Abstract
Cochlear implants containing iridium platinum electrodes are used to transmit electrical signals into the inner ear of patients suffering from severe or profound deafness without valuable benefit from conventional hearing aids. However, their placement is invasive and can cause trauma as well as local inflammation, harming remaining hair cells or other inner ear cells. As foreign bodies, the implants also induce fibrosis, resulting in a less efficient conduction of the electrical signals and, thus, potentially decreased system performance. To overcome these obstacles, dexamethasone has recently been embedded in this type of implants: into the silicone matrices separating the metal electrodes (to avoid short circuits). It has been shown that the resulting drug release can be controlled over several years. Importantly, the dexamethasone does not only act against the immediate consequences of trauma, inflammation and fibrosis, it can also be expected to be beneficial for remaining hair cells in the long term. However, the reported amounts of drug released at “early” time points (during the first days/weeks) are relatively low and the in vivo efficacy in animal models was reported to be non-optimal. The aim of this study was to increase the initial “burst release” from the implants, adding a freely water-soluble salt of a phosphate ester of dexamethasone. The idea was to facilitate water penetration into the highly hydrophobic system and, thus, to promote drug dissolution and diffusion. This approach was efficient: Adding up to 10% dexamethasone sodium phosphate to the silicone matrices substantially increased the resulting drug release rate at early time points. This can be expected to improve drug action and implant functionality. But at elevated dexamethasone sodium phosphate loadings device swelling became important. Since the cochlea is a tiny and sensitive organ, a potential increase in implant dimensions over time must be limited. Hence, a balance has to be found between drug release and implant swelling.
Keywords: Cochlear implant, Dexamethasone, Dexamethasone phosphate, Burst release, Silicone
Graphical abstract
1. Introduction
The treatment of diseases and disorders of the inner ear (cochlea) is highly challenging, because of the blood-cochlea-barrier (Juhn, 1988; El Kechai et al., 2015a; Dai et al., 2018; Chin and Diaz, 2019). The latter is similar to the blood-brain-barrier and effectively protects this tiny and sensitive organ (Swan et al., 2008): Upon administration using the classical routes (e.g., oral, i.v. or i.m.), only very minor drug amounts reach the target site (Szeto et al., 2020). This leads to the failure of the treatment, even though the drug would be highly efficient if it was able to reach its target site. Increasing the amount of drug reaching the inner ear by elevating the administered drug dose is generally not possible, because of toxic side effects caused by high drug concentrations in the rest of the human body.
To overcome these obstacles, different types of local drug delivery systems have been proposed for the treatment of ear ailments (Takumi et al., 2014; Aksit et al., 2020; Kita et al., 2020; Jaudoin et al., 2021; Lehner et al., 2019, Lehner et al., 2021). Generally, they are administered intratympanically (Borden et al., 2011; Borkholder et al., 2014; Plontke et al., 2014; El Kechai et al., 2015b, El Kechai et al., 2016; Liebau et al., 2018) or intracochlearly (Salt and Plontke, 2005, Salt and Plontke, 2009; Farahmand Ghavi et al., 2010; Douchement et al., 2015; Liu et al., 2015, Liu et al., 2016; Astolfi et al., 2016). In the first case, the system is administered into the middle ear and once the drug is released, it diffuses through the round window into the perilymph (the main liquid in the cochlea) (Engleder et al., 2014). In the case of intracochlear systems, the drug is directly administered into the inner ear (Takemura et al., 2004; Farhadi et al., 2013; Bas et al., 2016; Plontke et al., 2017; Maeder et al., 2018; Hao and Li, 2019). The advantage of intratympanic approaches is that the administration is less invasive. However, the residence time of gels, liquids or particles in the middle ear is uncertain, since more or less liquid can be present at this site. This results in unreliable drug exposure. Also, drug transfer through the round window might be hindered and limit the efficacy of the treatment. Since the placement of drug delivery systems directly into the inner ear is invasive and can cause trauma and inflammation, drug release from this type of devices should be controlled over very long time periods: Ideally, one single administration is sufficient for the life time of the patient. A kind of compromise between “invasiveness of administration” and “reliability of drug exposure” are offered by so-called “Ear Cubes” (Sircoglou et al., 2015; Gehrke et al., 2016, Gehrke et al., 2019): Miniaturized implants, which are placed into a tiny hole drilled through the round or oval window. The largest part of the implant (“cuboid”, containing most of the drug) is located in the middle ear (and acts as a drug reservoir), whereas a very small cylindrical extension is placed into the tiny hole, assuring fixation and direct access to the perilymph.
In clinical practice, miniaturized implants containing metal electrode contacts are placed into the inner ear of patients suffering from severe hearing loss. The electrode contacts conduct electrical signals to stimulate the spiral ganglion residual population (first relay of the cochlear nerve). Several electrode contacts are placed into the inner ear, corresponding to different acoustic frequencies and according to the cochlear tonotopy. To avoid short circuits between these electrodes, they are separated by a polymeric matrix, for instance based on silicone. It has previously been proposed to incorporate dexamethasone into these polymeric matrices (Krenzlin et al., 2012; Douchement et al., 2015) and provide local long term controlled drug release in order to: (i) limit potential damage resulting from the trauma and inflammation caused by the placement of the implant into the inner ear, and (ii) limit the formation of fibrotic tissue around the systems (which are recognized as foreign bodies) (Jia et al., 2016; Wilk et al., 2016). The fibrotic tissue reduces the efficacy of the transmittance of the electrical signals and, thus, may decrease system performance. Please note that the strategy to locally release dexamethasone during prolonged periods of time to maintain the efficacy of electrical signal transmittance from electrodes implanted into the human body, has also proven to be successful for a different type of application: It has been shown that dexamethasone releasing silicone rings located in the vicinity of pacemaker electrodes keep the simulation threshold values low for many years upon implantation (Mond and Stokes, 1996). The authors concluded that only small quantities of the steroid are needed for this effect, which was observed to be clinically relevant for at least 10 years. Dexamethasone release from the above mentioned intracochlear implants was shown to be slow and likely controlled over several years in vitro (Krenzlin et al., 2012). Furthermore, the in vivo efficacy of these systems seems to be promising, although non-optimal. For example, it was reported that 4–6 weeks post-implantation, the residual hearing of gerbils treated with dexamethasone-loaded intracochlear implants was more preserved compared to placebo implants (Douchement et al., 2015). However, these effects were non-optimal, which might in part be attributable to the slow drug release rate at “early” time points. During the first days/weeks, higher dexamethasone concentrations are likely needed to assure a more pronounced effect against the consequences of trauma, inflammation and fibrosis.
