Abstract
Background
Strategies to mitigate the impact of COVID‐19 in special populations are complex and challenging. Few studies have addressed the impact of COVID‐19 on pediatric patients with cancer in low‐ and middle‐income countries.
Methods
Multicenter observational cohort study with prospective records and retrospective analyses starting in April 2020 in 21 pediatric oncology centers distributed throughout Brazil. Participants: Patients under 18 years of age who are infected by the SARS‐CoV‐2 virus (confirmed diagnosis through reverse transcriptase‐polymerase chain reaction [RT‐PCR]) while under treatment at pediatric oncology centers. The variables of interest included clinical symptoms, diagnostic and therapeutic measures. The repercussions of SARS‐CoV‐2 infection on cancer treatment and general prognosis were monitored.
Results
One hundred seventy‐nine patients were included (median age 6 [4–13] years, 58% male). Of these, 55.9% had acute leukemia and 34.1% had solid tumors. The presence of SARS‐CoV‐2 was diagnosed by RT‐PCR. Various laboratory markers were analyzed, but showed no correlation with outcome. Children with low or high BMI for age had lower overall survival (71.4% and 82.6%, respectively) than those with age‐appropriate BMI (92.7%) (p = .007). The severity of presentation at diagnosis was significantly associated with outcome (p < .001). Overall mortality in the presence of infection was 12.3% (n = 22).
Conclusion
In children with cancer and COVID‐19, lower BMI was associated with worse prognosis. The mortality in this group of patients (12.3%) was significantly higher than that described in the pediatric population overall (∼1%).
Keywords: BMI, cancer, COVID‐19, pediatric oncology, SARS‐CoV‐2
Abbreviations
- BMI
body mass index
- COVID‐19
coronavirus disease 2019
- HCPA
Hospital de Clínicas de Porto Alegre
- ICU
intensive care unit
- LMIC
low‐ and middle‐income countries
- MIS‐C
multisystem inflammatory syndrome
- OS
overall survival
- RT‐PCR
reverse transcriptase‐polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation.
1. INTRODUCTION
In late 2019, a novel coronavirus was identified by Chinese scientists in Wuhan, China.1 Named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) due to its phylogenetic proximity to the already known causative agent of severe acute respiratory syndrome (SARS) first identified in 2003, the virus spread quickly to other countries. 2 The arrival of coronavirus disease 2019 (COVID‐19) in Brazil in February 2020 represented a great challenge in establishing adequate strategies to mitigate the impact caused by the disease, as the country has continental dimensions and significant regional differences. One of these obstacles is the possibility of compromising access by children and adolescents with cancer to pediatric oncology centers, which is already constrained by the fact that these facilities are almost exclusively located in urban regions such as state capitals and regional economic centers. 3 Although Brazil has one of the world's largest and most complex publicly funded, free‐at‐the‐point‐of‐care universal health system, the Sistema Único de Saúde (SUS), 4 the economic and social challenges faced by large segments of the population can increase the risk of exposure to SARS‐CoV‐2, as well as cause a worse prognosis. 5
Few studies have addressed the impact of COVID‐19 on the pediatric population with cancer, and even fewer have been conducted with children from low‐ and middle‐income countries (LMICs). 6 , 7 It is already known that all age groups are susceptible to SARS‐CoV‐2. 8 In the oncology setting, Liang et al. suggest that adults with cancer have an increased risk of infection with this virus. 9 However, Boulad, de Rojas, and Ferrari and their respective coauthors believe that pediatric cancer patients, although managed as high risk, may be no more vulnerable to infection or morbidity resulting from SARS‐CoV‐2 than are other children. 10 , 11 , 12
Given this scenario, the present study describes the impact of COVID‐19 on this population. Through a nationwide multicenter registry, 13 we identified factors related to worse prognosis in children with cancer, such as body mass index (BMI), age, and initial clinical presentation. In addition, we evaluated the mortality/fatality rate in comparison with the general pediatric population.
