Key Points
Question
Does the addition of camrelizumab to chemotherapy improve outcomes when used as first-line treatment for patients with advanced or metastatic esophageal squamous cell carcinoma?
Findings
In this randomized clinical trial that included 596 patients with advanced or metastatic esophageal squamous cell carcinoma, camrelizumab combined with chemotherapy, compared with placebo and chemotherapy, significantly improved overall survival (15.3 vs 12.0 months, respectively; hazard ratio for death, 0.70) and progression-free survival (6.9 vs 5.6 months, respectively; hazard ratio for disease progression or death, 0.56).
Meaning
Among patients with advanced or metastatic esophageal squamous cell carcinoma, an initial treatment strategy of camrelizumab combined with chemotherapy, compared with placebo and chemotherapy, resulted in improved overall survival and progression-free survival.
Abstract
Importance
Standard first-line therapy for advanced or metastatic esophageal carcinoma is chemotherapy, but the prognosis remains poor. Camrelizumab (an anti–programmed death receptor 1 [PD-1] antibody) showed antitumor activity in previously treated advanced or metastatic esophageal squamous cell carcinoma.
Objective
To evaluate the efficacy and adverse events of camrelizumab plus chemotherapy vs placebo plus chemotherapy as a first-line treatment in advanced or metastatic esophageal squamous cell carcinoma.
Design, Setting, and Participants
This randomized, double-blind, placebo-controlled, multicenter, phase 3 trial (ESCORT-1st study) enrolled patients from 60 hospitals in China between December 3, 2018, and May 12, 2020 (final follow-up, October 30, 2020). A total of 751 patients were screened and 596 eligible patients with untreated advanced or metastatic esophageal squamous cell carcinoma were randomized.
Interventions
Patients were randomized 1:1 to receive either camrelizumab 200 mg (n = 298) or placebo (n = 298), combined with up to 6 cycles of paclitaxel (175 mg/m2) and cisplatin (75 mg/m2). All treatments were given intravenously every 3 weeks.
Main Outcomes and Measures
Coprimary end points were overall survival (significance threshold, 1-sided P < .02) and progression-free survival (significance threshold, 1-sided P < .005).
Results
Of the 596 patients randomized (median age, 62 years [interquartile range, 56-67 years]; 523 men [87.8%]), 1 patient in the placebo-chemotherapy group did not receive planned treatment. A total of 490 patients (82.2%) had discontinued the study treatment. The median follow-up was 10.8 months. The overall survival for the camrelizumab-chemotherapy group was a median of 15.3 months (95% CI, 12.8-17.3; 135 deaths) vs a median of 12.0 months (95% CI, 11.0-13.3; 174 deaths) for the placebo-chemotherapy group (hazard ratio [HR] for death, 0.70 [95% CI, 0.56-0.88]; 1-sided P = .001). Progression-free survival for camrelizumab plus chemotherapy was a median of 6.9 months (95% CI, 5.8-7.4; 199 progression or deaths) vs 5.6 months (95% CI, 5.5-5.7; 229 progression or deaths) for the placebo-chemotherapy group (HR for progression or death, 0.56 [95% CI, 0.46-0.68]; 1-sided P < .001). Treatment-related adverse events of grade 3 or higher occurred in 189 patients (63.4%) in the camrelizumab-chemotherapy group and 201 (67.7%) in the placebo-chemotherapy group, including treatment-related deaths among 9 patients (3.0%) and 11 patients (3.7%), respectively.
Conclusions and Relevance
Among patients with advanced or metastatic esophageal squamous cell carcinoma, the addition of camrelizumab to chemotherapy, compared with placebo and chemotherapy, significantly improved overall survival and progression-free survival.
Trial Registration
ClinicalTrials.gov Identifier: NCT03691090
This clinical trial compares overall and progression-free survival of patients with advanced or metastatic esophageal squamous cell carcinoma randomized to camrelizumab plus chemotherapy or to placebo plus chemotherapy.
Introduction
In 2018, esophageal cancer was the seventh most frequently diagnosed cancer and the sixth most common cause of cancer-related death among the global population.1 The histological subtype of esophageal cancer varies widely by region, with esophageal squamous cell carcinoma (ESCC) being the predominant subtype in Asia and esophageal adenocarcinoma the major subtype in Australia, the United States, and some western European countries with rare exception.2,3 Patients with esophageal cancer commonly have advanced disease or metastases at diagnosis,2 and the current recommended standard first-line therapy for this advanced or metastatic disease is chemotherapy.4 However, the overall survival of patients receiving the standard of care, 2-drug cytotoxic agents, remains limited with a median of 7.0 to 13.0 months based on data from several prospective clinical studies.5,6,7 Therefore, novel drugs and strategies are required to improve clinical outcomes.
Inhibition of programmed death receptor 1 (PD-1) and its ligand (PD-L1) have been effective in treating a number of cancers.8 Camrelizumab (Jiangsu Hengrui Pharmaceuticals Co, Ltd), a humanized, selective IgG4-κ monoclonal antibody against PD-1, exerted antitumor activity in a wide range of tumors.9,10,11,12,13 In the randomized phase 3 ESCORT study, camrelizumab significantly improved overall survival and response rates over chemotherapy as a second-line therapy in patients with advanced or metastatic ESCC,9 leading to its approval by the China National Medical Products Administration as a second-line treatment in this population.
The combination of immunotherapy with cytotoxic agents has shown encouraging antitumor activity in multiple tumor types,14,15 but data are lacking to support this approach as a first-line treatment strategy for advanced ESCC. In this context, this randomized, double-blind, placebo-controlled, multicenter phase 3 trial (ESCORT-1st study) was conducted to evaluate the efficacy and adverse events of camrelizumab plus chemotherapy compared with placebo plus chemotherapy for untreated advanced ESCC.
Methods
Trial Oversight
The ESCORT-1st study was a randomized, double-blind, placebo-controlled, phase 3 trial conducted in 60 hospitals in China. The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guideline. The study protocol, protocol amendments, and statistical analysis plan (Supplement 1) were approved by the institutional review board or independent ethics committee of each study site. All patients provided written informed consent before enrollment.
Patients
Patients were eligible if they were aged 18 through 75 years; had histologically or cytologically confirmed ESCC; had unresectable, locally advanced, or recurrent disease that precluded esophagectomy or definitive chemoradiation, or distant metastatic disease; had received no previous systemic therapy (patients who had progressed ≥6 months after [neo]adjuvant therapy or definitive chemoradiation were eligible); had an Eastern Cooperative Oncology Group performance status score of 0 or 1; had at least 1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; had a life expectancy of at least 12 weeks; and had adequate organ function. Patients were required to provide fresh or archival tumor samples for PD-L1 expression assessment.
