Abstract
Coronavirus disease-19 (COVID-19) infection causing severe gastrointestinal complications is rare. A 9-year-old child after recovering from mild COVID-19 infection developed small bowel gangrene due to superior mesenteric artery thrombosis. He required resection of entire necrotic small bowel along with caecum causing ultra-short bowel syndrome. Reverse transcriptase-polymerase chain reaction (RT-PCR) done on the resected specimen was positive for COVID-19. He was maintained on individualized parenteral nutrition for 3 months. A living donor intestinal transplant was performed using 200 cm of ileum donated by the patient’s father. The graft function was satisfactory and was not complicated with thrombosis, infection, reactivation of latent COVID-19 or rejection. He could be weaned off completely from parenteral nutrition by postoperative day 21. The donor had an uneventful recovery. Six month follow-up was satisfactory with the child achieving complete enteral autonomy as well as target goal nutrition. Thrombotic phenomena associated with COVID-19 infection can affect larger vessel-like superior mesenteric artery leading to small bowel gangrene. Intestine transplant could be done safely after 3 months of recovery from COVID-19 without any adverse outcomes. Further studies are required to establish optimal timing and safety of small bowel transplant in this situation.
KEYWORDS: complication: surgical/technical, health services and outcomes research, infection and infectious agents - viral, infectious disease, intestine/multivisceral transplantation
Abbreviations: AASLD, American Association for the Study of Liver Disease; ATG, anti thymocyte globulin; CMV, Cytomegalovirus; CRBSI, central line related blood stream infection; CT, computed tomography; DDIT, deceased donor intestine transplant; GIA, gastrointestinal anastomosis; HLA, human leucocyte antigen; IFALD, intestinal failure associated liver disease; IVC, inferior vena cava; LDIT, living donor intestinal transplant; PCP, Pneumocystis carinii pneumonia; RT-PCR, reverse transcription-polymerase chain reaction; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; SIT, small intestine transplant; SMA, superior mesenteric artery; SMV, superior mesenteric vein; TPN, total parenteral nutrition; UW, University of Wisconsin
1. INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic, which originated from Wuhan, China, led to large-scale morbidity and mortality across the world.1 Apart from respiratory complications, gastrointestinal complications have been reported in various studies.2, 3, 4, 5 Predominant gastrointestinal symptoms reported were nausea, diarrhoea, and generalized abdominal pain. Catastrophic complication like massive bowel gangrene secondary to acute mesenteric artery thrombosis have also been described.6, 7, 8 These patients need extensive resection followed by either long-term total parenteral nutrition (TPN), intestinal lengthening procedures, or small intestine transplant (SIT). The latter is done in extreme cases depending on the extent of resection and in patients who develop life-threatening complication secondary to prolonged administration of TPN to offer long-term survival. COVID-19 pandemic brought the global transplant activity to a standstill. This was mainly due to diversion of life-saving resources for management of patients suffering from COVID infections. Second, there were concerns regarding the increased transmission of virus to the recipients along with safety of recipients receiving immuno-suppression during the pandemic. This case report highlights the successful management of a patient by living donor intestine transplant (LDIT) who suffered from massive small bowel gangrene and discusses the outcome of the same.
