Abstract
Objective
To describe the experiences of three women with blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) who desired to pursue planned oocyte cryopreservation.
Design
Case series.
Setting
An academic institution and a private clinic.
Patient(s)
Three nulligravid women aged 23, 25, and 34 years who desired to pursue planned oocyte cryopreservation. Two women had BPES diagnosed when they were infants and one had BPES diagnosed after presenting to discuss oocyte cryopreservation.
Intervention(s)
All three women underwent ovarian stimulation. One woman underwent three oocyte retrievals.
Main Outcomes Measure(s)
Vitrification of metaphase II oocytes.
Result(s)
One woman had a total of eight metaphase II oocytes vitrified. In addition, she underwent genetic testing that confirmed type 1 BPES. The other two women, who had BPES diagnosed when they were newborns, each underwent two cycles of ovarian stimulation. Neither of these two women responded to ovarian stimulation and both cycles were cancelled before oocyte retrieval.
Conclusion(s)
BPES is a rare condition that can lead to primary ovarian insufficiency. Early identification of this condition is important to allow for timely reproductive counseling so that oocyte cryopreservation can be offered at a young age before oocyte depletion. Careful counseling is critical for these patients, because this case series demonstrated that not all women with BPES will respond to stimulation. Further, outcomes with cryopreserved oocytes have not yet been described in women with BPES.
Key Words: Blepharophimosis-ptosis-epicanthus inversus syndrome, diminished ovarian reserve, premature ovarian failure, primary ovarian insufficiency, oocyte vitrification
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-21-00036
Primary ovarian insufficiency (POI), historically termed premature ovarian failure or premature menopause, is recognized as amenorrhea in the setting of elevated gonadotropins and hypoestrogenism in women <40 years old (1). Some women with POI present with ovarian failure. Others who will develop POI will still be ovulatory on initial presentation but will have diminished ovarian reserve (DOR) (2, 3). The workup for POI generally focuses on genetic etiologies and testing for adrenocortical or thyroid antibodies (1). A rare cause of POI that can easily be overlooked is blepharophimosis-ptosis-epicanthus inversus syndrome (BPES) (4).
Blepharophimosis-ptosis-epicanthus inversus syndrome is primarily recognized by the phenotypic eye findings that were first identified as an inherited disorder by Owens et al. (5) in the 1960s. These include blepharophimosis (narrowing of the eye opening), ptosis (drooping eyelids), epicanthus inversus (upward fold of the medial, lower eyelid), telecanthus (widening of medial border of the eyes), strabismus, and refractory errors (6, 7). Two types of BPES have been clinically recognized. Type 1 is associated with POI in addition to the phenotypic eye findings, and type 2 is limited to the eye findings (4, 6). Blepharophimosis-ptosis-epicanthus inversus syndrome-associated female infertility was first described in the 1970s, which subsequently led to the delineation of BPES into two types in the 1980s (8, 9). In 2001, a mutation in the FOXL2 gene on chromosome 3 was found to cause BPES (10). The FOXL2 gene plays a role in both eyelid development as well as ovarian development (11). Although still under investigation, the current understanding is that FOXL2 is necessary for granulosa cell differentiation and folliculogenesis (11, 12).
Blepharophimosis-ptosis-epicanthus inversus syndrome primarily follows an autosomal dominant inheritance pattern; however, de novo mutations have been discovered. The true prevalence of BPES is unknown, because there can be significant variability in the phenotypic inheritance (13). It was shown to affect families that are of diverse ethnicities and occurs in families without consanguinity (14, 15).
We report a case series of three patients with BPES. First, we describe a 34-year-old woman with DOR who had BPES diagnosed on presentation to discuss planned oocyte cryopreservation and who subsequently underwent ovarian stimulation that led to cryopreservation of eight metaphase II (MII) oocytes. Next, 2 siblings (aged 23 and 25 years) with known diagnoses of BPES who attempted to cryopreserve oocytes but failed to respond to ovarian stimulation are described. At our institution, Institutional Review Board approval was not required for this case series. Informed consent was obtained from the patients.
Case report
Patient 1
A 34-year-old nulligravida presented for care to the Northwestern Medicine Center for Fertility and Reproductive Medicine because of a desire to pursue planned oocyte cryopreservation. She experienced menarche at 12 years old and had been taking combined levonorgestrel/ethinyl estradiol oral contraceptive pills (OCPs) for 10 years before her initial consultation. Her medical history was unremarkable. Her surgical history was notable for a muscle transfer to her eyelids for ptosis when she was 5 years old. She denied a history of known familial genetic disorders.