Exclusion criteria included the presence of other malignancies, having an active or a history of autoimmune disease, central nervous system metastases, and use of antitumor therapies or live vaccine within the 4 weeks preceding study enrollment.
Randomization and Interventions
Eligible patients were randomly assigned in a 1:1 ratio to either the camrelizumab-chemotherapy group or the placebo-chemotherapy group. Camrelizumab (200 mg) or placebo were given on day 1 until disease progression, defined by the RECIST guideline, unacceptable toxic effects, withdrawal of consent, death, or initiation of new antitumor therapy, whichever occurred first (Figure 1). Paclitaxel (175 mg/m2) and cisplatin (75 mg/m2) were given on day 1 for up to 6 cycles after randomization. All treatments were given intravenously in 3-week cycles. Randomization was done using a centralized interactive web-response system with the block size randomly generated as 4 or 6 and stratified by liver metastases (yes vs no) and previous definitive chemoradiation (yes vs no). Patients, investigators, and the sponsor’s study team were masked to treatment assignment. Treatment interruption of camrelizumab or placebo was allowed to manage toxic effects but dose reduction of them was not allowed. Dose adjustment and treatment or retreatment criteria of paclitaxel and cisplatin was determined by the investigators according to clinical practice.
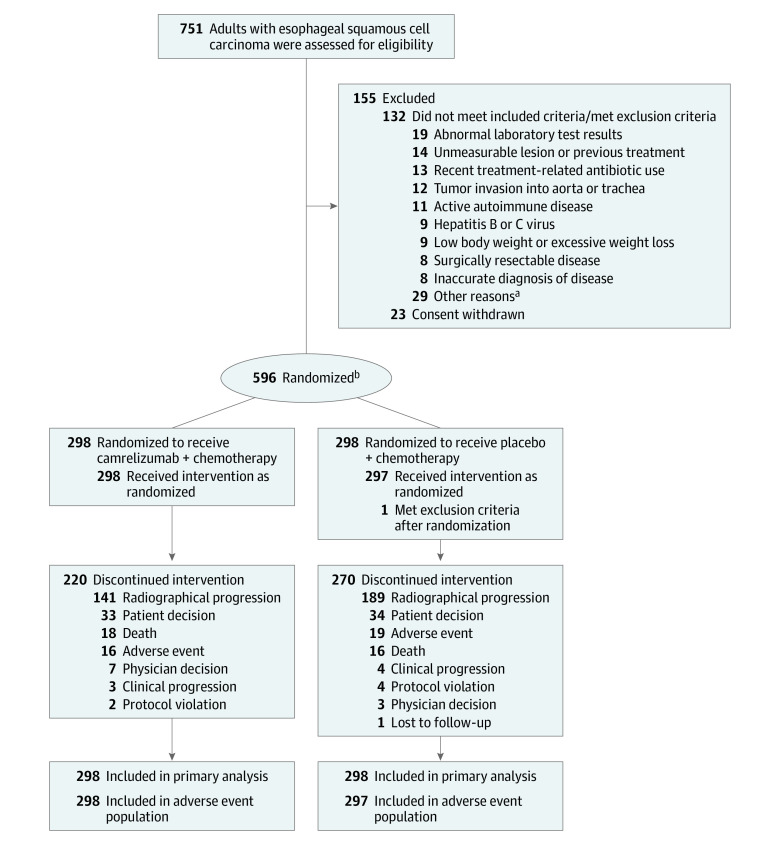
Figure 1. Assessment, Randomization, and Flow in a Trial of Camrelizumab in the Treatment of Esophageal Squamous Cell Carcinoma.
aSee Table 2 in Supplement 2 for detailed reasons.
bPatients were randomized in a 1:1 ratio stratified by liver metastases (yes vs no) and previous definitive chemoradiation (yes vs no).
Assessments
Tumor response was assessed every 6 weeks using radiographic examination by both the independent review committee and investigator. Patients who experienced radiographic progression but were clinically stable could continue to receive the assigned treatment until their disease progression was confirmed by imaging examination at least 4 weeks after first detection. Survival was assessed every 30 days until death. Adverse events were assessed up to 90 days after the last dose. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. PD-L1 expression in tumor samples was assessed at a central laboratory (Shuwen Biotech, Deqing, Zhejiang, China) using a PD-L1 immunohistochemistry kit (6E8 antibody: Abcam) and characterized according to tumor proportion score.16 Health–related quality of life was evaluated every 6 weeks from the start of treatment for 30 days after the last dose, according to European Organization for Research and Treatment of Cancer quality-of-life core 30 (QLQ-C30) and QLQ esophageal cancer 18 (QLQ-OES18) scales.17,18 All of the scales and single-item measures range in score from 0 to 100. A high score for the global health status and a functional scale of QLQ-C30 represents good condition, but a high score for a constitutional cancer symptom scale or item of QLQ-C30 and an esophageal cancer–specific symptom scale or item from QLQ-OES18 represents worse condition.
Outcomes
The coprimary end points were progression-free survival assessed by the independent review committee (the time from randomization to disease progression or death from any cause, whichever occurred first) and overall survival (the time from randomization to death from any cause). Secondary end points included progression-free survival assessed by investigator, objective response rate (proportion of patients whose best overall response was complete or partial response), disease control rate (proportion of patients whose best overall response was complete response, partial response, or stable disease), duration of response (the time from the first response to disease progression or death from any cause, whichever occurred first), probability of overall survival, adverse events, and health–related quality of life (QLQ-C30 and QLQ-OES18).
Statistical Analysis
The overall type I error was controlled at a 1-sided α level of .025 and was allocated as follows: α = .005 for progression-free survival per independent review committee and α = .02 for overall survival. An increase of 2.5 months (from 5.0 months in placebo-chemotherapy group to 7.5 months in camrelizumab-chemotherapy group, corresponding to a hazard ratio [HR] of 0.67 under the exponential model assumption) was assumed for progression-free survival and 3.7 months (from 10.0 months in placebo-chemotherapy group to 13.7 months in camrelizumab-chemotherapy group, corresponding to an HR of 0.73 under the exponential model assumption) was assumed for overall survival, and these assumptions were deemed as clinically meaningful based on consensus among the study investigators. It was calculated that 378 progression-free survival events would provide 90% power to detect an HR of 0.67 at a 1-sided α of .005, and 408 overall survival events would provide more than 85% power to detect an HR of 0.73 at a 1-sided α of .02. Duration of enrollment was supposed to be 18 months and 36 months for the study. Originally, 548 patients were planned to be enrolled under the assumption of a 5% dropout rate; however, because of the outbreak of the COVID-19 epidemic, 596 patients were ultimately enrolled under the assumption of a 15% dropout rate.