2. CASE
A 9-year-old male child suffered with symptoms of cough and fever which lasted for 3 days. His nasopharyngeal swab for SARS-CoV-2 was positive for which he was hospitalized in a peripheral hospital. One week later, after his symptoms subsided, he felt acute onset generalized pain in his abdomen with guarding and board-like rigidity. Then he underwent emergency exploratory laparotomy in the same peripheral hospital during which he was found to have massive small bowel gangrene secondary to superior mesenteric artery thrombosis. This necessitated resection of entire small bowel starting from 10 cm distal to duodeno-jejunal flexure extending up to caecum. Proximal jejunum was brought out as an end jejunostomy, and distal end was closed and placed intraperitoneally. Stomal gangrene with retraction of stoma occured on day 6 of the index surgery, for which reexploratory laparotomy was performed at our tertiary care hospital. His height, weight, and body mass index (BMI) were 31 kg, 130 cm, and 18.3 kg/m2, respectively, at the time of admission. His blood investigation, inflammatory markers, and hyper coagulable work-up at the time of admission revealed elevated inflammatory markers but did not show any preexisting prothrombotic state ( Table 1). Gangrenous remnant jejunum was resected, stump was closed using GIA staplers (Medtronic Covidien DST series GIA 6038s blue color) and tube gastrostomy was performed for biliary drainage. The resected specimen showed ischemic necrosis of all the layers of intestine along with areas of hemorrhage and fibrin. It revealed acute perivisceral inflammation; the mesenteric vessel showed complete thrombus with dense inflammatory infiltrates in the endothelium. The tissue subjected for COVID-19 RT-PCR (reverse transcriptase polymerase chain reaction) revealed the presence of significant copies of viral RNA. He was managed by total parenteral nutrition and anticoagulation (low molecular weight heparin) for 3 months, however, this period was complicated by two episodes of central line–related blood stream infection (CRBSI) including 1 episode of fungal sepsis. This necessitated listing him for deceased donor small intestine transplant. After a waiting period of 2 months, his father decided to donate partial intestine for his son. He was a 40-year-old healthy donor without any addictions, comorbidity, or a history of previous surgery. His height, weight, and BMI were 160 cm, 64 kg, and 25 kg/m2, respectively. His preliminary blood test and test to look for malabsorption (D xylose test, Serum VitB12 levels, and ferritin level) were within normal limits. Crossmatch for B cells and T cells were negative. CT mesenteric angiogram was done to evaluate mesenteric vasculature of the donor ( Figure 1), which revealed suitable anatomy for donation. Size matching was suitable as the child was planned for a partial intestine graft and there was no loss of domain in the child. Repeat nasopharyngeal swab by COVID-19 RT-PCR was negative for both the patient and the donor 48 hr prior to transplantation.
TABLE 1.
Patient parameters including basic blood investigations, hyper coagulable work-up, and inflammatory markers at the time of hospital admission
| Patient value | Normal range | |
|---|---|---|
| Hemoglobin | 7.9 | 13–18 g/dl |
| WBC counts | 2900 | 4000–11 000/μL |
| Platelet counts | 200 | 150–450 103/μL |
| Serum sodium | 136 | 136–148 mEq/L |
| Serum potassium | 4.3 | 3.5–5.1 mEq/L |
| Serum chloride | 99 | 98–107 mEq/l |
| Serum creatinine | 1.4 | 0.7–1.3 mg/decilitre |
| Coagulation parameters | ||
| aPTT (activated partial thromboplastin time) | 26.8 | 30–40 s |
| Prothrombin time | 16.7 | 8.3–12.3 s |
| International normalized ratio | 1.41 | 0.8–1.3 |
| Hypercoagulable work-up | ||
| Anti-Cardiolipin IgM | Negative(2.28)units/ml | 0.000–7 |
| Anti-Cardiolipin IgG | Negative(1.35) units/ml | Negative <10 |
| Beta–2-Glycoprotein 1-IgG | Negative(2) RU/ml | Negative <20 |
| Lupus anticoagulant | Absent | Absent |
| Protein C activity | 93% | 70–130 |
| Protein S activity | 109% | 77–143 |
| Factor V leiden, mutation detection | Not detected | Absent |
| Anti thrombin III | 90% | 80–130 |
| Inflammatory markers | ||
| C reactive protein | 357.5 | <10 mg/L |
| D dimer | 2.4 | <0.4 mg/mL |
FIGURE 1.
