Abstract
Objective
To compare the pregnancy outcomes of lesbian women undergoing donor sperm intrauterine insemination (IUI) with that of heterosexual women undergoing IUI using partner or donor sperm.
Design
Retrospective cohort analysis.
Setting
Two academic fertility practices.
Patient(s)
All IUI cycles between 2007 and 2016.
Intervention(s)
None.
Main outcome measure(s)
Primary outcomes included clinical pregnancy (CP) rates and live birth/ongoing pregnancy (LB) rates. The baseline characteristics and cycle characteristics were compared between the two groups using absolute standardized differences (ASDs). To account for the correlation between cycles per patient, a generalized estimating equation method for multivariable logistic regression was used.
Results
A total of 11,870 IUI cycles were included, of which 393 were in lesbian women using donor sperm and 11,477 were in heterosexual women with infertility using either partner or donor sperm. The CP rates were similar between the lesbian and heterosexual groups (13.2% vs. 11.1%, respectively, ASD = 0.06). In addition, the LB rates were similar between the two groups (10.4% vs. 8.3%, respectively, ASD = 0.10). After implementing the generalized estimating equation in a multivariable logistic regression, the lesbian group had an overall higher odds of CP (adjusted odds ratio 1.40, 95% confidence interval: [1.04–1.88]) and LB (adjusted odds ratio 1.59, 95% confidence interval [1.15–2.20]) compared with the heterosexual group. The clinical miscarriage rate was higher in the heterosexual group compared with that in the lesbian group (23.8% vs. 15.4%, respectively, ASD = 0.21).
Conclusion
Although the unadjusted rates were similar between the two groups, the adjusted CP and LB odds were significantly higher for lesbian women undergoing IUI for procreative management than those for heterosexual women undergoing IUI for infertility.
Key Words: Donor sperm, intrauterine insemination, infertility, lesbian, pregnancy
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00215
There is a general lack of knowledge regarding the unique health experiences and needs of the lesbian, gay, bisexual, transgender, queer and gender nonconforming populations, which is particularly relevant in the field of reproductive endocrinology and infertility (1). A steady increase has been noted in lesbian couples seeking and using reproductive services for family-building purposes, yet there is limited data regarding the optimal fertility treatments and outcomes for lesbian couples (2). According to the American College of Obstetrics and Gynecology, persistent stigmatization of the lesbian, gay, bisexual, transgender, queer and gender nonconforming communities may result in difficulty finding physicians to assist with achieving pregnancy. Insurance coverage for these services can be challenging for many populations, and additional barriers, such as documenting infertility using traditional definitions, further impede access (3). Additional considerations specific to lesbian women and couples include important decisions such as which partner will carry the pregnancy and the selection of a donor sperm source. Choosing the ideal treatment plan that minimizes time and cost as well as optimizes outcomes can be challenging for lesbian patients, because data regarding fertility outcomes in this population are limited and conflicting (4). Often, lesbian women seeking treatment do not have an infertility diagnosis, yet they often undergo a treatment plan similar to that of heterosexual patients with infertility such as intrauterine insemination (IUI). This study aimed to characterize the IUI experience of lesbian women and to identify their true pregnancy rates while taking into account the available potential confounders associated with IUI success.
Materials and methods
Patient Selection
This retrospective cohort analysis included all women who underwent IUI treatment at fertility centers of the University of California, San Francisco from 2007–2016 and Stanford University from 2016–2017. This project was approved by the Stanford Institutional Review Board. Lesbian and heterosexual women who underwent natural cycle or medicated IUI cycles were identified in an electronic medical record and included in this study. Lesbian women who underwent IUI with a cryopreserved donor sperm source and heterosexual women who underwent IUI with partner sperm for various indications, including an ovulatory disorders, tubal factor, unexplained infertility, or with donor sperm for male factor infertility were included. If the sperm source was documented as “unknown”, it was assumed to be of a donor source for the lesbian group (13% of lesbian cycles had unknown sperm source) and of a partner source for the heterosexual group (15%). Lesbian women who were included in the study self-identified as a female with a female partner. Heterosexual women who were included in the control group self-identified as a female with a male partner. Cancelled cycles and spontaneous pregnancies were excluded from this study.
