Abstract
The COVID‐19 pandemic led to major changes in public policies to address supply chain disruption and escalated the price of consumer disinfectant products. To address market demands on alcohol‐based hand rubs and disinfectants, Health Canada implemented major changes to the regulations regarding composition, handling, transportation, and packaging to insure product availability. Furthermore, accelerated licensing of ingredients and packaging did not meet standard medical quality guidelines yet were authorized for manufacturing and packaging of alcohol‐based hand rubs and disinfectants. The accountability associated with these policy changes were reactive, including industry self‐reporting, consumer reporting, and Health Canada advisories and recalls that were responsive to products after they were available in the market. Nonetheless, Canadian public health policy increased hand sanitizers availability. However, some of the interim policies have raised major public health concerns associated with ethanol quality, packaging, and labeling, and enforcement of regulations. In this paper, we review the changes in the Canadian regulations amid the current pandemic and we evaluate the unintended health risks that might arise from these changes.
Keywords: COVID‐19, ethanol, hand sanitizer, pandemic, public policy, regulations
INTRODUCTION
Covid‐19 crisis and hand hygiene
In December 2019, Chinese authorities (Wuhan, China) alerted the World Health Organization (WHO) of pneumonia‐like infections later attributed to the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2; aka COVID‐19). COVID‐19 has high person‐to‐person transmission and demonstrates increased mortality rate compared the seasonal flu (World Health Organization [WHO], 2020). By March 2020, the WHO declared the COVID‐19 outbreak a global pandemic and many Governments subsequently declared states of emergency and tightened restrictions on travel and public gatherings.
Governments and health‐care agencies have recommended behavioral modification (e.g., social distancing), the use of hygiene products, and personal protective equipment to slow the spread of the virus. Personal hygiene products are critically important for frontline workers, and the general public in mitigating the spread of COVID‐19. This has led to stockpiling and panic buying of essential items (e.g., soaps and hand sanitizers) (Statistics Canada, 2020). Approximately 77% of people preferred using hand sanitizers, over handwashing, to maintain hand hygiene (Grand View Research, 2020). In Canada, alcohol‐based hand rubs (ABHRs) and soap sales during the first week of March 2020 increased by 792% and 68%, respectively, compared to 2019 (Statistics Canada, 2020). An increase in ABHR sales resonated globally, with increases of 255%, 751%, 485%, and 23.15% in the United Kingdom, Germany, United States of America, and China, respectively (Korea Trade‐Investment Promotion Agency [KOTRA], 2020). In 2017, the global ABHR market was valued at 2.4B USD and projected to increase to 5.5B USD by 2024 (Ridder, 2020). However, due to the COVID‐19 pandemic, the market exhibited an exponential growth of 600% from 2019 to 2020 (Research and Markets, 2021).
This unprecedented demand for ABHRs has disrupted supply chains and put pressure on policy makers to amend regulations to address current shortages. Additional considerations that were included in the policy change were fake claims of homemade sanitizers, terminology regarding alcohol quality (e.g., technical and pharmaceutical grade) and the impact of COVID‐19 on previous enforcement protocols. In Canada, ABHRs are classified as natural health products (NHPs) and are regulated by Health Canada. Typically, pharmaceutical‐ or food‐grade ethanol must be used in the preparation of ABHRs. However due to the onset of COVID‐19, Health Canada has amended regulations to permit the use of technical‐grade ethanol, including higher levels of allowable impurities, to alleviate the growing demand for ABHRs (Health Canada, 2020f). Changes in the legislation can also lead to undesirable consequences, such as nonconformance with newly imposed labeling requirements and the added risk of contaminants present in the ethanol ingredient. These added risks to the consumer are related to ABHR use as labeled, for example, elevated levels (1000 ppm) of acetaldehyde inhalation are linked to headaches and carcinogenicity, and changes to reproductive tissue in rats and humans (Lui et al., 2014; Maxwell et al., 2010; Woutersen et al., 1986). Ethyl acetate removes oil from the skin and can cause dry skin and irritation (400 ppm; Nelson et al., 1943). Further risk is added in the case of accidental ingestion of ABHR through confusion over inadequate labeling, nontraditional packaging in food containers, and abuse. Methanol and ethanol poisoning are the highest risk, although elevated levels of impurities pose a risk when ingested as well (Health Canada, 2013; Tse, Purdy, et al., 2021). The levels of these impurities were controlled in the interim ABHR policy, however, some commercially available ABHRs were noncompliant (Tse, Nelson, et al., 2021). In this paper, we analyze changes to Health Canada's policies related to ABHRs intended for domestic and personal use during the COVID‐19 pandemic.