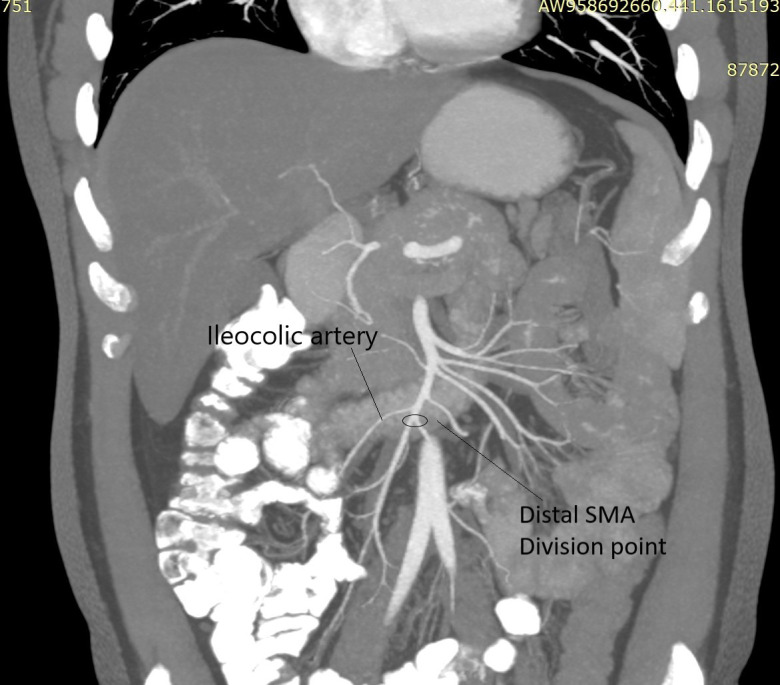
Mesenteric angiogram showing point of division at distal superior mesenteric artery (SMA) after take-off of Ileocolic artery [Color figure can be viewed at wileyonlinelibrary.com]
2.1. Donor surgery
Donor surgery was done by standardized technique as described by Beneditti et al.9 using 200 cm distal small bowel based on distal superior mesenteric artery and vein after the takeoff of ileo-colic pedicle, sparing 20 cm of terminal ileum.
2.2. Recipient surgery
The midline incision was taken. Adhesiolysis was performed to expose the distal most duodenum. Gastrostomy was brought down and the insertion site was closed in two layers. Bed was prepared by exposing the infrarenal aorta and the inferior vena cava (IVC). Small bowel graft brought to the operative field. Vascular clamps applied to the infrarenal aorta and IVC. Arterial anastomosis performed in end to side fashion using prolene 7–0 (Ethicon Blue 2X18” BV175-8) intermittent sutures. Venous anastomosis performed in end to side fashion using continuous prolene 6–0 (Ethicon BLUE 4X30” C-1 EVP DOUBLE ARMED) sutures. Uniform reperfusion was seen after releasing the clamps ( Figure 2). Proximal end of the graft was anastomosed to duodenum of the recipient in side-to-side fashion. Distal end of the graft was anastomosed to the transverse colon of the recipient in side-to-side fashion in two layers. Diversion loop ileostomy done in right iliac fossa. Incision was closed after placing a 23 Fr size tube drain.
FIGURE 2.
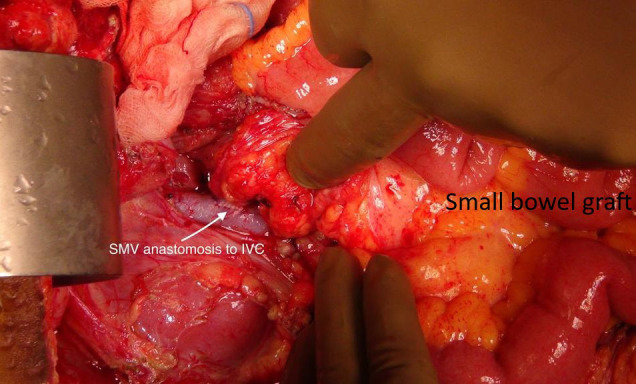
Intraoperative picture showing uniformly perfused graft with graft superior mesentric vein (SMV) to recipient infrarenal inferior vena cava [Color figure can be viewed at wileyonlinelibrary.com]
2.3. Immunosuppression
Recipient received four doses of antithymocyte globulin (ATG)/ day at 1.5 g/kg body weight starting as induction therapy given intraoperatively. CD3 lymphocyte counts were monitored to achieve a target range of <10 cells/μl. Methyl prednisone 500 mg was given before implantation of the graft. Tacrolimus was administered enterally from the first postoperative day and the dose titrated to maintain a trough level of 10–15 ng/ml. The methyl prednisone was tapered to 10 mg about 2 weeks after the transplant and tab mycophenolate was introduced on day 10 when the oral feeds were initiated.