The aim of this study was to increase the release rate of dexamethasone from silicone based intracochlear implants during this initial “burst release” phase. Please note that in this context (when a total release period of many years, eventually a patient's life time, is targeted), the term “burst release” refers to a period of “several days/weeks”. The basic strategy was to incorporate also a prodrug of dexamethasone into the polymeric matrix, which exhibits a higher affinity to water: Dexamethasone sodium phosphate (in the following abbreviated “dexamethasone phosphate” or “Dex-P"). The latter is a sodium salt of a phosphate ester of dexamethasone (Cazares-Delgadillo et al., 2016). The rationale was the following: The reported very low release rates of dexamethasone from silicone-based intracochlear implants can in great part be attributed to the limited amounts of water penetrating into the highly hydrophobic systems, combined with the low water-solubility of dexamethasone. Importantly, only dissolved drug can diffuse. Non-dissolved drug particles remain effectively trapped within the polymeric matrix. Since dexamethasone phosphate is much more hydrophilic than the parent drug dexamethasone, it is expected that the water penetration into the implants can thus be enhanced, facilitating drug dissolution and subsequent drug diffusion out of the device. The hydrolysis of the phosphate ester, generating the drug dexamethasone can occur within the implant or after its release. Since miniaturized inner ear implants are not straightforward to prepare and characterize, macroscopic films of identical composition as the polymer matrices separating the metal electrodes were studied as surrogates. Based on the obtained results, selected formulations were used to prepare also intracochlear implants. Optical and scanning electron microscopy, X-ray diffraction, in vitro drug release measurements, drug stability studies, and gravimetric analysis were applied to characterize the films and miniaturized implants before and after exposure to artificial perilymph at 37 °C.
2. Materials and methods
2.1. Materials
Kits for the preparation of silicone elastomers (MED-4735; NuSil Technology, Carpinteria, USA); dexamethasone and dexamethasone sodium phosphate (dexamethasone phosphate) (Discovery Fine Chemicals, Dorset, UK); calcium chloride dihydrate, magnesium sulfate tetrahydrate, potassium chloride, sodium chloride and 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES) (HEPES Pufferan; Carl Roth, Lauterbourg, France); acetonitrile (HPLC grade; Fisher Scientific, Illkirch, France); MilliQ water (obtained with a Millipore Integral 5 apparatus; Millipore Corporation, Billerica, USA).
The dexamethasone sodium phosphate powder was used as received, or (if indicated) milled as follows: 1 g drug was milled in a stainless-steel jar with a stainless-steel ball for 3 min at 30 Hz (Retsch MM400; Retsch, Haan, Germany).
2.2. Preparation of drug loaded films
Equal amounts of MED-4735 Parts A and B (approximately 5 g each) were passed separately 10 times through a two-roll mill (Chef Premier KMC 560/AT970A; Kenwood, Havant, UK). To initiate polymer crosslinking, both parts were manually blended and the mixture was passed 10 times through the mill. Subsequently, appropriate amounts of dexamethasone powder (as received) and/or dexamethasone sodium phosphate powder (as received or milled) were added and the mixture was passed another 40 times through the mill to obtain a homogenous film. Crosslinking was completed by a thermal treatment in an oven at 60 °C for 24 h. The thickness of the resulting films was determined with a micrometer gauge (Digimatic Micrometer; Mitutoyo, Tokyo, Japan).
2.3. Preparation of drug loaded cochlear implants
Blends of MED-4735 Parts A & B and dexamethasone/dexamethasone sodium phosphate were prepared as described in Section 2.2. Preparation of drug loaded films. The obtained mass was injected into a stainless-steel mold, containing glued iridium platinum electrode contacts with wires (Oticon Medical, Vallauris, France). The implant dimensions were suitable for use in humans (Krenzlin et al.). The mold was placed under a hydraulic press at 4.5 bars and heated to 110 °C for 10 min. Ethanol (96% v/v) was injected into the mold in order to dissolve the glue and allow for implant removal.
2.4. Scanning Electron Microscopy (SEM)
SEM pictures of drug powders were obtained with a JEOL Field Emission Scanning Electron Microscope (JSM-7800F, Tokyo, Japan). Samples were fixed with a ribbon carbon double-sided adhesive and covered with a fine chrome layer.
2.5. X-ray diffraction
A Panalytical X'pert Pro diffractometer (PANalytical, Almelo, Netherlands) in transmission mode with an incident beam parabolic mirror (λ Cu, Kα = 1.54 Å) was used to record X-ray diffraction patterns. The samples were placed inside Lindemann glass capillaries (diameter 1 mm; Hilgenberg, Malsfeld, Germany), which were fixed on a spinning sample holder.
2.6. Drug release measurements
2.6.1. From thin films
Film pieces (1 × 1 cm) were placed into amber glass flasks containing 10 mL (if not otherwise stated) artificial perilymph: an aqueous solution of 1.2 mmol calcium chloride dihydrate, 2 mmol magnesium sulfate tetrahydrate, 2.7 mmol potassium chloride, 145 mmol sodium chloride and 5 mmol HEPES Pufferan. The flasks were horizontally shaken in an incubator (80 rpm; GFL 3033; Gesellschaft fuer Labortechnik, Burgwedel, Germany) at 37 °C. At predetermined time points, 1 mL samples were withdrawn and replaced with fresh artificial perilymph (unless otherwise stated). The drug concentration in the withdrawn samples was determined by HPLC analysis using an Alliance e2695 apparatus (Waters Division, Milford, USA), equipped with an UV detector. Samples (50 μL) were injected into a reverse phase column C18 (Gemini 3 μm, 110 Å, 100 × 4.6 mm, Phenomenex, Le Pecq, France) (mobile phase = acetonitrile:water 33:67 V:V, flow rate = 1.2 mL/min). Dexamethasone and dexamethasone sodium phosphate were detected at λ = 220 nm. If indicated, the release medium was completely renewed every day or every week.