Hand sanitizers
ABHRs are crucial in mitigating the spread of infectious disease when water and soap are unavailable (Jing et al., 2020; Widmer, 2000). Hand hygiene products can be divided into washes and rubs. Washes (e.g., antibacterial soaps) are intended for use on wet hands and designed to be rinsed off with water. They are effective on soiled hands, removing oil and dirt that can retain infectious materials. When hands are not visibly dirty, and water is unavailable, ABHRs can be used as an alternative (Archer et al., 2007; Girou et al. 2002; Widmer, 2000).
There are two categories of hand sanitizer rubs: Alcohol‐based and alcohol‐free. ABHRs must contain 62%–95% (v/v) of alcohol (as ethanol, isopropanol or n‐propanol). These sanitizers are commercially available as foam, gel, spray, cream, or wipes. Alcohol‐free hand rubs typically consist of nonalcoholic active components (e.g., chlorhexidine, hexachlorophene, povidone‐iodine, quaternary ammonium compounds, triclosan, or chloroxylenol) (Jing et al., 2020).
The WHO has recommended two ABHR formulations, which were later modified, by the European Committee for Standardization, in 2010 to improve efficacy by increasing alcohol content and decreasing glycerol content (WHO, 2010). Current formulations require a minimum of 80% alcohol or 75% isopropyl alcohol as they have potent viricidal efficacy (EN 1500, 2013; Kratzel et al., 2020; Siddharta et al., 2017; Suchomel et al., 2013).
Canadian alcohol‐based hand sanitizer regulations during COVID‐19
In Canada, ABHRs are classified under the Non‐prescription Natural Health Products Directorate, and are regulated by Health Canada. Any entity that manufactures, processes, packages, labels, imports, and/or stores ABHRs must have a site license, and each ABHR formula must also obtain a product license and be assigned a natural product number (NPN).
Health Canada temporarily eased existing regulations on the manufacturing, formulation, and distribution of ABHRs to expand available ethanol resources and meet market demand. Permittance of lower quality technical‐grade ethanol helped to alleviate growing demand for ABHRs. However, this lower grade of ethanol contains elevated concentrations of contaminants. In March and April 2020, Health Canada published modified guidelines and supporting documents that included:
Guide on Health Canada's interim expedited licensing approach for production and distribution of alcohol‐based hand sanitizers (Health Canada, 2020h)
Hard‐surface disinfectants and hand sanitizers (COVID‐19): Information for manufacturers (Health Canada, 2020c)
Interim guide for production of ethanol for use in alcohol‐based hand sanitizers (Health Canada, 2020f)
Health Canada's decision on technical‐grade ethanol for the manufacture of hand sanitizers: Notice to industry (Health Canada, 2020d)
The interim measures enacted by Health Canada included seven major components (timelines are illustrated in Figure 1 and the impacts of interim legislation are highlighted in Figure 2).
Figure 1.
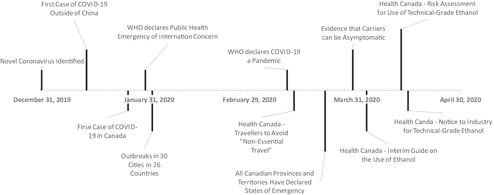
A timeline of COVID‐19 events and Health Canada response with respect to Sanitizers
Figure 2.
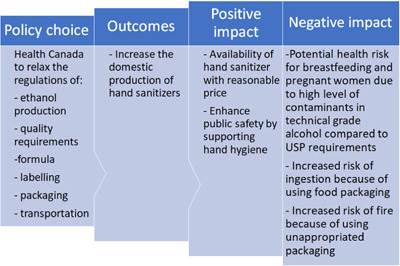
Impact of the new regulations of alcohol‐based hand sanitizers on public safety
Simplified application for both site licenses and product licenses and expedited product approvals
The amended application process is intended to foster conditions that increase ethanol and ABHR products by permitting market entry for new manufacturers and distributors. Before the pandemic, pharmaceutical companies, household product companies, and personal care product entities were primary suppliers of ABHRs, in Canada. Since simplification of the application process, microbreweries, fuel ethanol producers, animal feed facilities, and so forth were approved to produce ABHRs. This regulatory change led to a significant increase in the number of ABHRs registered by Health Canada from March to July 2020 (Health Canada, 2020a). The simplified license approach encouraged producers to prioritize ABHRs production. Under interim guidelines, products must comply with recommendations from Health Canada's Antiseptic Skin Cleansers monograph, for personal use, with time‐intensive requirements (e.g., time‐to‐effect requirement) waived (Health Canada, 2020h). For example, antiseptic products are expected to have a minimum time‐to‐effect of 30 s (for waterless hand rubs) to 1 min (for washes or scrubs using water). Health Canada must be notified of the intent to use interim licensed products for medical and commercial environments.