Intrauterine Insemination Cycle Types—Natural and Medicated
The induction of ovulation for IUI was performed according to the standard protocols of each center. During a natural cycle, the patients monitored ovulation with home detection kits and/or by an ultrasound to confirm follicular growth and maturity. During a medicated cycle, oral or injectable medications, such as clomiphene citrate (CC), letrozole, or gonadotropins, were used to induce ovulation; CC (50–100 mg/day, Clomid [Patheon Pharmaceuticals Inc., San Francisco, CA]) and letrozole (2.5–7.5 mg daily) were given for 5 days from the second or third day of the menstrual cycle. Gonadotropins, follicle-stimulating hormone and luteinizing hormone (75–300 IU), were given from the second or third day of the menstrual cycle. Ovulation was monitored with transvaginal pelvic ultrasound starting on the eighth day from the start of the ovulation induction medication. When a follicle reached a diameter of at least 18 mm, the standard protocol included a trigger injection of human chorionic gonadotropin (hCG; 5,000 IU) or recombinant hCG (250 mcg; Ovidrel, EMD Serono, Darmstadt, Germany), followed by the IUI procedure approximately 36 hours later.
Outcome Measures
The cycle characteristics including the patient age at the time of the cycle, IUI cycle type, and total motile count (TMC) were collected. Some basic patient demographics and cycle characteristics were largely unavailable in the electronic database, including ethnicity, body mass index, gravidity, parity, smoking status, antral follicle count, and thickness of the endometrial lining. Our primary outcomes of interest were clinical pregnancy (CP), defined as the presence of a gestational sac on transvaginal ultrasound, and live birth/ongoing pregnancy (LB), defined as a live infant born after 24 weeks of gestation or ongoing pregnancy after 24 weeks of gestation. The CP rate was calculated as the total number of CPs divided by the total number of IUI cycles, and the LB rate was calculated as the total number of live births/ongoing pregnancies divided by the total number of IUI cycles. Our secondary outcomes of interest included the incidence of positive beta human chorionic gonadotropin (βhCG) result, biochemical miscarriages, pregnancies of unknown location/ectopic pregnancy, and clinical miscarriages. We defined positive βhCG as a βhCG serum level >5 mIU/mL. A biochemical miscarriage was a rise and fall in βhCG level without evidence of a CP. A pregnancy of unknown location/ectopic pregnancy was diagnosed with a rising βhCG level without evidence of an intrauterine pregnancy. Lastly, clinical miscarriage was considered to be the loss of a CP before 20 weeks gestational age. We hypothesized that there was no difference in pregnancy outcomes between lesbian women who underwent IUI with donor sperm and heterosexual women who underwent IUI with either partner or donor sperm.
Statistical Analysis
We calculated descriptive statistics at the patient level and cycle level, stratified by cohort (lesbian and heterosexual). Means and standard deviations were reported for continuous variables, and frequencies and percentages were reported for categorical variables. We compared patient and cycle characteristics between the lesbian and heterosexual women using ASDs, a measure of the difference in means or proportions between two groups divided by the pooled standard deviation and expressed in units of standard deviations (5, 6). The ASD for continuous variables is equivalent to Cohen’s d and the commonly used guide for interpreting Cohen’s d can be applied to ASD as well; that is, 0.2, 0.5, and 0.8 correspond to small, medium, and large differences between the groups, respectively. To account for the correlation between cycles per patient, we implemented the generalized estimating equation (GEE) method for multivariable logistic regression to assess differences in CP and LB rates between the two groups while adjusting for patient age, TMC, year of the procedure, and cycle type. We calculated adjusted odds ratios (aORs) and 95% confidence intervals (CIs) to evaluate the relative odds for CP and LB for lesbian women vs. heterosexual women. Additionally, we performed sensitivity analyses comparing lesbian women with heterosexual women who used donor sperm and lesbian women with heterosexual women who used partner sperm. Lastly, because IUI requires normal tubal function, we conducted a sensitivity analysis that excluded known tubal factor infertility patients.
To address missing values of TMC and cycle type, we created 10 datasets using multiple imputations that were then combined using Rubin’s rules. All analyses were conducted with R version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria). The library geepack was used for GEE analysis (7, 8, 9, 10), and the library mitml was used for multiple imputations in multilevel modeling (11). All statistical tests were two-sided and performed at the .05 significance level.
