Abstract
Background
Several new variants of SARS‐CoV‐2 have emerged since fall 2020 which have multiple mutations in the receptor‐binding domain (RBD) of the spike protein. It is unclear which mutations affect receptor affinity versus immune recognition.
Methods
We produced wild type RBD, RBD with single mutations (E484K, K417N, or N501Y) or with all three mutations combined and tested their binding to ACE2 by biolayer interferometry (BLI). The ability of convalescent sera to recognize RBDs and block their interaction with ACE2 was tested as well.
Results
We demonstrated that single mutation N501Y increased binding affinity to ACE2 but did not strongly affect its recognition by convalescent sera. In contrast, single mutation E484K had almost no impact on the binding kinetics, but essentially abolished recognition of RBD by convalescent sera. Interestingly, combining mutations E484K, K417N, and N501Y resulted in a RBD with both features: enhanced receptor binding and abolished immune recognition.
Conclusions
Our data demonstrate that single mutations either affect receptor affinity or immune recognition while triple mutant RBDs combine both features.
Keywords: ACE2, affinity, neutralization, RBD, SARS‐CoV‐2
In this study, we tested RBD of the spike protein with single mutations or with all three mutations combinations for binding to sera from naïve donors and convalescent COVID‐19 patients. Convalescent sera recognize RBD of the early SARS‐CoV‐2 emerged from Wuhan, China. Single mutations (E484K or N501Y) either affect receptor affinity to ACE2 or immune recognition of RBD by convalescent sera. Combining three‐point mutations (K417N, E484K, and N501Y) in RBD increased binding to ACE2 and abolished recognition of RBD by sera of convalescent patients.
Abbreviations: ACE2, angiotensin‐converting enzyme 2; B.1.1.7., SARS‐CoV‐2 UK variant; B.1.351, SARS‐CoV‐2 Japan/Brazil variant; B.1.6.1.7, SARS‐CoV‐2 India variant; COVID‐19, coronavirus disease 2019; P.1, SARS‐CoV‐2 South Africa variant; RBD, receptor‐binding domain; RBDWT, RBD wild type; RBDTRIP, RBD N501Y, E484K, K417N mutations; RBDN501Y, RBD N501Y mutation; RBDE484K, RBD E484K mutation; RBDK417N, RBD K417N mutation; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; WT, wild type.

1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has caused a pandemic that is characterized in many countries by several waves of infection. 1 , 2 While the origin of these infection waves may differ in different regions of the world, the latest increase seen in numbers of infected individual is apparently caused by the occurrence of mutated viral strains. 3 Recent advances in genome sequencing have allowed to establish nucleotide databases of SARS‐CoV‐2 genome in real time (https://www.ncbi.nlm.nih.gov/sars‐cov‐2) and to identify mutations of the different SARS‐CoV‐2 isolates. 4 The most prominent mutated strains are the following variants: B.1.1.7. (UK variant), P.1 (Japan/Brazil variant), and B.1.351 (South Africa variant) and the newly emerging India variant, B.1.6.1.7, 5 , 6 which show mutations in the receptor‐binding domain (RBD) and receptor‐binding motif (RBM) of the spike (S) glycoprotein (Table 1, Figure 1). RBD and in particular RBM are responsible for interaction with the cellular receptor ACE2 and are the primary target of neutralizing antibodies 7 (Figure 1). Mutations altering the RBD conformation have been shown to permit SARS‐CoV‐2 to escape antibody neutralization and for the rapid infectivity and transmission of SARS‐CoV‐2. 8 Mutant viruses may spread more efficiently because they show increased affinity for the receptor or because they escape neutralizing antibody responses. 9 The importance of receptor affinity has been illustrated by SARS‐CoV‐1, which showed a fourfold lower affinity for ACE2 compared to SARS‐CoV‐2 and also was much less contagious and showed strongly reduced transmission than SARS‐CoV‐2. 10
TABLE 1.