Interim flexibility pertaining to ethanol quality
Regulations before the COVID‐19 pandemic consisted of using pharmaceutical‐grade or food‐grade ethanol for ABHR formulas. Under Canadian interim guidelines, ethanol producers should ensure products are free of foreign chemicals, that might influence color, clarity, specific gravity, UV absorbance or leave a residue. Ethanol that does not meet United States Pharmacopeia (USP) or Food Chemical Codex specifications, and contains less than 1000 ppm of acetaldehyde, could be marketed as “technical‐grade” and was authorized for its production in the manufacture of ABHR products on a case‐by‐case basis (Health Canada, 2020d, 2020g). As the Canadian ethanol production plants reduced the acetaldehyde concentrations in their products, and as 2020 progressed, Health Canada repeated its risk assessment of technical‐grade ethanol. The limit for acetaldehyde was reduced to 400 ppm in June and 75 ppm in September (Health Canada, 2020g). Industrial‐grade (i.e., more than 1000 ppm acetaldehyde) ethanol was never permitted by Health Canada for formulation into ABHRs and products with higher concentrations of acetaldehyde have been recalled. Manufacturers of technical‐grade ethanol in Canada are required to prioritize production of high‐quality ethanol and to prepare and retain certificates of analysis for all production lots. These manufacturers must inform users of elevated contaminant concentrations and their inherent risks (e.g., use by children, pregnant or breastfeeding women, or use on damaged skin). Technical‐grade ethanol is not to be resold and its production is expected to be discontinued after the COVID‐19 interim response measures are lifted.
As of June 12, 2020, Health Canada has authorized 45 companies to manufacture ABHRs using technical‐grade ethanol supplied by eight approved ethanol manufacturers. These manufacturers have vastly increased the availability of ABHRs in the Canadian market, providing these essential products for use in homes and businesses to combat the current COVID‐19 crisis. However, as of January 15, 2021, Health Canada is no longer accepting new notification forms for the exceptional release of hand sanitizers (Health Canada, 2020a).
On November 26, 2020 Health Canada updated their guidelines on technical‐grade ethanol by requiring all technical alcohol for use in hand sanitizers contain no more than 75 ppm of acetaldehyde. On January 15, 2021 Health Canada discontinued the interim licensing measures for ABHRs but not surface sanitizers. The standard licensing process was reinstated (Health Canada, 2020a).
Recommended formulas
Health Canada monograph requires the use of 60%–80% ethanol (v/v), or 75% isopropyl alcohol (v/v) in ABHRs (Health Canada, 2020f). However, to ensure the efficacy of ABHRs, the WHO formulation was recommended for the preparation of ABHRs, which includes the addition of glycerol and hydrogen peroxide (WHO, 2010).
List of alternatives for rheology modifiers
Carbomer is a common thickening agent used in production of gelled ABHRs. Like many other raw materials, the availability of these materials suffered from disrupted supply chains. In response to the unavailability of carbomer and other rheologic modifiers, Health Canada approved certain substitutions such as alkyl acrylate cross‐polymer, ammonium acryloyldimethyltaurate, and microcrystalline cellulose (Health Canada, 2020e).
Labeling requirements
The labeling must clearly state the blending of technical‐grade alcohol in the formulation of ABHR products and the risks associated with their use. Labeling checklist and claims must be verbatim as they occur in applicable product licenses. This condition was used to prevent false or misleading claims (e.g., broad claims of effectiveness against coronavirus without submission of additional supporting documents) (Health Canada, 2020d).
Flexibility for the packaging materials and sizes
Only one application and NPN is required for different sizes of ABHR product with the same formula and dose recommendation. Any container that is chemically resistant to the ABHR ingredients is currently authorized for packaging, including food or pharmaceutical‐grade packaging. Although packaging in food containers could, and has, lead to accidental ingestion of these products (British Columbia Centre for Disease Control [BC‐CDC], 2020; United States Food and Drug Administration [US‐FDA], 2020). It is recommended that the size of the opening be minimized to prevent evaporation. Packages that are non‐resealable should only be used for single‐use applications and labeled as “refills.” The packaging is also not required to have security features such as safety seals (Health Canada, 2020c).
Flexibility regarding good manufacturing practises (GMP) requirements applied on NHPs
The stability test, quality assurance report, microbiological contaminants or other forms of evidence required as part of the standard process for the NHPs are waived for ABHRs that contain more than 50% alcohol as a final concentration (Health Canada, 2020c, 2020h).