Results
A total of 11,870 IUI cycles were included, 393 of which were cycles of lesbian women using donor sperm and 11,477 of which were cycles of heterosexual women with infertility using either partner or donor sperm. The total number of lesbian women included was 109 and the total number of heterosexual women included was 3,725.
Patient demographics and cycle characteristics are presented in Table 1. For lesbian women, the mean ± SD maternal age was 36.8 ± 3.7 years old, and for heterosexual patients, the mean ± SD maternal age was 36.8 ± 4.1 years old. For lesbian women, the mean TMC of the donor sperm used was 18.4 ± 10.1 × 106, and for heterosexual women, the mean TMC was 83.0 ± 94.1 × 106 (ASD = 0.97). Although this difference between the two groups was large, the mean TMC for the heterosexual group using only donor sperm was 18.7 ± 19.2 × 106 sperm, which was comparable to that of the lesbian group using only donor sperm and corresponded to the sperm quality standards provided by the banks.
Table 1.
Patient demographics and cycle characteristics.
| Patient demographics and cycle characteristics | Lesbian | Heterosexual | |
|---|---|---|---|
| Patient demographics | n = 109 | n = 3,725 | ASDa |
| Age at first IUI (y)b | 36.79 (3.7) | 36.76 (4.1) | 0.01 |
| Cycle characteristics | n = 393 | n = 11,477 | |
| Total motile count (× 106)b | 18.4 (10.1) | 83.0 (94.1) | 0.97 |
| Missing TMC, n (%) | 44 (11.2) | 1,354 (11.8) | 0.02 |
| Cycle type, n (%) | 0.73 | ||
| Clomiphene citrate | 126 (32.1) | 6,401 (55.8) | |
| FSH/LH | 2 (0.5) | 40 (0.3) | |
| Letrozole | 76 (19.3) | 3,171 (27.6) | |
| Natural cycle | 187 (47.6) | 1,825 (15.9) | |
| Unknown | 2 (0.5) | 40 (0.3) | |
| Semen source, n (%) | 8.00 | ||
| Donor | 393 (100.0) | 349 (3.0) | |
| Partner | 0 (0.0) | 11,128 (97.0)) |
Note: ASD = absolute standardized difference; FSH = follicle-stimulating hormone; IUI = intrauterine insemination; LH = luteinizing hormone; TMC = total motile count.
ASD calculated between the heterosexual and lesbian groups; this represents the difference in means or proportions between the two groups divided by the pooled standard deviation; 0.2, 0.5, and 0.8 correspond to small, medium, and large differences, respectively. Therefore, smaller standardized differences represent less difference between the two groups.
Means and standard deviations reported for continuous variables.
The most common cycle type for both the lesbian and heterosexual women were medicated cycles (51.9% and 84.1%, respectively). Of the medicated cycles in lesbian women, CC was most commonly used at 32.1% (n = 126) followed by letrozole at 19.3% (n = 76). Among cycles of heterosexual women, only 15.9% (n = 1,825) were natural cycles and 84.1% (n = 9,652) were medicated cycles, of which 55.8% were CC cycles and 27.6% were letrozole cycles. Gonadotropins were used in the smallest proportion of cycles, 0.5% (n = 2) of lesbian patient cycles and 0.3% (n = 40) of heterosexual patient cycles.
Pregnancy outcomes are presented in Table 2. Lesbian women had a positive βhCG rate of 14.8% per cycle, and heterosexual women had a positive βhCG rate of 12.2% per cycle (ASD = 0.08). Lesbian women had a CP rate of 13.2% per cycle, and heterosexual women had a CP rate of 11.1% per cycle (ASD = 0.06). Lesbian women had a LB rate of 10.4% per cycle, and heterosexual women had a LB rate of 8.3% per cycle (ASD = 0.10).
Table 2.
Cycle outcomes for lesbian and heterosexual patients.
| Outcome | Lesbian N = 393 | Heterosexual N = 11,477 | ASDa |
|---|---|---|---|
| Positive βhCG result, n (%) | 58 (14.8) | 1,396 (12.2) | 0.08 |
| Clinical pregnancy, n (%) | 52 (13.2) | 1,279 (11.1) | 0.06 |
| Live birth/ongoing pregnancy, n (%) | 41 (10.4) | 939 (8.3%) | 0.10 |
| Biochemical miscarriage, n (%) | 5 (1.3) | 95 (0.8) | 0.07 |
| Clinical miscarriage, n (%) | 8 (15.4) | 305 (23.8) | 0.21 |
| Ectopic pregnancy, n (%) | 1 (0.3) | 22 (0.2) | 0.01 |
Note: ASD = absolute standardized difference; βhCG = beta human chorionic gonadotropin.