Efficacy was assessed in all patients who underwent randomization and was analyzed according to randomization group allocation. Adverse events were assessed among patients who received at least 1 dose of the study treatment and were analyzed by the treatment received. Patients were censored at the last tumor assessment for progression-free survival and at the last time known to be alive for overall survival. If a patient did not undergo a postbaseline tumor assessment, the best overall response of the patient was judged as not assessable. Missing data of health–related quality of life was accounted for using the mixed-model repeated-measures method.
The Kaplan-Meier method was used to estimate median overall survival, progression-free survival, and duration of response; 95% CIs were calculated using the Brookmeyer-Crowley method. Between-group differences in progression-free survival and overall survival were assessed using stratified log-rank test. HRs for progression-free survival and overall survival as well as 2-sided 95% CIs were assessed using stratified Cox proportional-hazards models. To take account of the group sequential design of the study, the prespecified repeated CIs method was used for the HR of overall survival at the interim analysis.19 The proportional hazard assumption was assessed based on the Grambsch-Therneau test and the plot of Schoenfeld residuals.20,21
Objective response and disease control were presented with 95% CIs (Clopper-Pearson method), and the comparisons between the groups were made using the stratified Cochran-Mantel-Haenszel test. The consistency of the treatment effect for each prespecified subgroup was evaluated using the stratified Cox proportional-hazards model with treatment as the only covariate. For the post hoc analyses, the homogeneity of treatment effects on the primary end points across prespecified subgroups was evaluated by adding treatment by subgroup interaction into a Cox proportional-hazards model, and the site effect was evaluated by adding site into a Cox proportional-hazards model. Changes from baseline in health–related quality of life between the 2 treatment groups were compared using the mixed-model repeated-measures method.
Two interim analyses of overall survival (planned to be conducted with about 66% and 85% of the total expected overall survival events) were prespecified. The superiority boundary in the interim and final analyses of overall survival was predefined by using the O’Brien-Fleming type Lan-DeMets alpha spending function and could be adjusted on the basis of observed number of overall survival events at the time of each analysis. The final analysis of progression-free survival was planned to be performed when approximately 378 events of progression or death occurred, and the first interim analysis of overall survival was planned to be conducted at the same time (when approximately 269 deaths were anticipated to have occurred). When the number of events of progression-free survival were close to the planned 378 events based on the periodic review of the independent review committee in September 2020, data as of October 30, 2020 (planned cutoff date) were planned to be used for analysis and the independent review committee was informed of the date. After the adjudication procedure of the independent review committee, a total of 428 events of progression or death and 309 deaths had occurred by the planned cutoff date. On the basis of the observed number of deaths, the threshold of the 1-sided α level for the first interim analysis of overall survival was .0075. The threshold for the final analysis of progression-free survival was .005. The independent data monitoring committee oversaw the results and reported that the efficacy boundaries for overall survival and progression-free survival had been crossed. The trial was continued to assess survival and adverse events with longer follow-up.
All the statistical tests for secondary end points had a 2-sided significance level of .05. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
From December 3, 2018, through May 12, 2020, 751 patients from 60 hospitals in China (eTable 1 in Supplement 2) were screened and 596 eligible patients were randomly assigned to either the camrelizumab-chemotherapy group (298 patients) or the placebo-chemotherapy group (298 patients). The 1 patient in the placebo-chemotherapy group who did not receive planned treatment was excluded from the adverse event population (Figure 1; eTable 2 in Supplement 2). Demographic and disease characteristics at baseline of the study groups are presented in Table 1.
Table 1. Baseline Patients Characteristics.
| No. (%) of patients | ||
|---|---|---|
| Camrelizumab + chemotherapy (n = 298) | Placebo + chemotherapy (n = 298) | |
| Age, y | ||
| Median (IQR) | 62 (56-66) | 62 (56-67) |
| <65 | 201 (67.4) | 185 (62.1) |
| Sex | ||
| Men | 260 (87.2) | 263 (88.3) |
| Women | 38 (12.8) | 35 (11.7) |
| Eastern Cooperative Oncology Group performance status scorea | ||
| 0 | 71 (23.8) | 66 (22.1) |
| 1 | 227 (76.2) | 232 (77.9) |
| Histological grade | ||
| Well or moderately differentiated | 107 (35.9) | 86 (28.9) |
| Poorly differentiated | 87 (29.2) | 82 (27.5) |
| Indeterminate | 104 (34.9) | 130 (43.6) |
| No. of organs with metastases | ||
| 1 | 138 (46.3) | 157 (52.7) |
| ≥2 | 160 (53.7) | 141 (47.3) |
| Sites of metastases | ||
| Lymph node | 276 (92.6) | 274 (91.9) |
| Lung | 92 (30.9) | 87 (29.2) |
| Liver | 69 (23.2) | 68 (22.8) |
| Bone | 31 (10.4) | 21 (7.0) |
| PD-L1 expression, %b | ||
| <1 | 126 (42.3) | 130 (43.6) |
| ≥1 | 166 (55.7) | 163 (54.7) |
| <5 | 145 (48.7) | 155 (52.0) |
| ≥5 | 147 (49.3) | 138 (46.3) |
| <10 | 188 (63.1) | 195 (65.4) |
| ≥10 | 104 (34.9) | 98 (32.9) |
| Indeterminate | 6 (2.0) | 5 (1.7) |
| Previous therapies | ||
| Surgery | 119 (39.9) | 99 (33.2) |
| Esophagectomy | 116 (38.9) | 95 (31.9) |
| Other | 3 (1.0)c | 4 (1.3)d |
| Antitumor medication | 75 (25.2) | 73 (24.5) |
| Adjuvant therapy | 54 (18.1) | 56 (18.8) |
| Definitive chemoradiation | 15 (5.0) | 16 (5.4) |
| Neoadjuvant therapy | 15 (5.0) | 8 (2.7) |
| Other | 1 (0.3)e | 1 (0.3)f |
| Radiotherapy | 54 (18.1) | 42 (14.1) |
Abbreviation: IQR, interquartile range; PD-L1, programmed death receptor 1 ligand 1.