DISCUSSION
Compliance and enforcement of the new regulations
Health Canada is consistently updating the interim ABHR regulations with respect to current scientific evidence (e.g., toxicity and risk assessments). Compliance is being achieved by combining different enforcement strategies. Usually, the Regulatory Operations and Enforcement Branch and Health Canada inspectors are required to enforce regulations by visiting production sites and analyzing available products. In the site licence application, Health Canada requires an attestation certifying that the manufacturing site complies with applicable GMPs for NHPs, and undergoes routine inspections ensuring compliance with federal and provincial regulations. It is unclear whether site inspections are being conducted under travel and workplace restrictions. Ethanol producers are required to maintain certificates of analyses demonstrating product compliance with the Antiseptic Skin Cleansers monograph. Technical‐grade alcohol producers are required to provide a certificate of analyses to Health Canada, detailing impurities and deviations from the USP monographs (USP, 2020). Producers are also required to inform consumers of the technical‐grade classification, formulae, and recommended use and restrictions for the products. Consequently, consumers play a vital role in enforcement by reporting adverse effects related to the use of ABHR products to the Canada Vigilance regional office. In general, Health Canada gives a period of 15 days for ABHR manufacturers to report any adverse reactions related to the use of their products. Approval for use of technical‐grade ethanol comes with the explicit condition that Health Canada may issue immediate notices to cease sale of ethanol and its products, issue recalls, and perform on‐site visits and product seizures. It is expected that companies would follow regulations as deviations might severely impact their businesses and licensing. Currently, 52 out of 5050 products have been listed for recall by Health Canada due to use of industrial‐grade ethanol or of noncompliant technical‐grade ethanol (e.g., contamination with methanol and ethyl acetate) (Health Canada, 2020i, 2020j).
Public safety impact of the new regulations
The outcome from interim COVID‐19 ABHR regulations has encouraged local manufacturers and distributors to increase ABHRs supply to address growing demand. Availability of ABHRs is paramount in providing protection and maintaining public health. However, some of the new ABHR regulations have raised concerns regarding ethanol quality, inadequate labeling, packaging in food containers, and enforcement efforts. Changing policy due to an emerging crisis can be difficult, especially when there is insufficient scientific data to support strong decisions. There have been insufficient efforts devoted to exploring possible consequences, (e.g., nonconforming label requirements of technical‐grade ethanol, impurities in technical‐grade ethanol), and little evidence of a structured approach to such analyses (Callele et al., 2018). The added risk to the population based on the use of technical‐grade ethanol for ABHRS has been calculated to be acceptable by Health Canada as part of their ongoing risk assessments in April, June, and September 2020, provided that duration of use is limited (Health Canada, 2020g). Added risk due to inadequate labeling and nontraditional food packaging has been observed in cases of accidental ingestion (BC‐CDC, 2020), but reports are not tracked or released by all jurisdictions in Canada. The added benefit of access to ABHRs is in higher rates of hand hygiene practice and reduced rates of worker or student absenteeism (Grand View Research, 2020; Hübner et al., 2010; White et al., 2005; Widmer, 2000).
Risks associated with ethanol quality
Contaminants produced during ethanol production can arise as a result of feedstock used or process conditions. Alcohols with similar boiling points are difficult to separate using single distillation methods, resulting in contaminants in the final product. High concentrations and prolonged exposure to these contaminants can be associated with adverse health effects. Ethanol grade is classified depending on impurity levels. Typically, pharmaceutical grade ingredients are required to formulate ABHRs, however, technical‐grade ethanol is permitted during the current pandemic (Health Canada, 2020b, 2020d, 2020f; USP, 2020).
On April 17, 2020, Health Canada's Decision on technical‐grade ethanol for the manufacture of hand sanitizers: Notice to industry permitted use of non‐USP compliant ethanol in ABHRs, with written authorization (Health Canada, 2020d). The guide does not provide specific information regarding impurities, only indicating that producers notify consumers of increased impurities and maintain records for Health Canada.
Permission guidelines that governed the use of technical‐grade alcohol in ABHR formulations confused both manufacturers and consumers. Health Canada has recently recalled 11 ABHRs that were produced with technical‐grade ethanol confirming the challenges in producing ABHRs according to interim guidelines. Flexibility in the new regulations and constant evolution complicated compliance and enforcement activities. In addition, consumers were concerned about health risks associated with contaminants found in “technical‐grade” ethanol.
In response, Health Canada released a risk assessment, that resulted in new labeling requirements for ABHRs formulated with “technical‐grade” alcohol (Health Canada, 2020g). The risk assessment study concluded that applying ABHRs containing acetaldehyde, a known carcinogen and teratogen (Lui et al., 2014; Woutersen et al., 1986), at concentrations up to 1000 ppm (v/v) was tolerable for causal and short‐term domestic use. This was later updated in June 2020 when new regulations required that acetaldehyde levels be reduced below 400 ppm. The benefits of ABHRs during the COVID‐19 pandemic outweighs the risk associated with contracting COVID‐19. However, this ongoing risk assessment does not provide cumulative effects of other ethanol contaminants, or concurrent exposure from other food and chemical sources (Liu & Pilone, 2000; Uebelacker & Lachenmeier, 2011). Nevertheless, Health Canada requires that health‐care workers and ABHR manufacturers add warning labels to inform consumers of risks of using such products to children or, pregnant or breastfeeding women.