ASD calculated between heterosexual and lesbian groups; this represents the difference in means or proportions between the two groups divided by the pooled standard deviation. 0.2, 0.5, and 0.8 correspond to small, medium, and large differences respectively. Therefore, smaller standardized differences represent less difference between the two groups.
Lesbian women had a biochemical miscarriage rate of 1.3% per cycle, and heterosexual women had a biochemical miscarriage rate of 0.8% per cycle (ASD = 0.04). Lesbian women had a clinical miscarriage rate of 15.4% per cycle, and heterosexual women had a clinical miscarriage rate of 23.8% per cycle (ASD = 0.21). Lastly, lesbian women had a pregnancy of unknown location/ectopic rate of 0.3% per cycle, and heterosexual women had a pregnancy of unknown location/ectopic rate of 0.2% (ASD = 0.01).
Overall, the CP and LB rates were similar between the lesbian group and the heterosexual group. However, after implementing GEE in a multivariable logistic regression adjusting for patient age, TMC, year of the procedure, and cycle type, the lesbian group had a higher odds of CP (aOR 1.40, 95% CI: [1.04–1.88], P-value =.02) and LB (aOR 1.59, [1.15–2.20], P-value =.005) compared with the heterosexual group, demonstrating higher pregnancy success for the lesbian group.
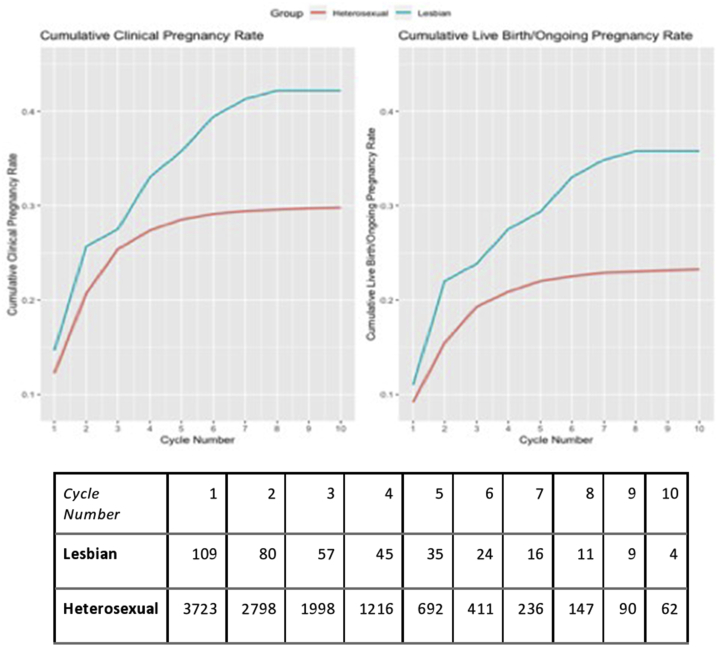
On a patient level, lesbian women completed more IUI cycles on average, as the mean number of cycles undergone was 3.6 ± 2.7 in the lesbian group and 3.1 ± 2.1 in the heterosexual group (ASD = 0.22) (Table 3). Cumulatively, lesbian women were more likely to ever have a CP through IUI compared with heterosexual women (42.2% vs. 29.8%, ASD = 0.3) and more likely to ever have a LB compared with heterosexual women (36.7% vs. 23.3%, ASD = 0.3). Among lesbian and heterosexual women who did become pregnant, the average number of cycles until a CP was achieved was higher for lesbian women compared with that for heterosexual women (2.9 ± 2.0 vs. 2.2 ± 1.5, ASD = 0.4) This was in addition consistent for the average number of cycles until an LB was achieved (3.1 ± 2.4 vs. 2.3 ± 1.7, ASD = 0.4). The cumulative CP rate after 3 cycles was 27.5% for the lesbian group and 25.4% for the heterosexual group, and the cumulative LB rate after 3 cycles was 23.9% for the lesbian group and 19.3% for the heterosexual group (Fig. 1).
Table 3.