An antimüllerian hormone (AMH) level was drawn at her initial clinic visit and was 0.66 ng/mL. She stopped her OCPs and returned in the subsequent month for day 3 laboratory test results and an antral follicle count (AFC) that confirmed DOR. Her follicle-stimulating hormone (FSH) level was 27.2 mIU/mL, estradiol level was 15 pg/mL, and AFC was 10. Menses were regular after discontinuation of OCPs. Ovarian reserve testing was repeated in the subsequent two months and showed an FSH level of 18.7 mIU/mL, AMH level of 0.64 ng/mL, and an AFC of 8 in month 1 and an FSH level of 7.1 mIU/mL and an AFC of 11 in month 2. Given the presence of detectable AMH and visible antral follicles, the patient was offered the option to pursue oocyte cryopreservation and was carefully counseled regarding both the potential for cycle cancellation as well as the uncertainties regarding oocyte competence.
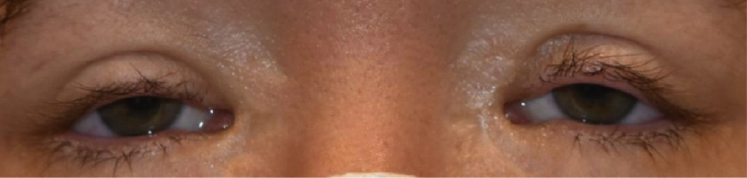
On presentation, the patient’s eyelid findings (Fig. 1) in conjunction with her family history and personal history of surgery prompted the question as to whether she had BPES. Her father and paternal grandmother had similar eye findings and her grandmother likely had secondary infertility after her first child was born. The patient was referred to the Division of Clinical Genetics at Northwestern Medicine to undergo testing for the FOXL2 gene to confirm the diagnosis of BPES. Genetic testing confirmed that she was heterozygous for a variant in the FOXL2 gene. The variant, c.148del, resulted in a frameshift mutation and premature protein termination and was predicted to be pathogenic. Given her clinical findings, which included DOR and eyelid findings, in combination with her genetic testing results, she met the criteria for a diagnosis of type 1 BPES.
Figure 1.
Patient 1’s eye findings are consistent with blepharophimosis-ptosis-epicanthus inversus syndrome.
Ovarian Stimulation, Oocyte Retrieval, and Oocyte Cryopreservation
The patient underwent three cycles of oocyte vitrification. There was concern for a poor response because of her elevated FSH level. Therefore, a microdose flare protocol with estrogen priming was selected for the initial cycle. In her first cycle, she underwent luteal estradiol priming with two transdermal estradiol patches (0.1 mg estradiol transdermal system; Novartis Pharmaceuticals, East Hanover, NJ, USA) placed every 2 days beginning 10 days after the luteinizing hormone surge. On cycle day 2, the patient had a transvaginal ultrasound, which revealed an AFC of 9, and stimulation was begun. The microdose flare protocol leuprolide acetate (10 units, 1 mg/0.2 mL, Lupron; Takeda Pharmaceuticals, Osaka, Japan) was administered twice daily beginning on cycle day 2 and continued through the day of trigger. Recombinant FSH (300 IU initial dose, Follistim; Merck & Co, Inc., Whitehouse Station, NJ, USA) and human menopausal gonadotropin (hMG, 150 IU initial dose, Menopur; Ferring Pharmaceuticals, Parsippany, NJ, USA) were administered to stimulate follicular development. On day 4, the estradiol level was 67 pg/mL and the dose of recombinant FSH was increased (to 450 IU). Serial transvaginal ultrasound monitoring was performed in conjunction with measurement of estradiol levels to determine the ovarian response. Choriogonadotropin alfa (Ovidrel; EMD Serono, Rockland, MA, USA) was used to induce final oocyte maturation on day 19 of gonadotropin stimulation with a peak estradiol level of 1,131 pg/mL and 4 follicles ≥15 mm. Transvaginal oocyte retrieval was performed 36 hours after the final maturation trigger under ultrasound guidance with monitored anesthesia care. Six oocytes were retrieved but only one mature (MII) oocyte was vitrified. The remaining oocytes (metaphase I [MI] × 1, germinal vesicle × 2, and empty zona pellucida × 2) were discarded.