2. METHODS
During the 1‐year period (from April 2020 to March 2021), pediatric patients (age 0–18 years) with cancer and infected by the SARS‐CoV‐2 virus seen at pediatric oncology centers were registered. All patients in this study were tested and had their confirmed diagnosis by reverse transcriptase‐polymerase chain reaction (RT‐PCR). Patients with suspected COVID‐19 based on clinical and/or radiological criteria whose molecular diagnostic tests were negative were excluded from the analysis. Children and adolescents who died due to causes unrelated to COVID‐19 were also excluded from the study.
Twenty‐one centers located in all five regions of Brazil registered patients in this study during the period of analysis. Data was recorded prospectively through Redcap software, thus ensuring confidentiality and allowing integration with other participating national institutions. Each center had access only to the information of its unit. Only the coordinating center had access to global data. The records were regularly assessed for compliance by the coordinating center team (Hospital de Clínicas de Porto Alegre (HCPA) ‐ Porto Alegre/RS).
The recorded variables include clinical symptoms, diagnostic method, therapeutic measures, and treatment setting. In addition, the repercussions of the infection on the initial treatment and on general prognosis are evaluated. BMI for age and sex was calculated, and nutritional status (underweight [< −2DP], adequate weight, overweight [> +1 standard deviation, SD, ‐ equivalent to BMI 25 kg/m2 at 19 years] or obesity [> +2 SD ‐ equivalent to BMI 30 kg/m2 at 19 years]) was defined for each child according to the World Health Organization (WHO) classification. 14
The severity of the clinical presentation at diagnosis was based on the parameters established by Qiu et al. 15 Briefly, mild disease is defined as the absence of the need for oxygen therapy; moderate or severe, there is hypoxemia that requires supplemental oxygen; and in critical condition, there is a need for mechanical ventilation and/or the presence of hemodynamic instability.
2.1. Statistical analysis
Continuous variables were assessed with the Shapiro–Wilk test of normality; those with asymmetric distribution were expressed as medians and interquartile ranges (IQR). Qualitative variables were summarized as absolute and relative frequencies. The significance level adopted was .05. Data were compiled in a Microsoft Excel spreadsheet and analyzed in PASW Statistics Version 18.0 and WinPepi version 11.65. Fisher's exact test and Pearson's chi‐square test were used for categorical variables and the Mann–Whitney U test for quantitative variables. Analysis of variance (ANOVA) was used to verify the distribution of data between three or more groups. Log rank tests (Mantel–Cox) were used to compare Kaplan–Meier survival curves between two or more groups.
2.2. Ethical statement and consent to participate
Ethical approval to conduct this study has been granted by the Ethics Committee of HCPA. The legal guardians of all participating minors provided written informed consent in duplicate, keeping one copy of the consent form for themselves.
3. RESULTS
One hundred seventy‐nine pediatric patients were included (Table 1) in 21 pediatric oncology centers, of whom 55.9% had acute leukemia, 8.4% had lymphoma, and 34.1% had solid tumors. Diagnosis of COVID‐19 was confirmed by RT‐PCR for all patients registered in this study. One hundred forty‐five (81%) had undergone some cancer treatment up to 30 days before diagnosis (85.5% chemotherapy, 8.3% surgery, and/or 6.2% radiotherapy).
TABLE 1.
