Over the last two decades, the large majority of clinical guidelines on the treatment of hyperglycemia in subjects with type 2 diabetes have suggested metformin as the first-line glucose-lowering treatment alongside lifestyle changes to reach personalized glycemic targets. Recently, the European Society of Cardiology recommended using glucagon-like peptide 1 receptor agonists (GLP-1RA) or sodium–glucose cotransporter 2 inhibitors (SGLT-2i) as first-line glucose-lowering therapy in subjects with type 2 diabetes at high or very high risk of cardiovascular disease, ahead of metformin treatment, to reduce cardiovascular events (1).
Following the European Society of Cardiology guidelines, several analyses have investigated whether the cardiovascular effects of GLP-1RA or SGLT-2i would differ in relation to the use of metformin. Some of these studies reported a “statistically significant” difference (i.e., interaction) in the cardiovascular effects of SGLT-2is, whereby subjects with metformin have a lower cardiovascular protection from SGLT-2i, leading to several hypotheses about the possible pharmacological mechanisms. Interpreting interaction results, however, may be difficult, as they suffer from well-known drawbacks, including limited statistical power (2). For overcoming this problem and identifying who may be most likely to benefit from a specific treatment, trial-specific interactions may be combined with a meta-analytical approach (3).
In this study, we systematically investigated the differences in the treatment effect of incretins (GLP-1RA and dipeptidyl peptidase 4 inhibitors [DPP-4i]) and SGLT-2i on cardiovascular outcomes according to metformin use. We included DPP-4i given their overlapping pharmacodynamics with GLP-1RAs and the previous evidence of interactions with metformin (4).
On 5 October 2020, we searched for randomized controlled trials (RCTs) in adults with type 2 diabetes reporting incretin or SGLT-2i treatment effect for the primary cardiovascular outcome (major adverse cardiovascular event [MACE]) stratified by baseline metformin use; details of the search, included trials, and risk of bias are available on request. We first estimated the differential cardiovascular treatment effect (i.e., interaction coefficients) by baseline metformin status within each RCT as the ratio of the hazard ratios (RHR) (5), with values <1 indicating a greater cardiovascular treatment effect in participants with metformin at baseline; then, RHRs were combined across RCTs (3). We used Stata, version 16.0, for the analysis.
Of the 22 full texts assessed, 10 reported cardiovascular treatment effects stratified by metformin. The risk of bias was deemed generally low; yet, in only three studies it was clearly stated that interactions were adjusted for potential confounders. The random-effects RHRs were 0.86 (95% CI 0.75, 0.99; I2 12.6%), 1.11 (0.89, 1.39; I2 28.2%), and 1.09 (0.91, 1.30; I2 39.8%) for combination of four DPP-4i, two GLP-1RA, and four SGLT-2i RCTs, respectively (Fig. 1). Fixed-effect estimates were virtually identical to the random-effects ones. These results suggested a larger effect of DPP-4i in patients on metformin at baseline; conversely, there was no statistical evidence that baseline metformin modified the cardiovascular effects of GLP-1RA and SGLT-2i.