Because of the poor oocyte maturity in the first cycle, an antagonist protocol (Ganirelix Acetate Injections; Merck & Co, Inc., Jersey City, NJ, USA) was used for her second cycle, because this afforded the ability to use a dual trigger that combined a gonadotropin-releasing hormone agonist and human chorionic gonadotropin (hCG). In women with DOR undergoing planned oocyte cryopreservation, a dual trigger may help improve maturation rates compared with those of hCG alone (16). Ovarian stimulation was initiated on cycle day 2 after baseline ultrasound showed an AFC of 13 and quiescent ovaries. The initial dosing was recombinant FSH (300 IU) with hMG (150 IU), and recombinant FSH was increased (to 450 IU) on stimulation day 8 with an estradiol level of 483 pg/mL. Ganirelix acetate was begun on stimulation day 6. The final oocyte maturation trigger was administered with both chorionic gonadotropin (10,000 IU, Novarel; Ferring Pharmaceuticals) and leuprolide acetate (40 units of 1 mg/0.2 mL) on day 14 of stimulation with a peak estradiol level of 2,529 pg/mL. On the day of trigger, 6 follicles were ≥15 mm. Oocyte retrieval was subsequently performed and 15 oocytes were retrieved. Three MIIs were vitrified and the remaining oocytes (MI × 5, germinal vesicle × 4, empty zona pellucida × 2, atretic ×1) were discarded.
Given the outcome from the second cycle, an antagonist protocol was again used for her third stimulation cycle. On cycle day 2, the AFC was 17, and she was started on recombinant FSH (300 IU) and hMG (150 IU). The dose of recombinant FSH was increased (to 375 IU on stimulation day 9 and to 450 IU on stimulation day 14). Ganirelix acetate was begun on stimulation day 7. The final oocyte maturation trigger was administered with both of chorionic gonadotropin (10,000 IU) and leuprolide acetate (80 units, 1 mg/0.2 mL) on day 16 of stimulation with a peak estradiol level of 2,639 pg/mL. Oocyte retrieval was subsequently performed, and 13 oocytes were retrieved. Four MIIs were vitrified whereas the remaining oocytes (MI × 5 and atretic ×4) were discarded
After all oocyte retrievals, cumulus-oocyte complexes were recovered in pre-equilibrated Quinn’s Advantage fertilization medium (SAGE, Trumbull, CT, USA) supplemented with 5 mg/mL human serum albumin (SAGE, Trumbull, CT, USA) under oil (Light Mineral Oil; Irvine scientific, Santa Ana, CA, USA) and maintained in 500 μL Quinn’s Advantage fertilization medium under oil at 37 C in an atmosphere containing 6.0% CO2, 5% O2, and balance N2 for 1–2 hours. Cumulus cells were then denuded enzymatically with ICSI Cumulase (SAGE) for assessment of nuclear maturity. Mature oocytes were vitrified using a commercial vitrification kit with the Cryolock device (Irvine Scientific).
Patient 2
A 25-year-old nulligravida who had BPES diagnosed when she was a newborn presented for consideration of oocyte cryopreservation to Pacific NW Fertility. In addition, her paternal grandmother, father, and sister had a diagnosis of BPES. Her grandmother gave birth to her father when she was 31 years old but was unable to have additional children.
The patient experienced menarche at age 14 and subsequently had irregular menses. She was started on OCPs at age 18 years, but self-discontinued them one year before she presented for care. When not taking OCPs, her menstrual cycle lengths ranged from 35 to 40 days, but were often >40 days. On presentation, her FSH level was 5.9 mIU/mL and her AMH level was 3.0 ng/mL. Repeat laboratory tests were performed one month later, which demonstrated an AMH level of 2.3 ng/mL and an FSH level of 16.6 mIU/mL. Because of the discrepancy in the results, ovarian reserve testing was repeated one month later and revealed an early follicular phase FSH level of 16.4 mIU/mL and an AMH level of 1.84 ng/mL. Given the patient’s elevated FSH level, she was presumed to have type 1 BPES, although genetic testing was performed before presentation with ongoing attempts to obtain these records.