IUI outcomes for lesbian and heterosexual patients.
| Outcome | Lesbian N = 109 | Heterosexual N = 3,725 | ASD |
|---|---|---|---|
| Number of IUI cyclesa | 3.6 (2.7) | 3.1 (2.1) | 0.22 |
| Number of ever CP, n (%) | 46 (42.2) | 1,111 (29.8) | 0.26 |
| Number of ever LB, n (%) | 40 (36.7) | 867 (23.3) | 0.34 |
| Number of IUI cycles until CP | 2.9 (2.0) | 2.2 (1.5) | 0.35 |
| Number of IUI cycles until LB | 3.1 (2.4) | 2.3 (1.7) | 0.38 |
| Cumulative ever CP after 3 cycles, n (%) | 30 (27.5) | 946 (25.4) | 0.05 |
| Cumulative ever LB after 3 cycles, n (%) | 0.15 | ||
| Yes | 26 (23.9) | 719 (19.3) | |
| No | 81 (74.3) | 2,976 (79.9) | |
| Missing follow up | 2 (1.8) | 30 (0.8) |
Note: ASD = absolute standardized difference; CP = clinical pregnancy; IUI = intrauterine inseminiation; LB = live birth.
Means and standard deviations reported for continuous variables.
Figure 1.
Cumulative clinical pregnancy and live birth rates.
Most of the cycles in the heterosexual group used partner sperm (97%). The lesbian group (limited to donor sperm use) had higher odds of CP and LB compared with the heterosexual group who used partner sperm (aOR 1.98, 95% CI: [1.45–2.69]; aOR 2.64, 95% CI: [1.87–3.70], respectively; P<.001). In a sensitivity analysis, IUI cycle outcomes for the lesbian group who used donor sperm were compared with those for heterosexual women who used donor sperm because of male factor infertility. Compared with heterosexual women who used donor sperm, lesbians had similar CP and LB outcomes (aOR 1.39, 95% CI: [0.84–2.31], P-value =.197; aOR 1.78, 95% CI: [1.00–3.18], P-value =.05, respectively). When patients with tubal factor infertility were excluded from the analysis, lesbian patients were still 1.38 times more likely to achieve CP (95% CI 1.03–1.85, P = .03) and 1.57 times more likely to achieve LB (95% CI 1.14–2.17, P = .006) compared with heterosexual patients.
Discussion
Increasing numbers of lesbian women are attempting to achieve pregnancy using IUI with donor sperm. The current study demonstrated that the odds of pregnancy success are higher for lesbian women undergoing IUI for procreative management compared with heterosexual women undergoing IUI with either partner or donor sperm for various causes of infertility, including anovulatory cycles, male factor infertility, or unexplained infertility. In this study, lesbian women had a CP rate of 13.2% per cycle and heterosexual women had a CP rate of 11.1% per cycle. Lesbian women had an LB rate of 10.4% per cycle, similar to that of heterosexual women who had an LB rate of 8.3%. After implementing GEE in a multivariable logistic regression, lesbian women had significantly higher odds of CP and LB compared with the heterosexual group. This association persisted after removing tubal factor infertility patients from the analysis. Another sensitivity analysis comparing outcomes of lesbian women with those of heterosexual women using partner sperm confirmed the increased odds of CP and LB in lesbian women. In addition, the CP and LB outcomes were comparable between lesbian women and heterosexual women using donor sperm. These data suggest that after correcting for severe male factor infertility among the heterosexual group with the use of donor sperm, this “now fertile” population has similar outcomes to those of the lesbian group, a mostly fertile group now with access to sperm.
Available data shows that the pregnancy success rates per IUI cycle have remained stable for years at approximately 12.4% (12). Overall, pregnancy rates for both groups in this study were comparable to nationally reported IUI success rates (13). Prior literature suggested mixed evidence regarding pregnancy success rates of lesbian women compared with the general population. One large meta-analysis similarly found that lesbian women had higher assisted reproduction success rates compared with heterosexual women with an odds ratio of 1.56 (95% CI 1.24–1.96) (14). Another randomized controlled trial reported CP rates as high as 57% and cumulative pregnancy rates as high as 70% after eight IUI cycles for lesbian women, higher than the pregnancy rates of 35% and 47%, respectively, for heterosexual women (15). However, that study was confounded by a statistically significant difference in age between the two groups—mean age was 34.5 years in the lesbian group and 38.5 years in the heterosexual group (P-value<.005)—without a multivariable regression analysis. A recent study, however, showed no difference in pregnancy outcomes between lesbian and heterosexual women undergoing IUI with CP rates reported between 7.2% and 11.6% (4). Our study specifically sought to identify the true pregnancy rates in a large, multiclinic population over an extended time period while taking into account all available potential confounders associated with IUI success.