2.4. Postoperative course
2.4.1. Donor
Donor surgery lasted for 4 hr. He was extubated on table and shifted to intensive care for monitoring. He had an uneventful recovery and was able to have adequate enteral feeds with 2–3 bowel movements per day till the time of discharge on day 14. Initial high frequency of bowel movement which is commonly seen after long segment bowel resection settled to one bowel movement per day on subsequent visit after 1 month of donor surgery.
2.4.2. Recipient
Patient was extubated on day 1. Postoperative course remained uneventful without episodes of sepsis or rejection. Protocol biopsy done on day 7 did not show any evidence of rejection ( Figure 3). Cytomegalovirus (CMV) and Pneumocystis carinii pneumonia (PCP) prophylaxis was started. Low residue diet was introduced on day 10 and slowly progressed till he achieved target calories without parenteral supplementation by day 21. Stoma output varied between 30 ml and 300 ml/day. Protocol biopsies were done weekly to look for any evidence of rejection till the time of discharge. He was discharged on day 36 on full enteral feed. Six month follow-up was satisfactory. He remained sepsis free without recurrence of symptoms related to COVID-19. Regular surveillance done for Cytomegalovirus, Ebstein-barr virus, and Adenovirus did not show any reactivation or de novo viral infection.
FIGURE 3.
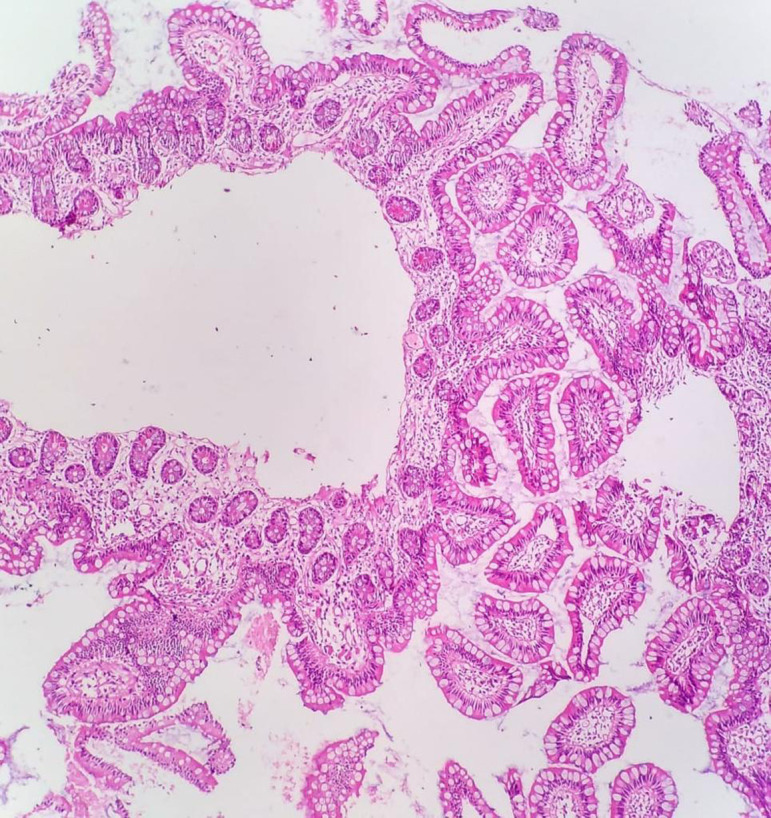
Graft ileal biopsy showing normal architecture without evidence of rejection [Color figure can be viewed at wileyonlinelibrary.com]
3. DISCUSSION
COVID-19 infection leading to gastrointestinal symptoms has been reported in up to 16% of patients.5 As per recent meta-analysis of 29 studies predominant gastrointestinal symptoms were anorexia (21%) nausea (7%), diarrhoea (9%), and generalized pain abdomen (3%). 10 Active shedding of virus has been confirmed in stool samples of the patients with COVID-19 infection. COVID-19 viral infection causing massive bowel gangrene secondary to superior mesenteric artery thrombosis is a rare devastating complication but has been reported by various authors.6, 7, 8 Despite the fact that thrombotic events are higher in patients suffering from severe disease but incidence of superior mesenteric artery thrombosis after mild COVID-19 infection have also been reported.11 Although the exact pathogenesis of COVID-19-related hyper-coagulopathy is unclear, elevated D-dimer levels and coagulation abnormalities have been frequently reported in COVID-19 patients.12 The temporal events, absence of any comorbidity, dense inflammatory infiltrates in the endothelium, as well as the presence of viral RNA in the endothelium of the resected specimen, suggest that causative factor could be COVID infection related coagulopathy. Managing dual complication of COVID infection as well as short gut syndrome in a resource limited setting can be very challenging. The absence of established total parenteral nutrition (TPN) services as well as scarcity of cadaveric donations in Asian countries led to nihilistic attitude toward such patients. Ultrashort gut syndrome requiring long-term TPN can be complicated by line infection, sepsis, central veins thrombosis, intestinal failure associated liver disease (IFALD). 