Abstract
The major aim of the current study was to assess thermal stability of red pigments produced by Monascus purpureus ATCC 16362/PTCC 5303 in submerged fermentation. Natural pigments were produced by Monascus purpureus using stirred tank bioreactor. Stability of Monascus purpureus pigments was assessed under various temperature (50.2–97.8°C), salt (0%–2.5%), and pH (4.3–7.7) values. Thermal degradation constant and half‐life value of the red Monascus purpureus pigments were analyzed using response surface methodology followed by a first‐order kinetic reaction. Results of this study showed that pH, temperature, and salt content could affect red color stability of Monascus purpureus. The pigment showed various stabilities in various thermal conditions (temperature, salt, and pH). At high temperatures, degradation constant of the red pigments increased with decreasing pH, revealing that the Monascus red pigment was destroyed at lower pH values and salt could affect stability of the red pigments at lower temperatures.
Keywords: Monascus purpureus, natural pigments, response surface methodology, stability
Thermal degradation and half‐life value of the red Monascus purpureus pigments were analyzed using response surface methodology followed by a first‐order kinetic reaction. The major aim of this study was to assess thermal stability of red pigments produced by Monascus purpureus ATCC 16362/PTCC 5303 in submerged fermentation processes. At high temperatures, degradation constant of the red pigments increased with decreasing pH, revealing that the Monascus red pigment was destroyed at lower pH values. Salt content could affect stability of the red pigments at lower temperatures.

1. INTRODUCTION
Color is one of the most important characteristics of foods. It includes visual signals for flavor identification and taste thresholds. Furthermore, color plays significant roles in consumer satisfaction of foods (Hutchings, 2021). Legally permitted pigments are divided into two major categories of natural and synthetic. Natural colors include beneficial and therapeutic characteristics. Use of natural colors is one of the current marketing trends because of consumer's concerns about the safety of artificial food dyes, facilitated by possible health benefits of the natural pigments (Rodriguez‐Amaya, 2016). However, natural pigments show more sensitivity and instability when exposed to environmental factors such as high temperature, oxidation, and pH than artificial colors (Coultate & Blackburn, 2018; Khoo et al., 2017; Minioti et al., 2007; Wahyuningsih et al., 2017). Microbial pigments, as natural pigments (biopigments), are produced by bacteria, algae, and fungi (Aruldass et al., 2018). Fungi are further useful for the industrial production of pigments because of their fast growth rate, easy culture, ability to grow on low‐cost substrates, independent of weather conditions, production of various shades of colors, high yield of the products, and feasibility of bioprocess development. Blakeslea trispora and Monascus purpureus are examples of fungi biopigments used in food industries (Tirumale & Wani, 2018). In 1884, Monascus was first named and classified by the French Scientist, van Tieghem (Chen et al., 2015). The Genus Monascus belongs to the Family Monascaceae, Order Eurotiales, class Ascomycetes, Phylum Ascomycota, and Kingdom fungi. Most strains belong to three species of M. pilosus, M. purpureus, and M. ruber (Pan and Hsu, 2014). For centuries, fermented rice products have been used in Asia and Indonesia as dietary staples and food (Domínguez‐Espinosa and Webb, 2003). Monascus pigments (Mps) are used for controlling blood cholesterol, diabetes, and obesity and preventing cancers (Hong et al., 2008; Lee et al., 2011; Lee & Pan., 2012; Shi & Pan, 2011). These pigments also include antioxidant, antimicrobial, and antifungal characteristics. Furthermore, Mps are used as nitrite or nitrate replacements of meats (Estiasih et al., 2020; Feng et al., 2012; Nateghi et al., 2020; Seong et al., 2017). The most important pigment produced by Monascus sp. is red. This pigment contains monacolin K, which includes several characteristics in red koji (Lee et al., 2001).
