To the Editor:
A recent study demonstrated that a single dose of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA-based vaccine is sufficient to mount a robust immunological response in immunocompetent subjects with a previous history of coronavirus disease 2019 (COVID-19).1 , 2 While research has suggested that immunocompromised kidney transplant recipients (KTRs) who received mRNA-based vaccines show low immunization rates,3, 4, 5 the question as to whether this also applies to KTRs with a past history of COVID-19 remains unanswered. The aim of this study was to describe the results of immunization after one dose of the mRNA-1273 SARS-CoV-2 vaccine in KTRs who were already seropositive at baseline because of previous exposure to SARS-CoV-2.
All KTRs (n = 41) tested positive for anti-SARS-CoV-2 antibodies on the day of vaccination. Of them, 11 were diagnosed by chance based on the results of serology testing. For the remaining 30 patients, the median interval between infection and vaccination was 306 days (interquartile range [IQR]: 171–316 days). The serological response was assessed after a median of 28 days (IQR: 28–31 days) from vaccination using the ARCHITECT IgG II Quant test (Abbott). Titers >50 arbitrary units (AUs)/mL were considered positive (detection range: 6.8–80000 AUs/mL). This assay has been reported to correlate with in vitro neutralization of SARS-CoV-2.1 The study protocol was approved by the local Ethics Committee (approval DC-2013–1990), and written informed consent was obtained.
KTRs were mainly men (74%) with a median age of 59 years (IQR: 51–66 years). Maintenance immunosuppression was based on the use of calcineurin inhibitors (95%), antimetabolites (86%), and steroids (57%). According to the WHO classification, 22 patients were asymptomatic or had mild disease and were managed at home. Nineteen patients were hospitalized (13 with moderate COVID-19 and six with critical/severe disease). Immunosuppression was reduced in 20 patients during the course of COVID-19. However, it was subsequently reintroduced and all of the participants were regularly receiving their immunosuppressive therapy at the time of vaccination.
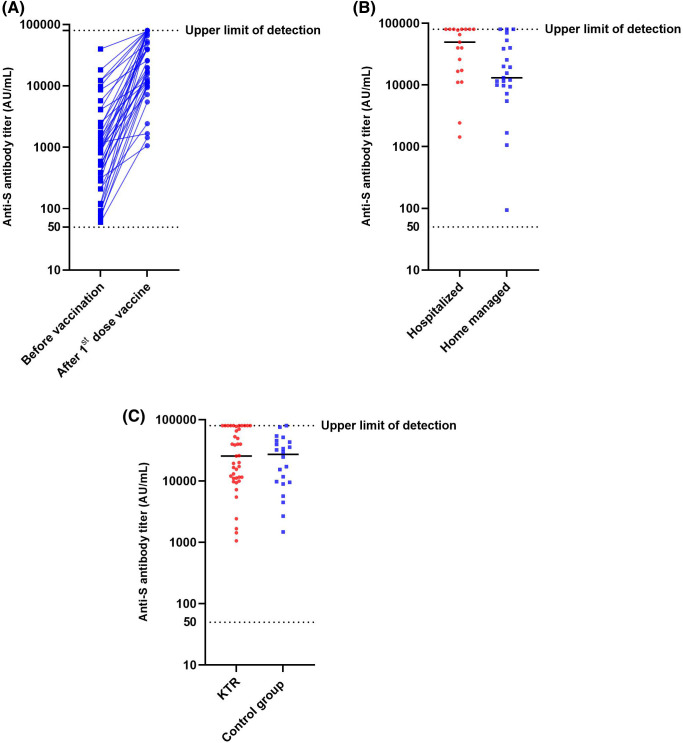
The median baseline antibody titer was 842 AUs/mL (IQR: 249–2234 AUs/mL), with a significant postvaccination increase being evident (median antibody titer: 22801 AUs/mL; IQR: 10768–78339 AUs/mL, p < 0.0001; Figure 1A). After vaccination, KTRs who required hospitalization (n = 19) had a higher antibody titer compared with those who were managed at home (n = 22; median antibody titer: 49531 AUs/mL vs. 14334 AUs/mL, respectively, p = 0.04, Figure 1B). However, no significant differences in prevaccination antibody titers were observed between the two groups (median antibody titer: 842 vs. 958 AUs/mL, respectively, p = 0.7). For comparison purposes, we also analyzed the antibody response of 22 health-care workers with a history of COVID-19 who received either the BNT162b2 (n = 19) or the mRNA-1273 vaccine (n = 3). This group mainly consisted of women (75%) with a median age of 47 years (IQR: 35–57 years). The antibody titer of health-care workers after a median of 19 days (IQR: 21–28 days) from vaccination did not differ significantly (median antibody titer: 27191 AUs/mL; IQR: 9370–42729 AUs/mL, p = 0.34, Figure 1C) from that observed in KTRs.
FIGURE 1.
(A) Kinetics of anti-Spike IgG antibody titers before and after one dose of the mRNA-1273 SARS-CoV-2 vaccine in 41 kidney transplant recipients (KTRs) who were already seropositive before vaccination. The dotted line (50 AUs/mL) denotes the cutoff for positivity. The blue lines indicate the antibody titers observed in each KTR. (B) Anti-Spike IgG antibody titers after one dose of the mRNA-1273 SARS-CoV-2 vaccine in hospitalized (n = 19; red color) and home managed KTRs (n = 22; blue color). The black lines denote the median antibody titers observed in the two groups. Antibody titer was significantly higher in hospitalized patients (median antibody titer: 49531 AUs/mL vs. 14334 AUs/mL, respectively, p = 0.04). (C) Anti-Spike IgG antibody titers after one dose of the mRNA-1273 SARS-CoV-2 vaccine in KTRs (n = 41; red color) and health-care workers (n = 22; blue color). The black lines denote the median antibody titers observed in the two groups. The antibody titer of health-care workers and KTRs did not differ significantly (median antibody titer: 27191 AUs/mL vs. 22801 AUs/mL, p = 0.34) [Color figure can be viewed at wileyonlinelibrary.com]
Despite their immunosuppression burden, KTRs with previous exposure to SARS-CoV-2 showed a marked increase in antibody titers even after a single-dose vaccine. Notably, the postvaccination antibody titers observed in these immunosuppressed patients were similar to those of immunocompetent subjects. A preexisting B cell memory in recovered individuals is likely to play a key role in this phenomenon2—which suggests that the immune response of KTRs to SARS-CoV-2 is similar to that observed in immunocompetent individuals. This response is strikingly different from that described for SARS-CoV-2-naïve KTRs who received SARS-CoV-2 mRNA-based vaccines.3, 4, 5 An “antigen dose” phenomenon may account for these discrepancies.
Acknowledgments
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr Caillard reports personal fees and nonfinancial support from Novartis, nonfinancial support from Sanofi, and nonfinancial support from Astellas, unrelated to the current study. The other authors have no conflict of interest to disclose.
DATA AVAILABILITY STATEMENT
Data supporting the findings from this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. The Lancet. 2021;397(10280):1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58):eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benotmane I, Gautier-Vargas G, Cognard N, et al. Weak anti–SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99(6):1487–1489. doi: 10.1016/j.kint.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings from this study are available from the corresponding author upon reasonable request.