In vivo, STZ‐induced type 2 diabetic C57BL/6 mice, Fed HFD | Ginsenoside Rk3, 10, 30, 60 mg kg−1 day−1, for 4 weeks |
↓ Hyperglycemia Improved GT ↓ IR |
Liu et al., (2019b) |
| In vivo, Diabetic rat | GP and Ginsenoside Rb1, for 30 days | Synergistic antidiabetic effect | Li, Li, et al., (2018) |
| In vivo, High‐fat and high‐sugar induced hepatic insulin resistance in rat | Ginsenoside Rg1, 10, 25, 50 mg kg−1 day−1, for 4 weeks | ↓ IR | Fan, Zhang, et al., (2019) |
| In vivo, C57BL/6J mice, Fed HFD | GLE, 250 and 500 mg kg−1 day−1, orally, for 8 weeks |
↓ BG, ↓ Hyperglycemia |
Yuan et al., (2010) |
| In vivo, Mice, Fed HFD | Cotreatment with FG, 150 mg kg−1 day−1 of with Levan, 100 mg kg−1 day−1 of for 11 weeks |
↓ FBG ↓ IR |
Oh, Kwon, et al., (2014) |
| In vivo, Diet‐Induced Obese mice | Ginsenoside Rb1, 20 mg kg−1 day−1, for 3 weeks | ↓ BG | Lin et al., (2014) |
| In vivo, Type 2 obese diabetic db/db mice | FSGB with Lactobacillus plantarum, 0.5 g kg−1 day−1, for 5 weeks |
↓ IR Improved GT |
Kim et al., (2012) |
| In vivo, Obese insulin‐resistant rat | PGVE, 300 mg kg−1 day−1 for 6 weeks | ↓ Fasting and postprandial glucose concentrations, Improved GT | Lim et al., (2009) |
| In vivo, Obese mice, Fed HFD | Ginsenoside Rb2 40 mg kg−1 day−1 for 9 weeks | Improved IS | Gu et al., (2019) |
| In vivo, Obese C57BL/6 mice, fed HFD | FGR 16 weeks |
FGR: ↓ Hyperglycemia ↓ IR |
Li, Li, et al., (2018) |
| In vivo, C57BL/6J mice, Fed HFD | Ginsenoside Rg1, 300 and 500 mg kg−1 day−1 for 8 weeks |
↓ IR Improved GT |
Li, Li, et al., (2018) |
| In vivo, C57BL/6J mice, Fed HFD | Ginseng‐plus‐Bai‐Hu‐Tang 0.5% (w/w) for 12 weeks |
↑ IS Improved GT |
Lu et al., (2018) |
| In vivo, C57BL/6 mice, Fed HFD | PG, 125 and 500 mg kg−1 day−1 for 12 weeks | ↓ BG | Chen et al., (2017) |
| In vivo, ovariectomized C57BL/6J mice | Ginseng 5% (w/w) for 15 weeks |
↓ IR ↓ Hyperglycemia |
Lee, Bae, et al., (2016) |
| In vivo, obese C57BL/6J mice, fed HFD | GE, 0.5 and 5 (w/w) daily for 8 weeks |
↑ IS Improved GT |
Lee, Kim, et al., (2013) |
| In vitro, HepG2 cells | Ginsenoside Rg1 |
↑ GU ↓ IR ↓ Output of glucose |
Fan, Tao, et al., (2019) |
| In vitro, C2C12 skeletal muscle cell | Ginsenoside Rb1, 0.1, 1, and 10 μM | ↑ GU | Tab andeh et al., (2017) |
| In vitro, HepG2 cells | Ginsenoside Rb3, 25 µM | ↓ Hepatic gluconeogenesis | Meng et al., (2017) |
| In vitro, C2C12 muscle cells | Ginsenoside Rg1 |
↑ GU ↓IR |
Lee, Lee, Kim, et al., (2012) |
| In vitro, 3T3‐L1 cells | Ginsenoside Re | ↓ IR | Gao et al., (2013) |
| In vitro, H4IIE cell line (rat hepatocytes) | Ginsenoside Rb2 |
↓ Abnormal hepatic gluconeogenesis on obesity‐induced |
Lee et al., (2011) |
| In vitro, Mouse C2C12 muscle cells and INS−1 pancreatic β‐cells | Extracts from white, Taegeuk, and red ginseng root |
TGRE: ↑ GU WGRE: ↑ Insulin‐induced glucose uptake |
Cha et al., (2010) |
| Human, Type 2 diabetic subject | AG, 3 g/day, for 8 weeks |
↓ FBG ↓ HbAlc |
Vuksan et al., (2019) |
| Human, 23 Subject | HGE, 960 mg/day, for 8 weeks |
↓ FBG ↓ Postprandial glucose |
Park et al., (2014b) |
| Human, Lately diagnosed type 2 diabetic subject | KRG, 5 g/day, for 12 weeks |
↓ BG |
Bang et al., (2014) |
| Human, 45 Subjects | RGC, 20 g/day for 8 weeks | ↓ FBG | Shin et al., (2011) |
| Human, 38 Subjects | FRG, 780 mg/day for 12 weeks |
↓ FBG, ↓ HbAlc ↓IR |
Kim et al., (2011) |
| Human, 93 postmenopausal women | FRG, for 2 weeks |
↓ HbAlc ↓ IR ↓ Hyperglycemia |
Lee et al., (2013) |
Abbreviations: AG, American ginseng; ARGME, American Red Ginseng methanol extract; BG, blood glucose; BGD, baihu ginseng decoction; BGE, black ginseng extract; BGE, black ginseng extract; Diol‐GF, diol‐ginsenoside fraction from Korean red ginseng; FBG, fasting blood glucose; FG, fermented ginseng; FG, fermented ginseng; FGR, fermented ginseng root; FKRG, fermented Korean red ginseng; FRG, fermented red ginseng; FSGB, fermented steam‐dried ginseng berries; GB, ginseng berry; GBE, ginseng berry extract; GE, ginseng extract; GK, Goto‐Kakizaki; GLE, ginseng leaf extract; GLE, ginseng leaf extract; GM, glucose metabolism; GP, ginseng polysaccharides; GR, ginseng root; GT, glucose tolerance; GTT, glucose tolerance test; GU, glucose uptake; HFD, high‐fat diet; HGE, hydrolyzed ginseng extract; IR, insulin resistance; IS, insulin sensitivity; KGB, Konjac‐glucomannan‐based fiber blend; KGE, Korean ginseng extracts; KRG, Korean red ginseng; KRGE, Korean red ginseng extract; KRGWE, Korean red ginseng water extract; PG, Panax ginseng; PGBE, Panax ginseng berry extracts; PGVE, Panax ginseng vinegar extract; PIL, plasma insulin levels; PPGR, pectinase‐processed ginseng radix; PRGP, puffed red ginseng powder; RGC, red ginseng Cheonggukjang; RGM, red ginseng marc; STZ, streptozotocin; TGRE, Taegeuk ginseng root extracts; WGRE, white ginseng root extracts.