Naturally, Mps are dissolved in water and fat (Carvalho et al., 2005), stable against pH 2–10 and high heats and usually affected by the production processes (Tseng et al., 2000). Monascus pigments produced by Monascus ruber include moderate stability when exposed at low pH or high temperatures (Silveira et al., 2013). Thermal processing is one of the most important methods of food preservation. Extreme thermal processes such as pasteurization, sterilization, and baking can affect stability of Mps (Velmurugan et al., 2011). Monascus pigments might tolerate pasteurization conditions. However, further studies are necessary to verify the interaction of Mps with food components on their stability (Silveira et al., 2013). Degradation constant (Dc) is an important factor to show instability of pigments during processing (Fernández‐López et al., 2013; Silveira et al., 2013; Vendruscolo et al., 2013). Design of kinetic models for Dc of Mp under processing conditions is necessary to prepare food products. However, availability of information on reaction kinetics is quite limited and modeling of the degradation kinetics for Mps in temperature ranges of heat processing is necessary. Based on the above‐mentioned facts, the aim of the current study was to investigate effects of salt content, temperature, and pH on the stability of Mps produced by Monascus purpureus ATCC 16362 in submerged fermentation using response surface methodology (RSM).
2. MATERIALS AND METHODS
2.1. Preparation of fungal strains
Monascus purpureus ATCC 16362/PTCC 5303 was provided by the Microbial Collection Center of Iran Scientific and Industrial Research Organization, Tehran, Iran. Mycelia were cultured on yeast powder‐soluble starch (YpSS) and resuspended every 30 days in fresh culture media and incubated at 30°C for 7 days. These were stored at 4°C until use (Keivani et al., 2020).
2.2. Preparation of spore suspensions
To prepare spore suspensions, the fungal strain was cultured on YpSS agar media and incubated at 30°C for 7–10 days and then rinsed with 5 ml of sterile distilled water (DW). Fungal cells in suspensions were counted using light microscope and cell‐counter slides (Keivani et al., 2020).
2.3. Preparation and inoculation of seed media
Seed culture media were prepared with specific compounds at pH 6 and sterilized using autoclave at 121°C for 15 min. Spore suspensions were used for the inoculation of seed media and incubated (Raiman Zist Fanavar, Iran) at 30°C for 30 hr at 120 rpm (Keivani et al., 2020).
2.4. Preparation and inoculation of the major culture media
Using seed culture media as the final media of 105 spores, inoculation was carried out in the major media optimized at 30°C for 21 days at 40 rpm (pH 4) using stirred tank bioreactor (MiniX, Yekta Tech, Iran) (Keivani et al., 2020).
2.5. Extraction of Monascus red pigments
Briefly, Mps were separated from the biomass using Whatman No. 1 filter papers. The mixing ratio of filtered materials to ethanol was 10:100. This was ultrasounded for 30 min using ultrasound device and transferred to shaking incubator for 1 hr at 180 rpm to extract red pigments (Aruldass et al., 2018).
2.6. Heat treatment
In general, Mps were mixed in citrate phosphate buffer to reach the selected pH values. Effects of temperature (50.2–97.8°C), salt (0%–2.5%), and pH (4.3–7.7) on Dc of the red pigments by M. purpureus ATCC 16362/PTCC 5303 were assessed using water bath. Mp solutions were heated for 2 hr using water bath (50.2–97.8°C), and samples were collected after 15‐min time intervals. Then, absorbance was measured at 500 nm using spectrophotometer (Spectronic UNICO 2100, USA) (Vendruscolo et al., 2013).
2.7. Kinetic calculations
The first‐order kinetic model could calculate heat Dcs. This parameter was expressed using Equation (1), and the regression lines were achieved by plotting logarithms of the remaining pigments.
| (1) |
where A was the absorbance (UA500nm), t was the time (h), and DC was the Dc (h−1). If boundary conditions in this formula linearized to A = A0 at t = 0 and A = A when t = t, results are shown in Equation (2).
| (2) |
where A0 was the initial absorbance (UA500 nm). Half‐life value (t 1/2) was calculated using various values of DC as follows:
| (3) |
where A/A0 was 2.
2.8. Experimental design
In the present study, temperature (A) (50.2–97.8°C), pH (B) (4.3–7.7), and salt content (C) (0%–2.5%) were analyzed as independent variables using central composite design (CCD) of RSM and Expert Design Software v.7.0.0. Levels of real variables in CCD are shown in Table 1. Effects of the significant independent variables were assessed in terms of DC (Y1) and t 1/2 (Y2) of the Mps using RSM at a temperature range of 50.2–97.8°C, pH range of 4.3–7.7, and salt content of 0%–2.5%. The CCD with 20 experiments (14 axial points and six replicates at the center point, α = 1.7) was used (Table 2). Response model was expressed using coded variables (Vendruscolo et al., 2013). The Mp solutions were heated for 2 hr using water bath (Table 2), and data were collected within 15‐min time intervals for the calculation of Dc and t 1/2.