Characteristics of patients
| Variable | N = 179 (%) | ||
|---|---|---|---|
| Age in years, median (IQR) | 6.0 (4–13) | ||
| Sex | Male | 103 (58) | |
| BMI | Underweight | 14 (8) | |
| Normal weight | 110 (62) | ||
| Overweight | 22 (12) | ||
| Obese | 24 (13) | ||
| Not reported | 9 (5) | ||
| Oncological diagnosis | Leukemia/MDS |
AML B T MDS |
22 (12) 69 (38) 8 (4) 1 (1) |
| Lymphoma |
Hodgkin Not Hodgkin Not reported |
4 (2) 10 (6) 1 (1) |
|
| Solid tumor |
Neuroblastoma Wilm's tumor Bone tumors Others |
10 (6) 9 (5) 8 (4) 34 (19) |
|
| Other | 1 (1) | ||
| Not reported | 2 (1) | ||
| Symptoms related to COVID‐19 a |
Absence of symptoms Cough Fever Rhinorrhea Respiratory dysfunction Diarrhea Headache Ageusia and/or anosmia Vomit |
25 (14) 64 (36) 57 (32) 40 (22) 37 (21) 21 (12) 10 (6) 10 (6) 5 (3) |
|
| Severity |
Asymptomatic Mild Moderate/severe Critical |
25 (14) 41 (23) 72 (40) 41 (23) |
|
| Initial oxygen therapy |
Nasal catheter High‐flow oxygen therapy Noninvasive ventilation Immediate invasive ventilation None Not reported |
22 (12) 2 (1) 7 (4) 10 (6) 137 (76) 1 (1) |
|
| n | Median (IQR) | ||
| Laboratory tests ‐ diagnosis |
Lymphocytes (/ml) C‐reactive protein (ng/ml) D‐dimers (mg/L) Ferritin (mcg/L) Fibrinogen (mg/dl) Creatinine Urea GOT |
137 96 33 36 35 121 118 102 |
691 (220–2071) 46 (10–161) 639 (306–1500) 1165 (669–2633) 403 (270–511) 0.4 (0.3–0.6) 21 (14–27) 32 (25–49) |
Some patients had more than one symptom.
Abbreviations: AML, acute myeloid leukemia; BMI, body mass index; COVID‐19, coronavirus disease 2019; GOT, glutamic‐oxalacetic transaminase; IQR, interquartile range; MDS, myelodysplastic syndromes.
Sixty‐six (36.9%) children were asymptomatic or had mild symptoms, 72 (40.2%) had moderate to severe symptoms, and 41 (22.9%) had a critical condition during the study. At the time of diagnosis, 34 (19.0%) were admitted to an intensive care unit (ICU) and 109 (60.9%) to a regular ward, while 35 (19.6%) remained at home; the disposition of one (0.5%) patient was not reported. Among the patients who did not require hospitalization, 19 (54.3%) were asymptomatic or had mild symptoms, eight (22.9%) were treated with antibiotics. The median follow‐up time was 44 (23–78.5) days. No asymptomatic patient or with mild symptoms died.
Type of neoplasm, lymphocyte count, inflammatory markers (ferritin, C‐reactive protein, and fibrinogen), and sex were not associated with severity or mortality. However, nutritional status defined by the BMI characterized three different prognostic groups. Children classified as underweight (< −2 SD) had the worst prognosis, with overall survival (OS) in the presence of infection of 71.4%, followed by children with overweight or obesity (> +1 SD) (OS 82.6%); those who had an adequate BMI had an OS of 92.7% (p = .007; Figure 1).
FIGURE 1.
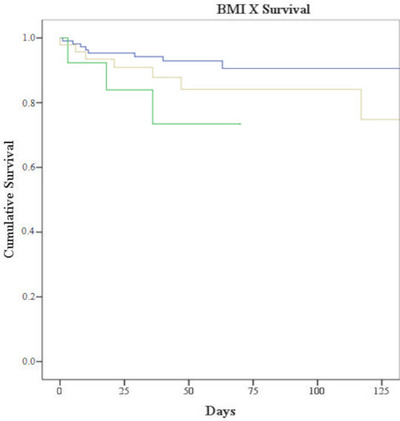
Survival according to bodymass index (BMI). Underweight: green line; Overweight and obesity: yellow line; Adequate BMI: blue line (p = .007)
We also observed that the group of patients whose initial severity was considered critical experienced worse outcomes when compared to the other groups (p < .001; Figure 2).
FIGURE 2.