7. CONCLUSION
The use of herbal medicines as supplementary drugs is prevalent and gaining global popularity. Ginseng has wide curative potentials including decreasing blood glucose level, blood lipids level, and blood pressure and enhancing insulin sensitivity. This review article summarizes a variety of in vitro, in vivo, and human studies on the role of ginseng and its active constituents in metabolic syndrome. The results of different studies have indicated that this plant exhibits useful effects in several components of metabolic syndrome including blood glucose, dyslipidemia, blood pressure, and obesity (Figure 2). Ginseng stimulates AMPK and activates lipolysis, so, it can improve hyperglycemia. Ginseng decreases adipose tissue mass and obesity. The possible mechanism is through the regulation of lipogenesis‐related gene expression. Treatment by ginseng can improve hyperlipidemia and stimulate lipolysis by AMPK activation. Ginseng is useful in decreasing high blood pressure through eNOS activation and enhances NO‐releasing. As ginseng does not induce important side effects, so it can be used as an herbal medicine for the treatment of various components of metabolic syndrome. However, more clinical studies need to be done for confirming the beneficial effects of ginseng in metabolic syndrome.
FIGURE 2.
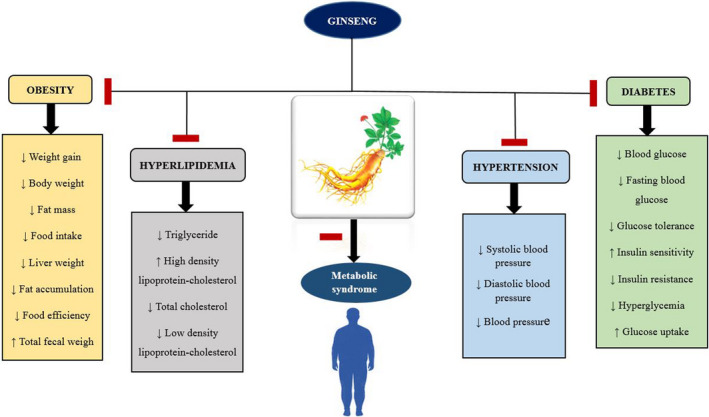
Schematic effects of ginseng in metabolic syndrome
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Tahereh Aminifard : Data curation (equal); Formal analysis (equal); Methodology (equal); Writing‐original draft (equal). Marjan Razavi: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Supervision (equal); Writing‐review & editing (equal). Hossein Hosseinzadeh: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Supervision (equal); Validation (equal); Writing‐review & editing (equal).
ACKNOWLEDGMENTS
We thank the Vice‐Chancellor of Research, Mashhad University of Medical Sciences, Iran.
Aminifard, T., Razavi, B. M., & Hosseinzadeh, H. (2021). The effects of ginseng on the metabolic syndrome: An updated review. Food Science & Nutrition, 9, 5293–5311. 10.1002/fsn3.2475
Contributor Information
Bibi Marjan Razavi, Email: hosseinzadehh@mums.ac.ir.
Hossein Hosseinzadeh, Email: hosseinzadehh@mums.ac.ir.
REFERENCES
- Akaberi, M., & Hosseinzadeh, H. (2016). Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytotherapy Research, 30(4), 540–556. [DOI] [PubMed] [Google Scholar]
- Akdoğan, M., Nasır, Y., Cengİz, N., & Bİlgİlİ, A. (2018). The effects of milled Tribulus terrestris, Avena sativa, and white ginseng powder on total cholesterol, free testosterone levels and testicular tissue in rats fed a high‐cholesterol diet. Ankara Üniversitesi Veteriner Fakültesi Dergisi, 65(3), 267–272. 10.1501/Vetfak_0000002856 [DOI] [Google Scholar]
- Arab, E., Ezz, A., El‐Mahdy, A., & Abbas, O. (2016). Nigella sativa and Panax ginseng supplementation ameliorate induced‐hyperlipidemia in male rats. Arab Journal of Nuclear Sciences and Applications, 49(3), 237–249. [Google Scholar]
- Arafa, R. M. (2013). Influence of ginseng, curcumin and their combination on rats suffering from diabetes and acute liver diseases. World Applied Sciences Journal, 26, 1391–1399. 10.5829/idosi.wasj.2013.26.10.13576 [DOI] [Google Scholar]
- Ayaz, N. O., & Alnahdi, H. S. (2018). Potential impact of Panax ginseng against ethanol induced hyperlipidemia and cardiac damage in rats. Pakistan Journal of Pharmaceutical Sciences, 31(3), 927–932. [PubMed] [Google Scholar]
- Bae, S., & Oh, T. (2019). Anti‐obesity effects of Korean red ginseng extract in healthy beagles. Polish Journal of Veterinary Sciences, 22(2), 385–389. [DOI] [PubMed] [Google Scholar]
- Bang, H., Kwak, J. H., Ahn, H. Y., Shin, D. Y., & Lee, J. H. (2014). Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. Journal of Medicinal Food, 17(1), 128–134. 10.1089/jmf.2013.2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, J.‐Y., Park, E.‐Y., Kim, H.‐J., Park, S.‐U., Nam, K.‐Y., Choi, J.‐E., & Jun, H.‐S. (2010). Effect of white, taegeuk, and red ginseng root extracts on insulin‐stimulated glucose uptake in muscle cells and proliferation of β‐cells. Journal of Ginseng Research, 34(3), 192–197. 10.5142/jgr.2010.34.3.192 [DOI] [Google Scholar]
- Cha, T. W., Kim, M., Kim, M., Chae, J. S., & Lee, J. H. (2016). Blood pressure‐lowering effect of Korean red ginseng associated with decreased circulating Lp‐PLA 2 activity and lysophosphatidylcholines and increased dihydrobiopterin level in prehypertensive subjects. Hypertension Research, 39(6), 449. 10.1038/hr.2016.7 [DOI] [PubMed] [Google Scholar]
- Chen, G., Li, H., Zhao, Y., Zhu, H., Cai, E., Gao, Y., Liu, S., Yang, H., & Zhang, L. (2017). Saponins from stems and leaves of Panax ginseng prevent obesity via regulating thermogenesis, lipogenesis and lipolysis in high‐fat diet‐induced obese C57BL/6 mice. Food and Chemical Toxicology, 106, 393–403. 10.1016/j.fct.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Cheon, J.‐M., Kim, D.‐I., & Kim, K.‐S. (2015). Insulin sensitivity improvement of fermented Korean Red Ginseng (Panax ginseng) mediated by insulin resistance hallmarks in old‐aged ob/ob mice. Journal of Ginseng Research, 39(4), 331–337. 10.1016/j.jgr.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, L. P. (2008). Ginsenosides: Chemistry, biosynthesis, analysis, and potential health effects. Advances in Food and Nutrition Research, 55, 1–99. [DOI] [PubMed] [Google Scholar]
- Chung, S., Rico, C., & Kang, M. (2014). Comparative study on the hypoglycemic and antioxidative effects of fermented paste (doenjang) prepared from soybean and brown rice mixed with rice bran or red ginseng marc in mice fed with high fat diet. Nutrients, 6(10), 4610–4624. 10.3390/nu6104610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration, N. R. F. (2016). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population‐based studies with 4· 4 million participants. The Lancet, 387(10027), 1513–1530. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, S., Roy, P., Pal, R., Auddy, R. G., Chakraborti, A. S., & Mukherjee, A. (2014). Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS One, 9(7), e101818. 10.1371/journal.pone.0101818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani, S., Mehri, S., & Hosseinzadeh, H. (2019). The effects of Crataegus pinnatifida (Chinese hawthorn) on metabolic syndrome: A review. Iranian Journal of Basic Medical Sciences, 22(5), 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Yeon Kim, J. S. P., Yuan, H.‐D., & Chung, S. H. (2009). Fermented ginseng attenuates hepatic lipid accumulation and hyperglycemia through AMPK activation. Food Science and Biotechnology, 18(1), 172–178. [Google Scholar]