Figure 1.
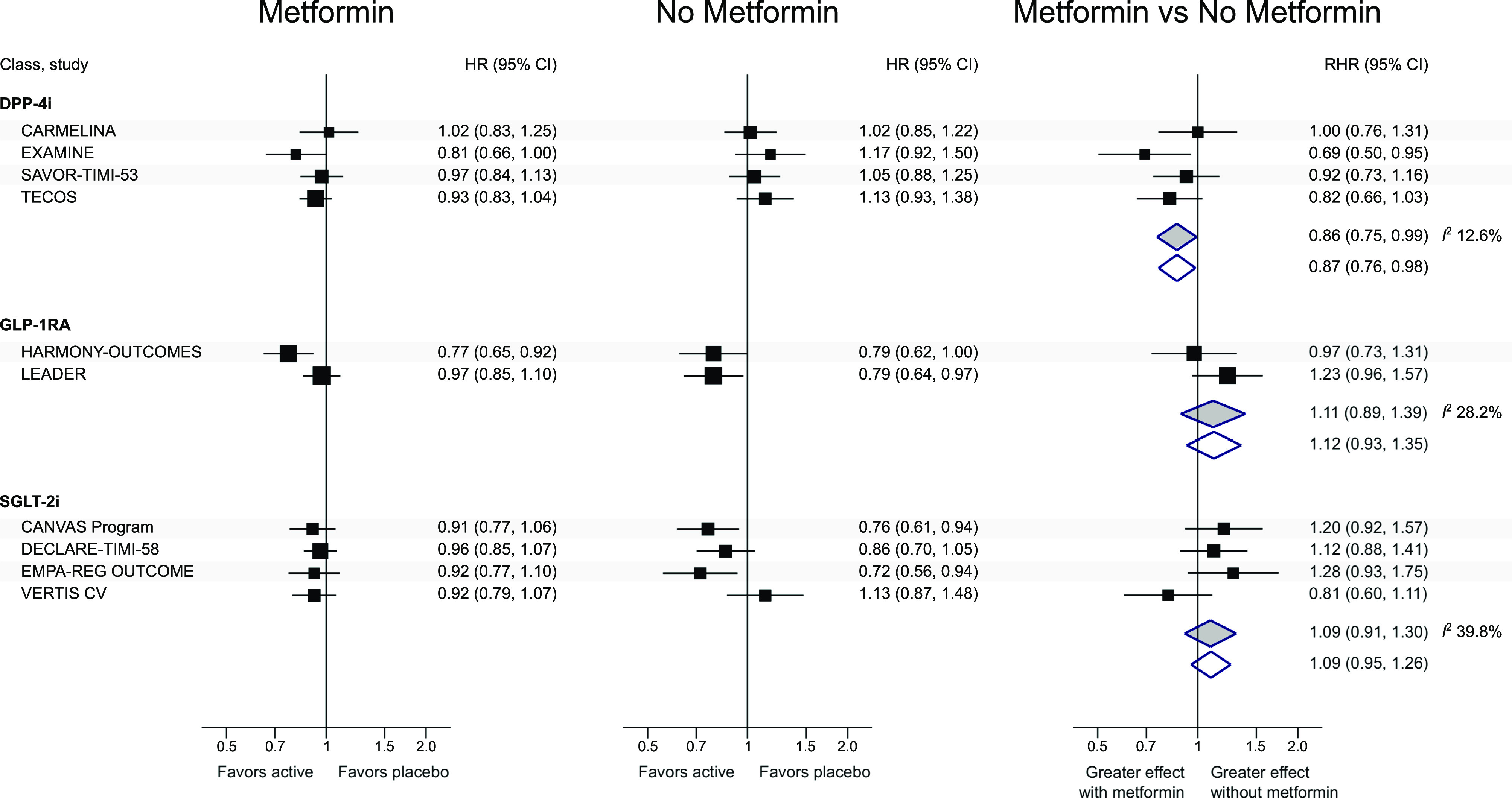
Cardiovascular treatment effect by baseline metformin use. HR, hazard ratio (active vs. placebo) of major adverse cardiovascular event (defined as the first occurrence of cardiovascular death, myocardial infarction, or stroke, except in Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS), which also included hospitalization for unstable angina); RHR, ratio between the hazard ratio in the subgroup of participants on metformin at baseline and the hazard ratio in the subgroup of participants not on metformin at baseline. An RHR <1 (i.e., a smaller hazard ratio in subjects with vs. those without metformin at baseline) indicates a greater treatment effect in subjects on metformin. White diamonds, inverse-variance fixed effect; gray diamonds, DerSimonian-Laird random effects. I2 (inconsistency across estimates): no evidence of heterogeneity for DPP-4i and GLP-1RA, moderate heterogeneity for SGLT-2i. CANVAS, Canagliflozin Cardiovascular Assessment Study; CARMELINA, CArdiovascular safety and Renal Microvascular outcomE with LINAgliptin in patients with Type 2 Diabetes mellitus at high vascular risk; DECLARE-TIMI 58, Dapagliflozin Effect on Cardiovascular Events trial; EMPA-REG OUTCOME, BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients trial; EXAMINE, Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care trial; Harmony Outcomes, Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER); SAVOR-TIMI 53, Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53; VERTIS CV, eValuation of ERTugliflozin efficacy and Safety CardioVascular outcomes trial.
The results of trial-specific subgroup analysis should be interpreted carefully. Indeed, several characteristics (such as kidney function, body weight, or diabetes duration) may differ between patients with and without metformin and may be the cause of the different cardiovascular effects. These confounders should be accounted for before it is concluded that metformin is the cause of any potential effect heterogeneity (2); however, we were only able to identify three studies that adjusted for confounding. Lastly, interaction estimates were not corrected for multiple testing, there were only a few RCTs with GLP-1RA, and the duration of metformin treatment at baseline could vary across RCTs. These limitations should be considered in the interpretation of the interactions for GLP-1RA, SGLT-2i, and DPP-4i, whose potentially larger effect in subjects with baseline metformin could be related to its bile acid–mediated effect on intestinal L cells (4).