2.6.2. From cochlear implants
Implants were placed into 2 mL HPLC glass vials (Screw-top amber glass; Sigma Aldrich, St. Quentin Fallavier, France) containing 0.2 mL inserts and 70 μL artificial perilymph. The vials were horizontally shaken at 80 rpm (37 °C, GFL 3033). At predetermined time points, the release medium was completely renewed. The drug content in the samples was determined as described for the thin films.
Each experiment was performed in triplicate, mean values +/− standard deviations are reported.
2.7. Monitoring of system swelling
Thin films and cochlear implants were treated as for the in vitro drug release measurements described in Section 2.6. Drug release measurements.
2.7.1. Thin films
Dynamic changes in the thickness and wet mass of the films upon exposure to artificial perilymph were measured using a micrometer gauge and a precision balance (Precisa 120A; Precisa, Dietikon, Switzerland). Measurements were performed before and after exposure to the release medium. At predetermined time points, film samples were withdrawn, surface water was carefully removed using Kimtech tissue paper (Kimberly-Clark, Reigate, UK), and the films' thickness and wet mass were determined.
2.7.2. Cochlear implants
Dynamic changes in the dimensions of the cochlear implants upon exposure to artificial perilymph were monitored using a Nikon Eclipse SMZ-U microscope, equipped with an AxioCam ICc 1 Zeiss camera (Zeiss, Oberkochen, Germany). At predetermined time points, samples were withdrawn and analyzed.
2.8. Drug stability in aqueous media
About 5 mg dexamethasone or dexamethasone sodium phosphate (as indicated) were dissolved in 100 mL MilliQ water, artificial perilymph, or aqueous solutions of either 1.2 mmol calcium chloride, 2 mmol magnesium sulfate tetrahydrate, 2.7 mmol potassium chloride, 145 mmol sodium chloride or 5 mmol HEPES. The samples were placed in a horizontal shaker at 37 °C (80 rpm; GFL 3033). At predetermined time points, 100 μL samples were withdrawn and their drug content was determined by HPLC-UV analysis, as described in Section 2.6. Drug release measurements.
3. Results and discussion
The aim of this study was to increase the initial “burst release” (drug release during the first days/weeks) from silicone-based cochlear implants allowing for controlled dexamethasone release for several years. The strategy was to add freely water-soluble dexamethasone sodium phosphate (“dexamethasone phosphate”), rendering the systems more hydrophilic and, thus, facilitating water penetration into the polymeric matrices. Increased water contents can be expected to accelerate drug dissolution and diffusion out of the implants. The chemical structures of dexamethasone and dexamethasone phosphate are illustrated at the top of Fig. 1. At the bottom, SEM pictures of the drug powder raw materials (as received) are shown.
Fig. 1.
Chemical structures of dexamethasone and dexamethasone phosphate (source: PubChem [Internet], 2004a, PubChem [Internet], 2004b), and SEM pictures of the drug powders (as received).
Since the manufacturing and characterization of miniaturized cochlear implants is not straightforward, experiments were also conducted with macroscopic films of identical composition as the polymeric matrices separating the metal electrodes (and controlling drug release).
3.1. Thin polymeric films
The optical macroscopy picture at the top of Fig. 2 shows a thin silicone film loaded with 1% w/w dexamethasone phosphate, which was prepared with drug powder as received. As it can be seen, large white drug agglomerates are visible (the pure silicone matrix being transparent). This can be explained by the hydrophilic character of the dexamethasone phosphate and the hydrophobic nature of the polymer: The 2 phases “do not like each other” and drug particle agglomeration reduces the surface of the interface. In the case of the less hydrophilic parent drug dexamethasone, it has previously been reported that the drug was homogeneously distributed within the same silicone matrix, in the form of tiny crystals using a similar preparation procedure (Krenzlin et al., 2012) (dexamethasone being less hydrophilic). In order to provide a more homogenous drug particle distribution within the polymeric system also for dexamethasone phosphate, the latter was milled for different time periods prior to incorporation into the silicone matrix. The idea was to start with smaller particles, eventually allowing to end up with smaller agglomerates. Optical macroscopy pictures of the obtained films are shown in the middle and at the bottom of Fig. 2. Clearly, the formation of large drug agglomerates could be substantially reduced by the milling step: With increasing milling time, the number and size of visible agglomerates decreased. Since milling can induce changes in the solid state of a drug (e.g., one polymorphic form might be transformed into another, or a crystalline drug might become amorphous), X-ray diffraction patterns of the milled and non-milled powders were recorded. As it can be seen in Fig. 3, no differences were visible: The drug remained crystalline, and kept its polymorphic form (Oliveira et al., 2018).
Fig. 2.
Optical macroscopy pictures of silicone films loaded with 1% w/w dexamethasone phosphate. The drug powder was optionally milled for different time periods before incorporation into the silicone (as indicated).
Fig. 3.

X-ray diffraction patterns of dexamethasone phosphate powder: as received and after 3 min milling.