TABLE 1.
Levels of the variables in central composite design (α = 1.7)
| Variable | Unit | Level and range | ||||
|---|---|---|---|---|---|---|
| −1.7 | −1 | 0 | +1 | +1.7 | ||
| Temperature (A) | °C | 50.2 | 60 | 74 | 88 | 97.8 |
| pH (B) | – | 4.3 | 5 | 6 | 7 | 7.7 |
| Salt (C) | % | 0 | 0.55 | 1.27 | 2 | 2.5 |
TABLE 2.
Degradation constants (DC) and half‐life value (t 1/2) for various heat treatments, pH, and salt percentages of red pigments produced by Monascus purpureus in submerged fermentation
| Experiment | Real variable | Response | |||
|---|---|---|---|---|---|
| Temperature (ºC) | pH | Salt (%) |
Dc (h−1) (Y1) |
t1/2 (h) (Y2) |
|
| 1 | 60 | 5 | 0.55 | 0.104 | 6.64 |
| 2 | 88 | 5 | 0.55 | 0.230 | 3.01 |
| 3 | 60 | 7 | 0.55 | 0.098 | 7.07 |
| 4 | 88 | 7 | 0.55 | 0.192 | 3.61 |
| 5 | 60 | 5 | 2 | 0.135 | 5.11 |
| 6 | 88 | 5 | 2 | 0.218 | 3.17 |
| 7 | 60 | 7 | 2 | 0.144 | 4.81 |
| 8 | 88 | 7 | 2 | 0.169 | 4.08 |
| 9 | 50.2 | 6 | 1.27 | 0.102 | 6.79 |
| 10 | 97.8 | 6 | 1.27 | 0.231 | 3.15 |
| 11 | 74 | 4.3 | 1.27 | 0.170 | 4.07 |
| 12 | 74 | 7.7 | 1.27 | 0.147 | 4.71 |
| 13 | 74 | 6 | 0.04 | 0.145 | 4.75 |
| 14 | 74 | 6 | 2.51 | 0.146 | 4.73 |
| 15 | 74 | 6 | 1.27 | 0.135 | 5.12 |
| 16 | 74 | 6 | 1.27 | 0.114 | 6.05 |
| 17 | 74 | 6 | 1.27 | 0.103 | 6.67 |
| 18 | 74 | 6 | 1.27 | 0.135 | 5.12 |
| 19 | 74 | 6 | 1.27 | 0.114 | 6.05 |
| 20 | 74 | 6 | 1.27 | 0.104 | 6.67 |
3. RESULTS AND DISCUSSION
In this study, effects of temperature (A) (50.2–97.8°C), pH (B) (4.3–7.7), and salt content (C) (0%–2.5%) on Dc and t 1/2 were investigated. Results presented in terms of A/A0 showed changes in Mp absorption. Since the graph was logarithmic, ln (A/A0) was used to linearize the graph. Based on Table 2, the highest Dc was linked to treatment 10 (97.8°C, pH 6, 1.27% salt) and the lowest Dc was associated with treatment 3 (60°C, pH 7, 0.55% salt). Regression analysis was used first for each isothermal experiment.
Figure 1 shows the curves of ln(A/A0) against heat treatment time. The kinetic parameters of Dc and t 1/2 of the red pigments are listed in Table 2. Clearly, Mp degradation increased with increasing temperatures. Furthermore, relationships revealed that degradation of the pigments was followed by a kinetic model with a good regression coefficient of .95 ≤ R 2 ≤ .99, resembling degradation behavior of monacolin K extracted from Monascus sp. (Ou et al., 2009). The adjusted R 2 and predicted R 2 included .9348 and .9124, respectively. Coefficient of variation (CV) of this model included 7.85%, which showed distribution of statistical data in the samples. The RSM is reported as an effective technique for the investigation of degradation kinetics. Effects of temperature and pH linearly and interactions of temperature–pH and temperature–salt concentration were statistically significant. Furthermore, square effects of each temperature, pH, and salt percentage were significant. For a better assessment of reaction kinetics, the second‐order polynomial model was coded to all data based on the following equation:
| (4) |
where DC was the degradation constant (h−1), A was the variable temperature, B was the variable pH, and C was the salt percentage.