- Eisvand, F., Razavi, B. M., & Hosseinzadeh, H. (2020). The effects of Ginkgo biloba on metabolic syndrome: A review. Phytotherapy Research, 34(8), 1798–1811. [DOI] [PubMed] [Google Scholar]
- El‐Khayat, Z., Hussein, J., Ramzy, T., & Ashour, M. (2011). Antidiabetic antioxidant effect of Panax ginseng . Journal of Medicinal Plants Research, 5(18), 4616–4620. [Google Scholar]
- Fan, X., Tao, J., Zhou, Y., Hou, Y., Wang, Y., Gu, D., Su, Y., Jang, Y., & Li, S. (2019). Investigations on the effects of ginsenoside‐Rg1 on glucose uptake and metabolism in insulin resistant HepG2 cells. European Journal of Pharmacology, 843, 277–284. 10.1016/j.ejphar.2018.11.024 [DOI] [PubMed] [Google Scholar]
- Fan, X., Zhang, C., Niu, S., Fan, B., Gu, D., Jiang, K., Li, R., & Li, S. (2019). Ginsenoside Rg1 attenuates hepatic insulin resistance induced by high‐fat and high‐sugar by inhibiting inflammation. European Journal of Pharmacology, 854, 247–255. 10.1016/j.ejphar.2019.04.027 [DOI] [PubMed] [Google Scholar]
- Gao, Y., Yang, M.‐F., Su, Y.‐P., Jiang, H.‐M., You, X.‐J., Yang, Y.‐J., & Zhang, H.‐L. (2013). Ginsenoside Re reduces insulin resistance through activation of PPAR‐γ pathway and inhibition of TNF‐α production. Journal of Ethnopharmacology, 147(2), 509–516. 10.1016/j.jep.2013.03.057 [DOI] [PubMed] [Google Scholar]
- Ghorbani, A. (2013a). Best herbs for managing diabetes: A review of clinical studies. Brazilian Journal of Pharmaceutical Sciences, 49(3), 413–422. 10.1590/S1984-82502013000300003 [DOI] [Google Scholar]
- Ghorbani, A. (2013b). Phytotherapy for diabetic dyslipidemia: Evidence from clinical trials. Clinical Lipidology, 8(3), 311–319. 10.2217/clp.13.26 [DOI] [Google Scholar]
- Gu, W., Kim, K.‐A., & Kim, D.‐H. (2013). Ginsenoside Rh1 ameliorates high fat diet‐induced obesity in mice by inhibiting adipocyte differentiation. Biological and Pharmaceutical Bulletin, 36(1), 102–107. 10.1248/bpb.b12-00558 [DOI] [PubMed] [Google Scholar]
- Gu, X., Hong, Y., Lin, Y., Si, Q., Yang, L., & Dong, W. (2019). Ginsenoside Rb2 alleviates obesity by activation of brown fat and induction of browning of white fat. Frontiers in Endocrinology, 10, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D.‐H., Kim, S. H., Higashida, K., Jung, S.‐R., Polonsky, K. S., Klein, S., & Holloszy, J. O. (2012). Ginsenoside Re rapidly reverses insulin resistance in muscles of high‐fat diet fed rats. Metabolism, 61(11), 1615–1621. 10.1016/j.metabol.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. Y., Kwon, Y. S., Yang, D. C., Jung, Y. R., & Choi, Y. E. (2006). Expression and RNA interference‐induced silencing of the dammarenediol synthase gene in Panax ginseng . Plant and Cell Physiology, 47(12), 1653–1662. 10.1093/pcp/pcl032 [DOI] [PubMed] [Google Scholar]
- Hassani, F. V., Shirani, K., & Hosseinzadeh, H. (2016). Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: A review. Naunyn‐Schmiedeberg's Archives of Pharmacology, 389(9), 931–949. 10.1007/s00210-016-1256-0 [DOI] [PubMed] [Google Scholar]
- Hong, S. Y., Kim, J. Y., Ahn, H. Y., Shin, J.‐H., & Kwon, O. (2012). Panax ginseng extract rich in ginsenoside protopanaxatriol attenuates blood pressure elevation in spontaneously hypertensive rats by affecting the Akt‐dependent phosphorylation of endothelial nitric oxide synthase. Journal of Agricultural and Food Chemistry, 60(12), 3086–3091. 10.1021/jf204447y [DOI] [PubMed] [Google Scholar]
- Hong, Y. J., Kim, N., Lee, K., Sonn, C. H., Lee, J. E., Kim, S. T., Ho Baeg, I., & Lee, K.‐M. (2012). Korean red ginseng (Panax ginseng) ameliorates type 1 diabetes and restores immune cell compartments. Journal of Ethnopharmacology, 144(2), 225–233. 10.1016/j.jep.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Hosseini, A., & Hosseinzadeh, H. (2015). A review on the effects of Allium sativum (Garlic) in metabolic syndrome. Journal of Endocrinological Investigation, 38(11), 1147–1157. 10.1007/s40618-015-0313-8 [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh, H., & Nassiri‐Asl, M. (2014). Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. Journal of Endocrinological Investigation, 37(9), 783–788. 10.1007/s40618-014-0096-3 [DOI] [PubMed] [Google Scholar]
- Hou, K., Zhong, W., Chen, C., Wu, B., Zhu, D., Wang, X., & Guo, Y. (2017). Effect of baihu ginseng decoction on treatment of type 2 diabetes. Biomedical Research, 28(19), 8190–8194. [Google Scholar]
- Im Chung, S., Nam, S. J., Xu, M., Kang, M. Y., & Lee, S. C. (2016). Aged ginseng (Panax ginseng Meyer) reduces blood glucose levels and improves lipid metabolism in high fat diet‐fed mice. Food Science and Biotechnology, 25(1), 267–273. 10.1007/s10068-016-0039-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanshahidi, M., & Hosseinzadeh, H. (2008). Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytotherapy Research, 22(8), 999–1012. 10.1002/ptr.2399 [DOI] [PubMed] [Google Scholar]
- Jang, S.‐H., Park, J., Kim, S.‐H., Choi, K.‐M., Ko, E.‐S., Cha, J.‐D., Lee, Y‐R., Jang, H., & Jang, Y.‐S. (2017). Red ginseng powder fermented with probiotics exerts antidiabetic effects in the streptozotocin‐induced mouse diabetes model. Pharmaceutical Biology, 55(1), 317–323. 10.1080/13880209.2016.1237978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, W. J., Oh, J. S., Park, M. S., & Ji, G. E. (2013). Anti‐hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phytotherapy Research, 27(2), 166–172. 10.1002/ptr.4706 [DOI] [PubMed] [Google Scholar]