Ovarian Stimulation
On cycle day 2, the patient's baseline estradiol was 23 pg/mL with an AFC of 3. She was started on a standard antagonist protocol and gonadotropin stimulation was begun with recombinant FSH (300 IU) and hMG (150 IU). This protocol was chosen because of her initially normal FSH and AMH levels. Serial transvaginal ultrasound monitoring was performed in conjunction with measurement of estradiol serum levels. The patient’s estradiol level rose to 65 pg/mL on stimulation day 4 but decreased to 30 pg/mL on stimulation day 6 with no follicles >10 mm present. The decision was made to cancel the cycle because of the lack of response.
The patient elected to attempt another stimulation cycle after careful counseling regarding her poor prognosis. Given her poor response to the first cycle, she was started on an antagonist protocol with an increased starting dose for patients with DOR of recombinant FSH (450 IU) and hCG (20 IU). At baseline, her estradiol level was 30 mg/mL and her AFC was 7. On stimulation day 4, her estradiol level was 68 pg/mL with no dominant follicles present. On stimulation day 6, the estradiol level decreased to 58 pg/mL, and no follicular growth was observed; the decision was made to proceed with cancellation.
Patient 3
This patient was the younger sister of Patient 2. She presented at age 23 years to discuss oocyte vitrification. Her medical history was notable for a diagnosis of BPES when she was a newborn. She underwent three eye surgeries as a child. When she was 17 years old, rheumatoid arthritis was diagnosed and has been well controlled with hydroxychloroquine.
The patient experienced menarche at age 14 years and was started on OCPs at age 16 years for abnormal uterine bleeding and severe dysmenorrhea. At age 23 years, she began experiencing hot flashes. At that time, her FSH level was 29.5 mIU/mL, her AFC was 10, and her AMH level was 0.99 ng/mL. She discontinued OCPs and repeated the ovarian reserve testing three months later with no significant differences observed; the FSH level was 30.1 mIU/mL and the AMH level was 1.17 ng/mL. Given her elevated FSH level and vasomotor symptoms, type 1 BPES was diagnosed, and attempts were made to obtain her previous genetic records. The patient was carefully counseled regarding her poor prognosis and the high likelihood of cycle cancellation, and she strongly desired to proceed.
Ovarian Stimulation
Because of her markedly elevated FSH, she was initiated on a higher gonadotropin dose. On cycle day 1, the patient was confirmed to have baseline estradiol and progesterone levels with an AFC of 8. Stimulation was begun with recombinant FSH (450 IU) and hCG (20 IU). Serial transvaginal ultrasound monitoring was performed in conjunction with measurement of estradiol serum levels. On cycle day 4, the estradiol level was 84 pg/mL, and there were no dominant follicles observed. On cycle day 6, the estradiol level rose to 121 pg/mL, and on stimulation day 8, it dropped to 100 pg/mL. Given the minimal follicular response, the decision was made on cycle day 10 to cancel the cycle.
The patient elected to proceed with a second stimulation attempt. Interestingly, her FSH level at baseline was 10.9 mIU/mL and her AMH level was 5.05 ng/mL. Given her improved ovarian reserve testing with this cycle, she was started on the same protocol as for her first cycle. On stimulation day 1, the patient had an AFC of 20, her estradiol level was 35 pg/mL, and stimulation was begun with recombinant FSH (450 IU) and hCG (50 IU). Serial transvaginal ultrasound monitoring was performed in conjunction with measurement of estradiol serum levels. On stimulation day 5, her estradiol level was 195 pg/mL. Her estradiol level increased to 231 pg/mL on stimulation day 8, and there were no dominant follicles visible. On stimulation day 10, her estradiol level decreased to 200 pg/mL, and no further follicular development was observed. Because of the lack of response, the decision was made to proceed with cycle cancellation.
Discussion
Although BPES is a rare cause of POI, early identification of the condition is critical to allow for comprehensive reproductive counseling. In addition, early confirmation of BPES may permit individuals to potentially cryopreserve oocytes at a younger age than is typically offered. This case series is of value for several reasons. First, it highlights that BPES should be considered in the differential diagnosis for POI or DOR in women with a childhood history of eye surgery, particularly those with a family history of eye surgery and similar physical findings, and especially in those with eye surgery and a family history of secondary infertility. Second, girls who have BPES identified at a young age should be referred to a reproductive endocrinology and infertility subspecialist for counseling on the timing of oocyte cryopreservation. Third, women with BPES who have a detectable ovarian reserve may be candidates for ovarian stimulation and oocyte cryopreservation, even in the setting of elevated FSH levels. Finally, this case series demonstrates that women with BPES may respond differently to ovarian stimulation, even when markers of ovarian reserve are similar. Despite detectable AMH levels and visible antral follicle counts, two of the women with BPES completely failed to respond to ovarian stimulation, and oocyte vitrification was not possible.