Before COVID‐19, there were significant concerns among the health‐care community regarding indiscriminate use of over‐the‐counter hand disinfectant products which may lead to the selection of bacteria with enhanced antibiotic resistance. Fears of contracting COVID‐19 is driving the trend of overuse, raising concerns that long‐term, daily use of these products may outweigh their benefits. Additional studies are needed to investigate effects of repeated use and daily recommended exposure of ABHRs formulated with technical‐grade ethanol. Risk assessments of prolonged exposure should not be limited to acetaldehyde but must also consider other potentially toxic impurities (e.g., methanol) (Chan & Chan, 2018; Onuki et al., 2016).
Risk associated with packaging
Traditionally, ABHRs were packaged in liquid pump dispensers or small squeezable containers. However, Heath Canada's interim regulations expanded packaging options used to store ABHRs. Under the interim regulations, any package that is chemically resistant to the alcohol is permitted, including food and beverage containers. It is recommended, that the size of the opening on the container should be as small as possible to reduce spills, inhalation, and evaporation. Currently, many Canadian ABHRs manufacturers (e.g., distilleries), have reused the color and design of their existing food product labels and packaging for ABHR containers. However, brightly colored labels and cartoon depictions can encourage exploratory behavior in children (Engel & Spiller, 2010) and can confuse consumers between food, beverage, and ABHR products, resulting in accidental poisoning. In response, Health Canada released an advisory reminding Canadians to be cautious when using hand sanitizers, disinfectants, or household cleaning products. Increased acute poisoning from intentional and unintentional ingestion of ABHRs has previously been reported (BC‐CDC, 2020; Chan & Chan, 2018; Gormley et al., 2012; Health Canada, 2013; Rayar & Ratnapalan, 2013), and these risks may increase with the use of technical‐grade ethanol.
Single‐use containers (e.g., non‐resealable) are permitted under the interim guidelines and should be labeled as “refill” products to be transferred into secondary containers after opening. However, the use of wide‐mouth ABHRs containers (e.g., glass jars), is discouraged by Health Canada, as these containers can increase the risk of spills, and subsequent exposure and ignition.
Further concerns arise with ABHRs packaged via beverage manufacturing processes. For example, carbonation can elicit unintentional chemical reactions (e.g., acidification). Acidification of ABHRs may cause dermatitis (Beiu et al., 2020; Gould et al., 2007), and affect the primary mechanisms involved in ethanol‐protein interactions and decrease ABHR efficacy. Carbonation of the sanitizers is not permitted in the interim guideline, as carbon dioxide is considered as an additional ingredient. However, nontraditional producers may not be aware of these chemical changes which can influence ABHR efficacy. To mitigate these potential risks, these additional ingredients should be avoided, and existing household cleaner or chemical containers should be employed.
CONCLUSION
Canadian public health policy has played a crucial role in increasing ABHRs availability and ensuring public safety during the COVID‐19 pandemic. Health Canada has released interim guidelines and regulations to accelerate ethanol production for the formulation of ABHRs. These interim measures remained in place, until the supply of high‐grade ethanol had stabilized. They continue to modify the guidelines in response to public need and industry activity, along with ongoing risk assessments of the interim approved product class. The interim policy changes were made in consultation with scientists and industry, and policy adaptations are responsive to real‐time cases. The modifications permitted by these policy changes included the use of nontraditional sources of ethanol, substitution of rheological ingredients, and flexibility in manufacturing, packaging, and labeling practices.
To reduce health risks associated with technical‐grade ethanol, regulations should explicitly display the threshold limitations of contaminants permitted in ABHR products. To discourage exploratory behavior and unintentional poisonings, chemical or household packaging and appropriate labeling must be mandatory for these products. Food packages should be prohibited, and critical information regarding usage and warnings must also be highlighted on the package label. Compliance with interim policies is incomplete as observed in the marketplace (e.g., inadequate labeling, unsuitable packaging, etc.). Though it is important to support local businesses producing ABHRs, policies must also focus on new‐to‐the‐market businesses involved in ABHR production. This includes improved communication with industry in regard to the proper handling and production of technical‐grade ethanol; as well as, informing consumers of potential hazards associated with these products. Clarification regarding impurity levels, stricter packaging and labeling requirements, and increased public education on compliance and risk regarding interim ABHRs will further mitigate risks and enhance public health measures in Canada.
CONFLICT OF INTERESTS
Dr. Martin J. T. Reaney is the founder of, and has an equity interest in, Prairie Tide Diversified Inc. (Saskatoon, SK, Canada: Previous company name is Prairie Tide Chemicals Inc.).
ETHICS STATEMENT
All authors contributed to the conceptualization and writing of this commentary.
ACKNOWLEDGMENTS
This study was supported by Mitacs (IT16156; IT19122) and the Government of Canada (Natural Sciences and Engineering Research Council of Canada: RGPIN‐2018‐06631 and Saskatchewan Agricultural Development Fund: 20170133; 20180248; 20180255; 20180281; 20190154; 20190155).
Biographies
Rana Mustafa is a research specialist for the Saskatchewan Ministry of Agriculture, Government of Saskatchewan.