The Eastern Cooperative Oncology Group performance status score 5-point scale defines 0 as fully active, able to carry on all predisease performance without restriction, and defines 1 as restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature.
For PD-L1 laboratory assessment tools see the Methods section. PD-L1 expression was quantified as tumor proportion score, which was defined as the percentage of viable tumor cells showing partial or complete membrane staining (≥1+), relative to all viable tumor cells present in the sample. A tumor proportion score of 1% was used as the cutoff of PD-L1 positive and negative.
One patient underwent abdominal exploration; 2 patients had residual primary lesion or lymph node lesion after esophagectomy.
One patient underwent neck mass resection; 3 patients had residual primary lesion or lymph node lesion after esophagectomy.
Intraoperative peritoneal perfusion with fluorouracil.
ENDOSTAR (a recombinant human endostatin injection) used during definitive chemoradiation.
The median follow-up duration was 10.8 months (interquartile range [IQR], 7.3-14.3 months). Relative dose intensity in each group for each drug was 100%. Of those who continued taking the study treatment, 78 patients (26.2%) of 298 patients were in the camrelizumab-chemotherapy group and 27 (9.1%) of 298 patients were in the placebo-chemotherapy group. The primary reason for treatment discontinuation was disease progression (141 [47.3%] vs 189 [63.4%], respectively) in each group. A total of 119 patients (39.9%) in the camrelizumab-chemotherapy group and 158 patients (53.0%) in the placebo-chemotherapy group received additional treatment after discontinuation of study treatment (eTable 3 in Supplement 2).
Coprimary Outcomes
A total of 309 deaths (51.8%) deaths occurred: 135 (45.3%) in the camrelizumab-chemotherapy group and 174 (58.4%) in placebo-chemotherapy group. Camrelizumab plus chemotherapy significantly improved overall survival compared with placebo plus chemotherapy (median, 15.3 months [95% CI, 12.8-17.3] vs 12.0 months [95% CI, 11.0-13.3]; HR for death, 0.70 [95% CI, 0.56-0.88; repeated CI, 0.53-0.93]; 1-sided P = .001; Figure 2A).
Figure 2. Kaplan-Meier Estimates of Overall Survival and Progression-Free Survival per Independent Review Committee.
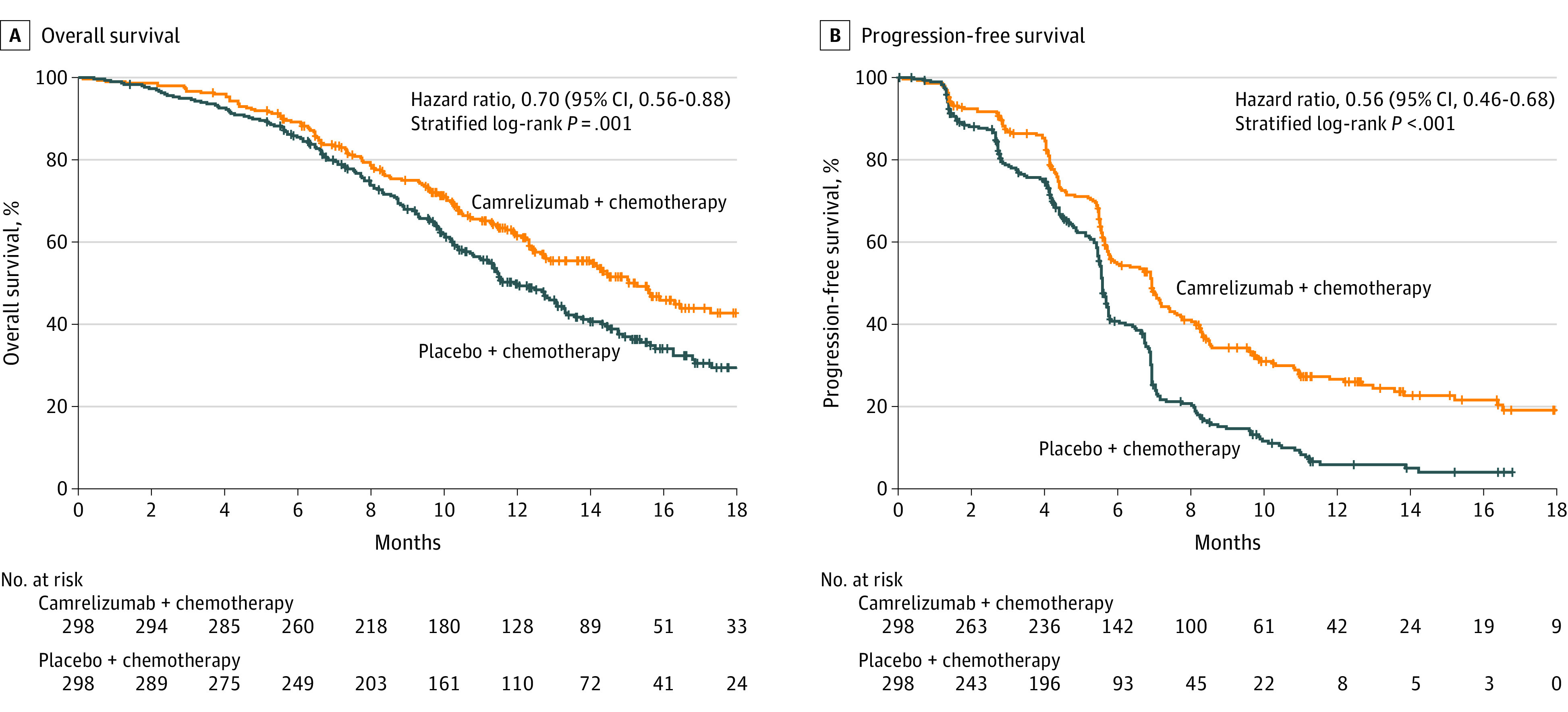
The tick marks indicate censored individuals. The median follow-up duration was 10.8 months (interquartile range, 7.3-14.3 months).
A, There were 135 deaths (45.3%) in the camrelizumab-chemotherapy group and 174 (58.4%) in the placebo-chemotherapy group, with median overall survival of 15.3 months (95% CI, 12.8-17.3) and 12.0 months (95% CI, 11.0-13.3), respectively.