BPES is the result of a mutation in the forkhead box L2 (FOXL2) gene located on chromosome 3q23 (10). Mutations include deletion, duplication, missense mutation, nonsense mutation, and rarely rearrangement (15). The FOXL2 protein is a helix-turn-helix structure that is often referred to as a “winged helix” because of its similarity to a butterfly (10, 17, 18). FOXL2 is a transcription factor with a polyalanine (polyA) tract (10). Patients with type 1 BPES often have a truncated polyA tract, and those with type 2 BPES have been found to have an expansion or frameshift mutations of the polyA tract (10, 19). Over 125 mutations of the FOXL2 gene have been described in patients with BPES (20). The FOXL2 gene is responsible for embryonic eyelid development, which explains the notable phenotypic eye findings. The effect on ovarian function is not fully understood. FOXL2 is expressed in the granulosa cells and may play a role in follicular growth and estrogen production (12). Mutations in FOXL2 are thought to cause either the rapid differentiation of granulosa cells, therefore leading to POI, or a decrease in ovarian reserve starting at birth (10, 18, 20). Perhaps most interesting is that these cases shed light on the mechanism of ovarian failure. Given the family histories of conception and subsequent secondary infertility, these points toward oocyte depletion as opposed to oocyte incompetence as the underlying etiology of ovarian failure.
To date, only one other case report has been published of ovarian stimulation in a patient with BPES who had a live birth of twins (21). This patient was a 30-year-old nulligravida with type 1 BPES who underwent a gonadotropin intrauterine insemination cycle with FSH (300 IU) and who conceived a dichorionic diamniotic twin pregnancy. Her pregnancy was complicated by preeclampsia and preterm labor and resulted in a cesarean section for arrest of descent and delivery of two male infants. One infant had phenotypic eye findings for BPES (21). A second case report described a patient who presented with infertility, irregular cycles, and elevated FSH levels. She had a presumed diagnosis of BPES but declined confirmatory genetic testing. This patient elected to use a donor embryo and successfully conceived (22).
Given the paucity of data on the use of fertility treatments in women with BPES and no published cases of oocyte vitrification in patients with BPES, it remains challenging to counsel affected patients on the likelihood of success with regard to gonadotropin stimulation and future oocyte competence. It is well established that elevated FSH level is a marker for poor ovarian response (23). All three patients had at least one elevated FSH level; however, the response to stimulation varied by patient. Limited follicular growth was observed in patients 2 and 3 despite detectable AMH levels and visible antral follicles. Patient 1 required long durations of gonadotropin stimulation to elicit follicle development but ultimately responded. The length of stimulation ranged between 9 and 14 days on each of her three cycles. She had a total of 34 oocytes retrieved; however, only 8 of these were MIIs. The prolonged stimulation and high immaturity rate was likely a consequence of the ovarian dysfunction seen in a FOXL2 mutation (12). This concept is supported by animal studies in which FOXL2 was shown to be involved in the formation of follicles and maintenance of follicle viability (24, 25). In addition, data suggest that FOXL2 may play a role in the differential expression of key steroidogenic enzymes within the ovary while growth and maturation of oocytes is occurring (25).
The varied response among these patients highlights the potential variability in the genotype/phenotype correlation and how response to ovarian stimulation may differ in patients with the same condition. Given that women with BPES may experience DOR over a large age range (20), a younger age in women with BPES may be less helpful in improving oocyte vitrification outcomes. There are >125 variants of the FOXL2 mutation, and it is possible that these variants, more so than age, may determine the timing of menopause and, in addition, may potentially influence individual responses to ovarian stimulation, although future research is needed to study this assumption (20). Despite the wide variation in response to ovarian stimulation seen in this case series and the fact that the younger patients responded less robustly, we can still assume that a prompt referral to a Reproductive Endocrinologist will lead to earlier testing for DOR. The early referral may provide patients with the option to cryopreserve the maximum number of oocytes at the youngest age possible. However, given the significant variation in ovarian reserve testing and poorer response to ovarian stimulation in younger patients as seen in this case series, we acknowledge that referral to a Reproductive Endocrinologist even at an early age may not improve outcomes.