Sarah K. Purdy is a postdoctoral fellow in the Department of Plant Sciences, University of Saskatchewan.
Fina B. Nelson is a research technician in the Department of Plant Sciences, University of Saskatchewan.
Timothy J. Tse is a postdoctoral fellow in the Department of Plant Sciences, University of Saskatchewan.
Daniel J. Wiens is a research technician in the Department of Plant Sciences, University of Saskatchewan.
Jianheng Shen is a professional research associate in the Department of Plant Sciences, University of Saskatchewan.
Martin J. T. Reaney is a professor and the Ministry of Agriculture Strategic Research Program Chair in Lipid Quality Utilization in the Department of Plant Sciences, University of Saskatchewan.
Mustafa, R. , Purdy, S. K. , Nelson, F. B. , Tse, T. J. , Wiens, D. J. , Shen, J. , & Reaney, M. J. T. (2021). Canadian policy changes for alcohol‐based hand rubs during the COVID‐19 pandemic and unintended risks. World Medical & Health Policy, 1–13. 10.1002/wmh3.463
[Article updated on June 24, 2021 after first online publication: The first author's affiliation was corrected, which required the other author affiliations be renumbered.]
Contributor Information
Timothy J. Tse, Email: timothy.tse@usask.ca.
Martin J. T. Reaney, Email: martin.reaney@usask.ca.
REFERENCES
- Archer, J. R. H. , Wood, D. M. , Tizzard, Z. , Jones, A. L. , & Dargan, P. I. (2007). Alcohol hand rubs: Hygiene and hazard. British Medical Journal, 335(7630), 1154–1155. 10.1136/bmj.39274.583472.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiu, C. , Mihai, M. , Popa, L. , Cima, L. , & Popescu, M. N. (2020). Frequent hand washing for COVID‐19 prevention can cause hand dermatitis: Management tips. Cureus, 12(4), e7506. 10.7759/cureus.7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Columbia Centre for Disease Control (BC‐CDC) . (2020, May 25). Poison control records spike in calls about children and adults accidentally ingesting hand sanitizer. http://www.bccdc.ca/about/news-stories/stories/2020/poison-control-records-spike-in-calls-about-children-and-adults-accidentally-ingesting-hand-sanitizer
- Callele, D. , Penzenstadler, B. , & Wnuk, K. (2018). Public policy challenges: An RE perspective. In: Chitchyan R., Penzenstadler B., & Venters C. C. (Eds.), Proceedings of the 7th International Workshop on Requirements Engineering for Sustainable Systems (RE4SuSy 2018) co‐located with the 26th International Conference on Requirements Engineering (RE 2018), Banff, Alberta, Canada, August 20, 2018 (Vol. 2223, pp. 24–33). CEUR Workshop Proceedings. [Google Scholar]
- Chan, A. P. L. , & Chan, T. Y. K. (2018). Methanol as an unlisted ingredient in supposedly alcohol‐based hand rub can pose serious health risk. International Journal of Environmental Research and Public Health, 15(7), 1440. 10.3390/ijerph15071440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EN 1500 . (2013). Chemical disinfectants and antiseptics. Hygienic hand rub. Test method and requirements (phase 2/step 2). European Committee for Standardization.
- Engel, J. S. , & Spiller, H. A. (2010). Acute ethanol poisoning in a 4‐year‐old as a result of ethanol‐based hand‐sanitizer ingestion. Pediatric Emergency Care, 26(7), 508–509. 10.1097/PEC.0b013e3181e5bfc9 [DOI] [PubMed] [Google Scholar]
- Girou, E. , Loyeau, S. , Legrand, P. , Oppein, F. , & Brun‐Buisson, C. (2002). Efficacy of hand rubbing with alcohol based solution versus standard handwashing with antiseptic soap: Randomised clinical trial. British Medical Journal, 325, 362. 10.1136/bmj.325.7360.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley, N. J. , Bronstein, A. C. , Rasimas, J. J. , Pao, M. , Wratney, A. T. , Sun, J. , Austin, H. A. , & Suffredini, A. F. (2012). The rising incidence of intentional ingestion of ethanol‐containing hand sanitizers. Critical Care Medicine, 40(1), 290–294. 10.1097/CCM.0b013e31822f09c0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, D. J. , Hewitt‐Taylor, J. , Drey, N. S. , Gammon, J. , Chudleigh, J. , & Weinberg, J. R. (2007). The CleanYourHandsCampaign: Critiquing policy and evidence base. Journal of Hospital Infection, 65(2), 95–101. 10.1016/j.jhin.2006.09.028 [DOI] [PubMed] [Google Scholar]
- Grand View Research . (2020, April). Hand sanitizer market size, share and trends analysis report by product (gel, foam, liquid), by distribution channel (hypermarket and supermarket, drug store, specialty store, online), by region, and segment forecasts, 2020–2027 (Market Analysis Report No. GVR‐3‐68038‐249‐5). https://www.grandviewresearch.com/industry-analysis/hand-sanitizer-market
- Health Canada . (2013, October 24). Two deaths linked to ingestion of hand sanitizer containing methanol. Government of Canada. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2013/36469a-eng.php