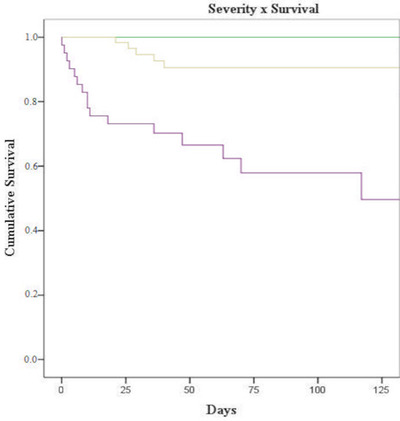
Survival according to clinical severity at diagnosis (p < .001) Assimptomatic or mild symptoms: green line; Moderate to severe symptoms: yellow line; Critical condition: purple line
Information on management of COVID‐19 was available for 122 children. Of these, 40.2% received antivirals (n = 49), 9.0% systemic antifungals, 95.1% antibiotics, and 17.2% corticosteroids. Oseltamivir was the most prescribed antiviral (42.9%). With regard to antibiotics, azithromycin was prescribed to 72 patients (62.1%), followed by cefepime (40.5%), vancomycin (25.9%), piperacillin/tazobactam (19.02%), and amoxicillin/clavulanate (3.4%). Corticosteroids were administered to 21 (17.2%) patients. Heparin (8.2%), intravenous (IV) immunoglobulin (2.5%), and hydroxychloroquine (2.5%) were also prescribed.
Information on the continuity of cancer treatment was available for 178 children. Of these, 65 (36.5%) experienced a delay of treatment (median 16 [10–28] days of delay), mainly in chemotherapy 42 (64.6%).
The overall lethality rate in the presence of infection for this sample was 12.3%, with a total of 22 deaths. The diagnosis of leukemia was presented in 10 (45.5%) of the cases, eight (36.4%) with a solid tumor, two (9.1%) with lymphoma, and two (9.1%) other. The median time to death was 19.5 (6.5–39) days and eight (36.4%) patients had some change in the underlying treatment of the disease due to SARS‐CoV‐2 infection.
4. DISCUSSION
Comparatively little data are available on the pediatric population with COVID‐19 in developing countries. There is even less information about children with cancer infected with SARS‐CoV‐2 in these countries. The present study described the clinical presentation and course of these patients in a middle‐income country, seeking to provide clues about possible risk factors for a less favorable prognosis. In the present national registration study, a high lethality rate was observed in children with cancer and factors such as severity of the condition at the time of presentation as well as nutritional status were associated with the final prognosis.
Combating COVID‐19 represents a major challenge for health authorities. Different protocols have been tested in order to find the best treatment to combat this disease. Thus, the fact that the patients in this study are treated in a heterogeneous manner was expected. Initiatives led by the International Pediatric Oncology Society (SIOP), Child Oncology Group (COG), the St. Jude Global and Childhood Cancer International program, and the Brazilian Society of Pediatric Oncology (Sociedade Brasileira de Oncologia Pediátrica [SOBOPE]) can allow a more uniform management of these patients. 16
Our data indicated a relationship between the severity of COVID‐19 at diagnosis and the likelihood of death in these patients. Children classified as critically ill at diagnosis had a higher mortality rate when compared to groups of children classified as asymptomatic or with mild or moderate/severe symptoms. This finding is unsurprising, as the critical classification is reserved for patients at advanced stages of COVID‐19, requiring ICU admission or mechanical ventilation and, often, with septic conditions.
Regarding biological markers, in a recent meta‐analysis, ElGohary et al. evaluated 22 studies with a total of 1018 adult patients and found that C‐reactive protein, D‐dimer, and prothrombin time were significantly higher in those with cancer. 17 In the pediatric population, a study conducted by Whittaker et al. evaluated 58 children admitted to eight hospitals in England. The objective was to seek clinical and laboratory characteristics of critically ill patients who developed a multisystem inflammatory syndrome (MIS‐C) during the COVID‐19 pandemic, and compare these data with other pediatric inflammatory diseases. The authors suggested that MIS‐C differs from other known pediatric inflammatory entities. 18 To date, we are not aware of a study that sought to describe inflammatory markers in children with cancer who developed COVID‐19. In our sample, there was no correlation between the evaluated markers and severity or prognosis.