The effects of drug milling for up to 3 min on the resulting drug release kinetics from thin silicone films loaded with 1% dexamethasone phosphate are shown in Fig. 4. Sink conditions were provided throughout the experiments. As it can be seen, in the case of non-milled drug powder: (i) the burst release was much more pronounced, and (ii) the standard deviations were much higher. This can be explained by the fact that the initial burst release is likely attributable to drug particles (and agglomerates of thereof), which are located close to the film surface and have either direct access to the latter from the beginning, or have/get rapidly access via small pores. Looking at the optical macroscopy pictures in Fig. 2, it becomes clear that in the case of non-milled dexamethasone phosphate powder, the access of a large particle agglomerate to the surface likely causes a relative important amount of drug to be released at early time points, compared to a much smaller drug particle having/getting such an access in the case of films prepared with pre-milled powder. Also, the likelihood that a large drug particle agglomerate has/gets direct surface access is higher than in the case of a tiny drug particle, if both are located in the same zone close to the surface (due to its larger dimensions). This overcompensates the higher number of numerous small particles compared to few larger particle agglomerates. The difference in the spatial drug distribution within films prepared with non-milled versus milled dexamethasone phosphate powder (Fig. 2) can also explain the difference in the variability of drug release (Fig. 4): The observed drug release rate is the sum of all the individual release events stemming from the dissolution of drug particles or agglomerates which are/get in contact with the surface (and hence, with the bulk fluid). In the case of large drug particle agglomerates, each of these individual events is relatively important. In contrast, in the case of numerous tiny drug particles, each individual “release event” is relatively less important. Since all these events are random, the variability of the sum is higher in the case of fewer events related to the large agglomerates compared to numerous small events associated with tiny drug particles. In practice, a reduced variability is highly desirable to assure more reliable therapeutic effects and a limited risk of potential toxic side effects. Thus, a milling time of 3 min was chosen for further experiments.
Fig. 4.

Cumulative absolute total drug release from silicone films loaded with 1% dexamethasone phosphate upon exposure to artificial perilymph at 37 °C. The drug powder was optionally milled before incorporation into the silicone (as indicated). Mean values +/− standard deviation are reported (n = 3).
3.2. Conversion of dexamethasone phosphate into dexamethasone
Please note that the absolute cumulative drug release curves shown in Fig. 4 encompass both: the prodrug dexamethasone phosphate as well as the parent drug dexamethasone (generated by the hydrolysis of dexamethasone phosphate upon contact with water). Both species were detected in the release medium (by HPLC-UV analysis) and considered for the calculation of the “total drug release”. To estimate the conversion rate of dexamethasone phosphate into dexamethasone under the given conditions, a solution of this drug in artificial perilymph was studied at 37 °C under horizontal shaking (80 rpm) (under the same conditions as for the in vitro drug release measurements). For reasons of comparison, the stability of dexamethasone phosphate was also monitored in pure water (MilliQ) and aqueous solutions of the different components of the artificial perilymph: 145 mmol sodium chloride, 1.2 mmol calcium chloride, 5 mmol HEPES, 2 mmol magnesium sulfate tetrahydrate, or 2.7 mmol potassium chloride. Fig. 5 shows the obtained results. About 5 mg drug were dissolved in 100 mL medium. The blue curve in the diagram at the top shows the degradation kinetics of dexamethasone phosphate in artificial perilymph. Clearly, the ester is relatively rapidly hydrolyzed. The brown curve in the same diagram shows the respective dexamethasone phosphate degradation in pure water: As it can be seen, in the latter case the ester hydrolysis is much slower. Thus, the presence of the co-dissolved salts in the artificial perilymph has an impact on the hydrolysis of the phosphate ester. The diagram in the middle of Fig. 5 differentiates between the impact of the different types of salts. The following rank order was observed with respect to the acceleration of dexamethasone phosphate degradation: NaCl < CaCl2 < HEPES < MgSO4 < KCl. For reasons of comparison, also the stability of the parent drug dexamethasone dissolved in artificial perilymph at 37 °C was studied (bottom diagram in Fig. 5): There were no signs for any noteworthy degradation during the observation period (1 month).
Fig. 5.
Stability of dexamethasone phosphate and dexamethasone in different aqueous media (as indicated) at 37 °C (n = 1).
Please note that in the case of controlled release silicone films or cochlear implants, the conversion of dexamethasone phosphate into dexamethasone can occur within the well-agitated bulk fluid (after the release of the prodrug), or within the drug delivery system (once water has reached the drug). It was beyond the scope of this work to study this aspect in more detail. For the therapeutic effects, most important is the conversion rate, not the location of this conversion.
Fig. 6 shows the HPLC chromatograms of samples of release medium withdrawn after 2 h or 11 days exposure of thin silicone films loaded with 11% dexamethasone phosphate to artificial perilymph at 37 °C (80 rpm). As it can be seen, after 2 h, only dexamethasone phosphate was detected, no dexamethasone. In contrast, after 11 d, a dexamethasone peak was clearly visible in addition to the dexamethasone phosphate peak. Please note that: (i) The dexamethasone phosphate peak was much larger than the dexamethasone peak after 11 d in the HPLC chromatogram, which was obtained during the drug release measurements from thin silicone films (Fig. 6). (ii) In contrast, most of the dexamethasone phosphate was degraded after 11 d when dissolved from the beginning in artificial perilymph at 37 °C (blue curve in the diagram at the top of Fig. 5). This indicates that the entrapment of the dexamethasone phosphate particles in the silicone matrix protects the drug from hydrolysis (avoiding the contact with water). However, this is not necessarily a 100% protection, since upon water penetration into the system, dexamethasone phosphate can be expected to be also hydrolyzed within the drug delivery system, prior to its release.
Fig. 6.
HPLC chromatograms of samples of release medium withdrawn after 2 h and 11 d during a drug release measurement: Silicone films loaded with 11% dexamethasone phosphate were exposed to artificial perilymph at 37 °C.
The left diagram in Fig. 7 shows the concentrations of the prodrug dexamethasone phosphate detected in samples, which were withdrawn from the release medium at different time points upon exposure of a silicone film loaded with 11% dexamethasone phosphate to artificial perilymph at 37 °C. The volume of the well agitated bulk fluid was 10 mL. At each sampling time point, 1 mL bulk fluid was withdrawn and replaced with 1 mL fresh medium. Thus, the observed decrease in the concentration of released dexamethasone phosphate can in part be attributed to a dilution effect. However, the observed decrease is much more pronounced than this dilution effect, indicating that dexamethasone phosphate conversion into dexamethasone in the release medium plays a major role, as expected from the discussion above. For reasons of comparison, the diagram on the right-hand side of Fig. 7 shows the dexamethasone concentrations measured in samples withdrawn from the release medium in the case of thin silicone films loaded with 11% dexamethasone. As it can be seen, in this case, the drug concentration monotonically increased with time, because the accumulation of drug in the bulk fluid due to its continuous release from the film was more important than the dilution effect due to sampling & medium replacement (and the drug was stable under the given conditions, bottom diagram in Fig. 5).