Characteristics of the main SARS‐CoV‐2 mutants
| Name | Origin | Spike mutations a | |
|---|---|---|---|
| Location | Date | ||
| B.1.1.7 | United Kingdom (UK) | February 2020 |
7 mutations: N501Y , A570D, D614G, P681H, T716I, S982A, D1118H 2 deletions: H69‐V70del, Y144del |
| B.1.351 | South Africa | October 2020 |
9 mutations: L18F, D80A, D215G, R246I, K417N, E484K, N501Y , D614G, A701V 1 deletion: LAL 242–244 del |
| P.1 | Japan/Brazil | January 2021 | 12 mutations: L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I, V1176F |
| B.1.6.1.7 | India | January 2021 | 6 mutations: D111D, G412D, L452R, E484Q , D614G, H655Y, P681R |
In bold the mutations in the receptor‐binding domain (RBD).
FIGURE 1.
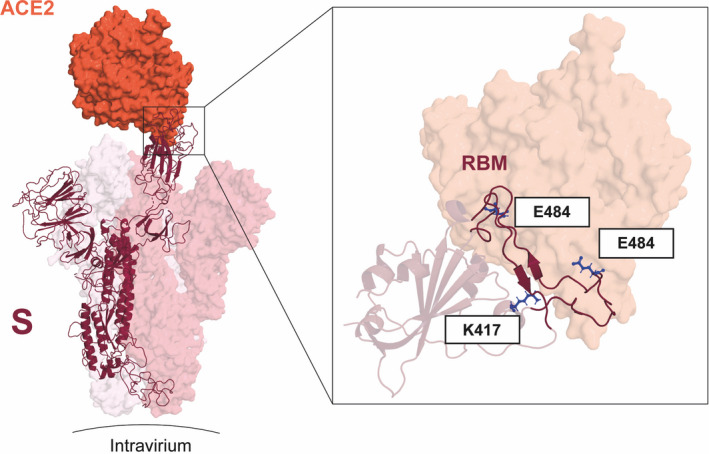
Key mutations of variants B.1.351 and P1 fall on the interface between the RBD and ACE2. (left) S monomer (purple ribbon) bound to ACE2 ectodomain (orange surface). In detail, the positions of residues K417, E484, and N501 (blue sticks) are highlighted. Mutations E484K and N501K occur on the RBM segments (dark purple ribbon), while K417N occurs on helix α4 of RBD. From PDB files 6ACG and 6M0J
Viruses that escape neutralization are typically called serotypes and usually may only occur when a large proportion of individuals show antibody‐based immunity against the original strain and further spread may only be possible by escape of neutralizing antibody responses. 11 For SARS‐CoV‐2, it remains to be shown definitively whether or not some variants are new serotypes; however, on a global scale, the number of the mutations present in the main variants and their infection rates in certain regions of the world with high previous infection rates are compatible with serotype formation.
Here we assessed the molecular basis for antibody escape and how the RBD mutations present in two variants of concern (B.1.1.7 and P.1) influence the affinity to the receptor.
2. MATERIAL AND METHODS
2.1. Protein expression and purification
The SARS‐CoV‐2 receptor‐binding domain of the wild‐type RBD (RBDWT), the single RBD mutants (RBDK417N, RBDE484K, and RBDN501Y,), and the triple RBD mutant (RBDTRIP) were expressed using Expi293F cells (Invitrogen, ThermoFisher Scientific). The genes that encode SARS‐CoV‐2 RBDWT (residues Arg319‐Phe541) or RBD mutants with a C‐terminal 6‐His‐tag were inserted into pTwist CMV BetaGlobin WPRE Neo vector (Twist Bioscience). The construct plasmids were transfected into Expi293F cells using ExpiFectatmine 293 transfection kit (Gibco, ThermoFisher Scientific). The supernatant of cell culture containing the secreted RBDs was purified using His‐Trap HP column (GE Healthcare). Collected RBDWT or RBD mutated proteins were equilibrated in PBS and kept at −20°C. ACE2‐mFc was purchased from Sino Biological. Biotinylated and non‐biotinylated soluble human ACE2 fused to mouse IgG2a Fc proteins were kindly provided by PD Dr. Alexander Eggel (University Clinic of Rheumatology and Immunology, Inselspital) who received the plasmid from Prof. Peter Kim (Stanford University).