Data on prognosis and outcome of pediatric cancer patients infected with SARS‐CoV‐2 remain scarce. A study carried out with 523 adult patients with COVID‐19 in four hospitals in Wuhan established an association of low BMI and protein levels with higher risk of death. In that study, patients with BMI under 20.5 kg/m2 were more prevalent among ICU nonsurvivors than those with a normal BMI. 19 In addition, obesity and overweight were described as risk factors in a meta‐analysis by Hussain et al. In 14 studies selected for quantitative analysis, BMI >25 was directly associated with higher mortality, as were factors such as advanced age (>70 years), severe comorbidities, and requiring advanced respiratory support. 20 Some studies in Brazil have addressed the impact of nutritional status on the survival of acute leukemias and found that low weight for age at diagnosis is associated with a worse prognosis due to a higher rate of recurrences. 21 In a cohort that analyzed obesity in lymphoblastic leukemia, the 5‐year event‐free survival in patients was significantly lower than that of the non‐overweight/obesity patients. 22 In this context, we observed that pediatric cancer patients with a BMI below the value considered appropriate for their age were also significantly more likely to die acutely when infected with SARS‐CoV‐2. Likewise, the group of patients classified as overweight/obese had a worse prognosis than patients with adequate nutritional status. Considering these analyses, our data suggest that the nutritional situation may have an impact on the clinical response and prognosis related to SARS‐CoV‐2 acute infection.
It can be observed that 49 (40.2%) patients of our sample (n = 122) received antiviral treatment, especially oseltamivir. Due to lack of evidence regarding effective treatments for COVID‐19 in the first few months of pandemic, it is believed that some treatment centers appealed to these antivirals in off‐label use. To the present day, oseltamivir is not recommended against SARS‐CoV‐2 infection. 23 Also, evidences of proven benefit from the use of corticosteroids was not clear until June 2020, when the preliminary data from the RECOVERY Trial was published. 24
Our study has some limitations, mostly inherent to the conduct of a multicenter registry in a country of continental dimensions with major regional differences. As the questionnaire was completed by local teams at each facility rather than centrally, some data required careful monitoring to ensure uniformity and consistency; despite our best efforts, some data could not be obtained for all patients. Another important point refers to the short follow‐up period. We were able to identify acute repercussions such as hospitalizations and survival. Only through continuous monitoring of these patients will we be able to understand the long‐term repercussions of infection in these children. In addition, it is possible that not all children diagnosed with COVID‐19 in the period of interest entered the register or there was a variation in systematic screening between institutions, causing a selection bias and, consequently, leading to a higher proportion of most serious patients. It is also important to mention that due to the small number of events, results of a multivariable analysis could be biased and therefore has not been performed. 25 , 26