Fig. 7.
Drug concentrations in samples withdrawn at different time points from the release medium upon exposure of silicone films loaded with 11% dexamethasone phosphate (3 min milling) or 11% dexamethasone upon exposure to artificial perilymph at 37 °C. The green bars are based on the detected dexamethasone phosphate only (not taking into account dexamethasone generated by hydrolysis). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Impact of the relative drug loadings
The basic idea of this study was to increase the dexamethasone release rate during the initial “burst phase” from silicone-based delivery systems by the addition of the more hydrophilic dexamethasone phosphate ester. In order to evaluate whether this strategy is successful, a series of thin silicone films was prepared, loaded with 11% total drug content, varying the concentrations of dexamethasone and dexamethasone phosphate as follows: 1 + 10, 2 + 9, 3 + 8, 4 + 7, 5 + 6, 6 + 5, 7 + 4, 8 + 3, 9 + 2 and 10 + 1%. Fig. 8 shows optical macroscopy pictures of the different films. The dexamethasone phosphate powder was milled for 3 min prior to incorporation into the silicone matrix to minimize the formation of drug particle agglomerates. Nevertheless, an increasing number of white agglomerates was visible with increasing dexamethasone phosphate contents. This can be attributed to the higher hydrophilicity of this drug compared to dexamethasone and the hydrophobic nature of the silicone, as discussed above. Please note that the pictures shown in Fig. 2 had a drug loading of 1% only.
Fig. 8.

Optical macroscopy pictures of silicone films loaded with different amounts of dexamethasone and dexamethasone phosphate (3 min milling). The total drug content was constant (11%).
The resulting total absolute drug release rates from the different films in artificial perilymph at 37 °C are illustrated in Fig. 9. The amounts of both, dexamethasone and dexamethasone phosphate, are considered. Importantly, this diagram clearly shows that the formulation strategy is successful: Despite the constant total drug loading, the release rate increased with increasing dexamethasone phosphate content. This can be expected to be beneficial for the patient, providing higher drug concentrations during the first hours/days/weeks after implant placement, when the risk of trauma, inflammation and fibrosis is particularly elevated. Please note that the shape of the drug release curve of films containing 1% dexamethasone phosphate and 10% dexamethasone (bottom curve in Fig. 9) is different from that of films containing only 1% dexamethasone phosphate (bottom curve in Fig. 4). This is because the presence of the additional 10% dexamethasone has an important effect on drug release: Upon leaching of dexamethasone phosphate or dexamethasone, the porosity of the films and their water content increase, facilitating the release of remaining drug. In the case of films containing only 1% dexamethasone phosphate, the “pore creating effect” of the 10% dexamethasone is missing. Thus, some kind of “plateau” is observed after a few days (Fig. 4): Drug particles/agglomerates with direct surface access have been released and it takes more time for drug particles/agglomerates located in deeper film regions to be released. In contrast, in films loaded with 1% dexamethasone phosphate and 10% dexamethasone (Fig. 9), the release of drug particles/agglomerates located in deeper film regions is facilitated by the presence of an important number of pores and channels in direct contact with the surface, created by drug leaching at earlier time points. The optical macroscopy pictures in Fig. 10 are consistent with this hypothesis: Films loaded with “10 % dexamethasone + 1 % dexamethasone phosphate” or with “1 % dexamethasone + 10 % dexamethasone phosphate” are illustrated before and after 32 d exposure to artificial perilymph: The initially clearly visible white drug particles/agglomerates of drug particles became water filled cavities. In the case of high dexamethasone phosphate loadings this effect was more pronounced, because this prodrug is more hydrophilic than dexamethasone, facilitating water penetration into the system and drug dissolution.
Fig. 9.
Cumulative total absolute drug release from silicone films loaded with different concentrations of dexamethasone and dexamethasone phosphate (3 min milling) in artificial perilymph at 37 °C. The total drug content was constant (11%).
Fig. 10.
Optical macroscopy pictures of silicone films loaded with varying concentrations of dexamethasone and dexamethasone phosphate (3 min milling) before and after 32 d exposure to artificial perilymph (37 °C). The red ovals indicate examples of drug agglomerates (left-hand side) and holes (right hand-side), respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
However, the addition of a more hydrophilic compound to a hydrophobic silicone matrix can also lead to a much more pronounced system swelling. In the case of miniaturized implants which are placed into the cochlea of a patient this effect must be limited, because the inner ear is a tiny and sensitive organ. Substantial implant swelling can be expected to cause damage. This is why also the dynamic changes in the wet mass and thickness of the silicone films loaded with different dexamethasone and dexamethasone phosphate contents was monitored upon exposure to artificial perilymph at 37 °C. As it can be seen in Fig. 11, film swelling became much more pronounced at higher dexamethasone phosphate contents. Thus, the strategy of adding a more hydrophilic prodrug to increase the initial burst release was successful, but must be used with caution: A compromise has to be found between the desired drug release rate and acceptable system swelling.
Fig. 11.
Dynamic changes in the wet mass and thickness of silicone films loaded with varying amounts of dexamethasone and dexamethasone phosphate (3 min milling) upon exposure to artificial perilymph at 37 °C. The total drug content was constant (11%).
Please note that even though sink conditions were maintained throughout the observation periods in the surrounding, well-agitated bulk fluid, limited drug solubility effects are likely playing a crucial role within the investigated drug delivery systems: The amounts of water penetrating into the silicone matrices can be expected to be insufficient to dissolve all drug immediately (even upon addition of up to 10% dexamethasone phosphate). The potential importance of limited drug solubility effects within a drug delivery system, in contrast to drug saturation effects in the surrounding release medium, has recently been highlighted and explained in more detail (Siepmann et al., 2017).