2.2. Human sera
Human sera were obtained from 11 COVID‐19 convalescent patients which were recruited at the University Hospital of Bern, Bern, Switzerland as described. 12 Participants were recruited via three different routes: (a) inpatients with a SARS‐CoV‐2 test result (real‐time PCR; RT‐PCR), (b) medical personnel of the Inselspital, and (c) residual material from patients stored at the Liquid Biobank Bern (www.biobankbern.ch). Inclusion criteria of inpatients are (a) hospitalization in Inselspital, (b) tested positive for SARS‐CoV‐2 using RT‐PCR (nasopharyngeal swab), (c) aged 18 or older, and (d) signed general consent.
2.3. ELISA assay
Corning half area 96‐well plates were coated with 1 μg/ml RBDWT or mutated RBDs in PBS overnight at 4°C and then blocked with PBS/0.15% casein. Convalescent human sera were added, serially diluted 1:3, and incubated on plates for 1 h at room temperature. Bound IgG antibodies were detected with goat anti‐human IgG‐POX antibody (Nordic MUbio). ELISA was developed with tetramethylbenzidine (TMB), stopped by adding equal 1 M H2SO4 solution, and read at OD450nm. Results are shown as endpoint titers (EPT) which were calculated as the maximum dilution factors for which 450‐nm absorbance was no less than 0.15 AU, the background baseline.
2.4. RBDwt and RBDmut kinetics by bio‐layer interferometry
The analysis of binding kinetics of RBDWT and RBDTRIP to ACE2‐mFc was performed by BioLayer Interferometry (BLI) using an Octet RED96e (Fortebio) instrument. High precision Streptavidin (SAX, ForteBio) biosensors were saturated with 7.5 μg/ml biotinylated ACE2‐mFc in BLI assay buffer (PBS, 0.1% BSA, 0.02% Tween 20) for 10 min. RBDWT and RBDTRIP were prepared as twofold serial dilution (typically 50, 25, 12.5, 6.25, and 3.125 nM) in BLI assay buffer plus buffer blanks. Kinetic values were calculated by ForteBio data analysis software using a 1:1 binding model.
2.5. Bio‐layer interferometry ‐based competitive assay
The ability of the sera of the COVID convalescent patient to compete with ACE2 for binding to RBDWT and RBDTRIP was tested in a sandwich format assay on the Octet RED96e (Fortebio). Anti‐penta‐His (HIS1K) biosensors were loaded for 10 min with RBDWT and RBDTRIP at a concentration of 7.5 μg/ml in BLI assay buffer followed by addition of samples (diluted 1:20 in BLI assay buffer) from convalescent human sera. To assess whether the sera can inhibit the binding of ACE2 to RBDWT and RBDTRIP, ACE2‐mFc (50 nM) was added to biosensor. For control two additional sensors with BLI buffer were used, one for baseline and one without serum sample to determine binding of ACE2‐mFc alone. The results are expressed of single individual. The response data were normalized using ForteBio data analysis software version1.2.0.1.55.
2.6. Data and statistical analysis
All statistical tests were performed using GraphPad PRISM 8.0 (GraphPad Software, Inc.). ELISA data in graphs are displayed as endpoint titers measured at a cutoff 0.15 OD 450 nm. Comparison between RBDWT and mutated RBDs were analyzed by two‐way ANOVA test for ELISA and paired two‐tailed Student's t‐test for BLI assay. α = 0.05 and statistical significance are displayed as p ≤ .05 (*), p ≤ .01 (**), p ≤ .005 (***), and p ≤ .001 (****).