As strategy-driven, head-to-head RCTs comparing SGLT-2i, GLP-1RA, or DPP-4i with metformin (and their combination with metformin) while ensuring glycemic and weight equipoise are unlikely to become available in the future, sharing and analyzing individual-level data from already conducted RCT would help to inform the evidence for the best first-line treatment(s) in subjects with type 2 diabetes and confirm or refute our findings. More importantly, these analyses would allow us to quantify the absolute treatment effect in relation to medication costs and multiple patient characteristics—in line with a personalized approach to diabetes treatment where other outcomes (i.e., glucose control, weight, and microvascular complications) alongside cardiovascular disease protection are at stake.
Article Information
Funding. F.Z., K.K., and M.J.D. acknowledge support from the National Institute for Health Research Applied Research Collaborations – East Midlands (NIHR ARC - EM) and the NIHR Leicester Biomedical Research Centre. J.B.B. is supported by grants from the National Institutes of Health (UL1TR002489, P30DK124723).
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Duality of Interest. F.Z. reports honoraria for lectures from Napp Pharmaceuticals and Boehringer Ingelheim. J.B.B. reports contracted consulting fees and travel support for contracted activities that are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Sanofi, Senseonics, vTv Therapeutics, and Zafgen as well as grant support from NovaTarg, Novo Nordisk, Sanofi, Tolerion, and vTv Therapeutics. J.B.B. is also a consultant to Cirius Therapeutics, CSL Behring, Fortress, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, and Stability Health and owns stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health. C.M. serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp & Dohme, Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Hanmi Pharmaceuticals, Roche, Medtronic, ActoBio Therapeutics, Pfizer, and UCB. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for C.M. from Medtronic, Novo Nordisk, Sanofi, Merck Sharp & Dohme, Eli Lilly and Company, Roche, Abbott, ActoBio Therapeutics, and Novartis; and C.M. serves or has served on the speakers bureau for Novo Nordisk, Sanofi, Merck Sharp & Dohme, Eli Lilly and Company, Boehringer Ingelheim, AstraZeneca, and Novartis. K.K. is a consultant for, on the advisory panel for, and on the speakers bureau for Amgen, AstraZeneca, Bayer, Napp Pharmaceuticals, Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche, Berlin-Chemie AG/Menarini Group, Boehringer Ingelheim, Sanofi, and Servier; is a board member for AstraZeneca, Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi; and has received grants from AstraZeneca, Novartis, Novo Nordisk, Sanofi, Lilly, Servier, Pfizer, Boehringer Ingelheim, and Merck Sharp & Dohme. M.J.D. is a consultant for, is an advisory board member of, and has received speaker fees from Novo Nordisk, Sanofi, Eli Lilly, and Boehringer Ingelheim; is an advisory board member of and has received speaker fees from AstraZeneca; has received advisory board member fees from Janssen and Gilead Sciences; has received consultant and advisory board member fees from Intarcia/Servier; has received speaker fees from Napp Pharmaceuticals, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, and Takeda Pharmaceuticals International, Inc.; and has received grants from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, Janssen, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. F.Z. contributed to data collection, statistical analysis, and writing the first draft of the manuscript. D.E.K. and M.J.D. contributed to data collection. D.E.K., J.B.B., C.M., K.K., and M.J.D. contributed to data interpretation and critical revision of the manuscript. F.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
References
- 1.Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group . 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323 [DOI] [PubMed] [Google Scholar]
- 2.Kent DM, van Klaveren D, Paulus JK, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement: explanation and elaboration. Ann Intern Med 2020;172:W1–W25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher DJ, Carpenter JR, Morris TP, Freeman SC, Tierney JF. Meta-analytical methods to identify who benefits most from treatments: daft, deluded, or deft approach? BMJ 2017;356:j573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley MJ, Williams JW Jr, Kosinski AS, D’Alessio DA, Buse JB. Metformin use may moderate the effect of DPP-4 inhibitors on cardiovascular outcomes. Diabetes Care 2017;40:1787–1789 [DOI] [PubMed] [Google Scholar]
- 5.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]