3.4. Drug release from cochlear implants
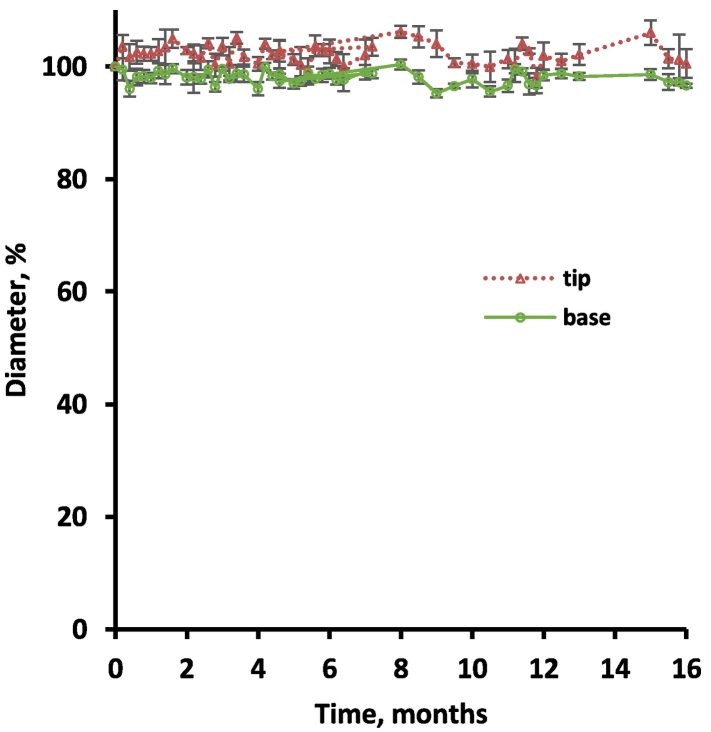
Based on the above described results obtained with thin silicone films, miniaturized inner ear implants were prepared with dimensions allowing for administration in human cochleae (Krenzlin et al., 2012). The systems were loaded with “10 % dexamethasone + 1 % dexamethasone phosphate” or with “10 % dexamethasone” for reasons of comparison. Please note that metal electrodes were not incorporated (but their impact on the investigated formulation effects, can be expected to be limited). Fig. 12 shows optical macroscopy pictures of the implants, which appeared to be rather homogenous. The resulting cumulative drug release kinetics in artificial perilymph are illustrated in Fig. 13: Importantly, the strategy was successful: The addition of only 1% dexamethasone phosphate significantly increased the initial burst release during the first few days/weeks. Please note that an average volume of only 76 μL perilymph has been reported for humans (Igarashi et al., 1986). Thus, the observed increase in the total drug release rate is likely relevant in vivo. At the same time, the addition of only 1% dexamethasone phosphate to the 10% dexamethasone containing silicone-based implants did not lead to noteworthy system swelling during at least 1 year, as illustrated in Fig. 14: The dynamic changes in the diameters of the “tips” and “bases” of the implants (shown in Fig. 12) were monitored upon exposure to artificial perilymph (37 °C, 80 rpm) by optical macroscopy. From a clinical perspective, this type of behavior can be expected to be acceptable.
Fig. 12.
Optical macroscopy pictures of cochlear implants loaded with “10 % dexamethasone” or “10 % dexamethasone + 1 % dexamethasone phosphate (milled for 3 min)”, before exposure to the release medium.
Fig. 13.
Impact of adding 1% dexamethasone phosphate (3 min milling) to cochlear implants loaded with 10% dexamethasone on the resulting cumulative absolute total drug release in artificial perilymph (37 °C).
Fig. 14.
Absence of noteworthy swelling or shrinking: Dynamic changes in the dimensions of cochlear implants loaded with 10% dexamethasone and 1% dexamethasone phosphate (milled for 3 min) upon exposure to artificial perilymph (37 °C).
4. Conclusion
The addition of small amounts of dexamethasone phosphate to silicone-based miniaturized cochlear implants for controlled dexamethasone release can effectively increase the initial burst release. This is expected to be beneficial for the patient, since it can help: (i) to limit local trauma and inflammation caused by the invasive insertion of the electrode array, and (ii) to limit the formation of fibrotic tissue around the foreign body (fibrosis decreasing the performance of the implants, as the transmission of electrical signals is hindered). The effect on drug release can be explained by the more hydrophilic nature of the prodrug dexamethasone phosphate compared to its parent drug dexamethasone, facilitating water penetration into the system and hence, drug dissolution and diffusion. Importantly, at limited dexamethasone phosphate contents, implant swelling remains clinically acceptable.
Declaration of Competing Interest
One of the authors is the Editor-in-Chief of this journal. The manuscript has been subject to all of the journal's usual procedures, including peer review, which has been handled independently of the Editor-in-Chief.
Acknowledgements
This project has received funding from the French National Research Agency (ANR-15-CE19-0014-01/03/05) and from the Interreg 2 Seas programme 2014-2020 co-funded by the European Regional Development Fund under subsidy contract “Site Drug 2S07-033”. The authors would also like to thank Mr. A. Fadel from the “Centre Commun de Microscopie” of the University of Lille (“Plateau techique de la Federation Chevreul CNRS FR 2638”) as well as Mr. J. Verin from the INSERM U1008 research group at the University of Lille for their valuable technical help with the SEM pictures.