Despite these, a very striking result is the case fatality rate in our sample. Unfortunately, it is similar to what was previously described by Montoya et al., who observed 10% of deaths in a pediatric cancer population with COVID‐19 (ages ranging from 0 to 16) from six tertiary centers in Peru, 6 but very different from what was described in other pediatric studies. In a nationwide register‐based study conducted in Brazil, it was found a rate of 0.7% of the deaths related to COVID‐19 in the healthy pediatric population. 27 In a systematic review that evaluated 131 studies across 26 countries, the case fatality rate was less than 1%. 8 It shows that despite socioeconomic differences between high‐income and low‐income countries, the mortality rate of COVID‐19 in healthy children is similar and very lower than those with cancer. In this context, the reasons why children with cancer in LMICs are at greater risk of developing severe forms of COVID‐19 and progressing to death remain to be clarified. It is possible that immunosuppression associated with treatment or underlying disease plays a role, but the relationship between survival and BMI found in our study suggests that social issues leading to a more precarious nutritional status may be important in the origin of this result. With the continuity of the record, we hope to be able to better understand how variables related to the socioeconomic context can interfere in the prognosis, as well as to determine the long‐term impact of SARS‐CoV‐2 infection in these children.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
The authors thank the investigators from all participating centers across the country, as well as the Brazilian Society of Pediatric Oncology (Sociedade Brasileira de Oncologia Pediátrica), the Brazilian Association of Hematology and Hemotherapy (ABHH), and the Brazilian Society of Bone Marrow Transplantation (Sociedade Brasileira de Transplante de Medula Óssea) for support. The authors listed below have actively contributed through data collection: Sandra Emília Almeida Prazeres (Hospital Infantil Albert Sabin, Fortaleza, Brazil); Maristella Bergamo Francisco dos Reis (Hospital das Clínicas de Ribeirão Preto, Ribeirão Preto, Brazil); Carmem Maria Costa Mendonça Fiori (Hospital do Câncer de Cascavel ‐ UOPECCAN, Cascavel, Brazil); Marcelo Otsuka (Hospital Infantil Darcy Vargas, São Paulo, Brazil); Mara Albonei Dudeque Pianovski (Hospital Erasto Gaertner, Curitiba, Brazil); Tatiana El Jaick Bonifacio Costa (Hospital Infantil Joana de Gusmão, Florianópolis, Brazil); Maria do Perpétuo Socorro Sampaio Carvalho (Fundação Hospitalar de Hematologia e Hemoterapia do Amazonas, Manaus, Brazil); Larissa Bueno Polis Moreira (Hospital Amaral Carvalho, Jaú, Brazil).
Corso MCM, Soares VJ, Amorim AMP, et al. SARS‐CoV‐2 in children with cancer in Brazil: Results of a multicenter national registry. Pediatr Blood Cancer. 2021;68:e29223. 10.1002/pbc.29223.
Contributor Information
Mariana Cristina M. Corso, Email: marianamcorso@gmail.com.
Victor J. Soares, Email: victorjablonski@hotmail.com.
Anna Maria P. Amorim, Email: annamprado17@hotmail.com
Rosana Cipolotti, Email: rosanaci@yahoo.com.
Isis Maria Q. Magalhães, Email: isisqmagalhaes@gmail.com
Mecneide M. Lins, Email: mecneide.mendes@imip.org.br.
Luciana N. Silva, Email: lucianansilva@yahoo.com.br
Ana Virginia L. de Sousa, Email: analopes@graacc.org.br
Seila I. do Prado, Email: seilaprado@hotmail.com
Pablo Santiago, Email: pablosantiago@terra.com.br.
Rebeca F. Marques, Email: rfmarques@hcpa.edu.br
Ciliana Rechenmacher, Email: crechenmacher@hcpa.edu.br.
Mariana B. Michalowski, Email: mmichalowski@hcpa.edu.br.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to information that could compromise the privacy of research participants.