- Jovanovski, E., Bateman, E. A., Bhardwaj, J., Fairgrieve, C., Mucalo, I., Jenkins, A. L., & Vuksan, V. (2014). Effect of Rg3‐enriched Korean red ginseng (Panax ginseng) on arterial stiffness and blood pressure in healthy individuals: A randomized controlled trial. Journal of the American Society of Hypertension, 8(8), 537–541. 10.1016/j.jash.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Ju, C., Jeon, S.‐M., Jun, H.‐S., & Moon, C.‐K. (2019). Diol‐ginsenosides from Korean Red Ginseng delay the development of type 1 diabetes in diabetes‐prone biobreeding rats. Journal of Ginseng Research. 10.1016/j.jgr.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, S., Lee, M.‐S., Shin, Y., Kim, C.‐T., Kim, I.‐H., Kim, Y. S., & Kim, Y. (2014). Anti‐obesity and anti‐inflammatory effects of high hydrostatic pressure extracts of ginseng in high‐fat diet induced obese rats. Journal of Functional Foods, 10, 169–177. 10.1016/j.jff.2014.06.007 [DOI] [Google Scholar]
- Kamyab, H., Hejrati, S., Khanavi, M., Malihi, F., Mohammadirad, A., Baeeri, M., Esmaily, H., & Abdollahi, M. (2010). Hepatic mechanisms of the walnut antidiabetic effect in mice. Open Life Sciences, 5(3), 304–309. 10.2478/s11535-010-0019-z [DOI] [Google Scholar]
- Kang, D., Li, Z., & Ji, G. E. (2018). Anti‐obesity effects of a mixture of fermented ginseng, Bifidobacterium longum BORI, and Lactobacillus paracasei CH88 in high‐fat diet‐fed mice. Journal of Microbiology and Biotechnology, 28(5), 688–696. 10.4014/jmb.1801.01016 [DOI] [PubMed] [Google Scholar]
- Kang, J. G., & Park, C.‐Y. (2012). Anti‐obesity drugs: A review about their effects and safety. Diabetes & Metabolism Journal, 36(1), 13–25. 10.4093/dmj.2012.36.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S.‐J., Lim, Y., & Kim, A.‐J. (2014). Korean red ginseng combined with nattokinase ameliorates dyslipidemia and the area of aortic plaques in high cholesterol‐diet fed rabbits. Food Science and Biotechnology, 23(1), 283–287. 10.1007/s10068-014-0039-y [DOI] [Google Scholar]
- Karaca, T., Uslu, S., & Yörük, M. (2011). Effects of green tea and ginseng on villus length and crypt depth and on the distribution of mast and goblet cells in the small intestine of rats with streptozotocin (stz)‐induced diabetes. Philippine Journal of Veterinary Medicine, 48(2). [Google Scholar]
- Karaca, T., Yoruk, M., Yoruk, I. H., & Uslu, S. (2010). Effects of extract of green tea and ginseng on pancreatic beta cells and levels of serum glucose, insulin, cholesterol and triglycerides in rats with experimentally streptozotocin‐induced diabetes: A histochemical and immunohistochemical study. Journal of Animal and Veterinary Advances, 9(1), 102–107. 10.3923/javaa.2010.102.107 [DOI] [Google Scholar]
- Karmazyn, M., Moey, M., & Gan, X. T. (2011). Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs, 71(15), 1989–2008. 10.2165/11594300-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Kim, C. Y., Kang, B., Suh, H. J., & Choi, H.‐S. (2018). Red ginseng‐derived saponin fraction suppresses the obesity‐induced inflammatory responses via Nrf2‐HO‐1 pathway in adipocyte‐macrophage co‐culture system. Biomedicine & Pharmacotherapy, 108, 1507–1516. 10.1016/j.biopha.2018.09.169 [DOI] [PubMed] [Google Scholar]
- Kim, E.‐J., Lee, H.‐I., Chung, K.‐J., Noh, Y.‐H., Ro, Y.‐T., & Koo, J.‐H. (2009a). The ginsenoside‐Rb2 lowers cholesterol and triacylglycerol levels in 3T3‐L1 adipocytes cultured under high cholesterol or fatty acids conditions. BMB Reports, 42(4), 194–199. 10.5483/BMBRep.2009.42.4.194 [DOI] [PubMed] [Google Scholar]
- Kim, H.‐J., Chae, I.‐G., Lee, S.‐G., Jeong, H.‐J., Lee, E.‐J., & Lee, I.‐S. (2010). Effects of fermented red ginseng extracts on hyperglycemia in streptozotocin‐induced diabetic rats. Journal of Ginseng Research, 34(2), 104–112. 10.5142/jgr.2010.34.2.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. Y., & Kim, K. (2012). Regulation of signaling molecules associated with insulin action, insulin secretion and pancreatic β‐cell mass in the hypoglycemic effects of Korean red ginseng in Goto‐Kakizaki rats. Journal of Ethnopharmacology, 142(1), 53–58. 10.1016/j.jep.2012.04.012 [DOI] [PubMed] [Google Scholar]
- Kim, H.‐O., Park, M.‐J., & Han, J.‐S. (2011). Effects of fermented red ginseng supplementation on blood glucose and insulin resistance in type 2 diabetic patients. Journal of the Korean Society of Food Science and Nutrition, 40(5), 696–703. 10.3746/jkfn.2011.40.5.696 [DOI] [Google Scholar]
- Kim, J. H., Pan, J. H., Cho, H. T., & Kim, Y. J. (2016). Black ginseng extract counteracts streptozotocin‐induced diabetes in mice. PLoS One, 11(1), e0146843. 10.1371/journal.pone.0146843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. M., Park, C. H., Park, S. K., Seung, T. W., Kang, J. Y., Ha, J. S., Lee, D. S., Lee, U., Kim, D‐O., & Heo, H. J. (2017a). Ginsenoside re ameliorates brain insulin resistance and cognitive dysfunction in high fat diet‐induced C57BL/6 mice. Journal of Agricultural and Food Chemistry, 65(13), 2719–2729. 10.1021/acs.jafc.7b00297 [DOI] [PubMed] [Google Scholar]
- Kim, K.‐S., Yang, H. J., Lee, I.‐S., Kim, K.‐H., Park, J., Jeong, H.‐S., Kim, Y., Seok Ahn, K., Na, Y‐C., & Jang, H.‐J. (2015). The aglycone of ginsenoside Rg3 enables glucagon‐like peptide‐1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice. Scientific Reports, 5, 18325. 10.1038/srep18325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M., Ahn, B. Y., Lee, J. S., Chung, S. S., Lim, S., Park, S. G., Jung, H. S., Lee, H. K., & Park, K. S. (2009b). The ginsenoside Rg3 has a stimulatory effect on insulin signaling in L6 myotubes. Biochemical and Biophysical Research Communications, 389(1), 70–73. 10.1016/j.bbrc.2009.08.088 [DOI] [PubMed] [Google Scholar]
- Kim, M.‐S., Kim, S.‐H., Park, S.‐J., Sung, M. J., Park, J., Hwang, J.‐T., & Shin, S. S. (2017b). Ginseng berry improves hyperglycemia by downregulating hepatic gluconeogenesis and steatosis in mice with diet‐induced type 2 diabetes. Journal of Functional Foods, 35, 295–302. 10.1016/j.jff.2017.05.050 [DOI] [Google Scholar]
- Kim, M.‐H., Lee, E.‐J., Cheon, J.‐M., Nam, K.‐J., Oh, T.‐H., & Kim, K.‐S. (2016). Antioxidant and hepatoprotective effects of fermented red ginseng against high fat diet‐induced hyperlipidemia in rats. Laboratory Animal Research, 32(4), 217–223. 10.5625/lar.2016.32.4.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.‐N., & Kang, S.‐J. (2009). Effects of black ginseng (9 times‐steaming ginseng) on hypoglycemic action and changes in the composition of ginsenosides on the steaming process. Korean Journal of Food Science and Technology, 41(1), 77–81. [Google Scholar]