References
- Aksit A., Rastogi S., Nadal M.L., Parker A.M., Lalwani A.K., West A.C., Kysar J.W. Drug delivery device for the inner ear: ultra-sharp fully metallic microneedles. Drug Deliv. Transl. Res. 2020 doi: 10.1007/s13346-020-00782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L., Simoni E., Giarbini N., Giordano P., Pannella M., Hatzopoulos S., Martini A. Cochlear implant and inflammation reaction: safety study of a new steroideluting electrode. Hear. Res. 2016;336:44–52. doi: 10.1016/j.heares.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Bas E., Bohorquez J., Goncalves S., Perez E., Dinh C.T., Garnham C., Hessler R., Eshraghi A.A., Van De Water T.R. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: a dose response study. Hear. Res. 2016;337:12–24. doi: 10.1016/j.heares.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Borden R.C., Saunders J.E., Berryhill W.E., Krempl G.A., Thompson D.M., Queimado L. Hyaluronic acid hydrogel sustains the delivery of dexamethasone across the round window membrane. Audiol. Neurotol. 2011;16:1–11. doi: 10.1159/000313506. [DOI] [PubMed] [Google Scholar]
- Borkholder D.A., Zhu X., Frisina R.D. Round window membrane intracochlear drug delivery enhanced by induced advection. J. Control. Release. 2014;174:171–176. doi: 10.1016/j.jconrel.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazares-Delgadillo J., Ganem-Rondero A., Merino V., Kalia Y.N. Controlled transdermal iontophoresis for poly-pharmacotherapy: Simultaneous delivery of granisetron, metoclopramide and dexamethasone sodium phosphate in vitro and in vivo. Eur. J. Pharm. Sci. 2016;85:31–38. doi: 10.1016/j.ejps.2016.01.027. [DOI] [PubMed] [Google Scholar]
- Chin O.Y., Diaz R.C. State-of-the-art methods in clinical intracochlear drug delivery. Curr. Opin. Otolaryngol. Head Neck Surg. 2019;27:381–386. doi: 10.1097/MOO.0000000000000566. [DOI] [PubMed] [Google Scholar]
- Dai J., Long W., Liang Z., Wen L., Yang F., Chen G. A novel vehicle for local protein delivery to the inner ear: injectable and biodegradable thermosensitive hydrogel loaded with PLGA nanoparticles. Drug Dev. Ind. Pharm. 2018;44:89–98. doi: 10.1080/03639045.2017.1373803. [DOI] [PubMed] [Google Scholar]
- Douchement D., Terranti A., Lamblin J., Salleron J., Siepmann F., Siepmann J., Vincent C. Dexamethasone eluting electrodes for cochlear implantation: effect on residual hearing. Cochlear Implants Int. 2015;16:195–200. doi: 10.1179/1754762813Y.0000000053. [DOI] [PubMed] [Google Scholar]
- El Kechai N., Agnely F., Mamelle E., Nguyen Y., Ferrary E., Bochot A. Recent advances in local drug delivery to the inner ear. Int. J. Pharmaceut. 2015;494:83–101. doi: 10.1016/j.ijpharm.2015.08.015. [DOI] [PubMed] [Google Scholar]
- El Kechai N., Bochot A., Huang N., Nguyen Y., Ferrary E., Agnely F. Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int. J. Pharmaceut. 2015;487:187–196. doi: 10.1016/j.ijpharm.2015.04.019. [DOI] [PubMed] [Google Scholar]
- El Kechai N., Mamelle E., Nguyen Y., Huang N., Nicolas V., Chaminade P., Yen-Nicolay S., Gueutin C., Granger B., Ferrary E., Agnely F., Bochot A. Hyaluronic acid liposomal gel sustains delivery of a corticoid to the inner ear. J. Control. Release. 2016;226:248–257. doi: 10.1016/j.jconrel.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Engleder E., Honeder C., Klobasa J., Wirth M., Arnoldner C., Gabor F. Preclinical evaluation of thermoreversible triamcinolone acetonide hydrogels for drug delivery to the inner ear. Int. J. Pharmaceut. 2014;471:297–302. doi: 10.1016/j.ijpharm.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmand Ghavi F., Mirzadeh H., Imani M., Jolly C., Farhadi M. Corticosteroid releasing cochlear implant: a novel hybrid of biomaterial and drug delivery system. J. Biomed. Mater. Res. 2010;94B:388–398. doi: 10.1002/jbm.b.31666. [DOI] [PubMed] [Google Scholar]
- Farhadi M., Jalessi M., Salehian P., Ghavi F.F., Emamjomeh H., Mirzadeh H., Imani M., Jolly C. Dexamethasone eluting cochlear implant: histological study in animal model. Cochlear Implants Int. 2013;14:45–50. doi: 10.1179/1754762811Y.0000000024. [DOI] [PubMed] [Google Scholar]
- Gehrke M., Sircoglou J., Gnansia D., Tourrel G., Willart J.F., Danede F., Lacante E., Vincent C., Siepmann F., Siepmann J. Ear cubes for local controlled drug delivery to the inner ear. Int. J. Pharmaceut. 2016;509:85–94. doi: 10.1016/j.ijpharm.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Gehrke M., Verin J., Gnansia D., Tourrel G., Risoud M., Vincent C., Siepmann F., Siepmann J. Hybrid ear cubes for local controlled dexamethasone delivery to the inner ear. Eur. J. Pharm. Sci. 2019;126:23–32. doi: 10.1016/j.ejps.2018.04.045. [DOI] [PubMed] [Google Scholar]
- Hao J., Li S.K. Inner ear drug delivery: recent advances, challenges, and perspective. Eur. J. Pharm. Sci. 2019;126:82–92. doi: 10.1016/j.ejps.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Igarashi M., Ohashi K., Ishii M. Morphometric comparison of endolymphatic and perilymphatic spaces in human temporal bones. Acta Otolaryngol. 1986;101:161–164. doi: 10.3109/00016488609132823. [DOI] [PubMed] [Google Scholar]
- Jaudoin C., Agnely F., Nguyen Y., Ferrary E., Bochot A. Nanocarriers for drug delivery to the inner ear: Physicochemical key parameters, biodistribution, safety and efficacy. Int. J. Pharmaceut. 2021;592(120038):1–31. doi: 10.1016/j.ijpharm.2020.120038. [DOI] [PubMed] [Google Scholar]
- Jia H., François F., Bourien J., Eybalin M., Lloyd R.V., Van De Water T.R., Puel J.L., Venail F. Prevention of trauma-induced cochlear fibrosis using intracochlear application of anti-inflammatory and antiproliferative drugs. Neuroscience. 2016;316:261–278. doi: 10.1016/j.neuroscience.2015.12.031. [DOI] [PubMed] [Google Scholar]
- Juhn S. Barrier systems in the inner ear. Acta Otolaryngol. Suppl. 1988;458:79–83. doi: 10.3109/00016488809125107. [DOI] [PubMed] [Google Scholar]