13 , 14 In pediatric patients, the situation can be worsened by growth failure. The quality of life for patients on long-term TPN is generally poor and can be challenging specially in paediatric age group.15 Most importantly, high mortality has been reported on the waiting list for deceased donor intestine transplant in a pediatric age group along with better patient survival in pediatric intestine recipients compared with adult patients (1- and 5-year survival, 89.1% and 76.4%, respectively). 16 This suggested that small intestine transplant (SIT) could be a better option in pediatric patients suffering from intestinal failure. However, this case occurred when COVID-19 pandemic was at its peak and the transplant activity in the country and world over was restricted to emergency cases only. The guideline for optimal timing for solid organ transplant in patients who has suffered from COVID-19 infection is still evolving. Although there are no reports of reactivation of SARS-CoV-2 virus after transplant, the optimal disease-free interval before transplantation is not known. American Association for the study of liver disease (AASLD) issued their recent guidelines for patients awaiting liver transplantation according to which transplantation in SARS-CoV-2-positive transplant candidates should be delayed for at least 14–21 days after symptom resolution and two negative SARS-CoV-2 diagnostic tests before the transplant for optimal outcomes.17 We at our institute followed state guideline for solid organ transplant issued by local authority according to which both the donor and the recipient were tested twice for COVID-19 naso-pharyngeal swabs 14 days apart and the last swab to be taken 24–48 hr prior to the scheduled transplant.18 In our case the patient had significant weight loss along with severe catheter related fungemia warranting an early SIT as a lifesaving procedure. Because Living donor intestine transplant (LDIT) virtually eliminates the waiting time, have short ischaemia time and better HLA matching a living donor was chosen. There are no studies reporting a LDIT for post COVID SMA thrombosis and also the outcome of transplant in this setting. Although there are reports of good outcomes in patients undergoing liver transplant after recent recovery from COVID19 infection19 , 20 but none have been reported for SIT to the best of our knowledge. Despite using higher immunosuppression compared with liver transplant recipients, there was no reactivation of the virus or additional complication related to the recent infection during the limited follow-up time in observation. The surveillance for other viruses like Epstein-Barr virus, cytomegalovirus, and adenovirus did not show any reactivation or a fresh infection during 6-month follow-up. Prospective organ transplant recipients who have recovered from COVID-19 can be considered for transplantation after careful pretransplant evaluation, donor selection, and individualized risk-benefit analysis. Further studies need to be done to establish the safety of solid organ transplant and optimum waiting time after recovery from COVID-19 infection and formulate the uniform guidelines accordingly.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article, as no data sets were generated or analyzed during the current study.