FIGURE 1.
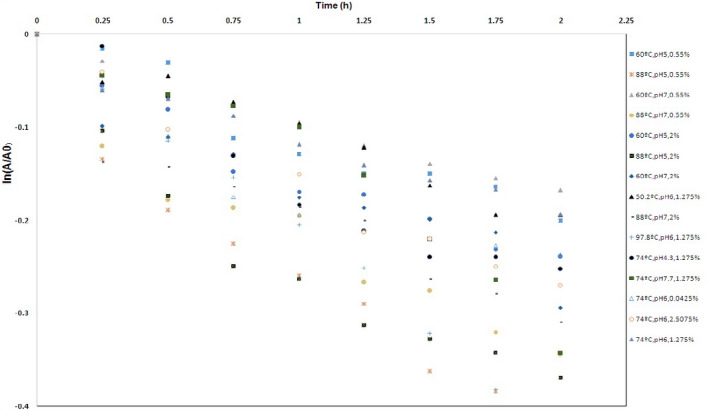
Degradation constant (Dc) of the Monascus pigments in aqueous solution versus various conditions [temperature (50.2–97.8°C), pH (4.3–7.7), and salt content (0%–2.5%)]
As shown in Equation 4, factors of temperature and salt content were linearly linked to positive coefficient, pH was linearly linked to negative coefficient, and temperature interacted with pH and salt percentage of decreasing effects. Those with positive coefficients additively affected red pigment instability. The three‐dimensional (3‐D) curve was an upward curve and graph showed that Dc of the aqueous Mps at high temperatures (75–88°C) was more stable at pH 7 than pH 5. At low temperatures (60–75°C), Dc of the Mps was mostly stable at pH 6. Moreover, graph showed that the highest instability occurred at the highest temperature, while the most stable conditions for the Mps included pH 6 and temperature of 50°C (Figure 2). This heat‐induced pigment degradation has been reported in red and orange pigments extracted from M. ruber (Vendruscolo et al., 2013).
FIGURE 2.

Effects of temperature and pH levels on the thermal degradation constant (Dc) of Monascus red pigments
Stability of the red pigments of Monascus added to rice has studied and achieved similar results in this model. Decreased color stability due to high temperatures is expected in most natural colors, but changes in pH can include various effects on natural colors (Priatni, 2015). The 3‐D diagram showed that at 88°C, the Dc of aqueous of Mps was more stable at 0.55 compared to 2% salt, although, at 60°C, the Dc of aqueous of Mps was more stable at 2 compared to 0.55% salt (Figure 3). Furthermore, red pigments of Monascus produced with corn wastes can be stable in 0.5% (wt%) sodium chloride solution and 0.5% (wt.%) ammonium chloride of the media (Velmurgan et al., 2011). Fransis (1987) reported that salt‐containing solutions of red pigments of Monascus sp. could affect the pigment stability. Red pigments of Monascus sp. were more sensitive to pH, compared to that yellow and orange pigments were. Greater stability of the yellow pigments could occur due to two reasons. The first reason was that the yellow pigments were less susceptible to red pigments and its true stability and the second reason was that degradation of yellow pigments and production of yellow compounds because of the color degradation caused further persistence of the yellow pigments (Francis, 1987). Half‐life value (t 1/2) assessments showed stability of the Monascus red pigments under process conditions. The t 1/2 of Mps was inversely linked to Dc. Half‐life value of Mps was another factor to show stability of Monascus red pigments. Values of t 1/2 were calculated as follows:
| (5) |
where t 1/2 was the half‐life value, A was the variable temperature, B was the variable pH, and C was the salt content. As shown in Equation 5, temperature and salt content were linearly linked to negative coefficient of decrease, pH was linearly linked to positive coefficient of additive effects, and temperature interacted with salt content of additive effects. Moreover, square of the three factors was individually linked to negative coefficients, including decreasing effects on t 1/2 of the Mps. The variance analysis showed significant effects of temperature, pH, and salt content on the t 1/2 of Mps (p < .01), insignificance of lack of fit (p = .9325) and R 2 of .8949 (Table 3). The most effective independent variable on t 1/2 was temperature. Effects of temperature linearly and square of temperature, salt, and pH on t 1/2 of Mps were significant; adjusted R 2 was .8204, and predicted R 2 was .7289. The coefficient of variation in this model was 11.06%.