B, There were 199 events (66.8%) of disease progression or death in the camrelizumab-chemotherapy group and 229 (76.8%) in the placebo-chemotherapy group, with median progression-free survival of 6.9 months (95% CI, 5.8-7.4) and 5.6 months (95% CI, 5.5-5.7), respectively.
A total of 199 of 298 patients (66.8%) in the camrelizumab-chemotherapy group and 229 of 298 patients (76.8%) in the placebo-chemotherapy group had disease progression or died as assessed by the independent review committee. The median progression-free survival was 6.9 months (95% CI, 5.8-7.4) in the camrelizumab-chemotherapy group vs 5.6 months (95% CI, 5.5-5.7) in the placebo-chemotherapy group (HR for progression or death, 0.56 [95% CI, 0.46-0.68]; 1-sided P < .001; Figure 2B).
Secondary Outcomes
The probabilities of overall survival in the camrelizumab-chemotherapy group at 6 months were 89.2% (95% CI, 85.1%-92.2%); at 12 months, 61.5% (95% CI, 55.4%-67.1%); and at 18 months, 42.7% (95% CI, 35.3%-50.0%) and in the placebo-chemotherapy group were 85.5% (95% CI, 80.9%-89.0%), 49.8% (95% CI, 43.6%-55.6%), and 29.5% (95% CI, 22.9%-36.3%), respectively. The Grambsch-Therneau test (2-sided P = .60) and the plot of Schoenfeld residuals (eFigure 1 in Supplement 2) indicated that the proportional hazard assumption of overall survival was maintained.
For both groups, the progression-free survival assessed by investigator was similar to that assessed by the independent review committee (eFigure 2 in Supplement 2). The median duration of response per investigator was 7.0 months (95% CI, 6.1-8.9) for the camrelizumab-chemotherapy group and 4.6 months (95% CI, 4.3-5.5) for the placebo-chemotherapy group (eFigure 3 in Supplement 2).
Objective responses per investigator were reported for 215 (72.1%; 95% CI, 66.7% to 77.2%) of the 298 patients in camrelizumab-chemotherapy group vs 185 (62.1%; 95% CI, 56.3% to 67.6%) of the 298 patients in the placebo-chemotherapy group, and the difference between groups was 10.1% (95% CI, 2.6% to 17.6%; 2-sided P = .009; eTable 4 in Supplement 2). The disease control rate was 91.3% (95% CI, 87.5% to 94.2%) and 88.9% (95% CI, 84.8% to 92.3%) in each group, respectively, and the difference between groups was 2.3% (95% CI, −2.4% to 7.1%; 2-sided P = .33; eTable 4 in Supplement 2).
Prespecified Subgroup Analyses and Post Hoc Analyses
Prespecified subgroup analyses showed that the overall survival benefit of camrelizumab plus chemotherapy relative to placebo plus chemotherapy did not vary significantly across most subgroups, although post hoc tests for interaction performed for the prespecified subgroups demonstrated significant interactions based on liver metastases and alcohol status. In patients with a baseline PD-L1 expression of less than 1% and those with 1% or higher, the HRs for death between the camrelizumab-chemotherapy group and the placebo-chemotherapy group were 0.79 (95% CI, 0.57-1.11) and 0.59 (95% CI, 0.43-0.80), respectively, with a P value for interaction of .32 (eFigures 4 and 5 in Supplement 2).
Benefits of progression-free survival with camrelizumab plus chemotherapy vs placebo plus chemotherapy were evident across subgroups. The HRs for progression or death between the study groups were 0.62 (95% CI, 0.46-0.83) in patients with a baseline PD-L1 of less than 1% and 0.51 (95% CI, 0.39-0.67) in patients with a PD-L1 of 1% or higher with a P value for interaction of .38 (eFigures 6 and 7 in Supplement 2). The objective response rate, disease control rate, and duration of response assessed by investigator in patients with PD-L1 of less than 1% and 1% or higher are presented in eTable 5 and eFigure 8 in Supplement 2.
The analyses for site effect as assessed by the independent review committee showed that there was no significant site effect on either progression-free survival (P = .48) or overall survival (P = .56).
Adverse Events
Treatment–related adverse events occurred in 296 (99.3%) of the 298 patients in the camrelizumab-chemotherapy group and 288 (97.0%) of the 297 patients in the placebo-chemotherapy group (Table 2). The treatment–related adverse events of grade 3 or higher were reported in 189 patients (63.4%) in the camrelizumab-chemotherapy group and 201 patients (67.7%) in the placebo-chemotherapy group, with the most common ones being decreased neutrophil count (119 [39.9%] vs 129 [43.4%]), decreased white blood cell count (72 [24.2%] vs 79 [26.6%]), and anemia (52 [17.4%] vs 40 [13.5%]). Treatment–related serious adverse events occurred in 90 patients (30.2%) in camrelizumab-chemotherapy group and 69 patients (23.2%) in placebo-chemotherapy group, with pneumonitis (17 [5.7%] vs 8 [2.7%]) being the most common (eTable 6 in Supplement 2).
Table 2. Adverse Events.
| No. (%) of patients | ||||
|---|---|---|---|---|
| Camrelizumab + chemotherapy (n = 298) | Placebo + chemotherapy (n = 297) | |||
| Any gradea | ≥Grade 3 | Any grade | ≥Grade 3 | |
| Treatment-related adverse eventsb | 296 (99.3)c | 189 (63.4) | 288 (97.0) | 201 (67.7) |
| Reactive cutaneous capillary endothelial proliferation | 238 (79.9) | 3 (1.0) | 32 (10.8) | 0 |
| Anemia | 229 (76.8) | 52 (17.4) | 217 (73.1) | 40 (13.5) |
| White blood cell count decreased | 202 (67.8) | 72 (24.2) | 194 (65.3) | 79 (26.6) |
| Neutrophil count decreased | 201 (67.4) | 119 (39.9) | 186 (62.6) | 129 (43.4) |
| Nausea | 150 (50.3) | 4 (1.3) | 154 (51.9) | 5 (1.7) |
| Asthenia | 141 (47.3) | 6 (2.0) | 129 (43.4) | 8 (2.7) |
| Alopecia | 135 (45.3) | 1 (0.3) | 147 (49.5) | 0 |
| Decreased appetite | 129 (43.3) | 2 (0.7) | 134 (45.1) | 5 (1.7) |
| Vomiting | 117 (39.3) | 10 (3.4) | 106 (35.7) | 6 (2.0) |
| Platelet count decreased | 77 (25.8) | 8 (2.7) | 73 (24.6) | 6 (2.0) |
| Weight decreased | 77 (25.8) | 2 (0.7) | 67 (22.6) | 8 (2.7) |
| Blood creatinine increased | 69 (23.2) | 1 (0.3) | 54 (18.2) | 0 |
| Aspartate aminotransferase increased | 46 (15.4) | 3 (1.0) | 42 (14.1) | 3 (1.0) |
| Hypoalbuminemia | 46 (15.4) | 0 | 36 (12.1) | 0 |
| Hypoesthesia | 44 (14.8) | 0 | 48 (16.2) | 2 (0.7) |
| Immune-related adverse eventsd | 252 (84.6) | 98 (33.0) | ||
| Reactive capillary endothelial proliferation | 238 (79.9) | 32 (10.8) | ||
| Hypothyroidism | 34 (11.4) | 13 (4.4) | ||
| Pruritus | 20 (6.7) | 7 (2.4) | ||
| Hyperthyroidism | 16 (5.4) | 3 (1.0) | ||
| Rash | 16 (5.4) | 6 (2.0) | ||
| Pneumonitis | 15 (5.0) | 9 (3.0) | ||
| Blood thyroid stimulating hormone decreased | 10 (3.4) | 1 (0.3) | ||
Adverse events were classified according to Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Grading ranges from 1 through 5 (1, mild; 2, moderate; 3, severe; 4, life-threatening; and 5, death).