REFERENCES
- 1.Cao Y, Cai K, Xiong L. Coronavirus disease 2019: a new severe acute respiratory syndrome from Wuhan in China. Acta Virol. 2020;64(2):245–250. doi: 10.4149/av_2020_201. [DOI] [PubMed] [Google Scholar]
- 2.Lin LU, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 3.Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115(6):916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol. 2020;18(8):1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a hong kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung S, Quiwa JC, Pillai A, Onwu C, Tharayil ZJ, Gupta R. Superior mesenteric artery thrombosis and acute intestinal ischemia as a consequence of COVID-19 infection. Am J Case Rep. 2020;21:e925753. doi: 10.12659/AJCR.92575. Published 2020 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beccara Lia A, Pacioni Carlotta, Ponton Sara, Francavilla Simone, Cuzzoli Antonio. Arterial mesenteric thrombosis as a complication of SARS-CoV-2 infection. Eur J Case Rep Intern Med. 2020;7(5):001690. doi: 10.12890/2020_001690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Mahruqi G, Stephen E, Abdelhedy I, Al WK. Our early experience with mesenteric ischemia in COVID-19 positive patients. Ann Vasc Surg. 2021;73:129–132. doi: 10.1016/j.avsg.2021.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Testa G, Panaro F, Schena S, Holterman M, Abcarian H, Benedetti E. Living related small bowel transplantation: donor surgical technique. Ann Surg. 2004;240(5):779–784. doi: 10.1097/01.sla.0000143266.59408.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amaravathi U, Balamurugan N, Muthu Pillai V, Ayyan SM. Superior mesenteric arterial and venous thrombosis in COVID-19. J Emerg Med. 2021;60(5):e103–e107. doi: 10.1016/j.jemermed.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman SS, Atkinson JB, Bianchi A, et al. Indications for pediatric intestinal transplantation: a position paper of the American Society of Transplantation. Pediatr Transplant. 2001;5(2):80–87. doi: 10.1034/j.1399-3046.2001.005002080.x. [DOI] [PubMed] [Google Scholar]
- 14.Fishbein TM. The current state of intestinal transplantation. Transplantation. 2004;78(2):175–178. doi: 10.1097/01.tp.0000125133.21993.f1. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe B. Current indications for and prospects of living related intestinal transplantation. Curr Opin Organ Transplant. 2000;5:290–294. doi: 10.1097/00075200-200009000-00022. [DOI] [Google Scholar]
- 16.OPTN/SRTR. Annual Data Report: Intestine; 2018. Available at https://srtr.transplant.hrsa.gov/annual_reports/2018/Intestine.aspx.
- 17.Clinical best practice advice for hepatology and liver transplant providers during the covid-19 pandemic: Aasld expert panel consensus Statement. Available at: https://www.aasld.org/sites/default/files/2021-03/AASLD-COVID19-ExpertPanelConsensusStatement-March92021.pdf [DOI] [PMC free article] [PubMed]
- 18.Standard Operating Procedure for doing transplants during the COVID 19 Pandemic. Available at http://ztccmumbai.org/sop_by_ztcc.pdf
- 19.Dhand A, Bodin R, Wolf DC, et al. Successful liver transplantation in a patient recovered from COVID-19. Transpl Infect Dis. 2020;e13492. [published online ahead of print, 2020 Oct 10]. doi: 10.1111/tid.13492 [DOI] [PMC free article] [PubMed]
- 20.Goss MB, Munoz FM, Ruan W, et al. Liver transplant in a recently COVID-19 positive child with hepatoblastoma. Pediatr Transplant. 2020;e13880. [published online ahead of print, 2020 Sep 26]. 10.1111/petr.13880 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no data sets were generated or analyzed during the current study.