FIGURE 3.

Effects of temperature and salt content on the thermal degradation constant (Dc) of Monascus red pigments
TABLE 3.
Effects of temperature (A), pH (B), and salt (C) and their interactions on Dc and t 1/2 of the red pigments produced by Monascus purpureus using RSM
| Source | Sum of square Dc | df Dc | F‐value Dc | p‐value Dc | Sum of squares t 1/2 |
df t 1/2 |
F‐value t 1/2 |
F‐value t 1/2 |
|---|---|---|---|---|---|---|---|---|
| Model | 0.033 | 8 | 35.05 | <.0001 | 29.12 | 7 | 14.61 | <0.0001 |
| A‐temperature | 0.022 | 1 | 183.42 | <.0001 | 18.46 | 1 | 64.8 | <0.0001 |
| B‐pH | 0.001 | 1 | 9.30 | .0110 | 0.54 | 1 | 1.9 | 0.1937 |
| C‐salt | 0.000 | 1 | 1.20 | .2953 | 0.74 | 1 | 2.6 | 0.1329 |
| AB | 0.001 | 1 | 8.20 | .0154 | ||||
| AC | 0.002 | 1 | 13.08 | .0040 | 2.44 | 1 | 8.57 | 0.0126 |
| A2 | 0.005 | 1 | 37.96 | <.0001 | 1.63 | 1 | 5.72 | 0.034 |
| B2 | 0.003 | 1 | 26.84 | .0003 | 4.21 | 1 | 14.76 | 0.0023 |
| C2 | 0.002 | 1 | 13.49 | .0037 | 2.51 | 1 | 8.8 | 0.0118 |
| Residual | 0.001 | 11 | 3.42 | 12 | ||||
| Lack of fit | 0.000 | 6 | 0.22 | .9496 | 0.98 | 7 | 0.29 | 0.9321 |
| Pure error | 0.001 | 5 | 2.43 | 5 | ||||
| Total | 0.035 | 19 | 32.54 | 19 |
Figure 4 shows that the t 1/2 of Mps decreased by increasing temperature and decreasing salt content; hence, the red pigment could be used in high‐salt foods such as snacks and potato chips after heat treatments. Since the half‐life of this pigment is significantly high at 74°C and includes relatively good stability in the presence of salt, the red pigment could be used in meat products such as sausages.
FIGURE 4.

Effects of temperature and salt content on the on half‐life value (t 1/2) of Monascus red pigments
4. CONCLUSIONS
In conclusion, results of the current study have suggested stability statues of the red pigments produced by M. purpureus trough submerged fermentation under special conditions, in which various temperatures, salt content, and pH values resulted in pigment degradations. The kinetic model for thermal degradation of the aqueous Mps produced by M. purpureus in submerged fermentation was validated as the first order based on the correlations from the assessment of the degradation rate kinetic constant for each thermal processing condition. Furthermore, Mp assessments have shown that the pigments include various behaviors against various thermal processing conditions (temperature, salt, and pH). In general, Dc of Mps increased with increasing temperature but the pigment instability decreased with increasing pH at high temperatures. Moreover, results have demonstrated that the highest instability occurred at the highest temperature and the most stable conditions for the Mps included pH 6 and temperature of 50°C. Half‐life of the Mps decreased as temperature increased, while addition of salt decreased t 1/2 of the aqueous Mps. Moreover, RSM analysis and empirical results have shown behaviors of DC and t 1/2 of the pigments produced by M. purpureus ATCC 16362/PTCC 5303 in submerged fermentation, revealing that kinetic equations of the color variables have represented color changes of the pigments well and, therefore, can be used to describe color degradations during heat processing and storage.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ETHICAL APPROVAL
This study does not involve any human or animal testing.
INFORMED CONSENT
Written informed consent was obtained from all study participants.
ACKNOWLEDGMENTS
The authors would like to thank Plant Improvement and Seed Production Research Center, Isfahan (Khorasgan) Branch, Isfahan, Islamic Azad University, Isfahan, Iran.
