To the Editor:
A weak humoral response to two-doses of SARS-CoV-2 vaccine was observed in solid organ transplant (SOT) patients.1 , 2 Preliminary reports suggested the usefulness of a boost with a third dose.3 , 4 Herein, we report the humoral response in 396 SOT patients (mean age 59 ± 15 years, 65% men) who were given three doses messenger RNA-based vaccine (BNT162b2 vaccine [Pfizer-BioNTech]) (Table S1). Of these, 101 were included in our previous report.3 The two first doses were given one month apart, and the third dose was administered 59 (IQR25-75: 47–67) days after the second dose, that is, once the third dose was recommended by the French National Authority for Health. Anti-SARS-CoV-2 spike protein total antibodies were assessed the day of vaccination before the injection using either the Wantai enzyme-linked immunosorbent assay test (Beijing Wantai Biological Pharmacy Enterprise) (228 patients, 57.6%),5 or another anti-SARS-CoV-2 spike protein assay (n = 168) (Table S2). According to French law (loi Jardé), anonymous retrospective studies do not require institutional review board approval.
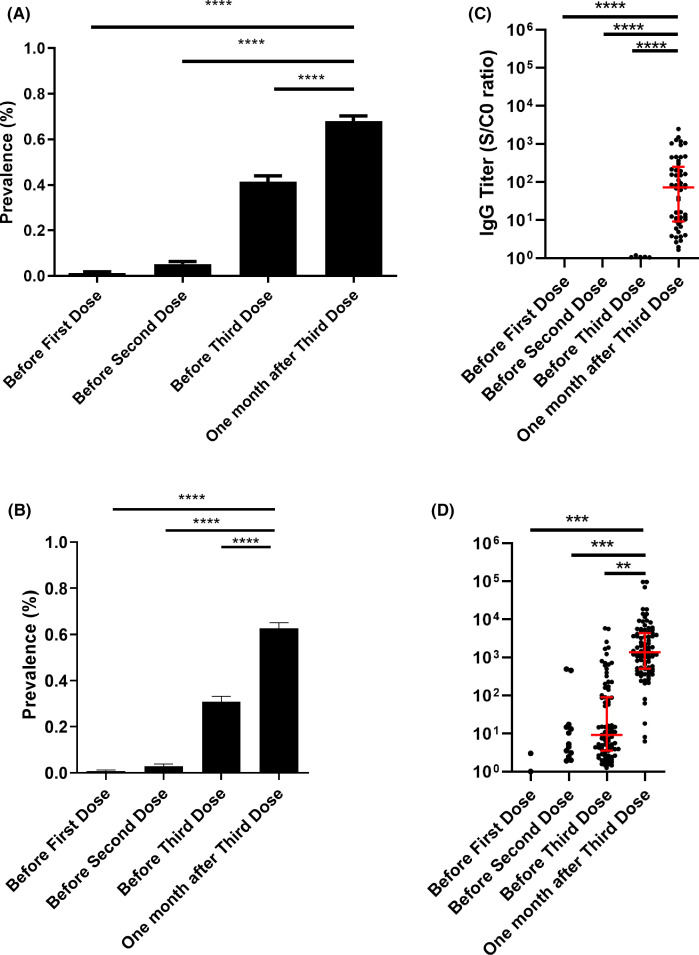
The prevalence of anti-SARS-CoV-2 antibodies was 1.3% (95% CI, 0.2% to 2.4%; n = 5) before the first injection, 5.1% (95% CI, 3.0% to 7.4%; n = 20) before the second one, 41.4% (95% CI, 36.5% to 46.3%; n = 164) before the third one, and 67.9% (95% CI, 63.3% to 72.6%; n = 269) 4 weeks after the third dose, p < .0001 ( Figure 1A). Among the 232 patients who were seronegative before the third dose, 105 (45.25%) turned positive. All patients who were seropositive before the third dose were still seropositive 4 weeks later.
FIGURE 1.
(A) Anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies before and after vaccination in patients tested with different assays. Results are expressed as means ± SEM. ****p < .0001. (B) Anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies before and after vaccination in patients tested with the Wantai enzyme-linked immunosorbent assay test. Results are expressed as means ± SEM. ****p < .0001. (C) Anti-SARS-CoV-2 antibodies titers before and after vaccination in patients who were tested with the Wantai test and who had no detectable antibodies titers before the third dose. A positive test using the Wantai assay was defined by a signal-to-cut-off ratio (S/CO) >1.1. ****p < .0001. (D) Anti-SARS-CoV-2 antibody titers before and after vaccination in patients who were tested with the Wantai test and who had detectable antibodies titers before the third dose. Results are expressed as means ± SD. A positive test using the Wantai assay was defined by a S/CO >1.1. ***p = .004. **p = .017 [Color figure can be viewed at wileyonlinelibrary.com]
By means of multivariate analysis (Table S3), younger patients (OR = 0.95, 95% CI [0.93–0.97], p < .0001) had a higher seroconversion rate, whereas patients receiving mycophenolic acid (OR = 0.28, 95% CI [0.14–0.54], p = .0002) or belatacept (OR = 0.14, 95% CI [0.43–0.46], p = .001), and patients that received at least a triple immunosuppression (OR = 0.42, 95% CI [0.21–0.86], p = .02) presented a lower seroconversion rate. After three doses, the seroconversion rate remained low in patients given belatacept with (5/16 [31%]) or without (3/9 [33%]) mycophenolic acid.
In the subgroup of patients tested with the Wantai test (n = 228), the seroconversion rate increased from 43.4% before the third dose to 69.3% one month later (Figure 1B). In this homogenous subgroup, antibodies titers were assessed and dramatically increased in patients who were seronegative (Figure 1C) or seropositive (Figure 1D) before the third dose. The increase and median titers were higher in seropositive than seronegative patients.
No serious adverse event or acute rejection episode was observed after the administration of the third dose.
In conclusion, this study confirmed that giving a third vaccine dose to SOT patients increases the humoral response and antibodies titers. Assessing the neutralizing antibodies’ prevalence and for how long antibodies will persist in SOT are required. Analysis of cell-mediated immunity is also necessary to comprehensively assess the increase on vaccine immunogenicity. Meanwhile, barriers measures should be maintained.
ACKNOWLEDGMENTS
We thank “La cellule de vaccination du CHU de Toulouse” and the nurses who vaccinated and performed the biological monitoring of all patients, as well as Mrs Célia Benzema and Marie Mattera who collected the data.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913–2915. doi: 10.1111/ajt.16607. https://orcid.org/0000-0002-5920-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marion O, Del "Bello A, Abravanel F, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021; [published online ahead of print May 25, 2021]. https://doi.org/10.7326/M21-1341 [DOI] [PMC free article] [PubMed]
- 3.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. http://www.ncbi.nlm.nih.gov/pubmed/34161700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021; [published online ahead of print June 15, 2021]. https://doi.org/10.7326/L21-0282 [DOI] [PMC free article] [PubMed]
- 5.Abravanel F, Miédouge M, Chapuy-Regaud S, Mansuy JM, Izopet J. Clinical performance of a rapid test compared to a microplate test to detect total anti SARS-CoV-2 antibodies directed to the spike protein. J Clin Virol. 2020;130:104528. doi: 10.1016/j.jcv.2020.104528. https://pubmed.ncbi.nlm.nih.gov/32771904/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material