3. RESULTS
3.1. Structural model and binding kinetics of SARS‐CoV‐2 RBD variants to ACE2
To address the questions of antibody binding strength and competition mechanistically, we have expressed the RBD of the early SARS‐CoV‐2 that emerged in Wuhan, China (RBDWT) which serves as positive control. In parallel, we have produced RBD of the isolate P.1 exhibiting RBD with three‐point mutations (RBDTRIP) namely K417N, a lysine (K) to asparagine (N) at position 417, E484K, a glutamate (E) to lysine at position 484 and N501Y, an asparagine (N) to tyrosine (Y) at position 501, two of which are located in the RBM (E484K, N501Y; shown in Figure 1A). 13 In order to assess the role of each of these mutations on the binding of the RBD to ACE2, we generated single RBD mutants each containing one of the above mentioned mutations (RBDK417N, RBDE484K, and RBDN501Y). All RBDs were purified to homogeneity, and the affinity to recombinant ACE2 was determined by Biolayer Interferometry using Octet technology. 14 The BLI assays showed that the affinity of ACE2 for RBDTRIP (shown in Figure 2B,F, Table 2, KD≈10 nM) was about twice as high as for RBDWT (shown in Figure 2A,F, Table 2, KD = 20.5 nM). The affinity of the SARS‐CoV‐2 for ACE2 has been reported to be only fourfold higher compared to SARS‐CoV‐1; thus, a difference of two reported here between RBDWT and RBDTrip is expected to be biologically significant and most likely reflects enhanced infectivity. In contrast, the introduction of a single E484K mutation in the RBD (RBDE484K) did not affect receptor affinity (shown in Figure 2D,F). For comparison, the affinity observed for RBDN501Y was threefold lower (KD≈6 nM, shown in Figure 2C,F, Table 2). Interestingly, K417N mutation in the single RBD mutant (RBDK417N) resulted in completely altered binding properties (shown in Figure 2E). RBDK417N showed much lower association rates and plateau levels and a non‐monovalent pattern of dissociation rates (shown in Figure 2E, Table 2 KD could not be determined in a meaningful way). Presence of aggregates was not responsible for this effect, as purification by size exclusion immediately before measurements did not alter the binding kinetics observed (data not shown). However, since K417N is not present in the RBM, we did not further investigate this effect.
FIGURE 2.
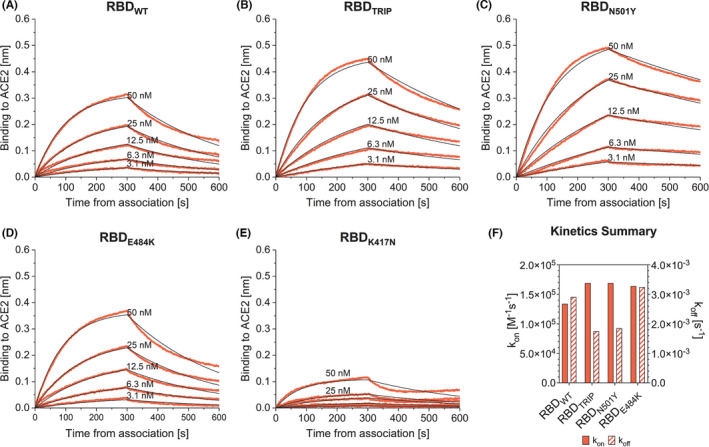
Binding kinetics of RBDWT (A), RBDTRIP (B), RBDN501Y (C), RBDE484K (D), and RBDK417N (E) to hACE2. In all assays, both association and dissociation were performed in 300 s. The resulting kon and koff values from each condition are compared in panel (F)
TABLE 2.