Treatment-related adverse events occurring in 15% or more of patients in either group are listed. Events are shown in descending order of frequency in the camrelizumab-chemotherapy group.
The numbers represent the number of patients with an adverse event.
Immune–related adverse events occurring in 3% or more of patients in either group are listed.
Treatment–related adverse events led to treatment interruption of any treatment component in 135 patients (45.3%) in the camrelizumab-chemotherapy group and 71 patients (23.9%) in the placebo-chemotherapy group. Thirty-six patients (12.1%) and 28 patients (9.4%) discontinued at least 1 treatment component due to treatment–related adverse events, respectively (eTable 7 in Supplement 2). Treatment–related adverse events led to death in 9 patients (3.0%) and 11 patients (3.7%) patients, respectively (eTable 8 in Supplement 2).
A total of 252 of the 298 patients (84.6%) in camrelizumab-chemotherapy group and 98 of the 297 patients (33.0%) in placebo-chemotherapy group had immune–related adverse events (Table 2, eTable 9 in Supplement 2), with 28 patients (9.4%) and 15 patients (5.1%) having had an event grade of 3 or higher. The most common immune–related adverse event was reactive capillary endothelial proliferation (238 [79.9%] vs 32 [10.8%]), which was generally associated with camrelizumab. This adverse event mainly occurred as grade 1 or 2 (235 [78.9%] vs 32 [10.8%]), with only 3 patients (1.0%) in the camrelizumab-chemotherapy group having a grade 3 event.
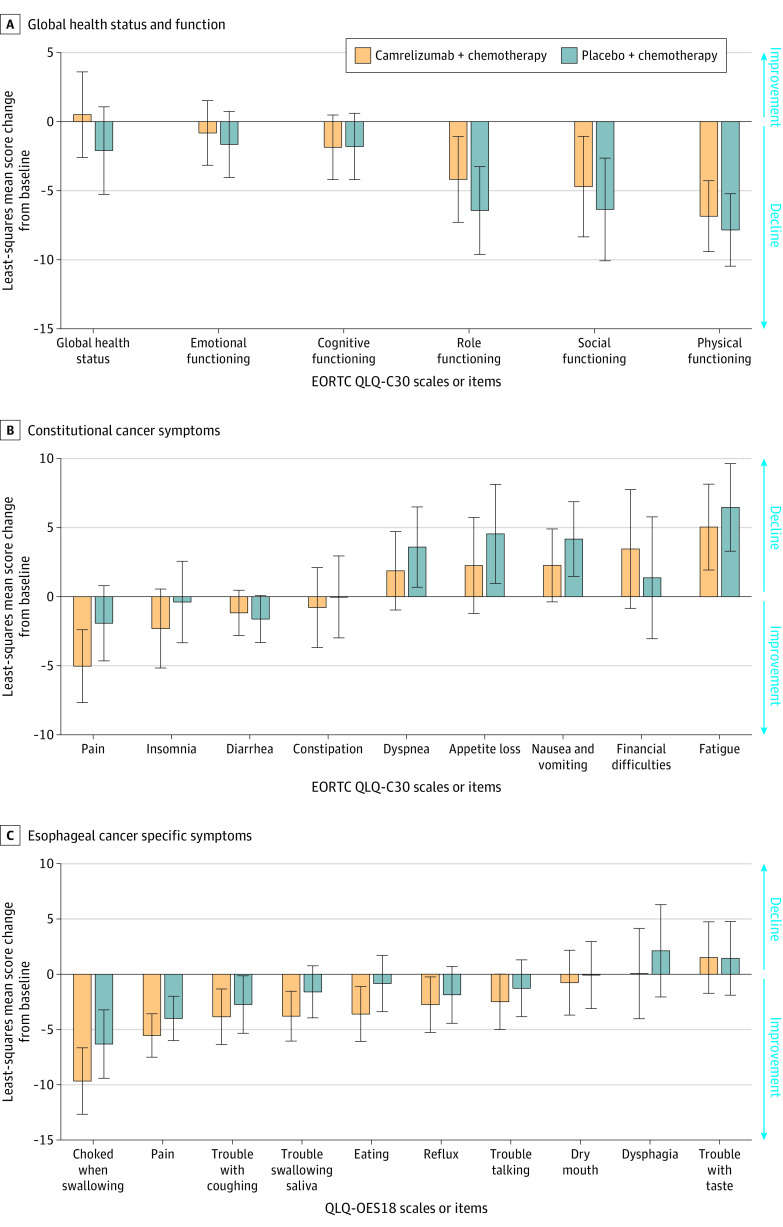
Health–Related Quality of Life
Of the total 15 health–related quality of life metrics of QLQ-C30 and 10 metrics of QLQ-OES18 assessed, 13 metrics of QLQ-C30 and 7 metrics of QLQ-OES18 showed no significant differences between groups.
However, health–related quality of life assessment up to 36 weeks showed statistically significant results in favor of the camrelizumab-chemotherapy group for some items from the QLQ-C30 scale: global health status (difference, 2.6; 95% CI, 0.0 to 5.2) and pain (difference, −3.1; 95% CI, −5.3 to −0.9); and from the QLQ-OES18 scale: eating (difference, −2.8; 95% CI, −4.8 to −0.7), trouble swallowing saliva (difference, −2.2; 95% CI, −4.1 to −0.3), and choked when swallowing (difference, −3.4; 95% CI, −5.9 to −0.8; Figure 3 and eTables 10 and 11 in Supplement 2).