In addition, the results of our study showed that the lesbian women were more likely to ever achieve a CP and LB than the heterosexual group. The higher success rates in the lesbian group cannot be explained by the fact that this group underwent more IUI cycles on average. Figure 1 best illustrates this point. It shows that the CP and LB rates in the heterosexual group were unlikely to increase after approximately five IUI cycles, whereas the CP and LB rates in the lesbian group continued to increase until approximately 10 IUI cycles. With a prior diagnosis of infertility, heterosexual couples may be less likely to continue IUI after a given number of failed cycles and switch to alternate treatments, such as in vitro fertilization, sooner. Given that sperm exposure is the limiting factor for lesbian couples, they may be encouraged to continue IUI treatments to a significantly higher number of IUI cycles.
Prior literature suggested that, compared with the prevalence in heterosexual women, lesbian women had an increased prevalence of risk factors for infertility, including smoking, obesity, and sexually transmitted diseases, all of which are known to affect fertility and perhaps IUI success (16). In addition, polycystic ovary syndrome (PCOS) was thought to be more prevalent in the lesbian population, although the literature is contradictory and inconclusive. One 2004 study evaluating PCOS in 396 women undergoing fertility treatment found that typical ultrasound features of polycystic ovaries were observed in 80% of the lesbian women and in 32% of the heterosexual women. Further analysis revealed that 38% of the lesbian women and 14% of the heterosexual women in that cohort had PCOS; this difference was statistically significant (P-value<.0001) (17). However, a more recent 2011 prospective study of 211 patients showed no difference between lesbian and heterosexual women in the prevalence of or risk factors for PCOS. In our study, the prevalence of PCOS among lesbian and heterosexual women was 5.5% and 5.8%, respectively (although 37% of diagnoses were missing in the database).
Fertility among lesbian patients cannot be assessed before presentation to the clinic because they cannot truly “try” without use of donor sperm. A lesbian woman’s experience in the fertility clinic is further complicated by poor access to health care and the discriminatory practices of medical professionals stemming from homophobia (18). Even when these patients do have access to health care, they are often undiagnosed or experience delay to diagnosis, which may predispose them to less effective treatment plans. In addition, home inseminations are an important aspect of fertility care, particularly for lesbian women. These are commonly used, cost-effective, and safe, although the true proportion of women who present to the clinic after failed home inseminations is unknown and not routinely documented. Although their success rates are not as high as IUI success rates (19), it is important for providers to elicit this history as prior failed home inseminations may guide subsequent treatment options and perhaps even allow for the possibility of insurance coverage. Screening lesbian patients to identify risk factors for infertility by thorough history taking without over-testing in the absence of an infertility diagnosis should be considered the standard of care.
Strengths and Limitations
The main strength of this study was the large sample size provided by the clinical database and the ability to quantify IUI cycle outcomes for lesbian women instead of inferring data from heterosexual women. However, the study was limited by the database itself, because some basic demographic information was unavailable/missing for a large number of the subjects, including body mass index, gravidity, parity, smoking status, antral follicle count, and endometrial lining thickness. The inability to control for these potentially confounding variables makes interpretation of the data more difficult. In addition, data regarding outcomes for pregnancies of multiple gestation were unavailable. This study is further limited by the retrospective nature of the chart review.
Conclusion
American College of Obstetrics and Gynecology urges obstetricians and gynecologists to “…take steps to ensure that clinical spaces are affirming and open to all patients, such that equitable and comprehensive reproductive health care can meet the needs of these communities” (3). In keeping with this recommendation, this study aimed to describe pregnancy success rates among lesbian women undergoing IUI and emphasize the need for individualized care in this patient population. Ultimately, larger prospective studies are needed to better guide the fertility care and experience for lesbian women, including counseling, management, and treatment options.
Footnotes
J.K.J. has nothing to disclose. R.M.G. has personal investment in Progyny. S.J.V. has nothing to disclose. E.G.J. has an EMD Serono investigator sponsored trial grant unrelated to the current endeavor. H.H. has nothing to disclose. L. A. has nothing to disclose.