REFERENCES
- 1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5(4):536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grabois MF, de Oliveira EXG, Sa Carvalho M. Assistencia ao cancer entre criancas e adolescentes: mapeamento dos fluxos origem‐destino no Brasil. Rev Saude Publica. 2013;47(2):368‐378. [DOI] [PubMed] [Google Scholar]
- 4. Castro MC, Massuda A, Almeida G, et al. Brazil's unified health system: the first 30 years and prospects for the future. Lancet. 2019;394(10195):345‐356. [DOI] [PubMed] [Google Scholar]
- 5. Demenech LM, de Dumith SC, Vieira MECD, Neiva‐Silva L. Desigualdade econômica e risco de infecção e morte por COVID‐19 no Brasil. Rev Bras Epidemiol. 2020;23. 10.1590/1980-549720200095 [DOI] [PubMed] [Google Scholar]
- 6. Montoya J, Ugaz C, Alarcon S, et al. COVID‐19 in pediatric cancer patients in a resource‐limited setting: national data from Peru. Pediatr Blood Cancer. 2021;68(2):e28610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vasquez L, Sampor C, Villanueva G, et al. Early impact of the COVID‐19 pandemic on paediatric cancer care in Latin America. Lancet Oncol. 2020;21(6):753‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoang A, Chorath K, Moreira A, et al. COVID‐19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boulad F, Kamboj M, Bouvier N, Mauguen A, Kung AL. COVID‐19 in children with cancer in New York City. JAMA Oncol. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Rojas T, Pérez‐Martínez A, Cela E, et al. COVID‐19 infection in children and adolescents with cancer in Madrid. Pediatr Blood Cancer. 2020;67(7):e28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrari A, Zecca M, Rizzari C, et al. Children with cancer in the time of COVID‐19: an 8‐week report from the six pediatric onco‐hematology centers in Lombardia, Italy. Pediatr Blood Cancer. 2020;67(8):e28410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corso M, Rechenmacher C, Jablonski V, Marques R, Daudt L, Michalowski M. RECOV‐Brasil: COVID‐19 in Children Undergoing Cancer Treatment or HSCT in Brazil . 2020. 10.22541/au.159415243.34937767 [DOI]
- 14. WHO Child Growth Standards. Geneva: World Health Organization; 2006. [Google Scholar]
- 15. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sullivan M, Bouffet E, Rodriguez‐Galindo C, et al. The COVID‐19 pandemic: a rapid global response for children with cancer from SIOP, COG, SIOP‐E, SIOP‐PODC, IPSO, PROS, CCI, and St Jude Global. Pediatr Blood Cancer. 2020;67(7):e28409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ElGohary GM, Hashmi S, Styczynski J, et al. The risk and prognosis of COVID‐19 infection in cancer patients: a systematic review and meta‐analysis. Hematol Oncol Stem Cell Ther. 2020. 10.1016/j.hemonc.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324(3):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li G, Zhou C, Ba Y, et al. Nutritional risk and therapy for severe and critical COVID‐19 patients: a multicenter retrospective observational study. Clin Nutr. 2021;40(4):2154‐2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hussain A, Mahawar K, Xia Z, Yang W, EL‐Hasani S. Obesity and mortality of COVID‐19. Meta‐analysis. Obes Res Clin Pract. 2020;14(4):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Viana MB, Murao M, Ramos G, et al. Malnutrition as a prognostic factor in lymphoblastic leukaemia: a multivariate analysis. Arch Dis Child. 1994;71(4):304‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gelelete CB, Pereira SH, Azevedo AMB, et al. Overweight as a prognostic factor in children with acute lymphoblastic leukemia. Obesity. 2011;19(9):1908‐1911. [DOI] [PubMed] [Google Scholar]
- 23. Falavigna M, Colpani V, Stein C, et al. Guidelines for the pharmacological treatment of COVID‐19. The task force/consensus guideline of the Brazilian Association of Intensive Care Medicine, the Brazilian Society of Infectious Diseases and the Brazilian Society of Pulmonology and Tisiology. Rev Bras Ter Intensiva. 2020;32(2):166‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horby P, Lim WS, Emberson J, et al. Dexamethasone in hospitalized patients with COVID‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Concato J, Peduzzi P, Holford TR, Feinstein AR. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol. 1995;48(12):1495‐1501. [DOI] [PubMed] [Google Scholar]
- 26. Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis II. J Clin Epidemiol. 1995;48(12):1503‐1510. [DOI] [PubMed] [Google Scholar]
- 27. Martins‐Filho PR, Quintans‐Júnior LJ, de Souza Araújo AA, et al. Socio‐economic inequalities and COVID‐19 incidence and mortality in Brazilian children: a nationwide register‐based study. Public Health. 2021;190:4‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to information that could compromise the privacy of research participants.