Kinetic parameters of RBDWT and mutated RBDs calculated by BLI
| Analyte | KD [M] | kon [M−1s−1] | koff [s−1] |
|---|---|---|---|
| RBDWT | 20.5 × 10−9 | 1.34 × 105 | 2.91 × 10−3 |
| RBDTRIP | 10.3 × 10−9 | 1.69 × 105 | 1.75 × 10−3 |
| RBDN501Y | 6.2 × 10−9 | 1.69 × 105 | 1.85 × 10−3 |
| RBDE484K | 19.7 × 10−9 | 1.64 × 105 | 3.24 × 10−3 |
| RBDK417N | ND | ND | ND |
Abbreviations: ND, not determined; RBDE484K, Receptor‐Binding Domain E484K mutation; RBDK417N, Receptor‐Binding Domain K417N mutation; RBDN501Y, Receptor‐Binding Domain N501Y mutation; RBDTRIP, Receptor‐Binding Domain N501Y, E484K, K417N mutations; RBDWT, Receptor‐Binding Domain wild type.
3.2. Reduced ability of convalescent sera to recognize RDB variants
To determine whether RBDWT‐specific immune sera might have a reduced ability to bind to mutated RBDs we performed ELISA and Biolayer Interferometry using sera from convalescent patients (shown in Figure 3). As expected RBDWT was well recognized by convalescent sera in ELISA experiments. In contrast, RBDK417N and RBDN501Y were recognized in a slightly impaired fashion (shown in Figure 3A). In marked contrast, mutation at position 484 essentially abolished recognition of both RBDE484K and RBDTRIP. Corresponding results were obtained using Biolayer Interferometry (shown in Figure 3B). RBD‐specific neutralizing antibodies typically block interaction of RBD with the viral receptor ACE2. We therefore assessed whether reduced binding of convalescent sera to RBDTRIP was paralleled by reduced ability of these antibodies to block binding of ACE2 to the triple mutant (shown in Figure 3C). These experiments demonstrate that human convalescent sera essentially failed to block binding of ACE2 to RBDTRIP, explaining why SARS‐CoV‐2‐induced antibodies largely fail to neutralize the triple mutant variants.
FIGURE 3.
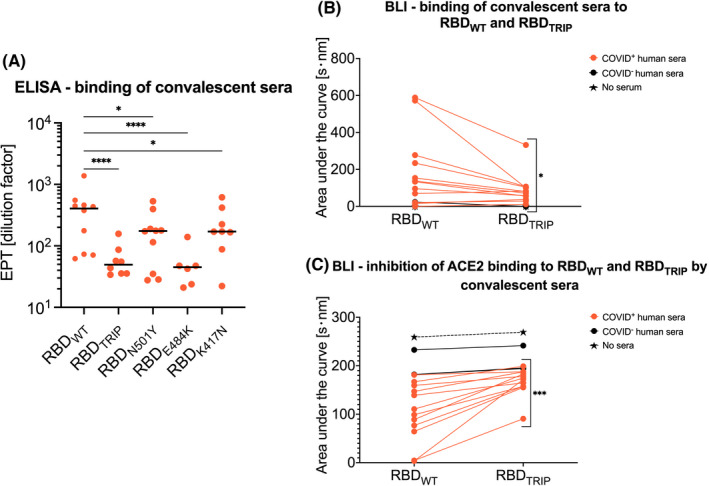
Sera of COVID‐19 convalescent patients recognize less mutated RBD (RBDTRIP, RBDN501Y, RBDE484K, and RBDK417N). A) Binding of sera from 12 COVID‐19 convalescent patients and one COVID negative individual to RBDWT and mutated RBDs was determined by ELISA. Endpoint titers (EPT; dilution factor) individuals are shown as dots. Direct binding of sera to RBDWT and mutated RBDs (B) and competitive inhibition of ACE2‐mFc interaction to RBDWT and mutated RBD (C) were assessed by BLI. The same sera (dilution 1:20) for each individual were usual. p ≤ .05 (*), p ≤ .01 (**), p ≤ .005 (***), and p ≤ .001 (****)
4. DISCUSSION
The newly emerging mutant RBDs may affect the affinity for the viral receptor. In addition, such mutations at the virus‐receptor interaction face may alter the ability of RBD‐specific antibodies—induced by previous infection—to neutralize the mutant viruses. When we investigated whether distinct mutations may affect receptor affinity, we found that N501Y mutation enhanced affinity for the viral receptor ACE2 both as a single mutation and as a triple mutation, while E484K mutation alone did not affect the interaction with ACE2.