Beyond this case series, there are no data demonstrating how patients with BPES respond to ovarian stimulation. It seems plausible that patients with BPES may have different phenotypes and thus experience variable reproductive outcomes. Given that there are limited data about the anticipated age of onset of ovarian failure in patients with BPES, it is possible that in patients with type 1 BPES there is a reproductive spectrum similar to that of patients with Turner syndrome, in which some individuals are able to spontaneously conceive whereas others experience POI at a very young age or never experience menarche (26).
There are limited data on pregnancy outcomes in women with BPES; therefore, it was difficult to counsel the patients on the likelihood of live birth with vitrified oocytes. Future success may be hampered by the expected age-related aneuploidy as well as the anticipated laboratory attrition with oocyte warming and embryo development. The rapidity of decline in ovarian reserve is not as well documented in women with FOXL2 mutations, adding to the challenge in counseling about fecundability in the setting of ovulatory cycles or conversely predicting time to menopause.
The uncertainties of ovarian stimulation and fertility outcomes were discussed at length with each of these patients before beginning gonadotropin stimulation, and for patient 1 before each subsequent cycle. Furthermore, this patient was counseled on the guarded prognosis for live birth with her aggregate eight oocytes. Realistic and thorough counseling is essential in this population and shared decision-making is encouraged. The economic, physical, and emotional ramifications of the oocyte vitrification process cannot be ignored, particularly when the oocyte yield is low or even unachievable. All of the patients were additionally counseled regarding transmission risk, preimplantation genetic testing for monogenic/single gene defects, and the potential to require donor oocytes in the future.
Conclusion
A diagnosis of BPES should be considered in women with DOR or POI who have eye findings characteristic of this condition. Early recognition of BPES is critical and should prompt early referral to a Reproductive Endocrinologist for counseling on reproductive potential and oocyte cryopreservation. Because this condition is often diagnosed in childhood, as seen with patients 2 and 3, increased education for ophthalmologists regarding early intervention is needed, specifically after menarche. As evidenced by this case series, ovarian stimulation and oocyte cryopreservation may be possible in women with BPES, even those with DOR. The ability to successfully retrieve mature oocytes cannot be assured, even in the setting of visible antral follicles and a detectable AMH level. This case series highlights that the mechanism of infertility in patients with BPES may be because of FOXL2-specific disordered follicular recruitment and/or more rapid oocyte depletion. Earlier referral might have led to a more robust response to stimulation and the potential to have more mature oocytes retrieved.
Footnotes
M.L.B. has nothing to disclose. R.P. has nothing to disclose. J.D.L. has nothing to disclose. E.C.F. has nothing to disclose. L.A.B. has nothing to disclose.
References
- 1.European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI. Webber L., Davies M., Anderson R., Bartlett J., Braat D. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 2.Practice Committee of the American Society for Reproductive Medicine Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103:e9–e17. doi: 10.1016/j.fertnstert.2014.12.093. [DOI] [PubMed] [Google Scholar]
- 3.Pastore L.M., Christianson M.S., Stelling J., Kearns W.G., Segars J.H. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 2018;35:17–23. doi: 10.1007/s10815-017-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlotogora J., Sagi M., Cohen T. The blepharophimosis, ptosis, and epicanthus inversus syndrome: delineation of two types. Am J Hum Genet. 1983;35:1020–1027. [PMC free article] [PubMed] [Google Scholar]
- 5.Owens N., Hadley R.C., Kloepfer H.W. Hereditary blepharophimosis, ptosis, and epicanthus inversus. J Int Coll Surg. 1960;33:558–574. [PubMed] [Google Scholar]