- Kita A., Saldate J., Chang C., Chellappa N., Jong J., Matsuda R., Schmidt A., Shih B., Shafqat I., Schoettler K., Acharya S., Seidlits S., Hoffman L. Implantable drug reservoir devices for inner ear delivery of pharmacotherapeutics. Otolaryngol. Head Neck Surg. 2020;163:791–798. doi: 10.1177/0194599820930229. [DOI] [PubMed] [Google Scholar]
- Krenzlin S., Vincent C., Munzke L., Gnansia D., Siepmann J., Siepmann F. Predictability of drug release from cochlear implants. J. Control. Release. 2012;159:60–68. doi: 10.1016/j.jconrel.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Lehner E., Guendel D., Liebau A., Plontke S., Maeder K. Intracochlear PLGA based implants for dexamethasone release: challenges and solutions. Int. J. Pharmaceut. X. 2019;1(100015):1–9. doi: 10.1016/j.ijpx.2019.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner E., Liebau A., Syrowatka F., Knolle W., Plontke S.K., Maeder K. Novel biodegradable round window disks for inner ear delivery of dexamethasone. Int. J. Pharmaceut. 2021;594(120180):1–9. doi: 10.1016/j.ijpharm.2020.120180. [DOI] [PubMed] [Google Scholar]
- Liebau A., Pogorzelski O., Salt A.N., Plontke S.K. Hearing changes after intratympanic steroids for secondary (salvage) therapy of sudden hearing loss: a meta-analysis using mathematical simulations of drug delivery protocols. Otol. Neurotol. 2018;39:803–815. doi: 10.1097/MAO.0000000000001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jolly C., Braun S., Janssen T., Scherer E., Steinhoff J., Ebenhoch H., Lohner A., Stark T., Kiefer J. Effects of a dexamethasone-releasing implant on cochleae: a functional, morphological and pharmacokinetic study. Hear. Res. 2015;327:89–101. doi: 10.1016/j.heares.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Liu Y., Jolly C., Braun S., Stark T., Schere E., Plontke S.K., Kiefer J. In vitro and in vivo pharmacokinetic study of a dexamethasone-releasing silicone for cochlear implants. Eur. Arch. Otorhinolaryngol. 2016;273:1745–1753. doi: 10.1007/s00405-015-3760-0. [DOI] [PubMed] [Google Scholar]
- Maeder K., Lehner E., Liebau A., Plontke S.K. Controlled drug release to the inner ear: Concepts, materials, mechanisms, and performance. Hear. Res. 2018;368:49–66. doi: 10.1016/j.heares.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Mond H.G., Stokes K.B. The steroid-eluting electrode: a 10-year experience. Pace. 1996;19:1016–1020. doi: 10.1111/j.1540-8159.1996.tb03407.x. [DOI] [PubMed] [Google Scholar]
- Oliveira P., Willart J.F., Siepmann J., Siepmann F., Descamps M. Using milling to explore physical states: the amorphous and polymorphic forms of dexamethasone. Cryst. Growth Des. 2018;18:1748–1757. [Google Scholar]
- Plontke S.K., Glien A., Rahne T., Maeder K., Salt A.N. Controlled release dexamethasone implants in the round window niche for salvage treatment of idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 2014;35:1168–1171. doi: 10.1097/MAO.0000000000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke S.K., Götze G., Rahne T., Liebau A. Intracochlear drug delivery in combination with cochlear implants: current aspects. HNO. 2017;65:19–28. doi: 10.1007/s00106-016-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PubChem [Internet] Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 2004. https://pubchem.ncbi.nlm.nih.gov/compound/Dexamethasone PubChem Compound Summary for CID 5743, Dexamethasone; [cited 2020 Sept. 13]. Available from.
- PubChem [Internet] Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 2004. https://pubchem.ncbi.nlm.nih.gov/compound/Dexamethasone-sodium-phosphate PubChem Compound Summary for CID 16961, Dexamethasone sodium phosphate; [cited 2020 Sept. 13]. Available from.
- Salt A.N., Plontke S.K. Local inner-ear drug delivery and pharmacokinetics. Drug Discov. Today. 2005;10:1299–1306. doi: 10.1016/S1359-6446(05)03574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt A.N., Plontke S.K. Principles of local drug delivery to the inner ear. Audiol. Neurootol. 2009;14:350–360. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepmann F., Karrout Y., Gehrke M., Penz F., Siepmann J. Limited drug solubility can be decisive even for freely soluble drugs in highly swollen matrix tablets. Int. J. Pharmaceut. 2017;526:280–290. doi: 10.1016/j.ijpharm.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Sircoglou J., Gehrke M., Tardivel M., Siepmann F., Siepmann J., Vincent C. Trans-oval-window implants, a new approach for drug delivery to the inner ear: Extended dexamethasone release from silicone-based implants. Otol. Neurotol. 2015;36:1572–1579. doi: 10.1097/MAO.0000000000000855. [DOI] [PubMed] [Google Scholar]
- Swan E.E., Mescher M., Sewell W., Tao S., Borensetin J. Inner ear drug delivery for auditory applications. Adv. Drug Deliv. Rev. 2008;60:1583–1599. doi: 10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto B., Chiang H., Valentini C., Yu M., Kysar J.W., Lalwani A.K. Inner ear delivery : challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2020;5:122–131. doi: 10.1002/lio2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura K., Komeda M., Yagi M., Himeno C., Izumikawa M., Doi T., Kuriyama H., Miller J.M., Yamashita T. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in Guinea pig. Hear. Res. 2004;196:58–68. doi: 10.1016/j.heares.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Takumi Y., Nishio S., Mugridge K., Oguchi T., Hashimoto S., Suzuki N., Iwasaki S., Jolly C., Usami S. Gene expression pattern after insertion of dexamethasoneeluting electrode into the Guinea pig cochlea. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk M., Hessler R., Mugridge K., Jolly C., Fehr M., Lenarz T., Scheper V. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS One. 2016;11:1–19. doi: 10.1371/journal.pone.0147552. [DOI] [PMC free article] [PubMed] [Google Scholar]