- Health Canada . (2020a, March 18). Hard‐surface disinfectants and hand sanitizers (COVID‐19): Disinfectants and hand sanitizers accepted under COVID‐19 interim measure. https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/products-accepted-under-interim-measure.html
- Health Canada . (2020b, March 20). Antiseptic skin cleansers (domestic/personal use). http://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=antiseptic_antiseptique%26lang=eng
- Health Canada . (2020c, March 29). Hard‐surface disinfectants and hand sanitizers (COVID‐19): Information for manufacturers. https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/information-manufacturers.html
- Health Canada . (2020d, April 17). Technical‐grade ethanol for use in hand sanitizers and hard‐surface disinfectants–Notice to industry. https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/covid19-technical-grade-ethanol-hand-sanitizer.html
- Health Canada . (2020e, April 23). List of alternative rheology modifiers for use in the production of hand sanitizers. https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/information-manufacturers/list-alternative-rheology-modifiers.html
- Health Canada . (2020f, May 9). Interim guide on the production of ethanol for use in alcohol‐based hand sanitizers: Background. https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/interim-guide-ethanol-hand-sanitizers.html
- Health Canada . (2020g, May 12). Technical‐grade ethanol for the manufacture of hand sanitizers during the COVID‐19 pandemic: Risk assessment summary report. https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/legislation-guidelines/covid19-technical-grade-ethanol-hand-sanitizer/risk-assessment-summary-report.html
- Health Canada . (2020h, July 31). Licensing approach to produce and distribute alcohol‐based hand sanitizers: Guidance document. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/covid-19-expediated-licensing-alcohol-hand-sanitizer.html
- Health Canada . (2020i, March 18). Hard‐surface disinfectants and hand sanitizers (COVID‐19): List of hand sanitizers authorized by Health Canada. https://www.canada.ca/en/health-canada/services/drugs-health-products/disinfectants/covid-19/hand-sanitizer.html
- Health Canada . (2020j, June 17). Recall of certain hand sanitizers that may pose health risks. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/73385a-eng.php
- Hübner, N.‐O. , Hübner, C. , Wodny, M. , Kampf, G. , & Kramer, A. (2010). Effectiveness of alcohol‐based hand disinfectants in a public administration: Impact on health and work performance related to acute respiratory symptoms and diarrhoea. BMC Infectious Diseases, 10(1), 250. 10.1186/1471-2334-10-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing, J. L. J. , Pei Yi, T. , Bose, R. J. C. , McCarthy, J. R. , Tharmalingam, N. , & Madheswaran, T. (2020). Hand sanitizers: A review on formulation aspects, adverse effects, and regulations. International Journal of Environmental Research and Public Health, 17(9), 3326. 10.3390/ijerph17093326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea Trade‐Investment Promotion Agency (KOTRA) . (2020). Hand sanitizer market trends caused by COVID‐19 in China, Germany, and USA. KOTRA and KOTRA Overseas Market News. https://www.kotra.or.kr/foreign/biz/KHENKO100M.html
- Kratzel, A. , Todt, D. , V'kovski, P. , Steiner, S. , Gultom, M. , Thao, T. , N., Holwerda, M. , Steinmann, J. , Niemeyer, D. , Dijkman, R. , Kampf, G. , Drosten, C. , Steinmann, E. , Thiel, V. , Pfaender, S. , & Ebert. (2020). Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO‐recommended hand rub formulations and alcohols. Emerging Infectious Diseases, 26(7), 1592–1595. 10.3201/eid2607.200915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S.‐Q. , & Pilone, G. J. (2000). An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. International Journal of Food Science and Technology, 35(1), 49–61. 10.1046/j.1365-2621.2000.00341.x [DOI] [Google Scholar]
- Lui, S. , Jones, R. L. , Robinson, N. J. , Greenwood, S. L. , Aplin, J. D. , & Tower, C. L. (2014). Detrimental effects of ethanol and its metabolite acetaldehyde, on first trimester human placental cell turnover and function. PLOS One, 9(2), 87328. 10.1371/journal.pone.0087328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell, R. C. , Spangenberg, R. J. , Hooek, J. B. , Silberstein, S. D. , & Oshinsky, M. L. (2010). Acetate causes alcohol hangover headache in rats. PLOS One, 31(12), 15963. 10.1371/journal.pone.0015963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, K. W. , Ege, J. F. , Ross, M. , Woodman, L. E. , & Silverman, L. (1943). Sensory response to certain industrial solvent vapours. Journal of Industrial Hygiene and Toxicology, 25, 282. [PubMed] [Google Scholar]