Figure 3. Health-Related Quality of Life.
A total of 298 patients in camrelizumab-chemotherapy group and 298 patients in placebo-chemotherapy group were included in the quality-of-life assessment. See the Methods section for calculating scores.
A, A high score based on the European Organization for Research and Treatment of Cancer (EORTC) quality of life core 30 (QLQ-C30) scale for the global health status and functional scale indicates good condition.
B, A high score for the constitutional cancer symptom scale or item of QLQ-C30 indicates a worse condition.
C, A high score in the esophageal cancer–specific symptom scale or item from QLQ esophageal cancer 18 (QLQ-OES18) indicates a worse condition.
Detailed descriptions of quality of life are provided in statistical analysis plan. Error bars indicate (95% CIs).
Discussion
In this randomized, double-blind, placebo-controlled, phase 3 trial, the addition of camrelizumab to standard chemotherapy with paclitaxel and cisplatin improved median overall survival and median progression-free survival, compared with placebo plus chemotherapy, in patients with untreated, locally advanced or metastatic ESCC.
The cytotoxic regimen in this study was chosen according to both international and local treatment guidelines, and paclitaxel-cisplatin was recommended especially for ESCC in China.4,5,6,22 Both primary end points in this study were met at the preplanned interim analysis of overall survival and final analysis of progression-free survival. The study was continued and the results of long-term assessment will be reported subsequently. Similar to findings of the KEYNOTE-590 study23 of pembrolizumab plus chemotherapy that used placebo plus fluorouracil and cisplatin as the control (HR for death, 0.72; HR for progression or death, 0.65; both in the ESCC subpopulation), the current study showed superiority of camrelizumab plus chemotherapy over placebo plus paclitaxel and cisplatin even though the control group had a relatively long survival (median 12.0 months) and a high response rate (62.1%).
Subgroup analyses suggested generally consistent benefits in both overall survival and progression-free survival with camrelizumab plus chemotherapy over placebo plus chemotherapy, although the small number of patients in some subgroups precluded accurate interpretation of the benefits. The point estimate of HR for death was less than 1 in both patients with baseline PD-L1 of 1% or higher and PD-L1 of less than 1% (HR for death, 0.59 [95% CI, 0.43-0.80] vs 0.79 [95% CI, 0.57-1.11], respectively). These data suggest a potentially better overall survival benefit in patients with baseline PD-L1 of 1% or higher than in patients with a PD-L1 of less than 1%, but the test for interaction was not statistically significant and no definite correlation between PD-L1 expression and efficacy of camrelizumab can be concluded on the basis of this study. This finding was consistent with results of the ESCORT study9 and ATTACTION-3 study24 (PD-L1 expression was quantified as tumor proportion score and measured using the IHC 28-8 pharmDx assay [Agilent Technologies]), but different from that of KEYNOTE-181, in which the survival benefit of pembrolizumab was only observed in patients with baseline PD-L1 combined positive score 10% of or more (assessed using IHC 22C3 pharmDx assay [Agilent Technologies]).25
In a disease with a short life expectancy and for a treatment with a small increment in life expectancy, the adverse effects of the treatment can have an important effect on quality of life. Therefore, the adverse events of treatment should be taken into consideration when making decisions in clinical practice. Results showed that the adverse event profile observed in the current trial was consistent with those previously observed with camrelizumab, paclitaxel-cisplatin,5,6,9,22 as well as that with camrelizumab plus chemotherapy in other tumor types.13 No new adverse event signal was identified. The incidence of treatment discontinuation due to treatment-related adverse events in the camrelizumab-chemotherapy group was higher than that in the placebo-chemotherapy group, which may have been due in part to the longer treatment exposure of this group. More patients in the camrelizumab-chemotherapy group experienced immune–related adverse events than those in the placebo-chemotherapy group. This phenomenon was mainly attributed to the high incidence of reactive capillary endothelial proliferation in the camrelizumab-chemotherapy group. Reactive capillary endothelial proliferation is a common adverse event associated with camrelizumab.9,26 Most patients with reactive capillary endothelial proliferation did not require special treatment, and it may spontaneously regress after discontinuation of camrelizumab. Reactive capillary endothelial proliferation is considered an immune response of capillary endothelial cells, and it has been reported that its occurrence is positively associated with tumor response.26
Patients with advanced or metastatic esophageal cancer commonly face a poor health–related quality of life caused by the disease itself and relevant treatments. Results showed that camrelizumab in combination with chemotherapy was associated with statistically significant improvements in some health-related quality-of-life metrics compared with placebo plus chemotherapy. This finding was also generally consistent with results from the prior second-line study in which camrelizumab monotherapy led to improved health–related quality of life vs chemotherapy.9
Limitations
This study has several limitations. First, some of the patients in the placebo-chemotherapy group received subsequent checkpoint inhibitor treatment after disease progression, which might affect the estimates of overall survival. Second, the correlation of PD-L1 expression status and efficacy of immunotherapy plus chemotherapy in ESCC remains unclear. Third, efficacy-related biomarker tests other than PD-L1 expression remain to be analyzed.
Conclusions
Among patients with advanced or metastatic esophageal squamous cell carcinoma, the addition of camrelizumab to chemotherapy, compared with placebo and chemotherapy, significantly improved overall survival and progression-free survival.