References
- 1.Institute of Medicine (US) Committee on Lesbian, Gay, Bisexual, and Transgender Health Issues and Research Gaps and Opportunities . National Academies Press; Washington (DC): 2011. The health of lesbian, gay, bisexual, and transgender people: building a foundation for better understanding. [PubMed] [Google Scholar]
- 2.Amato P., Jacob M. Providing fertility services to lesbian couples: the lesbian baby boom. Sex Reprod Menopause. 2004;2:83–88. [Google Scholar]
- 3.ACOG committee opinion no. 749: marriage and family building equality for lesbian, gay, bisexual, transgender, queer, intersex, asexual, and gender nonconforming individuals. Obstet Gynecol. 2018;132:e82–e86. doi: 10.1097/AOG.0000000000002765. [DOI] [PubMed] [Google Scholar]
- 4.Nazem T.G., Chang S., Lee J.A., Briton-Jones C., Copperman A.B., McAvey B. Understanding the reproductive experience and pregnancy outcomes of lesbian women undergoing donor intrauterine insemination. LGBT Health. 2019;6:62–67. doi: 10.1089/lgbt.2018.0151. [DOI] [PubMed] [Google Scholar]
- 5.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 6.Thomas L.E., Pencina M.J. Do not over (P) value your research article. J Am Med Assoc Cardiol. 2016;1:1055. doi: 10.1001/jamacardio.2016.3827. [DOI] [PubMed] [Google Scholar]
- 7.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ Available at:
- 8.Højsgaard S., Halekoh U., Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15:1–11. [Google Scholar]
- 9.Yan J., Fine J. Estimating equations for association structures. Stat Med. 2004;23:859–880. doi: 10.1002/sim.1650. [DOI] [PubMed] [Google Scholar]
- 10.Geepack Y.J. Yet another package for generalized estimating equations. R News. 2002;2:12–14. [Google Scholar]
- 11.Grund S., Robitzsch A., Luedtke O. Tools for multiple imputation in multilevel modeling. R package version 0.3-7, 2019. https://CRAN.R-project.org/package=mitml Available at:
- 12.Dinelli L., Courbière B., Achard V., Jouve E., Deveze C., Gnisci A. Prognosis factors of pregnancy after intrauterine insemination with the husband's sperm: conclusions of an analysis of 2,019 cycles. Fertil Steril. 2014;101:994–1000. doi: 10.1016/j.fertnstert.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Sicchieri F., Silva A.B., Silva ACJSRE., Navarro PAAS., Ferriani R.A., Reis R.M.D. Prognostic factors in intrauterine insemination cycles. JBRA Assist Reprod. 2018;22:2–7. doi: 10.5935/1518-0557.20180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodson K., Meads C., Bewley S. Lesbian and bisexual women's likelihood of becoming pregnant: a systematic review and meta-analysis. Br J Obstet Gynaecol. 2017;124:393–402. doi: 10.1111/1471-0528.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara I., Balet R., Grudzinskas J.G. Intrauterine donor insemination in single women and lesbian couples: a comparative study of pregnancy rates. Hum Reprod. 2000;15:621–625. doi: 10.1093/humrep/15.3.621. [DOI] [PubMed] [Google Scholar]
- 16.Nordqvist S., Sydsjö G., Lampic C., Åkerud H., Elenis E., Skoog Svanberg A. Sexual orientation of women does not affect outcome of fertility treatment with donated sperm. Hum Reprod. 2014;29:704–711. doi: 10.1093/humrep/det445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal R., Sharma S., Bekir J., Conway G., Bailey J., Balen A.H. Prevalence of polycystic ovaries and polycystic ovary syndrome in lesbian women compared with heterosexual women. Fertil Steril. 2004;82:1352–1357. doi: 10.1016/j.fertnstert.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 18.Buchmueller T., Carpenter C.S. Disparities in health insurance coverage, access, and outcomes for individuals in same-sex versus different-sex relationships, 2000-2007. Am J Public Health. 2010;100:489–495. doi: 10.2105/AJPH.2009.160804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll N., Palmer J.R. A comparison of intrauterine versus intracervical insemination in fertile single women. Fertil Steril. 2001;75:656–660. doi: 10.1016/s0015-0282(00)01782-9. [DOI] [PubMed] [Google Scholar]