In addition, such mutations at the virus‐receptor interaction interface may alter the ability of RBD‐specific antibodies to recognize and neutralize the mutant variants. 15 A previous study has shown that serum neutralization is not compromised by N501Y (also found in the strain B.1.1.7). 16 In contrast, E484K (found B.1.1.7 and in P.1 strains) was associated with reduced neutralization by monoclonal antibodies and reduced recognition as shown here. 17 , 18 , 19 Interestingly, studies applying in vitro pressure produced similar mutations as those that occurred naturally. 20 In this study, we showed that convalescent sera have reduced ability to recognize RDBTRIP variants explaining why the mutant SARS‐CoV‐2 strain P.1 is more infectious 21 and less susceptible to neutralization by antibodies induced with RBDWT. 22
In summary, our data demonstrate that distinct mutations may affect receptor affinity which likely affects viral infectivity versus recognition by convalescent sera which likely affects neutralization. These observations may shed light on the potential origin of the viral mutants. The variant with the mutation N501Y shows enhanced affinity but almost normal recognition by convalescent antibodies. This indicates that this variant spread largely by increased infectivity while recognition by antibodies of previously infected individuals was less relevant, a phenotype consistent with the epidemiology in the UK, where overall infection rates remain relatively low, rendering the previously infected individuals a relatively unimportant source of viral spread. 23 In contrast, the triple mutant variant shows enhanced infectivity and escape from antibody recognition. It may therefore not be a coincidence that this variant originated in Manaus, a region in Brazil, previously seen to have seroprevalence of >80%, forcing the virus to escape immunity for further spreading. 24
CONFLICT OF INTEREST
M. F. Bachmann is a board member of Saiba AG, involved in the development of RBD‐CuMV, a vaccine against COVID‐19. All other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
M.V., G.A., and X.C. designed, performed, and interpreted most experiments. X.L. M.M. provided serum sample. M.V., G.A. and D.S. wrote the manuscript. MF.B. designed experiments and wrote the manuscript.
ACKNOWLEDGEMENTS
We thank Marianne Zwicker for production of wild‐type and mutant RBDs. We thank PD Dr. Alexander Eggel and Dr. Daniel Brigger for providing biotinylated and non‐biotinylated ACE2‐mFc. Open Access Funding provided by Universitat Bern.
Vogel M, Augusto G, Chang X, et al. Molecular definition of severe acute respiratory syndrome coronavirus 2 receptor‐binding domain mutations: Receptor affinity versus neutralization of receptor interaction. Allergy. 2022;77:143–149. 10.1111/all.15002
Monique Vogel and Gilles Augusto equally contributed to this work.
Funding information
We thank Saiba AG, the Swiss National Science Foundation (SNF grant 31003A_185114) and the International Immunology Centre, Anhui Agricultural University, Hefei, China for financial support
Contributor Information
Xinyue Chang, Email: xinyue.chang@dbmr.unibe.ch.
Martin F. Bachmann, Email: martin.bachmann@dbmr.unibe.ch.