- 6.Oley C., Baraitser M. Blepharophimosis, ptosis, epicanthus inversus syndrome (BPES syndrome) J Med Genet. 1988;25:47–51. doi: 10.1136/jmg.25.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen C.E., Rubin P.A. Blepharophimosis-ptosis-epicanthus inversus syndrome (BPES): clinical manifestation and treatment. Int Ophthalmol Clin. 2008;48:15–23. doi: 10.1097/IIO.0b013e3181694eee. [DOI] [PubMed] [Google Scholar]
- 8.Moraine C., Titeca C., Delplace M.P., Grenier B., Lenoel Y., Ribadeau-Dumas J.L. Familial blepharophimosis and female sterility: pleiotropism or linked genes? [in French] J Genet Hum. 1976;24:125–132. [PubMed] [Google Scholar]
- 9.Townes P.L., Muechler E.K. Blepharophimosis, ptosis, epicanthus inversus, and primary amenorrhea. A dominant trait. Arch Ophthalmol. 1979;97:1664–1666. doi: 10.1001/archopht.1979.01020020232010. [DOI] [PubMed] [Google Scholar]
- 10.Crisponi L., Deiana M., Loi A., Chiappe F., Uda M., Amati P. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- 11.Cocquet J., Pailhoux E., Jaubert F., Servel N., Xia X., Pannetier M. Evolution and expression of FOXL2. J Med Genet. 2002;39:916–921. doi: 10.1136/jmg.39.12.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georges A., Auguste A., Bessière L., Vanet A., Todeschini A.L., Veitia R.A. FOXL2: a central transcription factor of the ovary. J Mol Endocrinol. 2014;52:R17–R33. doi: 10.1530/JME-13-0159. [DOI] [PubMed] [Google Scholar]
- 13.De Baere E., Beysen D., Oley C., Lorenz B., Cocquet J., De Sutter P. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype-phenotype correlation. Am J Hum Genet. 2003;72:478–487. doi: 10.1086/346118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L., Li T., Xing Y. Identification of a novel FOXL2 mutation in a single family with both types of blepharophimosis--ptosis-epicanthus inversus syndrome. Mol Med Rep. 2017;16:5529–5532. doi: 10.3892/mmr.2017.7226. [DOI] [PubMed] [Google Scholar]
- 15.Beysen D., Vandesompele J., Messiaen L., De Paepe A., De Baere E. The human FOXL2 mutation database. Hum Mutat. 2004;24:189–193. doi: 10.1002/humu.20079. [DOI] [PubMed] [Google Scholar]
- 16.Kim S.J., Kim T.H., Park J.K., Eum J.H., Lee W.S., Lyu S.W. Effect of a dual trigger on oocyte maturation in young women with decreased ovarian reserve for the purpose of elective oocyte cryopreservation. Clin Exp Reprod Med. 2020;47:306–311. doi: 10.5653/cerm.2020.03657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann O.J., Sowden J.C., Carlsson P., Jordan T., Bhattacharya S.S. Fox's in development and disease. Trends Genet. 2003;19:339–344. doi: 10.1016/S0168-9525(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 18.Uhlenhaut N.H., Treier M. Foxl2 function in ovarian development. Mol Genet Metab. 2006;88:225–234. doi: 10.1016/j.ymgme.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 19.De Baere E., Dixon M.J., Small K.W., Jabs E.W., Leroy B.P., Devriendt K. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype--phenotype correlation. Hum Mol Genet. 2001;10:1591–1600. doi: 10.1093/hmg/10.15.1591. [DOI] [PubMed] [Google Scholar]
- 20.Verdin H., De Baere E. In: GeneReviews. Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., editors. University of Washington, Seattle; Seattle (WA): 1993. Blepharophimosis, ptosis, and epicanthus inversus. [PubMed] [Google Scholar]
- 21.Roth L.W., Alvero R. Pregnancy in a woman with premature ovarian insufficiency associated with Blepharophimosis, ptosis, epicanthus inversus syndrome type I. A case report. J Reprod Med. 2014;59:87–89. [PubMed] [Google Scholar]
- 22.Siewert A.L., Stein Q., Flanagan J., Hansen K.A. Blepharophimosis-ptosis-epicanthus inversus syndrome and hypergonadotropic hypogonadism. Fertil Steril. 2008;90:2016.e11–2016.e12. doi: 10.1016/j.fertnstert.2008.07.1763. [DOI] [PubMed] [Google Scholar]
- 23.Ferraretti A.P., La Marca A., Fauser B.C., Tarlatzis B., Nargund G., Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 24.Mandon-Pepin B., Oustry-Vaiman A., Vigier B., Piumi F., Cribiu E., Cotinot C. Expression profiles and chromosomal localization of genes controlling meiosis and follicular development in the sheep ovary. Biol Reprod. 2003;68:985–995. doi: 10.1095/biolreprod.102.008557. [DOI] [PubMed] [Google Scholar]
- 25.Nagahama Y., Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50:S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 26.Bernard V., Donadille B., Zenaty D., Courtillot C., Salenave S., Brac de la Perrière A. Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Hum Reprod. 2016;31:782–788. doi: 10.1093/humrep/dew012. [DOI] [PubMed] [Google Scholar]