- Kim, S. T., Kim, H. B., Lee, K. H., Choi, Y. R., Kim, H. J., Shin, I. S., Gyoung, Y. S., & Joo, S. S. (2012). Steam‐dried ginseng berry fermented with Lactobacillus plantarum controls the increase of blood glucose and body weight in type 2 obese diabetic db/db mice. Journal of Agricultural and Food Chemistry, 60(21), 5438–5445. [DOI] [PubMed] [Google Scholar]
- Langin, D. (2006). Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacological Research, 53(6), 482–491. 10.1016/j.phrs.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Lee, H., Choi, J., Shin, S. S., & Yoon, M. (2016). Effects of Korean red ginseng (Panax ginseng) on obesity and adipose inflammation in ovariectomized mice. Journal of Ethnopharmacology, 178, 229–237. 10.1016/j.jep.2015.12.017 [DOI] [PubMed] [Google Scholar]
- Lee, H., Kim, M., Shin, S. S., & Yoon, M. (2014). Ginseng treatment reverses obesity and related disorders by inhibiting angiogenesis in female db/db mice. Journal of Ethnopharmacology, 155(2), 1342–1352. 10.1016/j.jep.2014.07.034 [DOI] [PubMed] [Google Scholar]
- Lee, H. M., Lee, O. H., Kim, K. J., & Lee, B. Y. (2012). Ginsenoside Rg1 promotes glucose uptake through activated AMPK pathway in insulin‐resistant muscle cells. Phytotherapy Research, 26(7), 1017–1022. 10.1002/ptr.3686 [DOI] [PubMed] [Google Scholar]
- Lee, S. H., Lee, H. J., Lee, Y. H., Lee, B. W., Cha, B. S., Kang, E. S., Ahn, C. W., Park, J. S., Kim, H. J., Lee, E., Lee, Y., & H. C. (2012). Korean red ginseng (Panax ginseng) improves insulin sensitivity in high fat fed Sprague‐Dawley rats. Phytotherapy Research, 26(1), 142–147. https://doi.org/10.1016/j.metabol.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Lee, H., Park, D., & Yoon, M. (2013). Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat diet‐induced obese C57BL/6J mice. Food and Chemical Toxicology, 53, 402–408. 10.1016/j.fct.2012.11.052 [DOI] [PubMed] [Google Scholar]
- Lee, K. H., Bae, I. Y., Park, S. I., Park, J.‐D., & Lee, H. G. (2016). Antihypertensive effect of Korean Red Ginseng by enrichment of ginsenoside Rg3 and arginine–fructose. Journal of Ginseng Research, 40(3), 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K.‐T., Jung, T. W., Lee, H.‐J., Kim, S.‐G., Shin, Y.‐S., & Whang, W.‐K. (2011). The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Archives of Pharmacal Research, 34(7), 1201. 10.1007/s12272-011-0719-6 [DOI] [PubMed] [Google Scholar]
- Lee, K. J., Lee, S. Y., & Ji, G. E. (2013). Diabetes‐ameliorating effects of fermented red ginseng and causal effects on hormonal interactions: Testing the hypothesis by multiple group path analysis. Journal of Medicinal Food, 16(5), 383–395. 10.1089/jmf.2012.2583 [DOI] [PubMed] [Google Scholar]
- Lee, M. J., Choi, H. R., Lee, J.‐H., Lee, S. J., Kwon, J. W., Choi, K.‐M., Cha, J‐D., Hwang, S‐M., Park, J. H., Lee, S. C., Park, P. J., & Lee, S. C. (2015). Co‐treatment with fermented black raspberry and red ginseng extracts improves lipid metabolism and obesity in rats fed with a high‐fat and high‐cholesterol diet. Korean Journal of Food Science and Technology, 47(3), 364–372. 10.9721/KJFST.2015.47.3.364 [DOI] [Google Scholar]
- Lee, M. R., Kim, B. C., Kim, R., Oh, H. I., Kim, H. K., Choi, K. J., & Sung, C. K. (2013). Anti‐obesity effects of black ginseng extract in high fat diet‐fed mice. Journal of Ginseng Research, 37(3), 308. 10.5142/jgr.2013.37.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. J., Lee, S. J., Choi, H. R., Lee, J.‐H., Jeong, J. T., Choi, K.‐M., Cha, J‐D., Hwang, S‐M., Park, J. H., Lee, J. H., & Lee, J. H. (2014). Cholesterol improvement effects of co‐treatment with black raspberry and red ginseng extracts in mice fed a high cholesterol diet. Journal of the Korean Society of Food Science and Nutrition, 43(10), 1491–1499. 10.3746/jkfn.2014.43.10.1491 [DOI] [Google Scholar]
- Lee, S. H., Lee, H. J., Lee, Y. H., Lee, B. W., Cha, B. S., & Kang, E. S., Ahn, C. W., Park, J. S., Kim, H. J., Lee, E. Y., & Lee, H. C. (2012). Korean red ginseng (Panax ginseng) improves insulin sensitivity in high fat fed Sprague‐Dawley rats. Phytotherapy Research, 26(1), 142–147. [DOI] [PubMed] [Google Scholar]
- Lee, S. G., Lee, Y. J., Jang, M.‐H., Kwon, T. R., & Nam, J.‐O. (2017). Panax ginseng leaf extracts exert anti‐obesity effects in high‐fat diet‐induced obese rats. Nutrients, 9(9), 999. 10.3390/nu9090999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.‐S., Cha, B.‐Y., Yamaguchi, K., Choi, S.‐S., Yonezawa, T., Teruya, T., Nagai, K., & Woo, J.‐T. (2010). Effects of Korean white ginseng extracts on obesity in high‐fat diet‐induced obese mice. Cytotechnology, 62(4), 367–376. 10.1007/s10616-010-9288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.‐M., Yoon, H., Park, H.‐M., Song, B. C., & Yeum, K.‐J. (2017). Implications of red Panax ginseng in oxidative stress associated chronic diseases. Journal of Ginseng Research, 41(2), 113–119. 10.1016/j.jgr.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, Y., Tao, L., & Wang, G. (2012). Effect of extracts from Panax ginseng, Panax notoginseng, and Ligusticum chuanxiong on vascular smooth muscle cells of aging and hypertension rats. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi= Chinese Journal of Integrated Traditional and Western Medicine, 32(10), 1374–1379. [PubMed] [Google Scholar]
- Leong, X.‐F., Rais Mustafa, M., & Jaarin, K. (2013). Nigella sativa and its protective role in oxidative stress and hypertension. Evidence‐Based Complementary and Alternative Medicine, 2013, 120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Li, R., Li, N., Zheng, F., Dai, Y., Ge, Y., Yue, H., & Yu, S. (2018). Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. Journal of Pharmaceutical and Biomedical Analysis, 158, 451–460. 10.1016/j.jpba.2018.06.024 [DOI] [PubMed] [Google Scholar]