Abdollahi, F., Jahadi, M., & Ghavami, M. (2021). Thermal stability of natural pigments produced by Monascus purpureus in submerged fermentation. Food Science & Nutrition, 9, 4855–4862. 10.1002/fsn3.2425
DATA AVAILABILITY STATEMENT
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
REFERENCES
- Aruldass, C. A., Dufossé, L., & Ahmad, W. A. (2018). Current perspective of yellowish‐orange pigments from microorganisms‐a review. Journal of Cleaner Production, 180, 168–182. 10.1016/j.jclepro.2018.01.093 [DOI] [Google Scholar]
- Carvalho, J. C. D., Oishi, B. O., Pandey, A., & Soccol, C. R. (2005). Biopigments from Monascus: Strains selection, citrinin production and color stability. Brazilian Archives of Biology and Technology, 48(6), 885–894. 10.1590/S1516-89132005000800004 [DOI] [Google Scholar]
- Chen, W., He, Y., Zhou, Y., Shao, Y., Feng, Y., Li, M., & Chen, F. (2015). Edible filamentous fungi from the species Monascus: Early traditional fermentations, modern molecular biology, and future genomics. Comprehensive Reviews in Food Science and Food Safety, 14(5), 555–567. 10.1111/1541-4337.12145 [DOI] [Google Scholar]
- Coultate, T., & Blackburn, R. S. (2018). Food colorants: Their past, present and future. Coloration Technology, 134(3), 165–186. 10.1111/cote.12334 [DOI] [Google Scholar]
- Domínguez‐Espinosa, R. M., & Webb, C. (2003). Submerged fermentation in wheat substrates for production of Monascus pigments. World Journal of Microbiology and Biotechnology, 19(3), 329–336. 10.1023/A:1023609427750 [DOI] [Google Scholar]
- Estiasih, T., Kuliahsari, D. E., & Widayanti, V. T. (2020). Increasing health benefit of wild yam (Dioscorea hispida) tuber by red mold (Angkak) Fermentation. In IOP Conference Series: Earth and Environmental Science (Vol. 515, No. 1, p. 12055). IOP Publishing. 10.1088/1755-1315/515/1/012055 [DOI] [Google Scholar]
- Feng, Y., Shao, Y., & Chen, F. (2012). Monascus pigments. Applied Microbiology and Biotechnology, 96(6), 1421–1440. 10.1007/s00253-012-4504-3 [DOI] [PubMed] [Google Scholar]
- Fernández‐López, J. A., Angosto, J. M., Giménez, P. J., & León, G. (2013). Thermal stability of selected natural red extracts used as food colorants. Plant Foods for Human Nutrition, 68(1), 11–17. 10.1007/s11130-013-0337-1 [DOI] [PubMed] [Google Scholar]
- Francis, F. J. (1987). Lesser‐Known food colorants. Food Technology, 41(4), 63–68. [Google Scholar]
- Hong, M. Y., Seeram, N. P., Zhang, Y., & Heber, D. (2008). Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. The Journal of Nutritional Biochemistry, 19(7), 448–458. 10.1016/j.jnutbio.2007.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings, J. B. (2021). Evolution and human's attraction and reaction to colour: Food and health. Color Research & Application, 10.1002/col.22582 [DOI] [Google Scholar]
- Keivani, H., Jahadi, M., & Ghasemisepero, N. (2020). Optimizing submerged cultivation for the production of red pigments by Monascus purpureus on soybean meals using Response Surface Methodology. Applied Food Biotechnology, 7(3), 143–152. 10.22037/afb.v7i3.28931 [DOI] [Google Scholar]
- Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research, 61(1), 1361779. 10.1080/16546628.2017.1361779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B. H., Hsu, W. H., Liao, T. H., & Pan, T. M. (2011). The Monascus metabolite monascin against TNF‐α‐induced insulin resistance via suppressing PPAR‐γ phosphorylation in C2C12 myotubes. Food and Chemical Toxicology, 49(10), 2609–2617. 10.1016/j.fct.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Lee, B. K., Park, N. H., Piao, H. Y., & Chung, W. J. (2001). Production of red pigments by Monascus purpureus in submerged culture. Biotechnology and Bioprocess Engineering, 6(5), 341–346. 10.1007/BF02933003 [DOI] [Google Scholar]