- Onuki, S. , Koziel, J. A. , Jenks, W. S. , Cai, L. , Grewell, D. , & van Leeuwen, J. H. (2016). Taking ethanol quality beyond fuel grade: A review. Journal of the Institute of Brewing, 121, 800. 10.1002/jib.364 [DOI] [Google Scholar]
- Rayar, P. , & Ratnapalan, S. (2013). Pediatric ingestions of household products containing ethanol: A review. Clinical Pediatrics, 52(3), 203–209. 10.1177/0009922812470970 [DOI] [PubMed] [Google Scholar]
- Research and Markets. (2021, March). Hand sanitizer market size, market share, application analysis, regional outlook, growth trends, key players, competitive strategies and forecasts, 2021 to 2029 (Market Analysis Report). https://www.researchandmarkets.com/reports/5308734/hand-sanitizer-market-size-market-share
- Ridder, M. (2020, November 23). Forecasted value of hand sanitizer market worldwide in 2017 and 2024 (in Billion U.S. Dollars). Statista. https://www.statista.com/statistics/888373/market-value-of-hand-sanitizer-global/
- Siddharta, A. , Pfaender, S. , Vielle, N. J. , Dijkman, R. , M., Friesland, Becker, B. , J., Engelmann, M. , Todt, D. , Windisch, M. P. , Brill, F. H. , Steinmann, J. , Steinmann, J. , Becker, S. , Alves, M. P. , Pietschmann, T. , Eickmann, M. , Thiel, V. , … Yang. (2017). Virucidal activity of World Health Organization—Recommended formulations against enveloped viruses, including zika, ebola, and emerging coronaviruses. The Journal of Infectious Diseases, 215(6), 902–906. 10.1093/infdis/jix046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada (StatsCan) . (2020, May 11). Canadian consumers adapt to COVID‐19: A look at Canadian grocery sales up to April 11. Statistics Canada. https://www150.statcan.gc.ca/n1/pub/62f0014m/62f0014m2020005-eng.htm
- Suchomel, M. , Kundi, M. , Pittet, D. , & Rotter, M. L. (2013). Modified World Health Organization hand rub formulations comply with European efficacy requirements for preoperative surgical hand preparations. Infection Control and Hospital Epidemiology, 34(3), 245–250. 10.1086/669528 [DOI] [PubMed] [Google Scholar]
- Tse, T. J. , Purdy, S. K. , Shen, J. , Nelson, F. , Mustafa, R. , Wiens, D. J. , & Reaney, M. J. T. (2021). Toxicology of alcohol‐based hand rubs formulated with technical‐grade ethanol. Toxicology Reports, 8, 785–792. 10.1016/j.toxrep.2021.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse, T. J. , Nelson, F. B. , & Reaney, M. J. T. (2021). Analyses of commercially available alcohol‐based hand rubs formulated with compliant and non‐compliant ethanol. International Journal of Environmental Research and Public Health, 18(7), 3766. 10.3390/ijerph18073766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelacker, M. , & Lachenmeier, D. W. (2011). Quantitative determination of acetaldehyde in foods using automated digestion with simulated gastric fluid followed by headspace gas chromatography. Journal of Automated Methods and Management in Chemistry, 2011, 907317. 10.1155/2011/907317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Food and Drug Administration (US‐FDA) . (2020, August 27). COVID‐19 update: FDA warns consumers about sanitizer packaged in food and drink containers. https://www.fda.gov/news-events/press-announcements/covid-19-update-fda-warns-consumers-about-hand-sanitizer-packaged-food-and-drink-containers
- United States Pharmacopeia (USP) . (2020, August 17). Excerpted USP‐NF and FCC standards: A hand sanitizer resource. https://www.usp.org/sites/default/files/usp/document/health-quality-safety/usp-hand-sanitizer-ingredients.pdf
- White, C. , Kolble, R. , Carlson, R. , & Lipson, N. (2005). The impact of a health campaign on hand hygiene and upper respiratory illness among college students living in Residence Halls. Journal of American College Health, 53(4), 175–181. 10.3200/JACH.53.4.175-181 [DOI] [PubMed] [Google Scholar]
- Widmer, A. F. (2000). Replace hand washing with use of a waterless alcohol hand rub? Clinical Infectious Diseases, 31(1), 136–143. 10.1086/313888 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2010, April). Guide to local production: WHO‐recommended handrub formulations. https://www.who.int/gpsc/5may/Guide_to_Local_Production.pdf
- World Health Organization (WHO) . (2020, March 3). WHO Director‐General's opening remarks at the media briefing on COVID-19. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---3-march-2020
- Woutersen, R. A. , Appelman, L. M. , Garderen‐Hoetmer, A. V. , & Feron, V. J. (1986). Inhalation toxicity of acetaldehyde in rats. III. Carcinogenicity study. Toxicology (Amsterdam), 41(2), 213–231. 10.1016/0300-483X(86)90201-5 [DOI] [PubMed] [Google Scholar]