- Li, J.‐B., Zhang, R., Han, X., & Piao, C.‐L. (2018). Ginsenoside Rg1 inhibits dietary‐induced obesity and improves obesity‐related glucose metabolic disorders. Brazilian Journal of Medical and Biological Research, 51(4). 10.1590/1414-431x20177139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Luo, J., Anandh Babu, P. V., Zhang, W., Gilbert, E., Cline, M., McMillan, R., Hulver, M., Alkhalidy, H., Zhen, W., Zhang, H., & Liu, D. (2014). Dietary supplementation of chinese ginseng prevents obesity and metabolic syndrome in high‐fat diet‐fed mice. Journal of Medicinal Food, 17(12), 1287–1297. 10.1089/jmf.2014.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., Kim, H. J., Park, M. S., & Ji, G. E. (2018). Effects of fermented ginseng root and ginseng berry on obesity and lipid metabolism in mice fed a high‐fat diet. Journal of Ginseng Research, 42(3), 312–319. 10.1016/j.jgr.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S., Yoon, J. W., Choi, S. H., Cho, B. J., Kim, J. T., Chang, H. S., Park, H. S., Park, K. S., Lee, H. K., Kim, Y‐B., & Jang, H. C. (2009). Effect of ginsam, a vinegar extract from Panax ginseng, on body weight and glucose homeostasis in an obese insulin‐resistant rat model. Metabolism, 58(1), 8–15. 10.1016/j.metabol.2008.07.027 [DOI] [PubMed] [Google Scholar]
- Lin, N., Cai, D.‐L., Jin, D., Chen, Y., & Shi, J.‐J. (2014). Ginseng panaxoside Rb1 reduces body weight in diet‐induced obese mice. Cell Biochemistry and Biophysics, 68(1), 189–194. 10.1007/s12013-013-9688-3 [DOI] [PubMed] [Google Scholar]
- Liu, H., Liu, M., Jin, Z., Yaqoob, S., Zheng, M., Cai, D., Liu, J., & Guo, S. (2019a). Ginsenoside Rg2 inhibits adipogenesis in 3T3‐L1 preadipocytes and suppresses obesity in high‐fat‐diet‐induced obese mice through the AMPK pathway. Food & Function, 10, 3603–3614. 10.1039/C9FO00027E [DOI] [PubMed] [Google Scholar]
- Liu, L., Anderson, G. A., Fernandez, T. G., & Dore, S. (2019b). Efficacy and mechanism of Panax ginseng in experimental stroke. Frontiers in Neuroscience, 13. 10.3389/fnins.2019.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R., Zhang, J., Liu, W., Kimura, Y., & Zheng, Y. (2010). Anti‐obesity effects of protopanaxdiol types of ginsenosides isolated from the leaves of American ginseng (Panax quinquefolius L.) in mice fed with a high‐fat diet. Fitoterapia, 81(8), 1079–1087. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Deng, J., & Fan, D. (2019c). Ginsenoside Rk3 ameliorates high‐fat‐diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food & Function, 10(5), 2538–2551. [DOI] [PubMed] [Google Scholar]
- Liu, Y.‐W., Zhu, X., Li, W., Lu, Q., Wang, J.‐Y., Wei, Y.‐Q., & Yin, X.‐X. (2012). Ginsenoside Re attenuates diabetes‐associated cognitive deficits in rats. Pharmacology Biochemistry and Behavior, 101(1), 93–98. 10.1016/j.pbb.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Liu, Z., Li, W., Li, X., Zhang, M., Chen, L., Zheng, Y.‐N., Sun, G.‐Z., & Ruan, C.‐C. (2013). Antidiabetic effects of malonyl ginsenosides from Panax ginseng on type 2 diabetic rats induced by high‐fat diet and streptozotocin. Journal of Ethnopharmacology, 145(1), 233–240. 10.1016/j.jep.2012.10.058 [DOI] [PubMed] [Google Scholar]
- Liu, Z., Wang, L. J., Li, X., Hu, J. N., Chen, Y., Ruan, C. C., & Sun, G. Z. (2009). Hypoglycemic effects of malonyl‐ginsenosides extracted from roots of Panax ginseng on streptozotocin‐induced diabetic mice. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 23(10), 1426–1430. [DOI] [PubMed] [Google Scholar]
- Lu, H.‐F., Lai, Y.‐H., Huang, H.‐C., Lee, I.‐J., Lin, L.‐C., Liu, H.‐K., Tien, H. H., & Huang, C. (2018). Ginseng‐plus‐Bai‐Hu‐Tang ameliorates diet‐induced obesity, hepatic steatosis, and insulin resistance in mice. Journal of Ginseng Research, 44(2), 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeva Rao, U., & Adinew, B. (2011). Remnant B‐cell‐stimulative and anti‐oxidative effects of persea Americana fruit extract studied in rats introduced into streptozotocin‐induced hyperglycaemic state. African Journal of Traditional, Complementary & Alternative Medicines, 8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F., Su, X., Li, W., & Zheng, Y. (2017). Ginsenoside Rb3 strengthens the hypoglycemic effect through AMPK for inhibition of hepatic gluconeogenesis. Experimental and Therapeutic Medicine, 13(5), 2551–2557. 10.3892/etm.2017.4280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollazadeh, H., & Hosseinzadeh, H. (2016). Cinnamon effects on metabolic syndrome: A review based on its mechanisms. Iranian Journal of Basic Medical Sciences, 19(12), 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollazadeh, H., Mahdian, D., & Hosseinzadeh, H. (2019). Medicinal plants in treatment of hypertriglyceridemia: A review based on their mechanisms and effectiveness. Phytomedicine, 53, 43–52. 10.1016/j.phymed.2018.09.024 [DOI] [PubMed] [Google Scholar]
- Moon, H.‐K., Kim, K.‐S., Chung, S.‐K., & Kim, J.‐K. (2015). Effect of wild Korean ginseng (Panax ginseng) extract on blood glucose and serum lipid contents in rats with multiple low‐dose streptozotocin‐induced diabetes. Food Science and Biotechnology, 24(4), 1505–1511. 10.1007/s10068-015-0194-9 [DOI] [Google Scholar]
- Mucalo, I., Jovanovski, E., Rahelić, D., Božikov, V., Romić, Ž., & Vuksan, V. (2013). Effect of American ginseng (Panax quinquefolius L.) on arterial stiffness in subjects with type‐2 diabetes and concomitant hypertension. Journal of Ethnopharmacology, 150(1), 148–153. [DOI] [PubMed] [Google Scholar]
- Mueller, M., Beck, V., & Jungbauer, A. (2011). PPARα activation by culinary herbs and spices. Planta Medica, 77(05), 497–504. [DOI] [PubMed] [Google Scholar]
- Nam, Y. H., Moon, H. W., Lee, Y. R., Kim, E. Y., Rodriguez, I., Jeong, S. Y., Castañeda, R., Park, J. H., Choung, S. Y., Hong, B. N., & Kang, T. H. (2019). Panax ginseng (Korea Red Ginseng) repairs diabetic sensorineural damage through promotion of the nerve growth factor pathway in diabetic zebrafish. Journal of Ginseng Research, 43(2), 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, J. S., Lee, S. R., Hwang, K. T., & Ji, G. E. (2014). The anti‐obesity effects of the dietary combination of fermented red ginseng with levan in high fat diet mouse model. Phytotherapy Research, 28(4), 617–622. 10.1002/ptr.5042 [DOI] [PubMed] [Google Scholar]
- Oh, Y.‐J., Kwon, S.‐H., Choi, K. B., Kim, T.‐S., & Yeo, I.‐H. (2014). Effect of vinegar made with hydroponic‐cultured Panax ginseng CA Meyer on body weight and lipid metabolism in high‐fat diet‐fed mice. Korean Journal of Food Science and Technology, 46(6), 743–749. 10.9721/KJFST.2014.46.6.743 [DOI] [Google Scholar]