- Lee, C. L., & Pan, T. M. (2012). Development of Monascus fermentation technology for high hypolipidemic effect. Applied Microbiology and Biotechnology, 94(6), 1449–1459. 10.1007/s00253-012-4083-3 [DOI] [PubMed] [Google Scholar]
- Minioti, K. S., Sakellariou, C. F., & Thomaidis, N. S. (2007). Determination of 13 synthetic food colorants in water‐soluble foods by reversed‐phase high‐performance liquid chromatography coupled with diode‐array detector. Analytica Chimica Acta, 583(1), 103–110. 10.1016/j.aca.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Nateghi, L., Maleki Kahaki, A., & Zarei, F. (2020). The Effect of replacing sodium nitrite with Monascus Purpureus pigment in German Sausage. Journal of Nutrition and Food Security, 5(4), 335–344. 10.18502/jnfs.v5i4.4435 [DOI] [Google Scholar]
- Ou, H. P., Wang, C. C., & Lai, L. S. (2009). Thermal degradation kinetics analysis of monacolin K in Monascus‐fermented products. LWT‐Food Science and Technology, 42(1), 292–296. 10.1016/j.lwt.2008.05.021 [DOI] [Google Scholar]
- Pan, T. M, & Hsu, W. H(2014). Monascus‐Fermented Products. In Encyclopedia of Food Microbiology, Second edition, (pp. 815–825). Elsevier Inc. 10.1016/B978-0-12-384730-0.00226-3 [DOI] [Google Scholar]
- Priatni, S. (2015). Encapsulation and stability study of Monascus fermented rice extract. Procedia Chemistry, 17, 189–193. 10.1016/j.proche.2015.12.118 [DOI] [Google Scholar]
- Rodriguez‐Amaya, D. B. (2016). Natural food pigments and colorants. Current Opinion in Food Science, 7, 20–26. 10.1016/j.cofs.2015.08.004 [DOI] [Google Scholar]
- Seong, P.‐N., Ba, H. V., Kim, Y.‐S., Kang, S.‐M., Cho, S.‐H., Kim, J.‐H., Park, B.‐Y., Kang, G.‐H., Moon, S.‐S., & Seo, H.‐W. (2017). Effects of additions of Monascus and laccaic acid on the color and quality properties of nitrite‐free emulsion sausage during refrigerated storage. Korean Journal for Food Science of Animal Resources, 37(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. C., & Pan, T. M. (2011). Beneficial effects of Monascus purpureus NTU 568‐fermented products: A review. Applied Microbiology and Biotechnology, 90(4), 1207. 10.1007/s00253-011-3202-x [DOI] [PubMed] [Google Scholar]
- Silveira, S. T., Daroit, D. J., Sant'Anna, V., & Brandelli, A. (2013). Stability modeling of red pigments produced by Monascus purpureus in submerged cultivations with sugarcane bagasse. Food and Bioprocess Technology, 6(4), 1007–1014. 10.1007/s11947-011-0710-8 [DOI] [Google Scholar]
- Tirumale, S., & Wani, N. A. (2018). Biopigments: Fungal pigments in fungi and their role in sustainable development: Current perspectives (pp. 413–426). Springer. [Google Scholar]
- Tseng, Y. Y., Chen, M. T., & Lin, C. F. (2000). Growth, pigment production and protease activity of Monascus purpureus as affected by salt, sodium nitrite, polyphosphate and various sugars. Journal of Applied Microbiology, 88(1), 31–37. 10.1046/j.1365-2672.2000.00821.x [DOI] [PubMed] [Google Scholar]
- Velmurugan, P., Hur, H., Balachandar, V., Kamala‐Kannan, S., Lee, K.‐J., Lee, S.‐M., Chae, J.‐C., Shea, P. J., & Oh, B.‐T. (2011). Monascus pigment production by solid‐state fermentation with corn cob substrate. Journal of Bioscience and Bioengineering, 112(6), 590–594. 10.1016/j.jbiosc.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Vendruscolo, F., Luise Müller, B., Esteves Moritz, D., de Oliveira, D., Schmidell, W., & Luiz Ninow, J. (2013). Thermal stability of natural pigments produced by Monascus ruber in submerged fermentation. Biocatalysis and Agricultural Biotechnology, 2(3), 278–284. 10.1016/j.bcab.2013.03.008 [DOI] [Google Scholar]
- Wahyuningsih, S., Wulandari, L., Wartono, M. W., Munawaroh, H., & Ramelan, A. H. (2017). The effect of pH and color stability of anthocyanin on food colorant. IOP Conference Series: Materials Science and Engineering, 193, 12047. 10.1088/1757-899X/193/1/012047 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.