REFERENCES
- 1. Choudhary S, Sreenivasulu K, Mitra P, Misra S, Sharma P. Role of genetic variants and gene expression in the susceptibility and severity of COVID‐19. Ann Lab Med. 2021;41(2):129‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bontempi E. The europe second wave of COVID‐19 infection and the Italy "strange" situation. Environ Res. 2021;193:110476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bakhshandeh B, Sorboni SG, Javanmard AR, et al. Variants in ACE2; potential influences on virus infection and COVID‐19 severity. Infect Genet Evol. 2021;90:104773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moustafa AM, Bianco C, Denu L, et al. Comparative analysis of emerging B.1.1.7+E484K SARS‐CoV‐2 isolates from Pennsylvania. bioRxiv. 2021. 10.1101/2021.04.21.440801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomez CE, Perdiguero B, Esteban M. Emerging SARS‐CoV‐2 variants and impact in global vaccination programs against SARS‐CoV‐2/COVID‐19. Vaccines 2021;9(3):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao S, Lou J, Cao L, et al. Quantifying the transmission advantage associated with N501Y substitution of SARS‐CoV‐2 in the United Kingdom: an early data‐driven analysis. J Travel Med. 2021;28(2):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yi C, Sun X, Ye J, et al. Key residues of the receptor binding motif in the spike protein of SARS‐CoV‐2 that interact with ACE2 and neutralizing antibodies. Cell Mol Immunol. 2020;17(6):621‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim JS, Jang JH, Kim JM, Chung YS, Yoo CK, Han MG. Genome‐wide identification and characterization of point mutations in the SARS‐CoV‐2 genome. Osong Public Health Res Perspect. 2020;11(3):101‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobson KB, Pinsky BA, Rath MEM, et al. Post‐vaccination SARS‐CoV‐2 infections and incidence of the B.1.427/B.1.429 variant among healthcare personnel at a northern California academic medical center. medRxiv. 2021(Apr 24). 10.1101/2021.04.14.21255431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS‐CoV‐2 spike protein: potential antivirus drug development for COVID‐19. Acta Pharmacol Sin. 2020;41(9):1141‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bachmann MF, Zinkernagel RM. The influence of virus structure on antibody responses and virus serotype formation. Immunol Today. 1996;17(12):553‐558. [DOI] [PubMed] [Google Scholar]
- 12. Brigger D, Horn MP, Pennington LF, et al. Accuracy of serological testing for SARS‐CoV‐2 antibodies: first results of a large mixed‐method evaluation study. Allergy. 2021;76(3):853‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faria NR, Mellan TA, Whittaker C, et al. Genomics and epidemiology of a novel SARS‐CoV‐2 lineage in Manaus, Brazil. Science. 2021;372(6544):815–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen RL. Strategies using bio‐layer interferometry biosensor technology for vaccine research and development. Biosensors. 2017;7(4):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smaoui MR, Yahyaoui H. Unraveling the stability landscape of mutations in the SARS‐CoV‐2 receptor‐binding domain. Sci Rep. 2021;11(1):9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie X, Liu Y, Liu J, et al. Neutralization of SARS‐CoV‐2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine‐elicited sera. Nat Med. 2021;27:620–621. [DOI] [PubMed] [Google Scholar]
- 17. Wibmer CK, Ayres F, Hermanus T, et al. SARS‐CoV‐2 501Y.V2 escapes neutralization by South African COVID‐19 donor plasma. Nat Med. 2021;27(4):622‐625. [DOI] [PubMed] [Google Scholar]
- 18. Greaney AJ, Starr TN, Gilchuk P, et al. Complete mapping of mutations to the SARS‐CoV‐2 spike receptor‐binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44‐57. e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jangra S, Ye C, Rathnasinghe R, et al. The E484K mutation in the SARS‐CoV‐2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post‐vaccination sera. medRxiv. 2021(Jan 29). 10.1101/2021.01.26.21250543 [DOI] [Google Scholar]
- 20. Andreano E, Piccini G, Licastro D, et al. SARS‐CoV‐2 escape in vitro from a highly neutralizing COVID‐19 convalescent plasma. bioRxiv. 2020(Dec 28). 10.1101/2020.12.28.424451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corum J, Zimmer C. Coronavirus variants and mutations. The New York Times. June 4, 2021.
- 22. Choudhary MC, Crain CR, Qiu X, Hanage W, Li JZ. SARS‐CoV‐2 sequence characteristics of COVID‐19 persistence and reinfection. Clin Infect Dis. 2021(Apr 27). 10.1093/cid/ciab380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. BBC news . Coronavirus infection levels continue to drop in the UK. March 5, 2021.
- 24. Candido DS, Claro IM, de Jesus JG, et al. Evolution and epidemic spread of SARS‐CoV‐2 in Brazil. Science. 2020;369(6508):1255‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]