- Organization, W. H. (2007). Prevention of cardiovascular disease: World Health Organization. [Google Scholar]
- Park, E.‐Y., Kim, H.‐J., Kim, Y.‐K., Park, S.‐U., Choi, J.‐E., Cha, J.‐Y., & Jun, H.‐S. (2012). Increase in insulin secretion induced by panax ginseng berry extracts contributes to the amelioration of hyperglycemia in streptozotocininduced diabetic mice. Journal of Ginseng Research, 36(2), 153. 10.5142/jgr.2012.36.2.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H.‐J., Kim, A.‐J., Cheon, Y.‐P., & Lee, M. (2015). Anti‐obesity effects of water and ethanol extracts of black ginseng. Journal of the Korean Society of Food Science and Nutrition, 44(3), 314–323. 10.3746/jkfn.2015.44.3.314 [DOI] [Google Scholar]
- Park, J., Jeon, Y.‐D., Kim, H.‐L., Lim, H., Jung, Y., Youn, D.‐H., Jeong, M. Y., Kim, H. J., Kim, S. H., Kim, S. J., Hong, S. H., & Um, J. Y. (2013). Interaction of Veratrum nigrum with Panax ginseng against obesity: A sang‐ban relationship. Evidence‐Based Complementary and Alternative Medicine, 2013, 732126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.‐B., Kwon, S. K., Nagar, H., Jung, S.‐B., Jeon, B. H., Kim, C. S., Oh, J.‐H., Song, H.‐J., & Kim, C.‐S. (2014a). Rg3‐enriched Korean Red Ginseng improves vascular function in spontaneously hypertensive rats. Journal of Ginseng Research, 38(4), 244–250. 10.1016/j.jgr.2014.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.‐H., Sung, K.‐S., Kim, S.‐S., Shim, G.‐S., & Han, C.‐K. (2012). Effects of puffed and fermented red ginseng on blood glucose‐related biomarkers in streptozotocin‐induced diabetic rats. Journal of the Korean Society of Food Science and Nutrition, 41(5), 630–637. 10.3746/jkfn.2012.41.5.630 [DOI] [Google Scholar]
- Park, S.‐H., Oh, M.‐R., Choi, E.‐K., Kim, M.‐G., Ha, K.‐C., Lee, S.‐K., Kim, Y.‐G., Park, B.‐H., Kim, D.‐S., & Chae, S.‐W. (2014b). An 8‐wk, randomized, double‐blind, placebo‐controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. Journal of Ginseng Research, 38(4), 239–243. 10.1016/j.jgr.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan, H.‐Y., Yuan, H.‐D., Jung, M. S., Ko, S. K., Park, Y. G., & Chung, S. H. (2012). Ginsenoside Re lowers blood glucose and lipid levels via activation of AMP‐activated protein kinase in HepG2 cells and high‐fat diet fed mice. International Journal of Molecular Medicine, 29(1), 73–80. [DOI] [PubMed] [Google Scholar]
- Razavi, B., & Hosseinzadeh, H. (2014). A review of the effects of Nigella sativa L. and its constituent, thymoquinone, in metabolic syndrome. Journal of Endocrinological Investigation, 37(11), 1031–1040. 10.1007/s40618-014-0150-1 [DOI] [PubMed] [Google Scholar]
- Razavi, B. M., & Hosseinzadeh, H. (2017). Saffron: A promising natural medicine in the treatment of metabolic syndrome. Journal of the Science of Food and Agriculture, 97(6), 1679–1685. 10.1002/jsfa.8134 [DOI] [PubMed] [Google Scholar]
- Razgonova, M. P., Veselov, V. V., Zakharenko, A. M., Golokhvast, K. S., Nosyrev, A. E., Cravotto, G., Tsatsakis, A., & Spandidos, D. A. (2019). Panax ginseng components and the pathogenesis of Alzheimer's disease. Molecular Medicine Reports, 19(4), 2975–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, M.‐Y., Cho, B., Kim, K.‐I., Kim, J., Kim, M. K., Lee, E.‐K., Kim, H.‐J., & Kim, C.‐H. (2014). Blood pressure lowering effect of Korea ginseng derived ginseol K‐g1. The American Journal of Chinese Medicine, 42(03), 605–618. 10.1142/S0192415X14500396 [DOI] [PubMed] [Google Scholar]
- Rhee, M.‐Y., Kim, Y.‐S., Bae, J.‐H., Nah, D.‐Y., Kim, Y.‐K., Lee, M.‐M., & Kim, H.‐Y. (2011). Effect of Korean red ginseng on arterial stiffness in subjects with hypertension. The Journal of Alternative and Complementary Medicine, 17(1), 45–49. 10.1089/acm.2010.0065 [DOI] [PubMed] [Google Scholar]
- Saba, E., Jeon, B. R., Jeong, D.‐H., Lee, K., Goo, Y.‐K., Kim, S.‐H., Sung, C.‐K., Roh, S.‐S., Kim, S. D., Kim, H.‐K., & Kim, H.‐K. (2016). Black ginseng extract ameliorates hypercholesterolemia in rats. Journal of Ginseng Research, 40(2), 160–168. 10.1016/j.jgr.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanati, S., Razavi, B. M., & Hosseinzadeh, H. (2018). A review of the effects of Capsicum annuum L. and its constituent, capsaicin, in metabolic syndrome. Iranian Journal of Basic Medical Sciences, 21(5), 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, E., Kim, S., Lee, S., Oh, B.‐C., & Jun, H.‐S. (2015). Ginseng berry extract supplementation improves age‐related decline of insulin signaling in mice. Nutrients, 7(4), 3038–3053. 10.3390/nu7043038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee‐Nick, R., Parizadeh, S. M. R., Zokaei, N., & Ghorbani, A. (2011). Effect of hydro‐alcoholic extract of Vaccinium arctostaphylos on insulin release from rat‐isolated langerhans islets. Koomesh, 12(4). [Google Scholar]
- Shafiee‐Nick, R., Parizadeh, S. M. R., Zokaei, N., & Ghorbani, A. (2012). Effect of Ganoderma lucidum hydroalcoholic extract on insulin release in rat‐isolated pancreatic islets. Avicenna Journal of Phytomedicine, 2(4), 206. [PMC free article] [PubMed] [Google Scholar]
- Shakib, Z., Shahraki, N., Razavi, B. M., & Hosseinzadeh, H. (2019). Aloe vera as an herbal medicine in the treatment of metabolic syndrome: A review. Phytotherapy Research, 33(10), 2649–2660. [DOI] [PubMed] [Google Scholar]
- Shang, W., Yu, X., Wang, G., & Zhao, J. (2013). Effect of ginsenoside Rb1 in ameliorating insulin resistance and ectopic fat deposition in obese mice induced by high fat diet. Zhongguo Zhong Yao Za Zhi= Zhongguo Zhongyao Zazhi=. China Journal of Chinese Materia Medica, 38(23), 4119–4123. [PubMed] [Google Scholar]
- Shen, L., Haas, M., Wang, D. Q. H., May, A., Lo, C. C., Obici, S., Tso, P., Woods, S. C., & Liu, M. (2015). Ginsenoside Rb1 increases insulin sensitivity by activating AMP‐activated protein kinase in male rats. Physiological Reports, 3(9), e12543. 10.14814/phy2.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, G.‐S., Seong, K.‐S., Lee, K.‐W., Cho, C.‐W., Lee, O.‐H., Lee, J.‐H., & Han, C.‐K. (2015). Effects of puffed red ginseng power and drink on blood glucose and serum lipid profile in streptozotocin‐induced diabetic rats. Journal of the Korean Society of Food Science and Nutrition, 44(10), 1415–1421. 10.3746/jkfn.2015.44.10.1415 [DOI] [Google Scholar]