Trial Protocols, Amendments, and Statistical Analysis Plan
eFigure 1. The plot of Schoenfeld residuals from stratified Cox proportional regression model for overall survival
eFigure 2. Kaplan-Meier curve of progression-free survival assessed by investigator
eFigure 3. Kaplan-Meier curve of duration of response assessed by investigator
eFigure 4. Forest plot analyses of overall survival in subgroups
eFigure 5. Kaplan-Meier curves of overall survival in patients with baseline PD-L1 <1% or ≥1%.
eFigure 6. Forest plot analyses of progression-free survival assessed by independent review committee in subgroups
eFigure 7. Kaplan-Meier curves of progression-free survival per independent review committee in patients with baseline PD-L1 <1% or ≥1%.
eFigure 8. Kaplan-Meier curves of duration of response per investigator in patients with baseline PD-L1 <1% or ≥1%
eTable 1. List of investigators
eTable 2. Detailed reasons of patients who did not meet inclusion criteria or met exclusion criteria
eTable 3. Summary of post-discontinuation therapy
eTable 4. Tumor response assessed by investigator
eTable 5. Tumor responses per investigator in patients with baseline PD-L1 <1% or ≥1%
eTable 6. Treatment-related serious adverse events
eTable 7. Treatment discontinuation caused by treatment-related adverse events
eTable 8. Deaths caused by treatment-related adverse events
eTable 9. Immune-related adverse events
eTable 10. Baseline values and least squares mean score changes from baseline of the EORTC QLQ-C30
eTable 11. Baseline values and least squares mean score changes from baseline of the EORTC QLQ-OES18
ESCORT-1st Investigators
Data Sharing Statement
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400-412. doi: 10.1016/S0140-6736(12)60643-6 [DOI] [PubMed] [Google Scholar]
- 3.Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31(6):281-286. doi: 10.5732/cjc.011.10390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . Esophageal and esophagogastric junction cancers. NCCN Clinical Practice Guidelines in Oncology. Version 2. Published March 9, 2021. Accessed May 5, 2021. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- 5.Petrasch S, Welt A, Reinacher A, Graeven U, König M, Schmiegel W. Chemotherapy with cisplatin and paclitaxel in patients with locally advanced, recurrent or metastatic oesophageal cancer. Br J Cancer. 1998;78(4):511-514. doi: 10.1038/bjc.1998.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Shen L, Li J, Li Y, Li J, Jin M. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. Am J Clin Oncol. 2008;31(1):29-33. doi: 10.1097/COC.0b013e3181131ca9 [DOI] [PubMed] [Google Scholar]
- 7.Sun S, Yu H, Wang H, et al. Phase II Study of S-1 plus cisplatin as first-line therapy in patients with metastatic esophageal carcinoma. Oncol Res Treat. 2019;42(3):115-122. doi: 10.1159/000495700 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto S, Kato K. Immuno-oncology for esophageal cancer. Future Oncol. 2020;16(32):2673-2681. doi: 10.2217/fon-2020-0545 [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Xu J, Chen Y, et al. ; ESCORT Study Group . Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832-842. doi: 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 10.Fang W, Yang Y, Ma Y, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338-1350. doi: 10.1016/S1470-2045(18)30495-9 [DOI] [PubMed] [Google Scholar]
- 11.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571-580. doi: 10.1016/S1470-2045(20)30011-5 [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Wu J, Chen X, et al. A single-arm, multicenter, phase II study of camrelizumab in relapsed or refractory classical Hodgkin lymphoma. Clin Cancer Res. 2019;25(24):7363-7369. doi: 10.1158/1078-0432.CCR-19-1680 [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Chen G, Huang Y, et al. ; CameL Study Group . Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305-314. doi: 10.1016/S2213-2600(20)30365-9 [DOI] [PubMed] [Google Scholar]
- 14.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 15.Burtness B, Harrington KJ, Greil R, et al. ; KEYNOTE-048 Investigators . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 16.Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330-337. doi: 10.5858/arpa.2018-0043-OA [DOI] [PubMed] [Google Scholar]
- 17.European Organization for Research and Treatment of Cancer . Quality of life. Accessed June 12, 2018. https://www.eortc.org.
- 18.Cocks K, King MT, Velikova G, Fayers PM, Brown JM. Quality, interpretation and presentation of European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 data in randomised controlled trials. Eur J Cancer. 2008;44(13):1793-1798. doi: 10.1016/j.ejca.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 19.Jennison C, Turnbull BW. Repeated confidence intervals for group sequential clinical trials. Control Clin Trials. 1984;5(1):33-45. doi: 10.1016/0197-2456(84)90148-X [DOI] [PubMed] [Google Scholar]
- 20.Wileyto EP, Li Y, Chen J, Heitjan DF. Assessing the fit of parametric cure models. Biostatistics. 2013;14(2):340-350. doi: 10.1093/biostatistics/kxs043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 22.Liu Y, Ren Z, Yuan L, et al. Paclitaxel plus cisplatin vs. 5-fluorouracil plus cisplatin as first-line treatment for patients with advanced squamous cell esophageal cancer. Am J Cancer Res. 2016;6(10):2345-2350. [PMC free article] [PubMed] [Google Scholar]
- 23.Kato K, Sun JM, Shah MA, et al. . LBA8_PR Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol. 2020;31:S1192-S1193. doi: 10.1016/j.annonc.2020.08.2298 [DOI] [Google Scholar]
- 24.Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506-1517. doi: 10.1016/S1470-2045(19)30626-6 [DOI] [PubMed] [Google Scholar]
- 25.Kojima T, Shah MA, Muro K, et al. ; KEYNOTE-181 Investigators . Randomized Phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138-4148. doi: 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13(1):47. doi: 10.1186/s13045-020-00886-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocols, Amendments, and Statistical Analysis Plan
eFigure 1. The plot of Schoenfeld residuals from stratified Cox proportional regression model for overall survival
eFigure 2. Kaplan-Meier curve of progression-free survival assessed by investigator
eFigure 3. Kaplan-Meier curve of duration of response assessed by investigator
eFigure 4. Forest plot analyses of overall survival in subgroups
eFigure 5. Kaplan-Meier curves of overall survival in patients with baseline PD-L1 <1% or ≥1%.
eFigure 6. Forest plot analyses of progression-free survival assessed by independent review committee in subgroups
eFigure 7. Kaplan-Meier curves of progression-free survival per independent review committee in patients with baseline PD-L1 <1% or ≥1%.
eFigure 8. Kaplan-Meier curves of duration of response per investigator in patients with baseline PD-L1 <1% or ≥1%
eTable 1. List of investigators
eTable 2. Detailed reasons of patients who did not meet inclusion criteria or met exclusion criteria
eTable 3. Summary of post-discontinuation therapy
eTable 4. Tumor response assessed by investigator
eTable 5. Tumor responses per investigator in patients with baseline PD-L1 <1% or ≥1%
eTable 6. Treatment-related serious adverse events
eTable 7. Treatment discontinuation caused by treatment-related adverse events
eTable 8. Deaths caused by treatment-related adverse events
eTable 9. Immune-related adverse events
eTable 10. Baseline values and least squares mean score changes from baseline of the EORTC QLQ-C30
eTable 11. Baseline values and least squares mean score changes from baseline of the EORTC QLQ-OES18
ESCORT-1st Investigators
Data Sharing Statement