- Shin, S.‐K., Kwon, J.‐H., Jeong, Y.‐J., Jeon, S.‐M., Choi, J.‐Y., & Choi, M.‐S. (2011). Supplementation of cheonggukjang and red ginseng cheonggukjang can improve plasma lipid profile and fasting blood glucose concentration in subjects with impaired fasting glucose. Journal of Medicinal Food, 14(1–2), 108–113. [DOI] [PubMed] [Google Scholar]
- Shin, S. S., & Yoon, M. (2018). Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet‐fed castrated mice. Journal of Ethnopharmacology, 210, 80–87. 10.1016/j.jep.2017.08.032 [DOI] [PubMed] [Google Scholar]
- Simu, S. Y., Ahn, S., Castro‐Aceituno, V., & Yang, D.‐C. (2017). Ginsenoside Rg5: Rk1 exerts an anti‐obesity effect on 3T3‐L1 cell line by the downregulation of PPARγ and CEBPα. Iranian Journal of Biotechnology, 15(4), 252. 10.15171/ijb.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R. K., Lui, E., Wright, D., Taylor, A., & Bakovic, M. (2017). Alcohol extract of North American ginseng (Panax quinquefolius) reduces fatty liver, dyslipidemia, and other complications of metabolic syndrome in a mouse model. Canadian Journal of Physiology and Pharmacology, 95(9), 1046–1057. [DOI] [PubMed] [Google Scholar]
- Siraj, F. M., SathishKumar, N., Kim, Y. J., Kim, S. Y., & Yang, D. C. (2015). Ginsenoside F2 possesses anti‐obesity activity via binding with PPARγ and inhibiting adipocyte differentiation in the 3T3‐L1 cell line. Journal of Enzyme Inhibition and Medicinal Chemistry, 30(1), 9–14. 10.3109/14756366.2013.871006 [DOI] [PubMed] [Google Scholar]
- Song, B., Ding, L., Zhang, H., Chu, Y., Chang, Z., Yu, Y., Guo, D., Zhang, S., & Liu, X. (2017). Ginsenoside Rb1 increases insulin sensitivity through suppressing 11β‐hydroxysteroid dehydrogenase type I. American Journal of Translational Research, 9(3), 1049. [PMC free article] [PubMed] [Google Scholar]
- Song, M.‐Y., Kim, B.‐S., & Kim, H. (2014). Influence of Panax ginseng on obesity and gut microbiota in obese middle‐aged Korean women. Journal of Ginseng Research, 38(2), 106–115. 10.1016/j.jgr.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabandeh, M. R., Hosseini, S. A., & Hosseini, M. (2017). Ginsenoside Rb1 exerts antidiabetic action on C2C12 muscle cells by leptin receptor signaling pathway. Journal of Receptors and Signal Transduction, 37(4), 370–378. 10.1080/10799893.2017.1286676 [DOI] [PubMed] [Google Scholar]
- Tabeshpour, J., Imenshahidi, M., & Hosseinzadeh, H. (2017). A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iranian Journal of Basic Medical Sciences, 20(5), 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeshpour, J., Razavi, B. M., & Hosseinzadeh, H. (2017). Effects of avocado (Persea americana) on metabolic syndrome: A comprehensive systematic review. Phytotherapy Research, 31(6), 819–837. [DOI] [PubMed] [Google Scholar]
- Tajmohammadi, A., Razavi, B. M., & Hosseinzadeh, H. (2018). Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review. Phytotherapy Research, 32(10), 1933–1949. [DOI] [PubMed] [Google Scholar]
- Takamura, Y., Nomura, M., Uchiyama, A., & Fujita, S. (2017). Effects of aerobic exercise combined with panaxatriol derived from ginseng on insulin resistance and skeletal muscle mass in type 2 diabetic mice. Journal of Nutritional Science and Vitaminology, 63(5), 339–348. 10.3177/jnsv.63.339 [DOI] [PubMed] [Google Scholar]
- Vassallo, P., Driver, S. L., & Stone, N. J. (2016). Metabolic syndrome: An evolving clinical construct. Progress in Cardiovascular Diseases, 59(2), 172–177. 10.1016/j.pcad.2016.07.012 [DOI] [PubMed] [Google Scholar]
- Vuksan, V., Xu, Z. Z., Jovanovski, E., Jenkins, A. L., Beljan‐Zdravkovic, U., Sievenpiper, J. L., Mark Stavro, P., Zurbau, A., Duvnjak, L., & Li, M. Z. C. (2019). Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double‐blind, randomized, crossover clinical trial. European Journal of Nutrition, 58(3), 1237–1245. [DOI] [PubMed] [Google Scholar]
- Woo, H.‐C., Shin, B.‐K., Cho, I., Koo, H., Kim, M., & Han, J. (2011). Anti‐obesity effect of carbon dioxide supercritical fluid extracts of Panax ginseng CA Meyer. Journal of the Korean Society for Applied Biological Chemistry, 54(5), 738–743. 10.1007/BF03253153 [DOI] [Google Scholar]
- Yang, H., Kim, M., Kwon, D., Kim, D., Zhang, T., Ha, C., & Park, S. (2018). Combination of aronia, red ginseng, shiitake mushroom and nattokinase potentiated insulin secretion and reduced insulin resistance with improving gut microbiome dysbiosis in insulin deficient type 2 diabetic rats. Nutrients, 10(7), 948. 10.3390/nu10070948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, K. M., Lee, C., Lo, Y. M., & Moon, B. (2012). The hypoglycemic effects of American red ginseng (Panax quinquefolius L.) on a diabetic mouse model. Journal of Food Science, 77(7), H147–H152. [DOI] [PubMed] [Google Scholar]
- Yuan, H.‐D., Kim, J. T., & Chung, S. H. (2012). Pectinase‐processed Ginseng radix (GINST) ameliorates hyperglycemia and hyperlipidemia in high fat diet‐fed ICR mice. Biomolecules & Therapeutics, 20(2), 220. 10.4062/biomolther.2012.20.2.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, H.‐D., Kim, S.‐J., Quan, H.‐Y., Huang, B., & Chung, S.‐H. (2010). Ginseng leaf extract prevents high fat diet‐induced hyperglycemia and hyperlipidemia through AMPK activation. Journal of Ginseng Research, 34(4), 369–375. 10.5142/jgr.2010.34.4.369 [DOI] [Google Scholar]
- Yun, T.‐K. (2001). Panax ginseng—a non‐organ‐specific cancer preventive? The Lancet Oncology, 2(1), 49–55. 10.1016/S1470-2045(00)00196-0 [DOI] [PubMed] [Google Scholar]
- Zaheri, S., & Marandi, S. M. (2016). The effect of ginseng supplement on heart rate, systolic and diastolic blood pressure to resistance training in trained males. Artery Research, 15, 6–11. 10.1016/j.artres.2016.06.001 [DOI] [Google Scholar]
- Zhou, S.‐S., Auyeung, K.‐ K.‐W., Yip, K.‐M., Ye, R., Zhao, Z.‐Z., Mao, Q., Xu, J., Chen, H‐B., & Li, S.‐L. (2018). Stronger anti‐obesity effect of white ginseng over red ginseng and the potential mechanisms involving chemically structural/compositional specificity to gut microbiota. Phytomedicine. 10.1016/j.phymed.2018.11.021 [DOI] [PubMed] [Google Scholar]


